Abstract
T cells are important in B-cell non-Hodgkin's lymphoma immunity, however the function of T cell subsets, including natural killer (iNKT), T helper (Th)17, and T regulatory cells remains to be elucidated. The present study analyzed the frequencies of iNKT, Th17 and T regulatory cells in the peripheral blood of 41 patients with B-cell non-Hodgkin lymphoma at diagnosis, then during and following immunochemotherapy R-CHOP/R-CVP. At lymphoma diagnosis, iNKT and Th17 frequencies were decreased and T regulatory cell frequencies were increased compared with healthy control group. The Th17 cell percentage was lower in patients with a worse prognosis and at a more advanced clinical stage and in contrast, the percentage of T regulatory cells was increased in patients at advanced stages of lymphoma, compared to earlier stages. There was an increase of iNKT and Th17 cells following R-CHOP/R-CVP therapy. In patients that responded, both prior to and following-treatment, percentages of iNKT and Th17 were higher and T regulatory cells were lower compared with patients with subsequent disease progression. Taken together, the results obtained demonstrated the opposing effects of T cell subsets in B-cell lymphoma immunity, with iNKT and Th17 inhibiting and T regulatory cells enhancing tumor growth. These alterations may be caused by malignant B-cells, however there may also be an axis of inverse feedback between T regulatory cells and their interaction with Th17 and iNKT cells.
Keywords: B-NHL, iNKT, Th17, Treg
Introduction
B-cell non-Hodgkin's lymphomas (B-NHL) are a heterogeneous group of malignancies with different etiopathogenesis, clinical presentation, course, prognosis and response to therapy. Many disorders in the immune system that may influence the tumor growth, development and progression have been described in patients with lymphoma. T-cells are considered to be of key importance in tumor immunity. However, while the role of cytotoxic CD8+ T cells, CD4+ Th1 cells and NK cells as the main effector cells with antitumor functions is well established, the functions of more recently discovered T cell populations, like NKT, Th17, and T regulatory cells still remain elusive.
NKT (natural killer T) cells are a subset of T cells sharing the features of T and NK cells. There are three defined NKT cell subtypes. Type I NKT cells (invariant NKT, iNKT) are characterized by canonical T-cell receptor (TCR) α chain (Vα24Jα18 in humans) and semi-invariant TCRβ chain (mainly Vβ11 in humans) that recognize glycolipid α-galactosylceramide-(GalCer) presented by MHC-like CD1d molecule (1). Type II NKT cells are also CD1d-dependent, but express a more diverse TCRα chain (2). Type III NKT cells (NKT-like) are CD1d-independent and express semiinvariant TCRs. iNKT that are predominant and the best characterized population of NKT cells were found to play an important role in tumor rejection. They exert anti-tumor responses mainly indirectly, by the secretion of Th1-type cytokines, activation and recruitment of other effectors, but can also directly kill CD1d-positive malignant cells in a CD1d-dependent manner (3). iNKT cells were demonstrated to suppress cancer cells growth in several tumor models (4–9). High numbers of tumor-infiltrating or circulating iNKT cells number were associated with improved disease outcome in patients with diverse types of cancer (10–12), including lymphoproliferative malignancies, but the data iNKT cells in patients with B-NHL are limited.
Th17 cells, named after their hallmark cytokine-IL-17, are effector T cells that play a pivotal role in the immune response against extracellular pathogens, they are also involved in the pathogenesis of autoimmune and allergic diseases. The studies on their role in cancer has brought divergent results. IL-17 was found to exert anti-tumor functions by recruitment of CD4+, CD8+ T cells, NK cell, neutrophils and dendritic cells to the tumor tissue and by enhancing the NK and cytotoxic T cell activity (13). However it might also promote tumor cell proliferation, angiogenesis, metastasis and invasion. In patients with lung (14) or ovarian cancer (15) the presence of Th17 cell infiltrates in tumor tissue correlated with better prognosis and longer overall survival. Conversely, infiltrations of Th17 cells in tumor microenviroment correlated with poor prognosis in hepatocellular carcinoma (16,17), pancreatic carcinoma (18) and colon cancer (19). In lymphoproliferative diseases, the data are inconsistent. Th17 cells were shown to promote tumor growth and correlate with higher tumor mass in patients with multiple myeloma (MM) (20,21) that probably might be connected the important contribution of inflammation and angiogenesis in pathogenesis of MM, taking into account proinflammatory and proangiogenic properties of IL-17. However in the study of Bryant et al, Th17 cell numbers were higher in patients surviving more than 10 years after the diagnosis of MM, than in patients with a shorter survival (22) and Th17 cells seem to play the protective role of in tumor immunity in patients with chronic lymphocytic leukemia (CLL) (23–25) as well as the other types of B-NHL (26), though there is still only a few studies published on this issue.
T regulatory cells (Tregs) are characterized by the expression of transcription factor FoxP and under physiological conditions their main function is maintaining the immune homeostasis by down-regulation of excessive adaptive immune reactivity. Tregs are the most extensively studied population of regulatory cells in cancer diseases. They were shown to promote cancer development and progression by suppressing anti-tumor immune responses, in some, but not all types of malignancies. The correlation between high numbers of Tregs in tumor microenvironment and short overall survival was described in patients with hepatocellular (27), breast (28) and ovarian cancer (29). Conversely, Treg infiltrates correlated with improved prognosis and survival in patients with colorectal and head and neck cancer (30–32) and some types of lymphoma like classical Hodgkin lymphoma or germinal center-like diffuse large B-cell lymphoma (33).
In the present study we analyzed the frequencies of iNKT, Th17 and Treg cells in peripheral blood samples from 41 patients with B-NHL, their interrelationships and the correlations both with disease activity parameters and tumor burden as well as the response to the first-line immunochemotherapy R-CHOP/R-CVP.
Materials and methods
Patients and samples
Peripheral blood samples were taken from 41 consecutive patients (23 male and 18 female) diagnosed with B-NHL and then treated in St. John of Dukla Lublin Region Cancer Center. In the study group there were 29 patients with diffuse large B-cell lymphoma (DLBCL) and 12 patients with indolent NHL (iNHL), including 6 patients with follicular lymphoma (FL) and 6 patients with marginal zone lymphoma (MZL). The median age of patients was 65 years (range: 24–81). Clinical stage of the disease was established according to the Ann Arbor staging system. Patients with DBCL were divided into risk groups according to the International Prognostic Index (IPI). Clinical characteristics of the study group are summarized in the Table I.
Table I.
