Abstract
Tauopathies are a group of disorders in which the cytosolic protein tau aggregates and accumulates in cells within the brain, resulting in neurodegeneration. A promising treatment being explored for tauopathies is passive immunization with anti-tau antibodies. We previously found that administration of an anti-tau antibody to human tau transgenic mice increased the concentration of plasma tau. We further explored the effects of administering an anti-tau antibody on plasma tau. After peripheral administration of an anti-tau antibody to human patients with tauopathy and to mice expressing human tau in the central nervous system, there was a dose-dependent increase in plasma tau. In mouse plasma, we found that tau had a short half-life of 8 min that increased to more than 3 hours after administration of anti-tau antibody. As tau transgenic mice accumulated insoluble tau in the brain, brain soluble and interstitial fluid tau decreased. Administration of anti-tau antibody to tau transgenic mice that had decreased brain soluble tau and interstitial fluid tau resulted in an increase in plasma tau, but this increase was less than that observed in tau transgenic mice without these brain changes. Tau transgenic mice subjected to acute neuronal injury using 3-nitropropionic acid showed increased interstitial fluid tau and plasma tau. These data suggest that peripheral administration of an anti-tau antibody results in increased plasma tau, which correlates with the concentration of extracellular and soluble tau in the brain.
Introduction
Tauopathies including Alzheimer disease (AD), Down syndrome dementia, progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), Pick disease, certain forms of frontotemporal lobar degeneration (FTLD), and chronic traumatic encephalopathy (CTE) are a group of neurodegenerative disorders characterized by the hyperphosphorylation and aggregation of the protein tau in the central nervous system (CNS) (1). Tau is a cytosolic microtubule-associated protein that under normal conditions is localized primarily in neuronal axons (1–3). Its aggregation in specific brain regions in different tauopathies is directly associated with neurodegeneration in those brain regions (4–7). Emerging evidence suggests that once tau aggregates in the specific brain regions that are characteristic for each tauopathy, pathological forms of tau then spread through synaptically connected neural networks (8, 9).
As tauopathies are currently untreatable, a variety of approaches have been taken attempting to target tau therapeutically. Despite the fact that tau is predominantly a cytosolic protein, it is normally released into the extracellular space from neural cells and its release is regulated by excitatory neuronal activity (10, 11). A number of studies in animal models have shown that both active and passive immunization targeting tau can have beneficial effects on reducing tau pathology and improving function (12–19). One mechanism that may account for these effects is via the ability of antibodies to block the intercellular spread of tau pathology. In recent studies, we found that a mouse monoclonal anti-tau antibody, HJ8.5, was able to block the cellular seeding activity of externally applied tau aggregates as well as decrease insoluble tau in the brain and improve function (12). In exploring the potential mechanisms by which this anti-tau antibody might exert its effects, we found that peripheral administration of HJ8.5 to tau transgenic mice that express human tau predominantly in the CNS, markedly increased plasma tau (20). This suggested that further exploration of the effects of anti-tau antibodies and plasma tau might provide insight into tau metabolism in the CNS.
To further determine both the origin of plasma tau as well as the potential utility of plasma tau measurements as a marker of CNS tau, we assessed plasma tau concentrations before and after anti-tau antibody administration in a variety of mice expressing human tau as well as in human subjects with PSP. Herein, we report that tau increased in the plasma of mice expressing human tau as well as in the plasma of PSP patients following administration of an anti-tau antibody.
Results
Anti-tau antibody dose-dependently increases plasma tau in transgenic mice and human patients with tauopathy
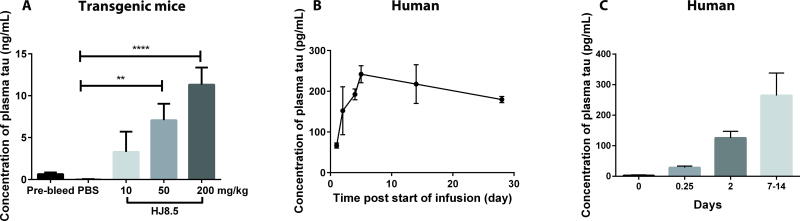
We previously assessed the effects of administering the mouse monoclonal anti-tau antibody, HJ8.5, to P301S Tau transgenic (Tg) mice that develop tau pathology in the brain beginning at ~5–6 months of age (21). HJ8.5 is a high affinity antibody specific to human tau that is directed to an epitope in the N-terminus amino acids 25–30 (12). In agreement with our previous report (20), intraperitoneal (i.p.) administration of HJ8.5 at doses ranging from 10–200 mg/kg to 3-month-old P301S Tau Tg mice resulted in a large, dose-dependent increase in plasma tau 48 hours after antibody injection (Fig. 1A). Plasma tau in Tg mice was assessed using single molecule array (Simoa) technology, a digital enzyme-linked immunosorbent assay (ELISA) detection system developed by Quanterix Inc. (22, 23). Given that HJ8.5 has strong effects on decreasing tau pathology and improving function when given chronically to P301S Tau Tg mice over months, a humanized version of HJ8.5 was developed as a potential treatment for tauopathies. It was administered to an individual with PSP as part of an expanded access treatment protocol. Several doses of the humanized antibody were administered monthly. Tau in human plasma was assessed using a plate-based human plasma tau ELISA (in contrast to the Simoa technology) both prior to dosing administration and following a dose of 7.5 mg/kg given intravenously. Consistent with peripheral administration of HJ8.5 to mice, intravenous administration of the humanized antibody to one PSP patient resulted in a robust increase in plasma tau (Fig. 1B). Baseline plasma tau in this individual was not detectable with the human plasma tau assay used (lower limit of detection, 10 pg/ml) prior to antibody administration. Following antibody administration, tau increased rapidly over 24 hours with concentrations of tau reaching ~250 pg/ml over several days (Fig. 1B). This is about 60-fold higher than the amount of tau previously reported in human plasma by others (~4 pg/ml) using a more sensitive assay (24). To confirm this observation, we also measured plasma tau in 3 additional participants receiving the same antibody in a phase 1 single dose study in subjects with PSP. These 3 individuals received a single dose of 15 mg/kg and their plasma tau was assessed (Fig. 1C). As with the first individual administered the antibody, baseline plasma tau was not detectable (< 10 pg/ml) with the human plasma tau assay utilized. However, following antibody administration, plasma tau increased to ~150–300 pg/ml after 7–14 days. Thus, consistent with our observations in Tg mice expressing human tau, plasma tau markedly increased 50–100 fold over baseline at the doses used after peripheral administration of an anti-tau antibody to humans.
Fig. 1. Dose-dependent increase in plasma tau after anti-tau antibody administration in transgenic mice and patients with PSP.
(A) Three month-old P301S Tau Tg mice were administered phosphate-buffered saline (PBS) or the anti-tau humanized antibody HJ8.5 at 10, 50 and 200 mg/kg doses (n=5 mice/group) by i.p injection. Plasma tau was measured before the injection (pre-bleed) and 48 hours after antibody injection. Plasma tau measurements were analyzed using the Simoa HD-1 Analyzer. **p≤0.01, ****p≤0.0001, one-way ANOVA followed by Dunnett’s post-hoc test for multiple comparisons. (B) Humanized anti-tau antibody HJ8.5 at a dose of 7.5 mg/kg was intravenously injected into a single individual with PSP and plasma tau was measured before (0 hour time) and after antibody injection at various time intervals (1, 2, 4, 5, 14, 28 days). (C) Humanized anti-tau antibody HJ8.5 at a dose of 15 mg/kg was intravenously injected into three human subjects with PSP and plasma tau was measured before and after antibody injection. Tau was not detected with this assay prior to antibody injection. Human plasma samples were analyzed with a human plasma tau ELISA assay. N=3 for each time point shown. Values represent mean ± SEM.
