Abstract
The upregulation of discoidin domain receptor tyrosine kinase 2 (DDR2) has been reported to be associated with poor prognosis and metastasis in numerous tumor types by inducing epithelial-mesenchymal transition (EMT); however, the expression profile of DDR2 in papillary thyroid carcinoma (PTC) with local metastasis and the effect of DDR2 on PTC cells remain unknown. The aim of the present study was to investigate the expression levels of DDR2 in tumor tissues of patients with PTC with local metastasis and cell lines and to determine the effect of DDR2 on EMT in PTC cells. In the present study, it was demonstrated that DDR2 was significantly increased in tumor tissues of patients with PTC with local metastasis and human PTC cell lines. The overexpression of DDR2 by lentiviral transfection decreased E-cadherin protein, increased Vimentin protein, and promoted cell migration and invasion. The inhibition of DDR2 reversed transforming growth factor-β- and collagen I-induced EMT. EMT induced by DDR2 overexpression was suggested to be dependent on increased Snail1 protein level following extracellular signal-regulated kinase (ERK)2 activation. The inhibition of Snail1 or ERK2 was sufficient to abrogate DDR2-induced PTC cell EMT. In conclusion, these results indicate that DDR2 is upregulated in PTC tissues with local metastasis. Overexpression of DDR2 induced EMT in PTC cells by activating ERK2 and stabilizing Snail1, making it a promising therapeutic target for reducing PTC local or distant metastasis.
Keywords: papillary thyroid carcinoma, discoidin domain receptor 2, epithelial mesenchymal transition, ERK2, Snail1
Introduction
Thyroid cancer is the most prevalent type of endocrine neoplasm globally (1). Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer accounting for ~80% of all thyroid cancer cases worldwide (2). The incidence of PTC has increased over the last four decades (3). The majority of patients with PTC have a good prognosis, and the 5-year survival can achieve >95% (2). However, a small fraction of patients develop PTC with aggressive features. These patients may develop local and distant metastasis, thus the prognosis of these cases is unsatisfactory and the 10-year survival falls to 10% (4,5). Despite the efforts to develop novel treatments for aggressive PTC, these patients have not benefited much (6). Therefore, a better understanding of the mechanisms underlying tumor progression in aggressive PTC is required.
It has been reported that epithelial-mesenchymal transition (EMT) is common in PTC and contributes to PTC metastasis (7). EMT has been extensively studied in tumor metastasis in recent years. The process of EMT is characterized by the loss of cell-to-cell contact, the remodeling of cytoskeleton and the gain of a migratory phenotype (8). EMT can be induced by various signals within the tumor microenvironment, which activate tumor cell intrinsic transcription factors, including Snail1, Snail2, Twist 1 and zinc finger E-box binding homeobox 1 (ZEB1) (9). The defining feature of EMT is the loss of E-cadherin and the acquisition of Vimentin (10). Numerous EMT regulators, including transforming growth factor (TGF)-β, Snail1, Snail2 and Twist have been analyzed in PTC, and are associated with PTC metastasis (11,12).
Discoidin domain receptor tyrosine kinase 2 (DDR2) is a member of the receptor tyrosine kinase (RTK) family that can be activated by collagen I, II, III and X (13,14). Multiple studies have demonstrated that DDR2 is implicated in several cancer cell behaviors, including tumor angiogenesis, cell adhesion and matrix remodeling (15–17). Recent studies have revealed that DDR2 upregulation is predictive of poor prognosis in breast cancer, hepatocellular carcinoma (HCC), head and neck squamous cell carcinoma, and gastric cancer. The possible mechanism is that DDR2 upregulation induces EMT in these cancer cells (15,18–20). Rodrigues et al (21) identified that DDR2 overexpression in aneuploidy PTC was associated with mortality from disease and distant metastasis. However, the expression profile of DDR2 in tumor tissues of PTC patients with local metastasis was not reported.