Clinical and laboratory characteristics of the study group.
| No of patients | Total number of patients |
|---|---|
| Sex: | |
| Male | 23 |
| Female | 18 |
| NHL subtype | |
| DLBCL/MCL | 29/4 |
| iNHL: | |
| FL | 6 |
| MZL | 6 |
| Bone marrow involvement | |
| (DLBCL/iNHL): | |
| Yes | 5 |
| No | 36 |
| B-symptoms | |
| (DLBCL/iNHL): | |
| Yes | 20/5 |
| No | 9/7 |
| Stage according to Ann Arbor | |
| (DLBCL/iNHL): | |
| I | 0/0 |
| II | 4/4 |
| III | 10/3 |
| IV | 15/5 |
| IPI (DLBCL): | |
| 1 | 3 |
| 2 | 15 |
| 3 | 6 |
| 4 | 1 |
| Median age (years) | 65 |
| Laboratory parameters | |
| (DLBCL); median (range); | |
| WBC (G/l) | 6.77 (3.6–13.8) |
| PLT (G/l) | 238 (125–1002) |
| Hgb (g/dl) | 13.1 (0.13–15.3) |
| LDH (IU/l) | 242 (156–971) |
| Laboratory parameters (iNHL); median (range): | |
| WBC (G/l) | 9.38 (4.45–30.89) |
| PLT (G/l) | 285 (116–876) |
| Hgb (g/dl) | 13 (10.4–15) |
| LDH (IUl) | 198 (148–717) |
| Response to the first line therapy (R-CHOP/R-CVP): DLBCL and Inhl: | |
| Complete response (CR) | 25 |
| Partial response (PR) | 11 |
| Progressive disease (PD) | 5 |
The control group consisted of 20 age-matched healthy donors (12 male, 8 female, median age 57; range: 36–83). Approval for this study was obtained from the Local Ethics Committee. All patients and donors had given their informed consent. None of the patients had autoimmune disease, and none had ongoing infections at the time of the sample taking or had had infections over the previous 3 months. All the patients received immunochemotherapy (6–8 cycles) with R-CHOP (rituximab, cyclophosphamide, vincristine, doxorubicin, prednisone)-31 patients or R-CVP (rituximab, cyclophosphamide, vincristine, prednisone)-10 patients. The blood samples were taken from all the patients at diagnosis before the start of anticancer treatment, before the 3rd cycle and after the completion of the R-CHOP/R-CVP treatment.
Ethics statement
This study was approved by the Ethics Committee of the Medical University of Lublin (No. KE-0254/66/2011). Written informed consent was obtained from all patients with respect to the use of their blood for scientific purposes.
Cell preparation
Peripheral blood samples were collected into heparinized tubes. Fresh samples were stained within 1–2 h and analyzed directly upon completion of staining process. Peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation on Biocoll Separating Solution (Biochrom) for 25 min at 400 × g at room temperature. Interphase cells were removed, washed twice, and resuspended in phosphate-buffered saline (PBS).
Assessment of iNKT cells
Flow cytometry analysis of iNKT cells was performed using monoclonal antibodies (MoAb) anti-iNKT FITC (anti-Vα24 FITC) and anti-CD3 PE (BD Pharmingen) (as described previously (24)).
Intracellular IL-17A analysis
For intracellular IL-17A staining PBMC (2×106/ml) were cultured in RPMI 1640 supplemented with 2 mmol/l L-glutamine, 5% human albumin, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells were stimulated with 25 ng/ml of PMA and 1 µg/ml of ionomycin (Sigma, Germany) in the presence of BD GolgiStop (BD Pharmingen, USA) for 5 h at 37°C in a 5% CO2 atmosphere. Flow cytometry analysis of Th17 cells was performed using MoAbs anti-CD4 FITC, anti-CD3 PE and anti-IL-17A PE (BD Pharmingen) (as described previously (24)).
Analysis of T regulatory (Treg) cells
The percentage of CD4+CD25+FoxP3+ Treg among CD4+ lymphocytes was determined using the Human Treg Flow kit (BioLegend, San Diego, CA, USA) according to the manufacturer's instructions.
Statistical analysis
Statistical analyses were performed with STATISTICA 10.0 PL and Graphpad Prism 5 (Graphpad Software, Inc.). Differences were considered statistically significant with a P-value ≤0.05. The Mann-Whitney U test was applied for statistical comparison of the results between DLBCL, iNHL patients and HV, as well as between patients in different stages of the disease. The Wilcoxon paired test was used to compare the results before during and after therapy. The Spearman rank correlation coefficient was used in correlation tests.
The percentages of circulatory iNKT, Th17 and T regulatory cells were analyzed depending on the clinical stage of lymphoma according to Ann Arbor, IPI, presence of B-symptoms, bone marrow involvement as well as the response to the first line therapy. They were also correlated with laboratory parameters, such as hemoglobin concentration, white blood cells (WBC), platelets (PLT) counts and lactate dehydrogenase (LDH) activity.
Results
Analysis of iNKT cell percentage
At lymphoma diagnosis, median percentage of iNKT cells was lower in patients with B-NHL than in healthy donors (0.40% vs. 0.75%, P<0.05). In patients with DLBCL median percentage of iNKT cells was 0.49% and in patients with indolent NHL was 0.37% (Fig. 1). There were no differences in iNKT numbers depending on the clinical stage of lymphoma or IPI (DLBCL). Pre-treatment iNKT cell percentage was higher in patients who subsequently achieved response to immunochemotherapy (0.56%) than in patients with disease progression (0.28%), P<0.01 (Fig. 2).
Figure 1.
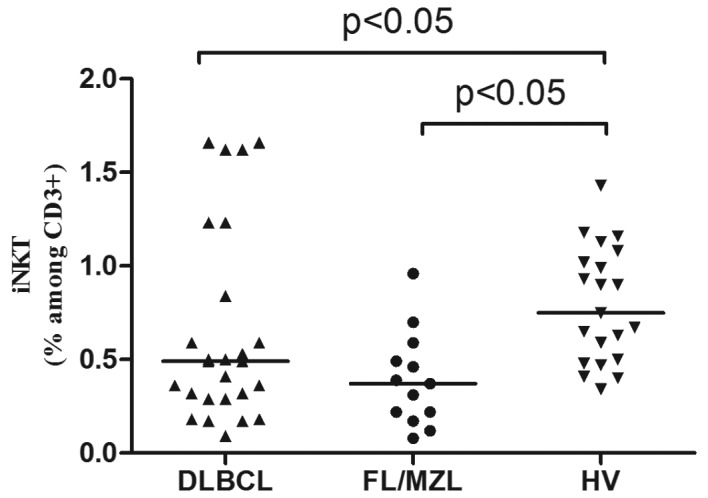
Percentages of iNKT cells in peripheral blood of patients with DLBCL, FL/MZL and healthy volunteers (HV).
Figure 2.