Antibody-mediated increase in plasma tau half-life enables tau detection
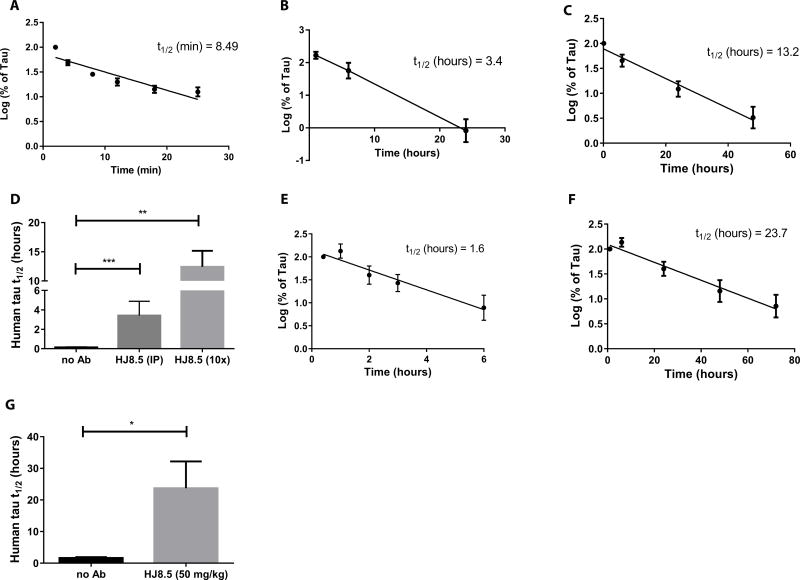
One possible mechanism by which tau antibodies could increase plasma tau is by altering the half-life of tau once it enters the plasma. To test this hypothesis, we injected recombinant human tau intravenously into the jugular vein of wild-type mice in the presence and absence of the anti-tau antibody HJ8.5 and assessed the clearance of tau from the plasma. Plasma tau was measured with the Simoa ELISA. We found that the half-life of intravenously injected human tau in plasma was 8.49 minutes (Fig. 2A, D). Administration of HJ8.5 (50 mg/kg, i.p.) one hour prior to jugular vein injection of recombinant human tau extended the half-life of tau to ~3.4 hours (Fig. 2B, D). We next co-incubated recombinant human tau with a 10-fold molar excess of HJ8.5 and administered the tau-antibody complex intravenously to wild-type mice. Co-incubation of HJ8.5 with tau increased the half-life of tau within the plasma to an even greater extent ~13.2 hours (Fig. 2C, D). These data suggested that the peripheral administration of an anti-tau antibody increased plasma tau by binding to tau and extending the half-life of tau in the plasma (Fig. 2B, C, D).
Fig. 2. Half-life of plasma tau in absence and presence of mouse anti-human tau antibody HJ8.5.
(A) Human tau was injected intravenously (i.v.) into wild-type B6C3 mice (n=6) and plasma tau was analyzed at 2, 4, 8, 12, 18 and 25 minutes (min). (B) Human tau was injected i.v. into wildtype B6C3 mice (n=9) 1 hour following i.p. administration of humanized anti-tau antibody HJ8.5 at 50 mg/kg and plasma tau was analyzed after 30 minutes, 6 hours, and 24 hours. (C) Human tau was pre-incubated for 1 hour with 10× molar excess of HJ8.5 and injected into the jugular vein of wildtype mice (n=8). Plasma was collected at 10 minutes, and 6, 24, 48, and 72 hours. (D) Half-life of plasma tau following jugular vein injection of human tau under the conditions studied in A, B, and C. (E) Human tau was injected into the cisterna magna of wildtype B6C3 mice (n=6) and plasma was collected at 25 minutes, and 1, 2, 3, and 6 hours. (F) Ten minutes after HJ8.5 antibody injection (50 mg/kg, i.p.), human tau was injected into the cisterna magna of wildtype mice (n=6) and plasma was collected at 10 minutes, and 1, 6, 24, 48, and 72 hours. (G) Half-life of plasma tau after injection into the cisterna magna of wildtype mice in the absence (E) and presence of HJ8.5 (F). Tau half-life was calculated by determining the slope from linear regression fit of semi-log plots of concentration versus time (41). Values in D and G represent mean ± SEM. *p≤0.05, **p≤0.01, ***p≤0.001. Values in D analyzed by ANOVA followed by post-hoc Dunnett’s test and values in G analyzed by unpaired t test.
To determine the extent to which tau in the extracellular space of the CNS enters the plasma and is measurable in the plasma, we injected recombinant human tau into the cisterna magna of wild-type mice in the presence or absence of peripherally administered HJ8.5. In control mice, plasma tau was detectable in the plasma within minutes after cisterna magna injection and exhibited a half-life of 1.6 hours (Fig. 2E, G). The increased half-life of CNS-injected tau versus venous-injected tau likely reflected continued entry of tau from the CSF into plasma over several hours following a single bolus injection. In HJ8.5-injected mice, human tau was detected in the plasma within minutes after CNS injection, but the half-life was increased to almost 24 hours (Fig. 2F, G). This again suggested that the HJ8.5-tau antibody complex had a prolonged half-life in plasma relative to tau alone, which likely accounted, at least in part, for the HJ8.5-dependent increase in plasma tau.
HJ8.5-induced increase in plasma tau occurs in different mouse models expressing human tau
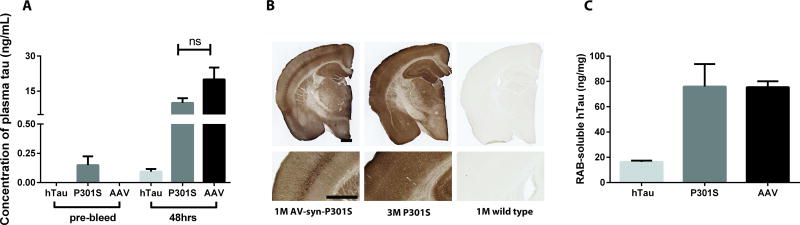
To further characterize the effects of HJ8.5 on plasma tau as well as to determine its origin, we administered HJ8.5 at a dose of 50 mg/kg i.p. to a variety of human tau Tg mice. P301S Tau Tg mice express human tau under the control of the prion promoter, which drives transgene expression predominantly in the CNS, but also in peripheral tissues such as muscle. Therefore, plasma tau is likely to be detectable under basal conditions in P301S Tau Tg mice due to some expression of tau in peripheral tissues outside of the CNS (Fig. 3A). We wanted to verify that the increase in plasma tau in Tg mice following HJ8.5 administration was attributable to CNS-derived tau. We administered HJ8.5 to two different mouse models, the hTau mouse, which expresses human tau under control of the endogenous tau promoter, and in another mouse model in which adeno-associated virus (AAV) 2/8-mediates expression of human tau with the neuronal-specific synapsin promoter in the brain (25). We injected AAV type 2/8 expressing human P301S tau (AAV-syn-P301S) intracerebroventricularly (ICV) into the brain of postnatal day 0 (P0) wild-type mice. This resulted in strong CNS expression of human tau by 1-month of age, at levels comparable to those observed in 3 month-old P301S Tau Tg mice (Fig. 3B). Unlike P301S Tau Tg mice, human tau was not detectable in the plasma of either 2-month old hTau mice or 1-month old AAV-tau mice under basal conditions (Fig. 3A). Plasma tau in different Tg mice was assessed using Simoa technology. The lower limit of detection in the plasma tau Simoa assay we utilized in mice was ~1 pg/ml. Injection with HJ8.5 increased plasma tau in hTau mice to 93 pg/ml and to ~20ng/mL for AAV-tau mice (Fig. 3A). Given the large difference between hTau and AAV-tau mice regarding plasma tau detected following HJ8.5 administration, we decided to measure soluble human tau in the mouse brain using a plate-based human tau-specific ELISA. We found that soluble tau was much lower in hTau mice compared to P301S and AAV-tau mice (Fig. 3C), suggesting that the different plasma tau concentrations observed following HJ8.5 administration reflected different concentrations of soluble tau within the CNS compartment. However, because of major differences in the promoters used in these mice and both the amount and location of tau expression between the human tau expressing mouse models, we decided to further test this idea in just one model.
Fig. 3. Increased plasma tau after HJ8.5 injection in different mouse models expressing human tau.