In the present research, the expression levels of DDR2 were detected in PTC tissues with local metastasis and human cell lines, and the effect of DDR2 on EMT was investigated in PTC cells. The present study aimed to identify the function of DDR2 on EMT in PTC and to reveal the underlying mechanism.
Materials and methods
Cell lines and reagents
The human normal follicular cell line Nthy-ori 3–1, and human PTC cell lines BCPAP, TPC-1 and GLAG-66 were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were maintained in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1% penicillin-streptomycin at 37°C in a humidified atmosphere with 5% CO2.
Primary antibodies against DDR2 (cat no. 12133; dilution, 1:1,000), GAPDH (cat no. 5174; dilution, 1:2,000), E-cadherin (cat no. 3195; dilution, 1:1,000), Vimentin (cat no. 5741; dilution, 1:1,000), phosphorylated (P)-extracellular signal-regulated kinase (ERK)1/2 (cat no. 4370, dilution, 1:1,000), ERK1/2 (cat no. 4695; dilution, 1:1,000), Snail1 (cat no. 3879, dilution, 1:1,000), Snail2 (cat no. 9586; dilution, 1:1,000), Twist1 (cat no. 46702; dilution, 1:1000) and ZEB1 (cat no. 3396; dilution, 1:1,000) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). The human recombinant TGF-β was purchased from PeproTech, Inc. (Rocky Hill, NJ, USA). Human collagen I was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Tissue samples
Human PTC tumor tissues (TT) and adjacent non-tumor tissues (NT) from 10 patients with PTC with local metastasis were obtained from the Department of Head and Neck Surgery, Zhejiang Cancer Hospital (Zhejiang, China). In total, 6 female patients and 4 male patients aged 45 to 73 years (mean age, 64.3 years) were included in the present study. No patients received previous neoadjuvant radiotherapy or chemotherapy. The clinical diagnosis was confirmed by pathological analysis. The present study was approved the Medical Ethics committee of Zhejiang Cancer Hospital and written informed consent was obtained from all patients.
Total RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from cell and tissue samples was extracted using TRIzol® reagent (Takara Bio, Inc., Otsu, Japan). The cDNA was synthesized by Primescript RT master mix (Takara Bio, Inc.). The temperature protocols were as follows: 37°C for 30 min, 85°C for 4 sec and 4°C for 2 min. The RT-qPCR was performed using the ABI PRISM 7300 Sequence Detector (Applied Biosystems; Thermo Fisher Scientific, Inc.) with the SYBR-Green PCR kit (Takara Bio, Inc., Otsu, Japan). The thermal cycling conditions for RT-qPCR was as follows: One cycle of 2 min at 95°C, 40 cycles of 15 sec at 95°C, 15 sec at 58°C and 30 sec at 72°C. The relative mRNA changes were calculated by 2−ΔΔCq method with GAPDH as an internal control (22). The primer sequences were as follows: DDR2 forward, 5′-CTCCCAGAATTTGCTCCAG-3′ and reverse, 5′-GCCACATCTTTTCCTGAGA-3′; E-cadherin forward, 5′-CGAGAGCTACACGTTCACGG-3′ and reverse, 5′-GGGTGTCGAGGGAAAAATAGG-3′; Vimentin forward, 5′-GACGCCATCAACACCGAGTT-3′ and reverse, 5′-CTTTGTCGTTGGTTAGCTGGT-3′; Snail1 forward, 5′-GCTCCACAAGCACCAAGAGT-3′ and reverse, 5′-ATTCCATGGCAGTGAGAAGG-3′; GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′, and reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′.