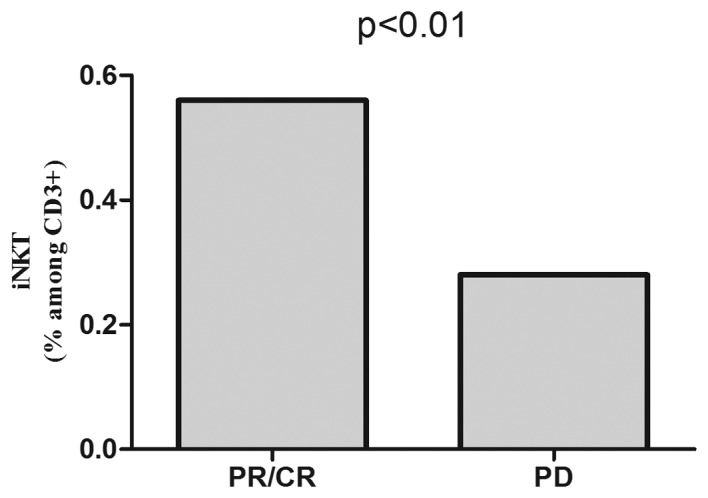
Pre-treatment percentages of iNKT cells in peripheral blood of patients with B-NHL depending on the subsequent response to the first-line immunochemotherapy R-CHOP/R-CVP.
After the completion of R-CHOP/R-CVP, iNKT cell percentage increased in the whole patients' group (0.65%) comparing to the values before (0.40%) or during treatment (0.35%) (Fig. 3A). In patients with response to R-CHOP/R-CVP, the percentage of iNKT cells was higher than in patients with disease progression (0.48% vs. 0.22%, P<0.01) (Fig. 3B) and similar to the values in control group.
Figure 3.
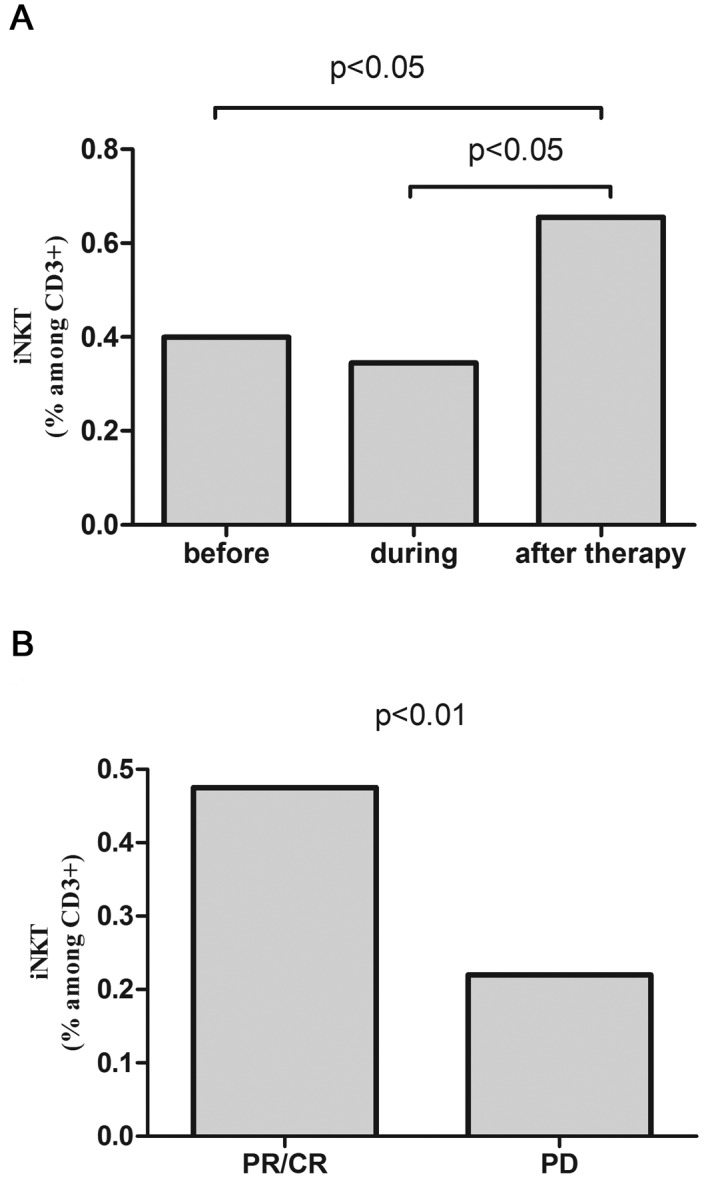
(A) Percentages of iNKT cells in peripheral blood of patients B-NHL before, during and after R-CHOP/R-CVP therapy and (B) iNKT cells percentages after the completion of therapy depending to the achieved response.
Analysis of Th17 cell percentage
Median percentage of Th17 cells in peripheral blood of patients with B-cell NHL was 0,39% and it was significantly lower than in healthy donors (2.95%, P<0.001). In patients with DLBCL the median percentage of Th17 cells was 0.74% and in patients with indolent NHL-0.39% (Fig. 4). Th17 cell percentage was higher in patients with DLBCL with better prognosis (IPI 1/2) than in patients with worse prognosis (IPI 3/4) (0.18% vs. 0.52%, P=0.17) (Fig. 5A) and in patients with iNHL in earlier clinical stages comparing to the advanced ones. Significant difference was noted in between patients with clinical stage II and IV according to Ann Arbor (0.47% vs. 0.12%, P<0.05) (Fig. 5B).
Figure 4.
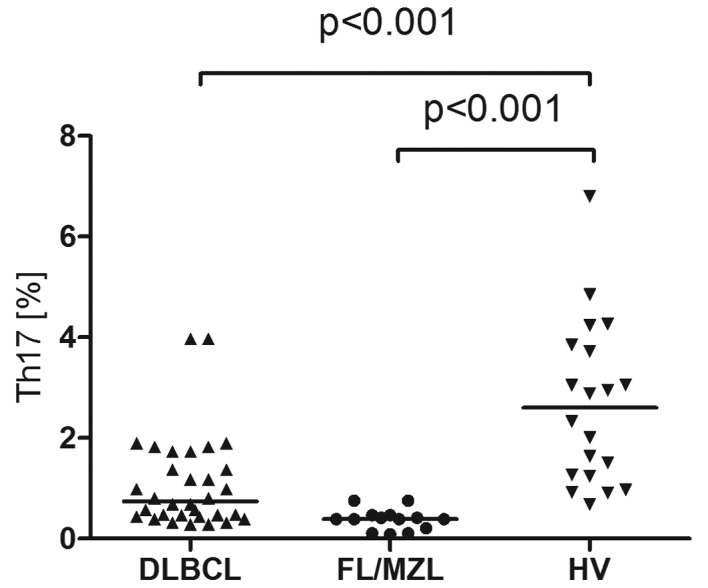
Percentages of Th17 cells in peripheral blood of patients with DLBCL, FL/MZL and healthy volunteers (HV).
Figure 5.