(A) Plasma tau was analyzed before antibody injection (pre-bleed) and 48 hours after HJ8.5 injection in 2 month-old hTau mice which express human tau under control of the endogenous tau promoter (n=5), 3 month-old P301S mice (n=8), and 1 month old wild-type mice injected ICV with AAV viral particles expressing P301S human tau under control of the synapsin promoter at post-natal day 0 (AAV-Syn-P301S) (n=8, AAV). (B) Representative coronal brain sections from a one month-old mouse with AAV2/8 mediated expression of P301S human tau under control of the synapsin promoter (AAV-Syn-P301S), from a 3 month-old P301S Tau transgenic mouse (P301S), and from a 1 month-old C57BL/6 wildtype (WT) mouse stained with biotinylated anti-human tau antibody HJ8.5. Scale bar 600 µm in length. (C) Human tau measured by human tau-specific ELISA in brain cortical salt-containing reassembly buffer (RAB)-soluble fractions from 2-month-old hTau mice, from 3-month-old P301S mice and from 1 month-old AAV-Syn-P301S mice (AAV). Values in A and C represent mean ± SEM.
HJ8.5-induced increase in plasma tau is correlated with soluble brain tau
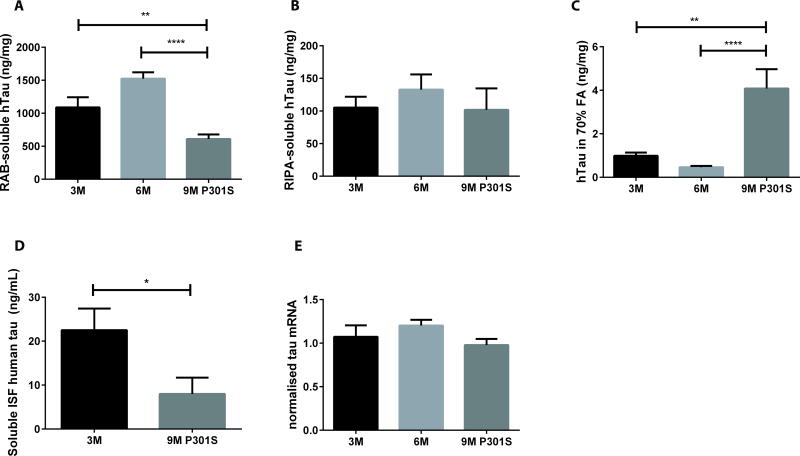
Given that we saw differences in plasma tau elevation upon antibody injection that appeared to be potentially related to differences in soluble tau in the brain, we hypothesized that we might be able to detect pathology-dependent alterations in soluble tau using antibody-dependent measurements in plasma tau. In previous studies, we characterized the amount of tau within the interstitial fluid (ISF) as well as brain soluble and insoluble tau in P301S Tau Tg mice (26). ISF tau represents tau present in the extracellular space of the brain that is released from neurons (11). It is only a small fraction of the soluble brain tau assessed biochemically, which is predominantly composed of cytosolic tau. In accordance with previous results, we observed an age-dependent decline in soluble tau, an age-dependent increase in insoluble tau, and no age-dependent change in the detergent-containing radioimmunoprecipitation assay (RIPA) buffer solubilized tau (Fig. 4A–C). Furthermore, we found higher ISF tau in young, pre-symptomatic P301S mice compared to aged 9-month-old P301S mice (Fig. 4D). The amount of ISF tau in 9-month-old mice was ~60% lower than in 3-month-old mice. No changes were observed in total human tau expression by qPCR (Fig 4E).
Fig. 4. Soluble brain tau and ISF tau decrease in P301S Tau Tg mice.
The concentrations of brain cortical reassembly buffer (RAB) soluble (A), radioimmunoprecipitation assay (RIPA) buffer soluble (B) and 70% formic acid (FA) soluble tau (C) are shown in 3-month old (n=10), 6-month old (n=15) and 9-month old (n=14) P301S Tau Tg mice. Nine month-old P301S mice had lower cortical RAB soluble and higher formic acid soluble tau compared to 3-month or 6-month old P301S mice. One-way ANOVA followed by post-hoc Dunnett’s test, **p≤0.01, ****p≤0.0001. (D) Soluble ISF human tau was measured by microdialysis in hippocampal tissue from 3 month old (n=9) and 9 month old (n=7) P301S mice that were not treated with an anti-tau antibody. Nine month old mice had lower ISF tau compared to 3 month old mice. *p≤0.05, 2-tailed t-test. (E) Human tau mRNA in cortex from 3-month old (n=10), 6-month old (n=14) and 9-month old (n=9) P301S Tau Tg mice. Tau mRNA was normalized to total cDNA. Human tau mRNA from 6 and 9 month-old P301S Tau Tg mice was normalized and compared to tau mRNA from 3-month old P301S Tau Tg mice. No difference in human tau mRNA between groups was observed (one-way ANOVA post-hoc Dunnett’s test, p>0.05). Values represent mean ± SEM.
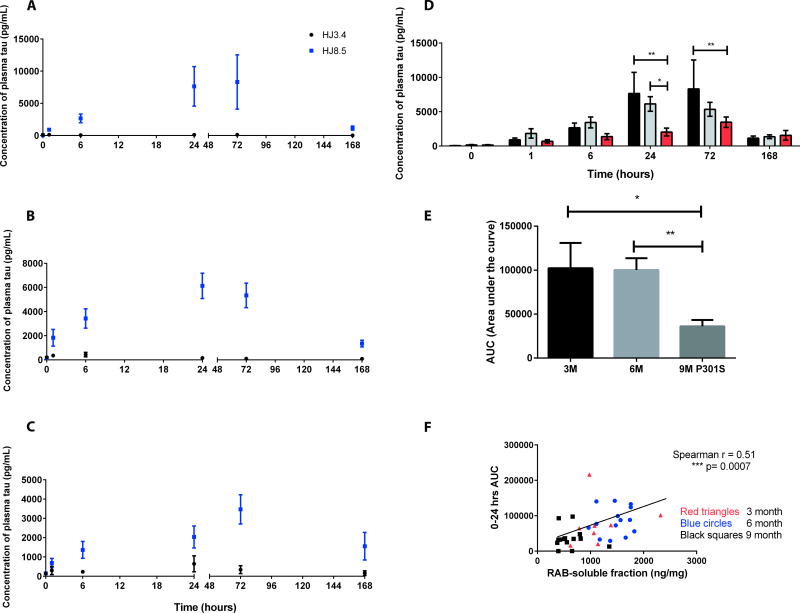
Having established that aged mice bearing tau pathology exhibited decreased total soluble and ISF tau, we next tested whether we would detect differences in antibody-dependent increases in plasma tau between P301S mice of different ages before and after the onset of tau pathology. After i.p. injection of HJ8.5 (50 mg/kg), plasma tau assessed with the Simoa assay increased over 1–6 hours and remained elevated for more than a week compared to basal conditions in 3-month, 6-month, and 9-month-old P301S mice (Fig. 5A–C). As a control group, anti-human Aβ antibody HJ3.4 at 50 mg/kg was injected into P301S Tau Tg mice. No change in plasma tau was observed in these groups (Fig. 5A–C). Interestingly, the amount of plasma tau in the HJ8.5-injected mice was decreased by 60% in the 9-month-old mice during the first 24 hours following HJ8.5 injection compared to the amount in the 3-month and 6-month-old groups (Fig. 5D, E). This result was in accordance with the observed decrease in total brain soluble tau and ISF tau in 9-month-old compared to 3-month-old P301S mice (Fig. 4A,D). To further investigate this relationship we plotted the amount of plasma tau in HJ8.5-treated mice as a function of soluble brain tau and found a positive correlation between the amount of soluble brain tau and plasma tau (Fig. 5F). Together, these results suggest that brain extracellular tau in ISF is in equilibrium with biochemically extractable salt-soluble pool of tau within the brain and that the ISF pool of tau is able enter the plasma where it was captured and detected by an anti-tau antibody.
Fig. 5. Anti-tau antibody mediated increases in plasma tau in P301S Tau Tg mice of different ages.