DDR2 overexpression
The pEGFP-DDR2 cDNA lentiviral vector was obtained from Shanghai GeneChem Co., Ltd. (Shanghai, China). The vector used was GV218 vector, with the elements of Ubi-MCS-EGFP-IRES-Puromycin (Shanghai GeneChem Co., Ltd., Shanghai, China). The colon site was AgeI/AgeI. The recombinant GV218-DDR2 was transfected into HEK293T cells using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). The supernants containing the lentivirus were collected 72 h after transfection. The lentiviral transfection was conducted following the manufacturer's protocol. Briefly, the cells were seeded on a 24-weel plate and cultured overnight. Prior to transfection, the lentivirus was diluted in Enhanced Infection Solution (Eni.S, Shanghai GeneChem Co., Ltd.) to the concentration of 1×107 TU/ml, and polybrene (Shanghai GeneChem Co., Ltd.) was diluted in complete medium to the final concentration of 50 µg/ml. To perform transfection, 300 µl fresh medium mixed with 10 µl lentivirus and 10 µl polybrene were added to the plates and cultured for 8 h. Then the medium was substituted with fresh medium. The cells were then incubated at 37°C for an additional 96 h.
Small interfering RNA (siRNA) transfection
The siRNA was transfected in to PTC cells using Lipofectamine RNAiMAX Regent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's protocol. The siRNA of DDR2 (cat. no. sc-39922), Snail1 (cat. no. sc-39922), ERK2 (cat. no. sc-35335) and scramble siRNAs (cat. no. sc-37007) were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The scramble siRNAs were used as a control. The concentration of all the siRNAs used was 100 nM/l.
Wound healing assay
The cell migration was assessed by performing wound healing assay. A total of 2×104 cells suspended in RPMI 1640 medium with 10% FBS were seeded on each side of a wound healing culture insert (Ibidi, Munich, Germany). The culture inserts were removed to create a cell-free area of ~500 µm following cultivation at 37°C and 5% CO2 for 24 h. Afterwards, the cells were incubated with 1 ml serum-free RPMI 1640 medium for another 48 h. Cell migration was imaged under an Olympus BX51 microscope (Olympus Corporation, Tokyo, Japan).
Transwell migration and invasion assays
Transwell migration assay was performed in 12-well plates with BioCoat control inserts with 8-µm diameter pores (BD Biosciences, Franklin Lakes, NJ, USA). A total of 1×105 cells suspended in 500 µl serum-free RPMI 1640 medium were seeded in the upper chamber. The lower chamber was filled with RPMI 1640 medium with 10% FBS. After 24 h of incubation, cells on the upper membrane were wiped out, while the cells on the opposite side were stained with crystal violet (MCE China, Shanghai, China) for 5 min at room temperature and imaged at magnification, ×40 under an Olympus BX51 microscope. As for the Transwell invasion assay, a BioCoat Matrigel invasion chamber (BD Biosciences) was utilized following the same protocols of the Transwell migration assay. To induce EMT, 6-well plates were coated with collagen I at the concentration of 2 mg/ml, and TPC-1 cells and TPC-1 with DDR2 siRNA transfection were incubated with 5 ng/ml TGF-β for 24 h at 37°C. The ERK1/2 inhibitor U0126 was obtained from Selleck Chemicals (Shanghai, China) and used at the concentration of 1 µM at 37°C for 24 h.
Statistical analysis
All the data are expressed as the mean ± standard deviation. Statistical significance was established using SPSS version 16.0 statistical software package (SPSS, Inc., Chicago, IL, USA). The differences between groups were analyzed using the Student's t-test and one-way analysis of variance with post hoc contrasts by Student-Newman-Keuls test. P<0.05 was considered to indicate a statistically significant difference.