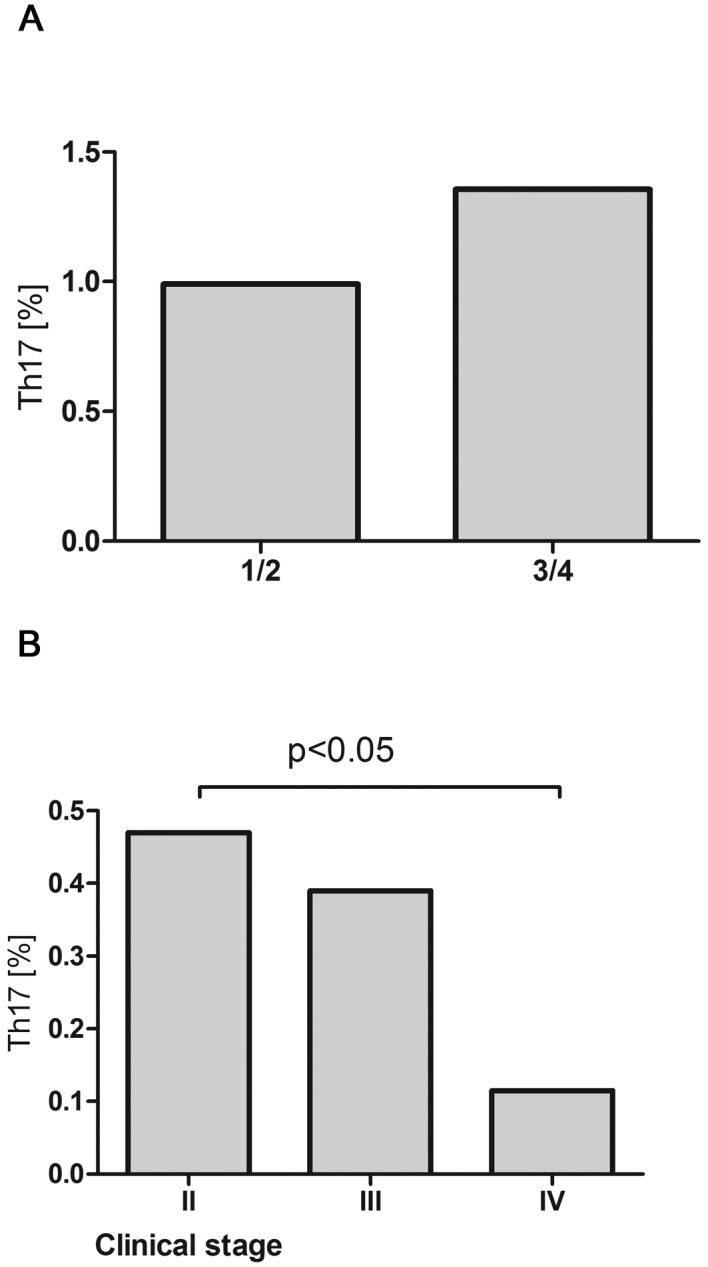
(A) Percentages of Th17 cell in peripheral blood of patients with DLBCL depending on IPI (1/2 vs. 3/4) and (B) in patients with iNHL depending to clinical stage according to Ann Arbor.
Pre-treatment Th17 cell percentages were higher in patients who subsequently achieved response to the therapy (CR/PR) than in patients with disease progression (0.63% vs. 0.28%, P<0.05) (Fig. 6).
Figure 6.
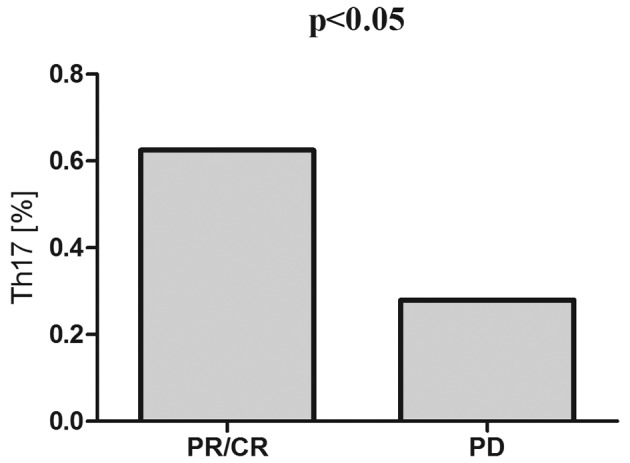
Pre-treatment Th17 cell percentages in patients with B-NHL depending on the subsequent response to first line immunochemotherapy R-CHOP/R-CVP.
During R-CHOP/R-CVP immunochemotherapy, in the whole patients group there was a gradual increase of the percentage of Th17 that after completion of the treatment was significantly higher than before the therapy (1.57% vs. 0.47%, P<0.05) (Fig. 7A). In patients with response to R-CHOP/R-CVP, the percentage of Th17 was higher than in patients with disease progression (1.17% vs. 0.76%, P<0.05) (Fig. 7B), however it was still lower than in the control group (2.95%).
Figure 7.
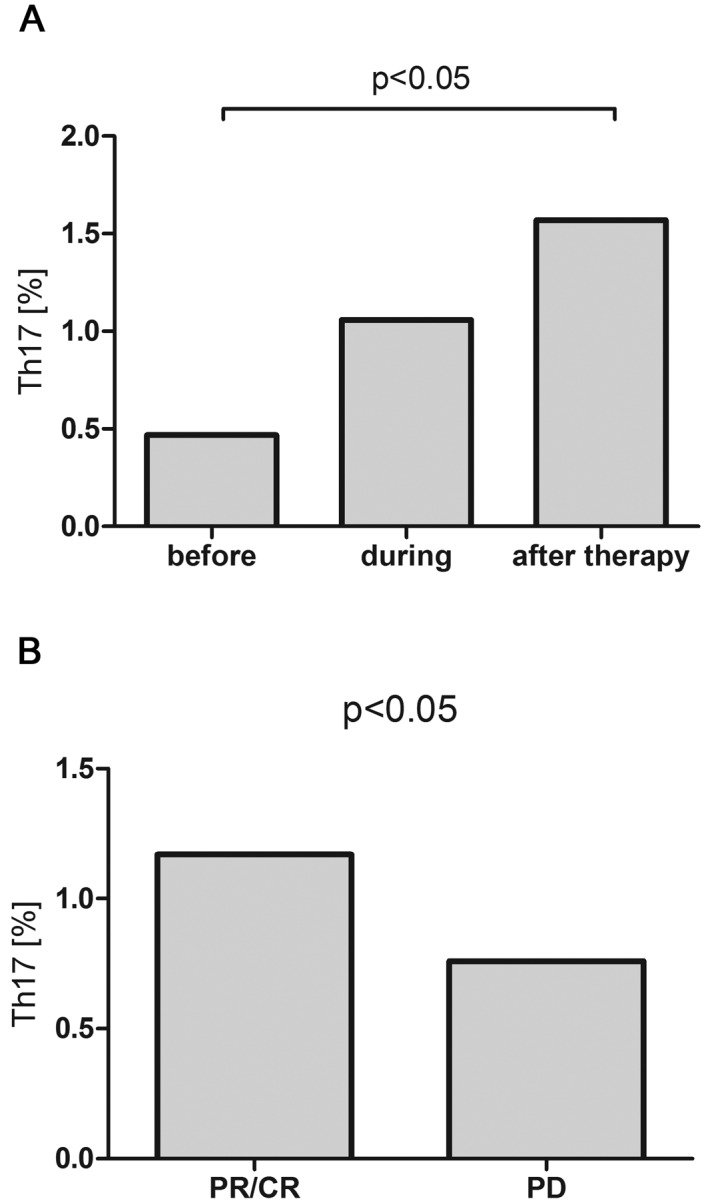
(A) Percentages of Th17 cells in peripheral blood of patients with B-NHL before, during and after R-CHOP/R-CVP therapy and (B) Th17 cells percentages after the completion of therapy depending to the achieved response.