Plasma tau was measured before (0 hour time point) and after i.p injection of HJ8.5 (50 mg/kg) or anti-human Aβ antibody HJ3.4 (50 mg/kg) at 1, 6, 24, 72 and 168 hours in 3-month old (A), 6-month old (B) and 9-month old (C) P301S Tau Tg mice (n=10–15 mice/group). (D) Summary of data from panels A, B, and C in bar graph showing plasma tau measured in 3-month old (black bars), 6-month old (gray bars) and 9-month old (red bars) P301S mice, before (0 hour time point) and after HJ8.5 i.p injection. *p≤0.05, **p≤0.01, 2-way ANOVA with post hoc Dunnett test. (E) Area under the curve (AUC) of plasma tau (pg/ml × hours) was calculated for the first 24 hours after HJ8.5 injection in 3, 6 and 9 month old P301S mice. *p=0.04, **p=0.004, 2-tailed t-test. Values represent mean ± SEM. All plasma samples were analyzed on a Simoa HD-1 Analyzer. (F) Positive correlation was seen between plasma tau 24 hours after HJ8.5 injection (y-axis) and brain cortical RAB soluble tau (x-axis). Spearman r, r=0.51, ***p=0.0007. Red triangles, 3 month old mice; Blue circles, 6 month old mice; Black squares, 9 month old mice.
Changes in mouse brain ISF tau and plasma tau detected by HJ8.5 after neuronal injury
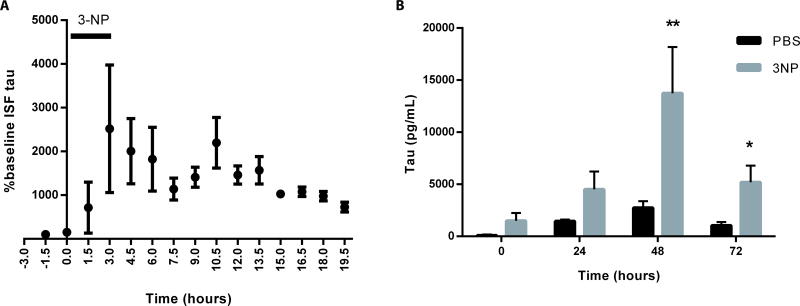
Whereas the data suggest that decreases in soluble brain tau and ISF tau that occur in 9-month-old versus 3-month-old P301S mice maybe reflected in plasma following HJ8.5 injection, we wanted to determine whether injury-induced increases in extracellular tau could also be detected in the blood following peripheral injection of the anti-tau antibody. We decided to assess the effects of unilateral striatal infusion of a neuronal toxin, 3-nitropropionic acid (3-NP), on ISF tau. Baseline striatal ISF tau was assessed in a group of P301S Tau Tg mice followed by infusion of 3-NP through reverse microdialysis. This treatment resulted in a 10–20 fold increase in striatal ISF tau over several hours (Fig 6A). We then stereotactically injected a group of P301S Tau Tg mice bilaterally with either 3-NP or PBS into the striatum as described previously (27). Four hours later, mice received an i.p. injection of HJ8.5 (50 mg/kg). Plasma tau was assessed prior to and after 3-NP or PBS injection using the Simoa tau assay. Plasma tau increased in all mice administered HJ8.5 (Fig. 6B). However, there was a significanty larger increase in plasma tau in mice given 3-NP compared to those given PBS (p<0.01) (Fig. 6B). This suggested that not only decreases but also increases in ISF tau could be detected in plasma after treatment with an anti-tau antibody.
Fig. 6. Increased human tau in plasma and ISF after anti-tau antibody administration following neuronal injury.
(A) Soluble ISF human tau was measured by microdialysis in the striatum of 8–9 month old P301S Tau Tg mice (n=4). After stable baseline collection, the neurotoxin 3-NP was administered via reverse microdialysis for 3 hours and ISF tau was measured. Injury to the mouse striatum caused an increase in ISF tau for 12 hours post 3-NP treatment. One-way ANOVA followed by post-hoc Dunnett’s test p<0.05. (B) Four to five month old P301S mice were injected bilaterally in the striatum with either PBS (n=5) or 3-NP (n=5, 100 nmol/µl). Four hours later, HJ8.5 at 50 mg/kg was administered i.p. and plasma tau was assessed before (0 hour) and after 3-NP or PBS injection at 24, 48 and 72 hours. Compared to PBS treated P301S Tau Tg mice, there was an increase in plasma tau at 48 and 72 hours in P301S Tau Tg mice after 3NP treatment (2-way ANOVA with post-hoc Dunnett test, **p<0.01, *p=0.029).
Discussion
Disease-modifying therapies including immunotherapies are emerging as potential treatments for neurodegenerative diseases. Antibodies targeting Aβ are furthest along in this process (28); however, antibodies targeting tau and α-synuclein have also shown promising results in animal models and have entered clinical trials (29, 30). A challenge moving forward in clinical trials for neurodegenerative diseases characterized by protein aggregation is screening for patients that have the aggregated form of the protein being targeted in the CNS, as well as determining whether the therapy being utilized is hitting its target. In this study, we found that peripheral administration of anti-tau antibody to mice expressing human tau as well as to humans with PSP resulted in a marked increase in plasma tau. Our data indicated that the antibody-dependent increase of plasma tau resulted primarily from CNS-derived tau and that plasma tau concentrations appeared to reflect soluble, extracellular tau in the brain. The results of this study in both humans and mouse models suggest a direct relationship between extracellular tau in the CNS and plasma tau.
Expression of tau is almost exclusively in neurons where it is present in the cytosol. It is also released by neurons in the brain physiologically and can be detected in soluble forms in brain ISF and CSF (10, 11). In brain ISF, tau has a long half-life, ~11 days in mice (31). In human plasma, it is detectable in only low amounts ~4 pg/ml in humans (24). Our data suggest that when tau enters the plasma from the CNS, it is rapidly cleared (Fig. 2A). In the presence of a tau antibody, tau that enters the plasma binds to the antibody and this extends the plasma half-life of detectable tau (Fig. 2). It is also possible that some antibody-tau complexes form initially in the CNS and then are cleared via the plasma with normal clearance of antibody from the brain to the blood. Interestingly, our data suggest that the amount of tau bound to anti-tau antibody in plasma is related to brain soluble tau and perhaps more directly to extracellular tau such as that in the ISF. For example, after neuronal injury, ISF tau and plasma tau both increased (Fig. 6), possibly due to extracellular release of cytosolic tau in the brain after injury. Also, we detected lower tau in the plasma of older P301S Tau Tg mice with tau pathology following anti-tau antibody administration reflecting lower ISF tau and brain soluble tau (Fig. 5). While there was a difference in salt-containing reassembly buffer (RAB) soluble tau in mice before versus after establishment of tau pathology between age groups, there was no correlation between RAB soluble tau and plasma tau after administration of anti-tau antibody within each age group. This may be similar to the observed relationship between CSF Aβ42 and amyloid deposition in the brains of human patients with Alzheimer’s disease pathology and mouse models of amyloidosis in which once Aβ has aggregated then CSF and ISF Aβ42 decreases (32–34). However, low CSF Aβ42 in individuals with amyloid deposition does not correlate with the absolute amount of Aβ deposition within this group. Even though CSF Aβ42 does not correlate with the absolute amount of amyloid deposition, it may be useful for clinical trial patient selection based on its ability to discriminate between the presence or absence of amyloid deposition. Whether increases in plasma tau following anti-tau antibody administration would be different in humans with a primary tauopathy compared to healthy controls is currently not known but should be tested in future studies.
We, and others, have found that administration of certain anti-Aβ antibodies results in a large increase in plasma Aβ in both mice and humans that is predominantly derived from the brain (35–39). The large increase in plasma Aβ seen with certain antibodies may be due to a prolongation of plasma Aβ half-life when bound to antibody, perhaps similar to what we report here with tau. Animal studies suggest that such changes in Aβ may also reflect the amount of extracellular soluble Aβ in the CNS that is entering the blood (36, 39). In fact, manipulations that alter brain to blood clearance of Aβ, such as the brain expression of the low-density lipoprotein receptor, affect the concentration of Aβ detectable in the blood following anti-Aβ antibody administration (40). There are, however, differences between extracellular CNS-derived Aβ and tau. The half-life of ISF Aβ in mice is ~1.5–3 hours; the half-life of ISF tau in mice is ~ 11 days (31, 41).