Results
Upregulation of DDR2 is frequent in PTC with local metastasis and human PTC cell lines
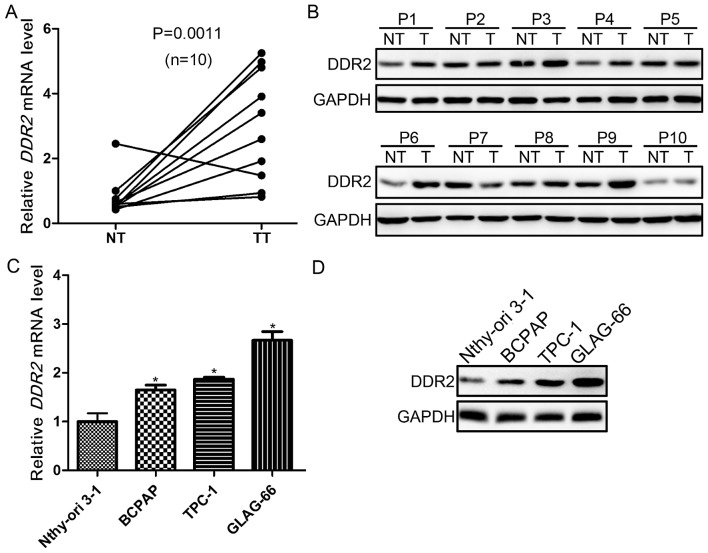
To explore the role of DDR2 in PTC with local metastasis, its expression was evaluated in tumor and adjacent non-tumor tissues from 10 patients with PTC with local metastasis using RT-qPCR, and western blotting. It was demonstrated that DDR2 was upregulated in 9/10 tumor samples. The expression of DDR2 was significantly higher in tumor compared with non-tumor tissues (P=0.0011; Fig. 1A). The protein expression levels were then detected in the tumor and non-tumor tissues. The DDR2 protein level was markedly higher in PTC tumor tissues compared with non-cancerous tissues (Fig. 1B). Furthermore, the DDR2 expression in normal follicular cell line (Nthy-ori 3-1) and PTC cell lines (BAPCP, TPC-1 and GLAG-66) was also determined using RT-qPCR, and western blotting. The DDR2 expression in PTC cell lines was significantly upregulated compared with Nthy-ori 3-1 cells at the mRNA and protein level (Fig. 1C and D).
Figure 1.
Expression of DDR2 in tumor tissues of PTC with local metastasis and human PTC cell lines. (A) The DDR2 mRNA expression level in tumor tissues and adjacent non-tumor tissues from 10 patients with PTC with local metastasis was detected using RT-qPCR (n=10; P=0.0011). (B) The DDR2 protein expression in tumor tissues and adjacent non-tumor tissues from 10 patients with PTC with local metastasis was detected using western blotting. (C) The DDR2 mRNA expression in human normal follicular cell line (Nthy-ori 3-1) and PTC cell lines (BAPCP, TPC-1 and GLAG-66) was detected by RT-qPCR (*P<0.05 vs. Nthy-ori 3-1). (D) The DDR2 protein expression in Nthy-ori 3-1, BAPCP, TPC-1 and GLAG-66 cell lines was detected by western blotting. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; DDR2, discoidin domain receptor tyrosine kinase 2; PTC, papillary thyroid carcinoma; NT, non-tumor tissue; TT, tumor tissue.
Overexpression of DDR2 induces EMT, and promotes invasion and migration of TPC-1 cells
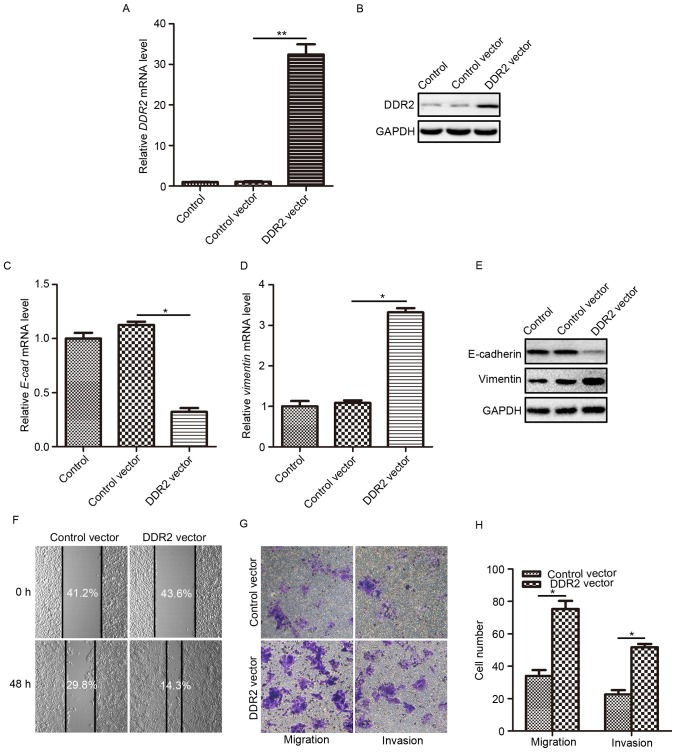
According to the research by Xie et al (18), the upregulation of DDR2 may induce EMT in HCC cells. To fully understand how DDR2 affects the behaviors of PTC cells and to explore whether DDR2 upregulation contributes to PTC cell EMT, the effects DDR2 overexpression on cell invasion and migration were evaluated. TPC-1 cells were infected with lentivirus carrying the human DDR2-expressing vector. The overexpression efficiency was verified using RT-qPCR and western blotting. It was demonstrated