Analysis of T regulatory cells
T regulatory cell percentage was higher in patients with B-NHL compared to healthy volunteers (3.15% vs. 2,1%, P<0.001). In patients with DLBCL, Treg percentage was 3,5% and in patients with iNHL it was 3.56% (Fig. 8). In patients with DLBCL with advanced clinical stage (III/IV), the percentage of T regulatory cells was higher as compared to the earlier stages (I/II) (3.95% vs. 3.15%, P<0.05) (Fig. 9).
Figure 8.
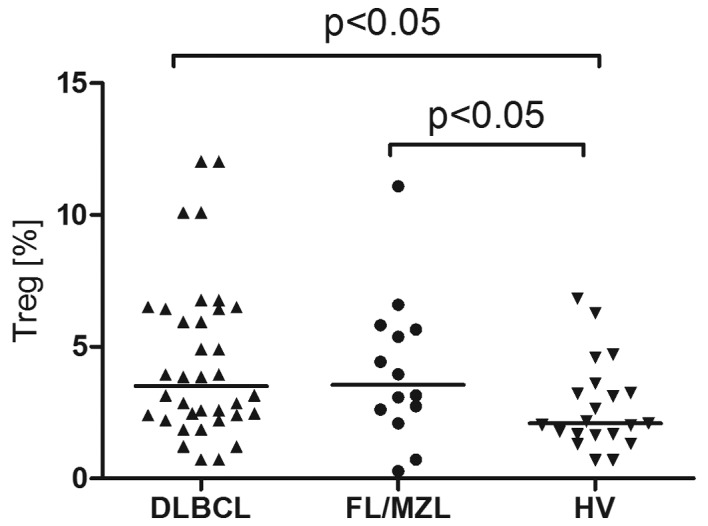
Percentages of T regulatory cells in peripheral blood of patients with DLBCL, FL/MZL and healthy volunteers (HV).
Figure 9.
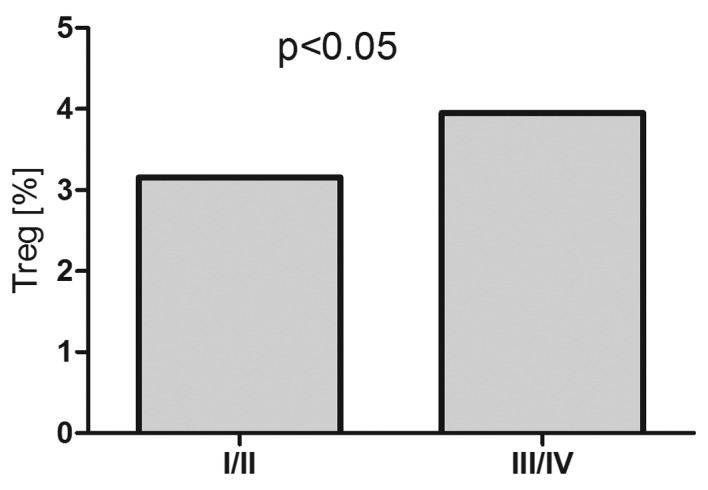
Percentages of T regulatory cells in peripheral blood of patients with DLBCL depending on clinical stage according to Ann Arbor classification.
Pre-treatment T regulatory cell percentage was lower in patients with B-NHL, who subsequently achieved response to the therapy (CR/PR), than in patients with disease progression (3.29% vs. 5.75%, P<0.05) (Fig. 10A). The percentage of T regulatory cells did not change significantly during therapy. After treatment, T regulatory cell percentage in DLBCL patients with CR/PR was significantly lower than in patients with disease progression (2.97% vs. 6.13%, P<0.01), but still higher than in control group (2.1%) (Fig. 10B).
Figure 10.
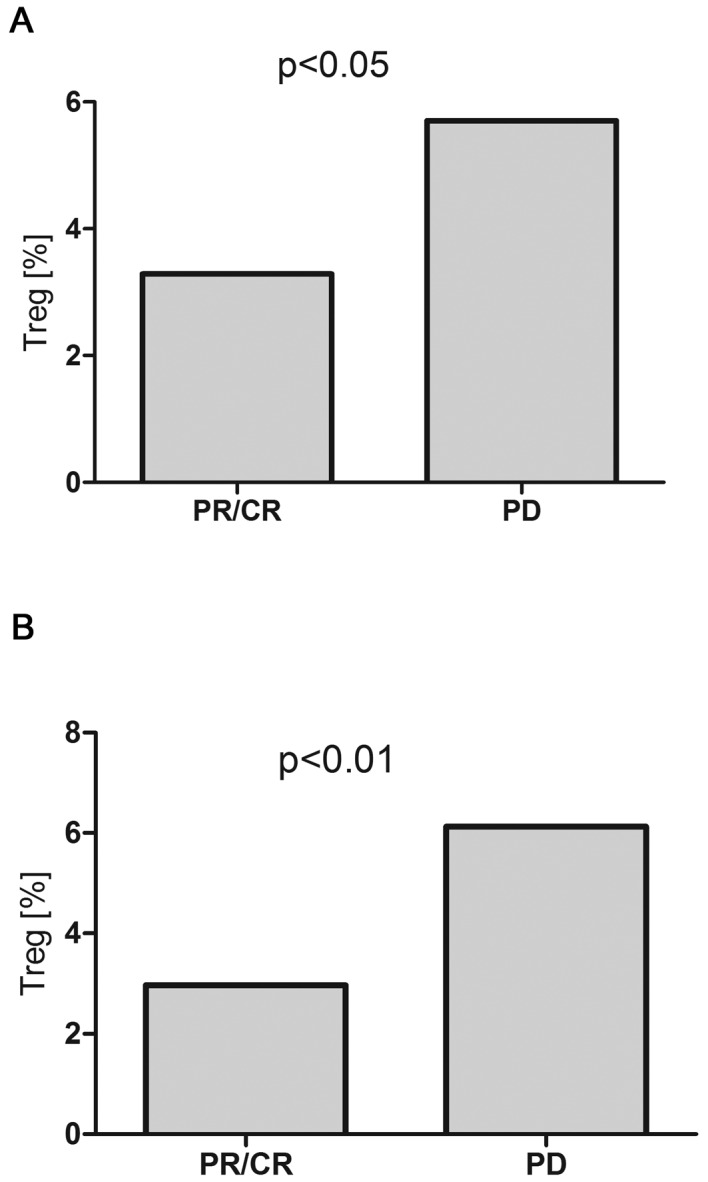
(A) Pre-treatment and (B) after-treatment T regulatory cell percentages in peripheral blood of patients with B-NHL depending on the response to the first line immunochemotherapy R-CHOP/R-CVP.