There are several limitations to this study. First, whether all anti-tau antibodies will increase plasma tau as we report here is not yet known although others have also observed increases in serum tau after administration of different anti-tau antibodies to human Tau Tg mice (16, 42). Second, the differences in plasma tau following anti-tau antibody administration that were observed in the presence or absence of tau pathology or after neuronal injury in mice have only been studied in one mouse model, P301S tau transgenic mice. Whether similar differences will be observed in other animal models of tauopathy is not clear. Third, it is not yet known whether increases in plasma tau in PSP patients that were observed following the administration of the anti-human tau antibody used herein will reflect brain extracellular tau in humans. Given these limitations, as anti-tau antibodies have now moved forward into clinical trials, it will be important to assess plasma tau across different patient populations with both primary tauopathies such as PSP, corticobasal degeneration, Pick disease, chronic-traumatic encephalopathy, and frontotemporal lobar degeneration (FTLD) with MAPT mutations versus AD in which there is both Aβ and tau accumulation and changes in CSF tau. Notably, whereas CSF tau is elevated in AD and following acute neuronal injury such as stroke or traumatic brain injury compared to controls, CSF tau is not elevated in the primary tauopathy PSP. In fact, CSF tau is lower in PSP patients than in age-matched controls (43–45).
A well-established observation is that there is an elevation of CSF tau in AD (46). This is likely a completely different phenomenon than what takes place in primary tauopathies. In AD, CSF tau is likely elevated due to neuronal and synaptic membrane damage secondary to amyloid deposition, not directly due to tau aggregation. This was demonstrated in a recent study where it was shown that murine CSF tau is normal in young amyloid precursor protein (APP) Tg mice prior to amyloid deposition, but then progressively increases once amyloid deposition begins to accumulate in the brain (47). Given that these mice do not develop neurofibrillary tangles or tau aggregation, it suggests that amyloid-linked neuronal/synaptic injury leads to an increase in ISF/CSF tau release independent of tau aggregation. We report a similar phenomenon following acute brain injury in P301S Tau Tg mice. In this mouse model of acute CNS neuronal injury following striatal 3-NP administration, we found an increase in brain ISF tau, and also higher plasma tau following anti-tau antibody administration compared to mice given an anti-tau antibody with no neuronal injury. Given these results, we would predict in patients with amyloid deposition and in individuals with acute brain injury from stroke or head trauma that there would be an increase in plasma tau compared to age-matched healthy controls following anti-tau antibody administration.
Given our findings, it will be important to determine whether differences in plasma tau following anti-tau antibody administration can be utilized to screen for individuals with primary tauopathies (where tau is the only major protein aggregating in the brain), for those with AD (where both Aβ and tau are 2 major proteins aggregating in the brain), and for those with different forms of acute versus chronic neuronal/synaptic damage. Finally, as clinical trials move forward with anti-tau antibodies, it will be important to determine whether a reduction in tau accumulation detected by tau imaging as well as any clinical response following anti-tau antibody therapy correlates with measurements of plasma tau. In addition, understanding how plasma and CSF tau correlate before and after anti-tau antibody administration may also provide insights into the mechanism of action of anti-tau antibodies.
Materials and Methods
Study design
The aim of this study was to assess concentrations of plasma tau in patients with PSP and in mouse models expressing human tau before and after administration of an anti-tau antibody. In mouse models, another goal was to determine the half-life of plasma tau in the presence and absence of the anti-tau antibody as well as any differences in plasma tau after antibody administration that were due to tau pathology. All mice and human subjects in the studies were randomly assigned to experimental groups. All studies were performed by investigators blinded to treatment groups and sample identity. Replication numbers for experiments are listed in the figure legends.
Administration of humanized HJ8.5 antibody to patients with PSP
The mouse monoclonal antibody was cloned from the hybridoma expressing HJ8.5 and the variable regions were cloned to a human IgG4 Fc domain followed by humanization of several amino acids outside of the CDR region. A humanized version of HJ8.5 was administered to an individual with PSP following a treatment protocol under an expanded access program (expanded access IND 119404) by C2N Diagnostics. Informed consent was obtained from the individual and all procedures were approved by an Institutional Review Board. The individual was administered multiple doses of the antibody once per month. Blood samples were collected at various time points before and after start of drug infusion for the purpose of measuring drug concentrations as well as biomarkers. The same antibody was also administered to individuals as part of a phase I single dose study entitled “Safety, Tolerability, and Pharmacokinetics of C2N-8E12 in subjects with Progressive Supranuclear Palsy”, NCT02494024. Informed consent was obtained from all participants and all procedures were approved by an Institutional Review Board.
Tau transgenic mice and treatment with anti-tau antibody
All animal procedure and experiments were performed by the guidelines approved by the animal studies committee at Washington University School of Medicine. We utilized several different human Tau Tg mice. P301S Tau Tg mice (Jackson Laboratories) overexpress the human T34 isoform of human tau (1N4R) with the P301S mutation under the control of prion promoter (21). These mice are on B6C3 background and develop tau pathology by 5–6 months of age. Human tau (hTau) mice (male and female) were utilized that express all six isoforms of hTau but do not express mouse tau (25). These mice develop hyperphosphorylated tau by 6 months of age (25). For adeno-associated virus (AAV) injections, postnatal day (P) 0 pups of C57BL/6 mice were used. Pregnant C57BL/6 mice were purchased from Charles River Labs. Plasma was collected from two month-old hTau mice, 1-month-old AAV injected mice, and 3-month-old male P301S Tau Tg mice one day before HJ8.5 injection and 48hrs after HJ8.5 (50 mg/kg) i.p. injection. For dose response experiments, 3 month-old male P301S mice were administered HJ8.5 at 10, 50 and 200 mg/kg by i.p injection. Control mice were administered phosphate buffer saline (PBS). All the plasma samples were stored at −80°C until use. For experiments involving P301S mice at different ages (3-months, 6-months and 9-months), only male mice were utilized and plasma samples were collected 1 day before HJ8.5 injection and at various time intervals (1, 6, 24, 72, 168 hours) after HJ8.5 (50 mg/kg) i.p injection. Anti-human Aβ antibody HJ3.4 was used as control antibody. All plasma samples were stored at −80°C until use.
AAV viral particle injection into mouse brain
P301S human tau was cloned from P301S transgenic mouse cDNA and inserted into an AAV-synapsin driven promoter vector. AAV 2/8 serotype was produced at the Hope Center Viral Vectors Core (Washington University in St. Louis). C57BL/6 mice post-natal day 0 (P0) pups were intraventricularly injected bilaterally with 2 µl (1 × 1013 vg/mL) of AAV2/8 carrying human tau with the P301S mutation (AAV-P301S) under the control of the synapsin promoter using a 10 µl Hamilton syringe (Sigma-Aldrich). One month after viral infection, plasma was collected from tail bleeds one day before injection and 48 hours after an i.p. injection of HJ8.5 (50 mg/kg). Plasma samples were stored at −80°C until use.
Collecting blood samples from mice
Blood samples were collected at various time points from tail bleeds for Tg mice and retro-orbital bleeds for wild-type mice. Heparinized capillary tubes (Kimble & Chase) were used to collect blood and transferred into 1.5 ml Eppendorf tubes along with 1 µl of 0.5M Ethylenediamine tetraacetic acid (EDTA). The last time point of blood collection was from anesthetized mice (50 mg/kg of sodium pentobarbital) from the right ventricle of the heart with a 1 ml insulin syringe filled with 20 µl of 0.5M EDTA. Blood samples were spun at 8000 rpm (6000 × g) for 10 minutes at 4°C to obtain plasma. Plasma samples were stored at −80°C until use.