that the transduction of the DDR2 vector caused a significant elevation in DDR2 mRNA expression (Fig. 2A) and protein level (Fig. 2B) compared with the control, and control vector groups. Then, the E-cadherin and Vimentin expression levels were determined in TPC-1 cells with elevated DDR2. The epithelial cell marker E-cadherin mRNA level was significantly reduced (Fig. 2C) and the mesenchymal cell marker Vimentin mRNA level was significantly increased (Fig. 2D) following DDR2 upregulation. The changes in E-cadherin and Vimentin protein level were consistent with that at the mRNA level (Fig. 2E).
Figure 2.
DDR2 overexpression induces EMT, and promotes migration and invasion of TPC-1 cells. (A) TPC-1 was transduced with lentivirus carrying the DDR2-expressing plasmid. The overexpression efficiency was verified using (A) RT-qPCR and (B) western blotting. (C) The relative mRNA changes of E-cadherin were detected by RT-qPCR in TPC-1 cells transfected with DDR2 vector or control vector. (D) The relative mRNA changes of Vimentin were detected by RT-qPCR in TPC-1 cells transfected with DDR2 vector or control vector. (E) Western blotting results of E-cadherin and Vimentin in TPC-1 cells transfected with DDR2 vector or control vector. (F) TPC-1 cells were transfected with DDR2 vector or control vector and were incubated in serum-free medium for 48 h. The cell migration capacity was examined using a wound healing assay. The percentage of the cell-free area was quantified and labeled. (G) TPC-1 cells were transfected with DDR2 vector or control vector. The cell migration and invasion capacities were measured using a Transwell assay. (H) Quantification of cells that migrated or invaded to the opposite membrane of Transwell chamber. *P<0.05; **P<0.01. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; DDR2, discoidin domain receptor tyrosine kinase 2; E-cad, E-cadherin.
Subsequently, the migration and invasion capacity of TPC-1 cells following DDR2 upregulation were assessed using wound healing and Transwell assays. The wound healing assay demonstrated that following incubation with serum-free medium for 48 h, cell migration was markedly enhanced by DDR2 upregulation (Fig. 2F). The migration assay and invasion assay conducted using the Transwell chamber system also revealed that DDR2 upregulation significantly promoted TPC-1 cell migration, and invasion (Fig. 2G and H) compared with the control vector-treated cells.
The aforementioned results demonstrated that DDR2 upregulation induced EMT in TPC-1 cells, which facilitated PTC cell migration and invasion.