Correlations between Th17 cells, iNKT cells and T regulatory cells
Both in patients with DLBCL and in patients with iNHL, there was a significant correlation between Th17 cells and iNKT cells (R=0.25; P<0,05; R=0.39; P<0,05, respectively) and in patients with DLBCL there was also inverse correlation between Th17 cells and T regulatory cells (R=−0.242; P<0,05).
Discussion
Extensive studies showed an ambiguous and divergent role of the immune system in cancer development, on one side protecting but on the other side promoting tumor cell growth. As compared to the solid tumors, the interactions between cancer and immune cells are more complicated in lymphoma where malignant cells themselves are derived from immune system cells and many issues concerning the influence of the immune system on lymphoma development remain unknown. In the present study, circulatory iNKT and Th17 cell percentages in patients with B-NHL at lymphoma diagnosis were decreased, and Treg percentage was increased comparing to the healthy control. Lower Th17 cell numbers and higher Treg numbers were observed in patients with more advanced disease, higher tumor mass and worse prognosis. Corresponding results were obtained in patients with both FL/MZL and DLBCL suggesting similar relationships between studied cell populations in indolent and aggressive types of lymphoma. Presented data indicate for an opposing role of studied cell populations in tumor immunity of B-NHL with iNKT and Th17 protecting from and Tregs promoting tumor growth. These results correspond with accumulated evidence showing iNKT cells as important mediators of antitumor immunity in different types of malignancies including B-NHL. iNKT cells were shown to be essential for the survival of mice against B-cell lymphoma (34). Treatment of lymphoma-bearing mice with potent iNKT cell agonist α-GalCer or tumor cell vaccine incorporating α-GalCer resulted in protection from tumor growth and prolonged survival (35,36). Though deficiencies in iNKT numbers and functions correlating with tumor progression was found in patients with diverse type of cancer, including prostate cancer, head and neck cancer, myelodysplastic syndrome and multiple myeloma (37–40), there is only a few data on iNKT cells in patients with B-NHL. Decrease of circulatory iNKT cell percentage was described in patients with hematological malignancies including patients with different types of malignant lymphoma (41). More studies concerned NKT-like cells. Decreased numbers of NKT-like cells in peripheral blood of patients with CLL and DLBCL were found by us and also by the other authors (42–44). Correlation with disease stage and progression indicated for the protective role of NKT-like cells from tumor growth. Further research exploring the role iNKT in B-NHL immunosurveillance would be of significance regarding the potential use of iNKT enhancing strategies in anticancer immunotherapy. Significant correlations between iNKT and Th17 cells frequencies in peripheral blood of patients with B-NHL found in this study suggest their congruous involvement in tumor immunosurveillance. Similarly to iNKT, there is not too much data on Th17 cells in patients with B-NHL, but the results of so far published studies are rather consistent and militate in favor their anti-lymphoma activity. In the recent paper, Lu et al, showed the decrease of circulatory Th17 cells in patients with B-NHL, that were lower at the relapse than at lymphoma diagnosis (26). In patients with CLL lower numbers of Th17 in peripheral blood cells correlated with worse prognosis, and there was a also decrease of Th17 cells along with disease progression (23,24). Similarly to the peripheral blood, in the study by Yang et al (45), Th17 cell numbers were lower in malignant B-cell lymphoma lymph nodes than in benign lymph nodes, and peripheral blood and tonsils of healthy individuals. Frequencies of IL-17 producing CD4+ T cells were lower in patients with FL, MZL and DLBCL compared to MCL, MALT and CLL/SLL (45). In the study of Galand et al (46), there was an adverse correlation between IL-17 production by Th17 cells in tumor tissue and tumor burden in mice primary intraocular B-cell lymphoma, suggesting a protective effect of this cell population from tumor development (46).
In opposition to iNKT and Th17 cells, circulatory Treg frequencies were increased in patients with B-NHL compared to healthy control and their higher numbers in more advanced stages of lymphoma suggest a supportive role in tumor development. These data are in line with earlier studies showing increased frequencies of Treg in peripheral blood of patients diagnosed with B-NHL (47,48) that correlated with tumor burden (49). Immunosupressive effect of Tregs on anti-tumor T-cell responses in lymphoma was demonstrated in several ex vivo studies (49–52). The role of T regulatory cells in B-cell lymphoma is, however ambiguous, because Tregs can also inhibit B-cell lymphoma growth in different mechanisms (53,54) and high tumor infiltrating Tregs were found to correlate with good prognosis in patients with B-NHL (55,56). In the present study, except the higher numbers of Tregs in more advanced clinical stages of lymphoma, we have also found an inverse correlation between circulatory Th17 and Treg cell percentages that might result from the effect of malignant B-cells on T cell differentiation-inhibiting Th17 and promoting Tregs. In vitro studies revealed that malignant B-cells not only induce the conversion of CD4+CD25− T cells into Treg cells (47,56), but also skew the balance between Th17 and Treg cells inhibiting Th17 cells and up-regulating Tregs (45). Moreover, in contrast to Th1 and Th2 cells that are irreversibly differentiated, a plasticity exists between Th17 cells and Tregs, so CD25highFoxP3+ Treg might transdifferentiate into Th17 cells and vice versa depending on the presence of lineage-specific polarizing factors (57). In this study there were no differences in circulating iNKT frequencies depending on the tumor mass and we did not observed direct relationship between Tregs and iNKT cells. However lower frequencies of iNKT in the presence of higher frequencies of Tregs might suggest inhibition of iNKT differentiation by Tregs. This suppressive effect of Tregs on iNKT proliferation and functions was therefore demonstrated in in vitro studies by Azuma et. al. (58). Activated iNKT cells seem also to modulate both numbers and functions of Tregs (59). Another finding in the present study was an increase of iNKT and Th17 cells after immunochemotherapy. In contrast to the Lu et al (26) study, where the numbers of Th17 cells in patients with B-NHL normalized after one or two cycles of chemotherapy, in our study the significant increase was observed after the completion of R-CHOP/R-CVP therapy. In patients with disease progression both iNKT and Th17 cells were significantly lower after therapy than in patients who achieved response, again suggesting possible suppressive effect of tumor on these cell populations. However, higher iNKT and Th17 cell frequencies observed both before and after the therapy in responding patients might also indicate for their important contribution in achieving disease control. Interestingly, Molling et al (60), did not find a restoration of iNKT numbers in patients with solid tumors after the therapy like surgery or radiotherapy, but this discrepancy might result both from different types of malignancy (B-NHL vs. solid tumors) as well as the treatment used (local surgery, radiotherapy vs. systemic immunochemotherapy) (60). In contrast, T regulatory cells percentage was higher before the therapy in patients in whom subsequently disease progression was observed, suggesting their negative impact for treatment results. These data show the potential predictive value of circulatory iNKT, Th17 and Treg in patients with B-NHL.