Measurements of plasma tau half-life in mice
For experiments involving assessment of human tau half-life in the plasma of mice, recombinant human tau (rPeptide), at 1 µg per 100 µl of saline, was injected into the jugular vein under isoflurane anesthesia in 3 month old male B6C3 wild type mice, and retro-orbital bleeds were collected at various time intervals (after 2, 4, 8, 12, 18, and 25 minutes). Recombinant human tau (1 µg, rPeptide) was pre-incubated at room temperature with 10 times molar excess of HJ8.5 (2.3 µM) and injected into the jugular vein of 3 month old B6C3 wild-type mice. Plasma was collected from tail bleeds at different time intervals of 10 minutes, 6, 24, 48, and 72 hours. In some experiments, 1 µg of recombinant human tau was injected into the jugular vein 1 hour following the i.p. administration of HJ8.5 (50 mg/kg) and plasma was collected at 10 minutes, 1 hour, 6 hours, and 24 hours after injection. To determine the half-life of human tau in plasma after administration into the cerebrospinal fluid in the cisterna magna, we anesthetized 3-month old wild type male B6C3 mice by i.p injection of xylazine (10mg/kg)/ ketamine (100mg/kg) in normal saline. Then mice were injected with recombinant human tau (2 µg/2 µl, rPeptide) into the cisterna magna compartment as described previously (48). Plasma was collected from tail bleeds at different time intervals of 25 minutes, 1, 2, 3, and 6 hours. For experiments in the presence of HJ8.5 antibody, 10 minutes after i.p. injection of HJ8.5 (50 mg/kg), human tau was injected into the cisterna magna. Plasma was collected at various time intervals of 10 minutes, 1, 6, 24, 48, 72 hours. All Plasma samples were analyzed on Simoa HD-1 analyzer. Half-life of tau was calculated by first order kinetics, determining slope of linear regression from semi-log plot of concentration vs. time. Elimination rate constant (Ke) and half-life t1/2 were calculated by using Ke= 2.0303/slope; t1/2=0.693/Ke as previously described (11, 41, 49).
Immunohistochemistry to human detect tau
Immunostaining was performed as described previously (12). To determine the expression of human tau in different transgenic mice, we stained brain sections with biotinylated HJ8.5 antibody. For staining, 3 brain sections of 50 µm thickness and 300 µm apart corresponding approximately to sections at Bregma coordinates −1.4, −1.7, and −2.0 mm in the mouse brain atlas were used (50). Brain sections were blocked with 3% milk in Tris-buffered saline and 0.25% (vol/vol) Triton-X followed by incubation at 4°C overnight with the biotinylated HJ8.5 antibody. C57BL/6 and B6C3 wild type mice brain sections were used as negative controls.
Measurements of plasma tau using the Simoa assay in mice
To measure tau in mouse plasma we utilized a Single molecule array – (Simoa) assay, a sensitive digital ELISA platform (51). Homebrew assays specific for total tau were developed according to the manufacturer’s recommendations (Quanterix Corp). All plasma tau levels measurements were analyzed using the Simoa HD-1 Analyzer (Quanterix Corp). Simoa HD-1 consumables were purchased from Quanterix Corp. The paramagnetic beads were pre-coated with a mouse monoclonal anti-tau HJ8.7 capture antibody. In addition to the coated beads, diluted plasma samples, biotinylated detector anti-tau antibody BT2 (Pierce), streptavidin β-galactosidase (sβg), and enzyme substrate (resorufin β-D galactopyranoside) were added to the Simoa HD-1 Analyzer. Recombinant human tau was included in each run to generate a standard curve. Assays were performed according to the manufacturer’s instructions (Quanterix Corp). The presence of HJ8.5 in this assay does not interfere with or influence the assay results.
ELISA assay for plasma tau in PSP patients
To determine tau in human plasma samples, we utilized an ELISA in which plates were first coated with 1 µg/mL of BT2 antibody (Thermo Scientific) in PBS supplemented with 20% glycerol and left to incubate overnight at 4°C. The next day, plates were blocked with 2% BSA in PBS supplemented with Tween-20 0.05% and 20% glycerol for 2 h at RT. Human plasma samples were diluted 1:5 in sample diluent (0.20% BSA/300mM Tris PH 8.0/0.05% Tween 20/20 µg/mL mIgG/0.05% Proclin-300/PBS) while calibrator (recombinant tau 412, rPeptide) was prepared in calibrator diluent (0.20% BSA/300mM Tris PH 8.0/0.05% tween-20/20 µg/mL mIgG/20% pooled normal plasma samples/0.05% Proclin-300/PBS). Calibrators and samples were incubated overnight at 4°C. The next day, plates were washed 4 times with PBS followed by incubation with biotinylated mouse monoclonal anti-tau antibody HJ8.7 (1 µg/mL) in 1% BSA in PBS for 2 h at RT. Plates were then washed 4 times with PBS followed by incubation with streptavidin-poly-horseradish peroxidase-40 (1:6000, Fitzgerald) for 1 h at RT. Plates were washed 4 times with PBS and then developed with Super Slow ELISA TMB (Sigma) and absorbance read at 650 nm.
Biochemical extraction of mouse brain tissue
Biochemical extractions were performed as described previously (12). Briefly, cortical brain samples were homogenized in 30 µl/mg (v/w) in reassembly buffer - RAB (100 mM MES, 0.5mM MgSO4, 1 mM EDTA, 2mM DTT, 0.75M NaCl, 1 mM Na3VO4, pH 6.8) supplemented with 1× protease and phosphatase inhibitors (Roche). Samples were spun at 50,000g for 20 minutes, and the supernatant was saved as a RAB-soluble fraction. Pellet was dissolved in 30 µl/mg (v/w) in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 50 mM Tris, 0.5% deoxycholic acid, 1% TritonX-100, 0.5% SDS, 25 mM EDTA, pH 8.0) supplemented with protease and phosphatase inhibitors (Roche) and spun at 50,000g for 20 minutes. The supernatant was collected as a RIPA-soluble fraction. The pellets were further dissolved in 70% formic acid (FA) at 10 µl/mg, sonicated, centrifuged at 50,000g for 20 minutes. The supernatant was collected as FA-soluble fractions. All fractions were stored at −80°C until analyzed. Samples were analyzed using a human tau specific ELISA.
Human tau specific ELISA to measure tau in mouse brain
Human tau specific enzyme-linked immunosorbent assay (ELISA) that was utilized to measure brain tissue tau levels was performed as described previously (20). For assessing human tau levels in Figure 4, biochemical cortical brain extraction samples were analyzed by coating ELISA 96 well plates with mouse monoclonal anti-tau antibody Tau5 (20 µg/ml, gift of L. Binder) overnight. Plates were washed 5 times with PBS and blocked with 4% BSA in PBS for 1 hr at 37°C. Cortical brain extraction samples were diluted in 0.25% BSA in BSA, 300 mM Tris, pH 8.0 supplemented with protease inhibitors and kept at 4°C for overnight on a shaker. The next day, plates were washed 8 times with PBS followed by the addition of mouse monoclonal anti-human tau-specific biotinylated HT7 antibody (0.2 µg/ml, Thermo Scientific) in 1% BSA in PBS for 1.5 hr at 37°C. Next, plates were washed 8 times with PBS followed by addition of streptavidin-poly-horseradish peroxidase-40 (1:6000, Fitzgerald) for 1.5 h in the dark at room temperature. Then plates were washed 8 times with PBS, developed with Super Slow ELISA TMB (Sigma) and absorbance read at 650 nm. For assessing human tau levels as shown in Figure 3C, cortical brain extracts of different transgenic mice were analyzed by coating ELISA plates with anti-tau antibody BT2 (1 µg/ml) in PBS supplemented with 20% glycerol and incubated overnight at 4°C. Plates were blocked with 2% BSA in PBS supplemented with Tween-20 0.05% and 20% glycerol for 2 h at RT. Next plates were washed 4 times with PBS followed by the addition of mouse monoclonal anti-human tau-specific biotinylated HT7 antibody (0.2 µg/ml, Thermo Scientific) in 1% BSA in PBS for 1.5 h at 37°C. Then plates were washed 4 times with PBS followed by incubation with streptavidin-poly-horseradish peroxidase-40 (1:6000 Fitzgerald) for 1 h at RT. Plates were washed 4 times with PBS and then developed with Super Slow ELISA TMB (Sigma) and absorbance read at 650 nm.