Downregulation of DDR2 reverses TGF-β- and collagen I-induced EMT in TPC-1 cells
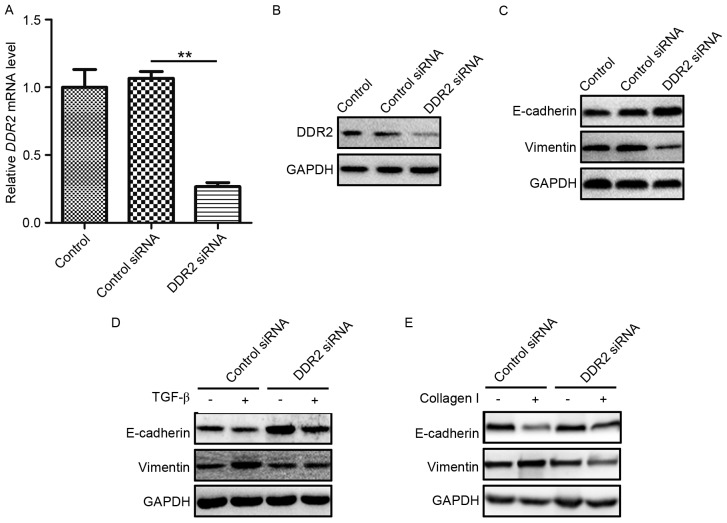
As DDR2 has been reported to possess a prominent role in the pathway of TGF-β-induced EMT, and as a RTK, DDR2 may directly bind to collagen I and induce EMT (15), in the present study, whether DDR2 was a key regulator in the TGF-β-and collagen I-induced EMT was evaluated in PTC. TPC-1 cells were transfected with DDR2 siRNA or a scrambled non-specific siRNA as a negative control. The knockdown efficiency of DDR2 siRNA was verified by RT-qPCR and western blotting (Fig. 3A and B). E-cadherin and Vimentin protein levels were then detected in TPC-1 cells transfected with DDR2 siRNA. DDR2 siRNA markedly increased E-cadherin and reduced Vimentin levels (Fig. 3C).
Figure 3.
Inhibition of DDR2 reverses TGF-β- and collagen I-induced EMT in TPC-1 cells. (A) TPC-1 cells were transfected with DDR2 siRNA. The knockdown efficiency was detected by RT-qPCR. (B) The interfering efficiency of DDR2 siRNA was verified by western blotting. (C) Western blotting results of E-cadherin and Vimentin in TPC-1 cells transfected with DDR2 siRNA or control siRNA. (D) TPC-1 cells transfected with DDR2 siRNA or control siRNA were incubated with 5 ng/ml of TGF-β for 48 h. The E-cadherin and Vimentin protein level was detected by western blotting. (E) TPC-1 cells transfected with DDR2 siRNA or control siRNA were incubated with 2 µg/ml collagen I for 48 h. The E-cadherin and Vimentin protein level was detected by western blotting. **P<0.01. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; DDR2, discoidin domain receptor tyrosine kinase 2; si, small interfering; TGF, transforming growth factor.
To assess the function of DDR2 in TGF-β-induced EMT in PTC cells, TPC-1 cells transfected with DDR2 siRNA were treated with recombinant TGF-β (5 ng/ml) for 24 h and western blotting was performed using lysates from the cells. The silencing of DDR2 by siRNA reversed TGF-β-induced EMT in PTC cells (Fig. 3D). To determine the role in DDR2 in collagen I-induced EMT in PTC cells, TPC-1 cells were transfected with DDR2 siRNA and cultured on plates pre-treated with collagen I (2 mg/ml) for 24 h. The western blotting results demonstrated that DDR2 siRNA reversed the decrease in E-cadherin and the increase in Vimentin protein levels (Fig. 3E).
These results demonstrated that the downregulation of DDR2 reversed TGF-β- and collagen I-induced EMT in PTC cells.