In conclusion- the results of the present study suggest an opposite role of iNKT, Th17 and Tregs in B-cell lymphoma immunity with iNKT and Th17 inhibiting and Tregs supporting tumor growth. Alterations in studied T cells subsets in peripheral blood of patients with B-NHL might be caused by malignant B-cells, but there might be also an axis of inverse feedbacks between Tregs on one side and Th17 and iNKT cells on the other. Higher baseline frequencies of iNKT and Th17 cells in patients with subsequent response for immunochemotherapy might suggest not only their predictive value but also their supporting role in achieving disease control. Further research on the role of T cells in B-cell lymphoma immunity, involving larger patients' groups and other types of B-cell malignancies would be essential for the understanding B-NHL biology especially in context of introducing novel targeted therapies that were demonstrated to influence T cell populations.
Acknowledgments
This work was supported by research grants of the Medical University of Lublin DS 174.
References
- 1.Metelitsa LS. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin Immunol. 2011;140:119–129. doi: 10.1016/j.clim.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: What's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 3.McEwen-Smith RM, Salio M, Cerundolo V. The regulatory role of invariant NKT cells in tumor immunity. Cancer Immunol Res. 2015;3:425–435. doi: 10.1158/2326-6066.CIR-15-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart TJ, Smyth MJ, Fernando GJ, Frazer IH, Leggatt GR. Inhibition of early tumor growth requires Jα18-positive (natural killer T) cells. Cancer Res. 2003;63:3058–3060. [PubMed] [Google Scholar]
- 6.Tagawa T, Wu L, Anraku M, Yun Z, Rey-McIntyre K, de Perrot M. Antitumor impact of interferon-γ producing CD1d-restricted NKT cells in murine malignant mesothelioma. J Immunother. 2013;36:391–339. doi: 10.1097/CJI.0b013e3182a801f2. [DOI] [PubMed] [Google Scholar]
- 7.Bassiri H, Das R, Guan P, Barrett DM, Brennan PJ, Banerjee PP, Wiener SJ, Orange JS, Brenner MB, Grupp SA, Nichols KE. iNKT cell cytotoxic responses control T-lymphoma growth in vitro and in vivo. Cancer Immunol Res. 2014;2:59–69. doi: 10.1158/2326-6066.CIR-13-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nur H, Rao L, Frassanito MA, De Raeve H, Ribatti D, Mfopou JK, Van Valckenborgh E, De Bruyne E, Vacca A, Vanderkerken K, Menu E. Stimulation of invariant natural killer T cells by α-Galactosylceramide activates the JAK-STAT pathway in endothelial cells and reduces angiogenesis in the 5T33 multiple myeloma model. Br J Haematol. 2014;167:651–663. doi: 10.1111/bjh.13092. [DOI] [PubMed] [Google Scholar]
- 9.Gebremeskel S, Clattenburg DR, Slauenwhite D, Lobert L, Johnston B. Natural killer T cell activation overcomes immunosuppression to enhance clearance of postsurgical breast cancer metastasis in mice. Oncoimmunology. 2015;4:e995562. doi: 10.1080/2162402X.2014.995562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metelitsa LS, Wu HW, Wang H, Yang Y, Warsi Z, Asgharzadeh S, Groshen S, Wilson SB, Seeger RC. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199:1213–1221. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, Imamura M. Increased intratumor Valpha24-positive natural killer T cells: A prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322–7327. doi: 10.1158/1078-0432.CCR-05-0877. [DOI] [PubMed] [Google Scholar]
- 12.Molling JW, Langius JA, Langendijk JA, Leemans CR, Bontkes HJ, van der Vliet HJ, von Blomberg BM, Scheper RJ, van den Eertwegh AJ. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862–868. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 13.Qian X, Chen H, Wu X, Hu L, Huang Q, Jin Y. Interleukin-17 acts as double-edged sword in anti-tumor immunity and tumorigenesis. Cytokine. 2017;89:34–44. doi: 10.1016/j.cyto.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, Tao XN, Shi HZ. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185:6348–6354. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 15.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greten TF, Zhao F, Gamrekelashvili J, Korangy F. Human Th17 cells in patients with cancer: Friends or foe? Oncoimmunology. 2012;1:1438–1439. doi: 10.4161/onci.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan J, Liu XL, Xiao G, Li NL, Deng YN, Han LZ, Yin LC, Ling LJ, Liu LX. Prevalence and clinical relevance of T-helper cells, Th17 and Th1, in hepatitis B virus-related hepatocellular carcinoma. PLoS One. 2014;9:e96080. doi: 10.1371/journal.pone.0096080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He S, Fei M, Wu Y, Zheng D, Wan D, Wang L, Li D. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci. 2011;12:7424–7437. doi: 10.3390/ijms12117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Simone V, Pallone F, Monteleone G, Stolfi C. Role of Th17 cytokines in the control of colorectal cancer. Oncoimmunology. 2013;2:e26617. doi: 10.4161/onci.26617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen CJ, Yuan ZH, Liu YX, Hu GY. Increased numbers of T helper 17 cells and the correlation with clinicopathological characteristics in multiple myeloma. J Int Med Res. 2012;40:556–564. doi: 10.1177/147323001204000217. [DOI] [PubMed] [Google Scholar]
- 21.Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W, Pai C, Amin S, Tai YT, Richardson PG, et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115:5385–5392. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryant C, Suen H, Brown R, Yang S, Favaloro J, Aklilu E, Gibson J, Ho PJ, Iland H, Fromm P, et al. Long-term survival in multiple myeloma is associated with a distinct immunological profile, which includes proliferative cytotoxic T-cell clones and a favourable Treg/Th17 balance. Blood Cancer J. 2013;3:e148. doi: 10.1038/bcj.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain P, Javdan M, Feger FK, Chiu PY, Sison C, Damle RN, Bhuiya TA, Sen F, Abruzzo LV, Burger JA, et al. Th17 and non-Th17 interleukin-17-expressing cells in chronic lymphocytic leukemia: Delineation, distribution, and clinical relevance. Haematologica. 2012;97:599–607. doi: 10.3324/haematol.2011.047316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hus I, Bojarska-Junak A, Chocholska S, Tomczak W, Woś J, Dmoszyńska A, Roliński J. Th17/IL-17A might play a protective role in chronic lymphocytic leukemia immunity. PLoS One. 2013;8:e78091. doi: 10.1371/journal.pone.0078091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lad DP, Varma S, Varma N, Sachdeva MU, Bose P, Malhotra P. Regulatory T-cell and T-helper 17 balance in chronic lymphocytic leukemia progression and autoimmune cytopenias. Leuk Lymphoma. 2015;56:2424–2428. doi: 10.3109/10428194.2014.986479. [DOI] [PubMed] [Google Scholar]