Quantitative PCR to measure human MAPT RNA in mouse brain tissue
For the isolation of RNA, brain tissues were homogenized in TRIzol (Invitrogen). Chloroform was added (1:5 ratio), samples were vigorously shaken for 15 seconds at room temperature, and then centrifuged at 12,000 × g for 15 minutes at 4°C. The aqueous phase of the sample was removed by angling the tube at 45° and pipetting the solution out. Then samples were diluted (1:1) in 100% isopropanol and incubated for 10 minutes at room temperature. Samples were centrifuged at 12,000 × g for 10 minutes at 4°C. The pellet was washed in 75% ethanol, and then centrifuged at 7500 × g for 10 minutes. Supernatant was discarded and the RNA pellet air-dried for 10 minutes before resuspension in RNase-free water. Reverse transcription was performed by using a high-capacity RNA-cDNA kit (Applied Biosystems [ABI]). Quantitative PCR was performed using an ABI TaqMan primer targeting human MAPT (Product no: Hs00902194_m1, Life Technologies) and reagents on an ABI Prizm 7500 thermocycler according to the manufacturer’s instructions. All mRNA measurements were normalized to the total cDNA
Microdialysis and 3-NP treatment of P301S Tau Tg mice
P301S hippocampal microdialysis was performed in P301S male mice with some modifications (11, 26). In brief, a microdialysis probe with 100 kDa cutoff (Amuza Inc.) was implanted in the left hippocampus through a guide cannula (Amuza Inc.). Artificial cerebral spinal fluid (ACSF) containing 4% human albumin solution was used as perfusion buffer for ISF collection. ISF was collected in 90-minute fractions for 24 hours following probe implantation. Striatal microdialysis with 3-nitropropionic acid (3-NP) treatment was performed in 8–9-month-old P301S mice using a microdialysis probe with 1000 kDa cutoff (Amuza Inc.) implanted in the left striatum. ACSF containing 4% human albumin solution was used as perfusion buffer for ISF collection. After stable baseline ISF collection 200 µM 3-NP (Sigma-Aldrich) was dissolved in perfusion buffer and administered directly in the striatum for 3 hours via reverse microdialysis. For bilateral striatal 3-NP injections combined with plasma tau measurements, a 100 nmol/µl solution of 3-NP (Sigma-Aldrich) was prepared in PBS and 0.5 µl of this solution was injected bilaterally in the striatum of 5 month old P301S mice via stereotaxic surgery at Bregma coordinates − +0.98 mm, +/− 1.5 mm mediolateral, and a depth of 2.6 mm as described (27).
Statistical analysis
The amount of plasma tau in P301S mice of different ages was compared by 2-way ANOVA with post hoc Dunnett test using GraphPad Prism 6.0 (GraphPad Software Inc.). Non-parametric Spearman correlation analyses were performed with Graph Pad Prism 6.0 software. The Area Under the Curve (AUC) for tau in plasma during the 24 hours following injection of anti-tau antibody was calculated using the basic non-comparmental pharmacokinetics (PK) package for R. R is a free software environment for statistical computing and graphics. The mean AUCs and error estimates for each group were calculated by PK and a 2-tailed t-test was used to determine statistical significance. One-way ANOVA followed by post-hoc Dunnett’s test was used to evaluate the qPCR data, plasma tau-half life data, as well as ISF human tau. 2-way ANOVA with post hoc Dunnett test was used to evaluate plasma tau measurements from mice administered the neurotoxin 3-NP. Statistical significance was set at p<0.05. Outliers were determined by performing Grubbs’ test using free web Graph Pad software. There was one outlier each in the 6 and 9 month old groups of mice analyzed in Figure 4 and 5 for plasma and brain tau. All values are presented as mean ± SEM.
Editor’s Summary.
Tauopathies such as progressive supranuclear palsy and Alzheimer’s disease are a group of neurodegenerative diseases characterized by the accumulation of aggregated forms of tau protein in the brain. Administration of anti-tau antibodies is a new treatment approach being tested for tauopathies. Tau is present at high levels in the brain and very low levels in the plasma. Peripheral administration of an anti-tau antibody markedly increased tau levels in the plasma in both transgenic mice expressing human tau as well as in patients with tauopathy. The increase in plasma tau in mice correlated with extracellar and soluble tau in brain.
Acknowledgments
Funding: This work was supported by research grants from C2N Diagnostics (DMH), the Tau Consortium (DMH), The JPB Foundation (DMH), and NIH R01AG048678 (DMH).
DMH co-founded and is on the scientific advisory board of C2N Diagnostics. DMH, HJ, and GG are inventors on a submitted patent “Antibodies to Tau”, PCT/US2013/049333, that is licensed by Washington University to C2N Diagnostics. This patent was subsequently licensed to AbbVie. ALB receives research support from C2N Diagnostics, Avid, Biogen, BMS, Eli Lilly, Forum, Genentech, Roche and TauRx. ALB consults for AbbVie, Alector, Asceneuron, Ionis, Delos, Janssen and Merck. DRK receives research support from C2N, AbbVie, and Roche and serves on a clinical advisory board for AbbVie. DMH consults for Genentech, AbbVie, Eli Lilly, Neurophage, and Denali. KY was at Washington University during the course of these studies and is now an employee at AbbVie. Tim West, Philip B. Verghese, Joel Braunstein, Stephanie Knapik, and Helen Hu are employees of C2N Diagnostics.
Footnotes
Author contributions: KY, TKP, and DMH designed the study. SS, JU, TW, and KY did statistical analysis. HJ, FS, MBF, JK, GR, TKP, and KY performed Simoa, ELISA and immunohistochemistry. PBV developed the formulation for humanized antibody and performed biochemical characterization, stability and compatibility studies. ALB, DRK, PBV, IF, TW, JB, SSK, and HH collected and analyzed human data. KY and DMH edited and wrote the manuscript with critical revisions from TKP, HJ, GG, BLM, FS, MBF, NC, JU, ALB, DRK, PBV, TK, TW, JB, SSK, HH, and GR.
Competing interests: The other authors declare no competing interests.
Data and material availability: The material in this study generated in the lab of David Holtzman, including anti-tau antibodies, can be requested through the office of technology management at Washington University (https://otm.wustl.edu/).