Overexpression of DDR2 increases Snail1 protein expression
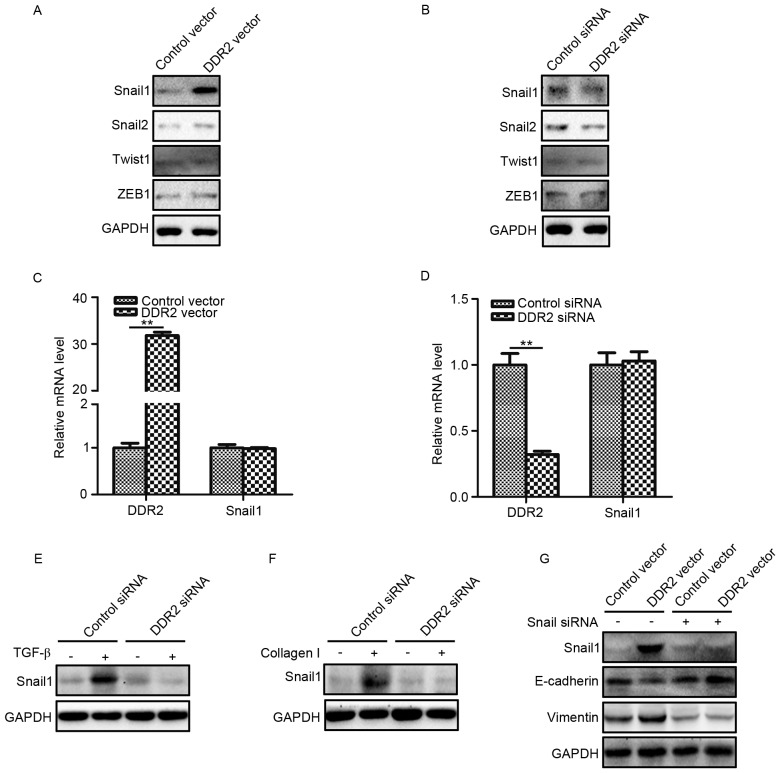
To explore the underlying mechanism by which DDR2 regulated EMT in PTC cells, the protein expression levels of four classical EMT transcription factors (Snail1, Snail2, Twist1 and ZEB1) were determined in TPC-1 cells with increased and decreased DDR2 expression. It was demonstrated DDR2 upregulation increased the Snail1 protein level, while the silencing of DDR2 reduced the Snail1 protein level (Fig. 4A and B). The changes of DDR2 had no significant effect on the levels of Snail2, Twist1 and ZEB1. The RT-qPCR results revealed that the overexpression or depletion of DDR2 did not significantly affect Snail1 mRNA expression (Fig. 4C and D).
Figure 4.
Overexpression of DDR2 increases Snail1 protein expression. (A) TPC-1 cells transfected with DDR2 vector or control vector were subjected to Snail1, Snail2, Twist1 and ZEB1. (B) TPC-1 cells transfected with DDR2 siRNA or control siRNA were subjected to western blotting for Snail1, Snail2, Twist1 and ZEB1 (C) RT-qPCR results of Snail1 in TPC-1 cells transfected with DDR2 vector or control vector. (D) RT-qPCR results of Snail1 in TPC-1 cells transfected with DDR2 siRNA or control siRNA. (E) TPC-1 cells transfected with DDR2 siRNA or control siRNA were incubated with 5 ng/ml of TGF-β for 48 h. The Snail1 protein level was detected by western blotting. (F) TPC-1 cells transfected with DDR2 siRNA or control siRNA were incubated with 2 µg/ml collagen I for 48 h. The Snail1 protein level was detected by western blotting. (G) TPC-1 cells transfected with DDR2 vector or control vector were transduced with Snail1 siRNA. The protein level of Snail1, E-cadherin and Vimentin was detected by western blotting. **P<0.01. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; DDR2, discoidin domain receptor tyrosine kinase 2; si, small interfering; TGF, transforming growth factor; ZEB, zinc finger E-box binding homeobox 1.
The expression of Snail1 in TGF-β- and collagen I-treated TPC-1 cells transduced with DDR2 siRNA were then determined. The results demonstrated that the depletion of DDR2 inhibited TGF-β- and collagen I-induced Snail1 elevation (Fig. 4E and F). To further confirm that Snail1 was the key transcription factor in DDR2 mediated EMT in PTC cells, Snail1 siRNA was adopted to downregulate Snail1 expression in TPC-1 cells transfected with DDR2 vector. It was revealed that Snail1 siRNA abrogated DDR2 overexpression-induced E-cadherin decreases and Vimentin increases (Fig. 4G).