- 26.Lu T, Yu S, Liu Y, Yin C, Ye J, Liu Z, Ma D, Ji C. Aberrant circulating Th17 cells in patients with B-cell Non-Hodgkin's lymphoma. PLoS One. 2016;11:e0148044. doi: 10.1371/journal.pone.0148044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 28.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 29.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 30.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 31.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 32.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V, Laccourreye O, Bruneval P, Fridman WH, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 33.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 34.Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host's innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111:5637–5645. doi: 10.1182/blood-2007-05-092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Sun W, Subrahmanyam PB, Page C, Younger KM, Tiper IV, Frieman M, Kimball AS, Webb TJ. NKT cell responses to B cell lymphoma. Med Sci (Basel) 2014;2:82–97. doi: 10.3390/medsci2020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattarollo SR, West AC, Steegh K, Duret H, Paget C, Martin B, Matthews GM, Shortt J, Chesi M, Bergsagel PL, et al. NKT cell adjuvant-based tumor vaccine for treatment of myc oncogene-driven mouse B-cell lymphoma. Blood. 2012;120:3019–3129. doi: 10.1182/blood-2012-04-426643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molling JW, Kölgen W, van der Vliet HJ, Boomsma MF, Kruizenga H, Smorenburg CH, Molenkamp BG, Langendijk JA, Leemans CR, von Blomberg BM, et al. Peripheral blood IFN-gamma-secreting Valpha24+ Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- 38.Schwemmer B. Natural killer T cells in patients with prostatic carcinoma. Urol Int. 2003;71:146–149. doi: 10.1159/000071836. [DOI] [PubMed] [Google Scholar]
- 39.Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, Dhodapkar MV. Severe and selective deficiency of interferon-gamma-producing invariantnatural killer T cells in patients with myelodysplastic syndromes. Br J Haematol. 2003;122:617–622. doi: 10.1046/j.1365-2141.2003.04465.x. [DOI] [PubMed] [Google Scholar]
- 40.Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, Krasovsky J. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoneda K, Morii T, Nieda M, Tsukaguchi N, Amano I, Tanaka H, Yagi H, Narita N, Kimura H. The peripheral blood Valpha24+ NKT cell numbers decrease in patients with haematopoietic malignancy. Leuk Res. 2005;29:147–152. doi: 10.1016/j.leukres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Bojarska-Junak A, Hus I, Sieklucka M, Wąsik-Szczepanek E, Mazurkiewicz T, Polak P, Dmoszyńska A, Roliński J. Natural killer-like T CD3+/CD16+ CD56+ cells in chronic lymphocytic leukemia: Intracellular cytokine expression and relationship with clinical outcome. Oncol Rep. 2010;24:803–810. doi: 10.3892/or_00000924. [DOI] [PubMed] [Google Scholar]
- 43.Gibson SE, Swerdlow SH, Felgar RE. Natural killer cell subsets and natural killer-like T-cell populations in benign and neoplastic B-cell proliferations vary based on clinicopathologic features. Hum Pathol. 2011;42:679–687. doi: 10.1016/j.humpath.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hus I, Starosławska E, Bojarska-Junak A, Dobrzyńska-Rutkowska A, Surdacka A, Wdowiak P, Wasiak M, Kusz M, Twardosz A, Dmoszyńska A, Roliński J. CD3+/CD16+ CD56+ cell numbers in peripheral blood are correlated with higher tumor burden in patients with diffuse large B-cell lymphoma. Folia Histochem Cytobiol. 2011;49:183–187. doi: 10.5603/FHC.2011.0025. [DOI] [PubMed] [Google Scholar]
- 45.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Malignant B cells skew the balance of regulatory T cells and Th17 cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 2009;69:5522–5530. doi: 10.1158/0008-5472.CAN-09-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galand C, Donnou S, Crozet L, Brunet S, Touitou V, Ouakrim H, Fridman WH, Sautès-Fridman C, Fisson S. Th17 cells are involved in the local control of tumor progression in primary intraocular lymphoma. PLoS One. 2011;6:e24622. doi: 10.1371/journal.pone.0024622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han Y, Wu J, Bi L, Xiong S, Gao S, Yin L, Jiang L, Chen C, Yu K, Zhang S. Malignant B cells induce the conversion of CD4+CD25− T cells to regulatory T cells in B-cell non-Hodgkin lymphoma. PLoS One. 2011;6:e28649. doi: 10.1371/journal.pone.0028649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fozza C, Corda G, Virdis P, Contini S, Barraqueddu F, Galleu A, Isoni A, Cossu A, Dore F, Careddu MG, et al. Derangement of the T-cell repertoire in patients with B-cell non-Hodgkin's lymphoma. Eur J Haematol. 2015;94:298–309. doi: 10.1111/ejh.12417. [DOI] [PubMed] [Google Scholar]
- 49.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 2006;66:10145–10152. doi: 10.1158/0008-5472.CAN-06-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mittal S, Marshall NA, Duncan L, Culligan DJ, Barker RN, Vickers MA. Local and systemic induction of CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma. Blood. 2008;111:5359–5370. doi: 10.1182/blood-2007-08-105395. [DOI] [PubMed] [Google Scholar]
- 52.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 53.Lindqvist CA, Christiansson LH, Thörn I, Mangsbo S, Paul-Wetterberg G, Sundström C, Tötterman TH, Simonsson B, Enblad G, Frisk P, et al. Both CD4+ FoxP3+ and CD4+ FoxP3−T cells from patients with B-cell malignancy express cytolytic markers and kill autologous leukaemic B cells in vitro. Immunology. 2011;133:296–306. doi: 10.1111/j.1365-2567.2011.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grygorowicz MA, Biernacka M, Bujko M, Nowak E, Rymkiewicz G, Paszkiewicz-Kozik E, Borycka IS, Bystydzienski Z, Walewski J, Markowicz S. Human regulatory T cells suppress proliferation of B lymphoma cells. Leuk Lymphoma. 2016;57:1903–1920. doi: 10.3109/10428194.2015.1121260. [DOI] [PubMed] [Google Scholar]
- 55.Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, Montserrat E, Campo E, Banham AH. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–2964. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 56.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. CD70+ non-Hodgkin lymphoma B cells induce Foxp3 expression and regulatory function in intratumoral CD4+ CD25 T cells. Blood. 2007;110:2537–2544. doi: 10.1182/blood-2007-03-082578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye J, Su X, Hsueh EC, Zhang Y, Koenig JM, Hoft DF, Peng G. Human tumor-infiltrating TH17 cells have the capacity to differentiate into IFN-g+ and FOXP3+ T cells with potent suppressive function. Eur J Immunol. 2011;41:936–951. doi: 10.1002/eji.201040682. [DOI] [PubMed] [Google Scholar]
- 58.Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63:4516–4520. [PubMed] [Google Scholar]
- 59.La Cava A, Van Kaer L, Fu-Dong-Shi CD4+CD25+ Tregs and NKT cells: Regulators regulating regulators. Trends Immunol. 2006;27:322–327. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Molling JW, Kölgen W, van der Vliet HJ, Boomsma MF, Kruizenga H, Smorenburg CH, Molenkamp BG, Langendijk JA, Leemans CR, von Blomberg BM, et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]