References and Notes
- 1.Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trinczek B, Biernat J, Baumann K, Mandelkow EM, Mandelkow E. Domains of tau protein, differential phosphorylation, and dynamic instability of microtubules. Mol Biol Cell. 1995;6:1887–1902. doi: 10.1091/mbc.6.12.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Probst A, Langui D, Lautenschlager C, Ulrich J, Brion JP, Anderton BH. Progressive supranuclear palsy: extensive neuropil threads in addition to neurofibrillary tangles. Very similar antigenicity of subcortical neuronal pathology in progressive supranuclear palsy and Alzheimer's disease. Acta Neuropathol. 1988;77:61–68. doi: 10.1007/BF00688244. [DOI] [PubMed] [Google Scholar]
- 5.Probst A, Tolnay M, Langui D, Goedert M, Spillantini MG. Pick's disease: hyperphosphorylated tau protein segregates to the somatoaxonal compartment. Acta Neuropathol. 1996;92:588–596. doi: 10.1007/s004010050565. [DOI] [PubMed] [Google Scholar]
- 6.Goedert M, Falcon B, Clavaguera F, Tolnay M. Prion-like mechanisms in the pathogenesis of tauopathies and synucleinopathies. Curr Neurol Neurosci Rep. 2014;14:495. doi: 10.1007/s11910-014-0495-z. [DOI] [PubMed] [Google Scholar]
- 7.Saito Y, Ruberu NN, Sawabe M, Arai T, Tanaka N, Kakuta Y, Yamanouchi H, Murayama S. Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol. 2004;63:911–918. doi: 10.1093/jnen/63.9.911. [DOI] [PubMed] [Google Scholar]
- 8.de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013;14:389–394. doi: 10.1038/embor.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, Cirrito JR, Patel TK, Hochgrafe K, Mandelkow EM, Holtzman DM. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211:387–393. doi: 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanamandra K, Kfoury N, Jiang H, Mahan TE, Ma S, Maloney SE, Wozniak DF, Diamond MI, Holtzman DM. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80:402–414. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011;118:658–667. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010;30:16559–16566. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Lasagna-Reeves CA, Gerson JE, Singh G, Estes DM, Barrett AD, Dineley KT, Jackson GR, Kayed R. Passive immunization with Tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J Neurosci. 2014;34:4260–4272. doi: 10.1523/JNEUROSCI.3192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckner N, Hanmer J, Davies P, O'Neill MJ, Hutton ML, Citron M. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol Chem. 2011;286:34457–34467. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troquier L, Caillierez R, Burnouf S, Fernandez-Gomez FJ, Grosjean ME, Zommer N, Sergeant N, Schraen-Maschke S, Blum D, Buee L. Targeting phospho-Ser422 by active Tau Immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr Alzheimer Res. 2012;9:397–405. doi: 10.2174/156720512800492503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder SK, Joly-Amado A, Gordon MN, Morgan D. Tau-Directed Immunotherapy: A Promising Strategy for Treating Alzheimer's Disease and Other Tauopathies. J Neuroimmune Pharmacol. 2016;11:9–25. doi: 10.1007/s11481-015-9637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanamandra K, Jiang H, Mahan TE, Maloney SE, Wozniak DF, Diamond MI, Holtzman DM. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann Clin Transl Neurol. 2015;2:278–288. doi: 10.1002/acn3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Rissin DM, Fournier DR, Piech T, Kan CW, Campbell TG, Song L, Chang L, Rivnak AJ, Patel PP, Provuncher GK, Ferrell EP, Howes SC, Pink BA, Minnehan KA, Wilson DH, Duffy DC. Simultaneous detection of single molecules and singulated ensembles of molecules enables immunoassays with broad dynamic range. Anal Chem. 2011;83:2279–2285. doi: 10.1021/ac103161b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L, Piech T, Patel PP, Chang L, Rivnak AJ, Ferrell EP, Randall JD, Provuncher GK, Walt DR, Duffy DC. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010;28:595–599. doi: 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, Hansson O. Plasma tau levels in Alzheimer's disease. Alzheimers Res Ther. 2013;5:9. doi: 10.1186/alzrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- 26.Yamada K, Cirrito JR, Stewart FR, Jiang H, Finn MB, Holmes BB, Binder LI, Mandelkow EM, Diamond MI, Lee VM, Holtzman DM. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci. 2011;31:13110–13117. doi: 10.1523/JNEUROSCI.2569-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musiek ES, Xiong DD, Patel T, Sasaki Y, Wang Y, Bauer AQ, Singh R, Finn SL, Culver JP, Milbrandt J, Holtzman DM. Nmnat1 protects neuronal function without altering phospho-tau pathology in a mouse model of tauopathy. Ann Clin Transl Neurol. 2016;3:434–442. doi: 10.1002/acn3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spencer B, Masliah E. Immunotherapy for Alzheimer's disease: past, present and future. Front Aging Neurosci. 2014;6:114. doi: 10.3389/fnagi.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valera E, Spencer B, Masliah E. Immunotherapeutic Approaches Targeting Amyloid-beta, alpha-Synuclein, and Tau for the Treatment of Neurodegenerative Disorders. Neurotherapeutics. 2016;13:179–189. doi: 10.1007/s13311-015-0397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer's disease. Trends Mol Med. 2015;21:394–402. doi: 10.1016/j.molmed.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Yamada K, Patel TK, Hochgrafe K, Mahan TE, Jiang H, Stewart FR, Mandelkow EM, Holtzman DM. Analysis of in vivo turnover of tau in a mouse model of tauopathy. Mol Neurodegener. 2015;10:55. doi: 10.1186/s13024-015-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 33.Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer's disease pathology. Sci Transl Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potter R, Patterson BW, Elbert DL, Ovod V, Kasten T, Sigurdson W, Mawuenyega K, Blazey T, Goate A, Chott R, Yarasheski KE, Holtzman DM, Morris JC, Benzinger TL, Bateman RJ. Increased in vivo amyloid-beta42 production, exchange, and loss in presenilin mutation carriers. Sci Transl Med. 2013;5:189ra177. doi: 10.1126/scitranslmed.3005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 37.Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, Friedrich S, Dean RA, Gonzales C, Sethuraman G, DeMattos RB, Mohs R, Paul SM, Siemers ER. Safety and biomarker effects of solanezumab in patients with Alzheimer's disease. Alzheimers Dement. 2012;8:261–271. doi: 10.1016/j.jalz.2011.09.224. [DOI] [PubMed] [Google Scholar]
- 38.Asami-Odaka A, Obayashi-Adachi Y, Matsumoto Y, Takahashi H, Fukumoto H, Horiguchi T, Suzuki N, Shoji M. Passive immunization of the Abeta42(43) C-terminal-specific antibody BC05 in a mouse model of Alzheimer's disease. Neurodegener Dis. 2005;2:36–43. doi: 10.1159/000086429. [DOI] [PubMed] [Google Scholar]
- 39.Winkler DT, Abramowski D, Danner S, Zurini M, Paganetti P, Tolnay M, Staufenbiel M. Rapid cerebral amyloid binding by Abeta antibodies infused into beta-amyloid precursor protein transgenic mice. Biol Psychiatry. 2010;68:971–974. doi: 10.1016/j.biopsych.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 40.Castellano JM, Deane R, Gottesdiener AJ, Verghese PB, Stewart FR, West T, Paoletti AC, Kasper TR, DeMattos RB, Zlokovic BV, Holtzman DM. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Abeta clearance in a mouse model of beta-amyloidosis. Proc Natl Acad Sci U S A. 2012;109:15502–15507. doi: 10.1073/pnas.1206446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.d'Abramo C, Acker CM, Schachter JB, Terracina G, Wang X, Forest SK, Davies P. Detecting tau in serum of transgenic animal models after tau immunotherapy treatment. Neurobiol Aging. 2016;37:58–65. doi: 10.1016/j.neurobiolaging.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arai H, Morikawa Y, Higuchi M, Matsui T, Clark CM, Miura M, Machida N, Lee VM, Trojanowski JQ, Sasaki H. Cerebrospinal fluid tau levels in neurodegenerative diseases with distinct tau-related pathology. Biochem Biophys Res Commun. 1997;236:262–264. doi: 10.1006/bbrc.1997.6908. [DOI] [PubMed] [Google Scholar]
- 44.Wagshal D, Sankaranarayanan S, Guss V, Hall T, Berisha F, Lobach I, Karydas A, Voltarelli L, Scherling C, Heuer H, Tartaglia MC, Miller Z, Coppola G, Ahlijanian M, Soares H, Kramer JH, Rabinovici GD, Rosen HJ, Miller BL, Meredith J, Boxer AL. Divergent CSF tau alterations in two common tauopathies: Alzheimer's disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2015;86:244–250. doi: 10.1136/jnnp-2014-308004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature. 2009;461:916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maia LF, Kaeser SA, Reichwald J, Hruscha M, Martus P, Staufenbiel M, Jucker M. Changes in amyloid-beta and Tau in the cerebrospinal fluid of transgenic mice overexpressing amyloid precursor protein. Sci Transl Med. 2013;5:194re192. doi: 10.1126/scitranslmed.3006446. [DOI] [PubMed] [Google Scholar]
- 48.DeMattos RB, Bales KR, Parsadanian M, O'Dell MA, Foss EM, Paul SM, Holtzman DM. Plaque-associated disruption of CSF and plasma amyloid-beta (Abeta) equilibrium in a mouse model of Alzheimer's disease. J Neurochem. 2002;81:229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 49.Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- 51.Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, Meyer RE, Fishburn MW, Cabrera C, Patel PP, Frew E, Chen Y, Chang L, Ferrell EP, von Einem V, McGuigan W, Reinhardt M, Sayer H, Vielsack C, Duffy DC. The Simoa HD-1 Analyzer: A Novel Fully Automated Digital Immunoassay Analyzer with Single-Molecule Sensitivity and Multiplexing. J Lab Autom. 2016;21:533–547. doi: 10.1177/2211068215589580. [DOI] [PubMed] [Google Scholar]