These results suggested that the Snail1 protein level increased by DDR2 may be responsible for DDR2-induced EMT in PTC cells.
Inhibition of ERK2 reverses DDR2-induced Snail1 upregulation and EMT
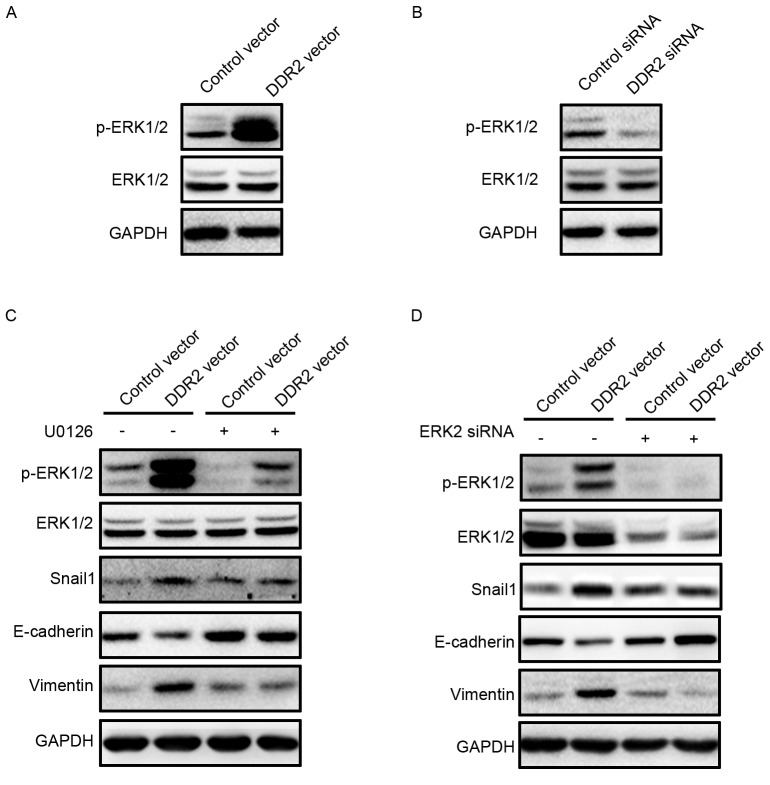
Zhang et al (15) reported that ERK2 may directly phosphorylate Snail1, thus lead to Snail1 nuclear accumulation and increase the Snail1 protein half-life. ERK activation in TPC-1 cells with DDR2 overexpression or depletion was investigated. It was demonstrated that p-ERK1/2 was activated in TPC-1 cells with increased DDR2 (Fig. 5A). DDR2 depletion in TPC-1 cells resulted in a downregulation of p-ERK1/2 (Fig. 5B). The inhibition of p-ERK1/2 by p-ERK1/2 inhibitor U0126 reversed Snail1 upregulation, and the subsequent E-cadherin decrease and Vimentin increase induced by DDR2 overexpression. To further confirm the role of ERK2 in DDR2-induced Snail1 upregulation, ERK2 siRNA was transfected into TPC-1 cells with DDR2 overexpression. ERK2 depletion attenuated the increase in Snail1, Vimentin and the decrease in E-cadherin.
Figure 5.
Inhibition of ERK2 reverses DDR2-induced Snail1 upregulation and EMT. (A) TPC-1 cells transfected with DDR2 vector or control vector were subjected to western blotting for p-ERK1/2. (B) TPC-1 cells transfected with DDR2 siRNA or control siRNA were subjected to western blotting for p-ERK1/2. (C) TPC-1 cells transfected with DDR2 vector or control vector were incubated with 1 µM of U0126 for 24 h. The p-ERK1/2, Snail1, E-cadherin and Vimentin protein level was detected by western blotting. (D) TPC-1 cells transfected with DDR2 vector or control vector were transfected with ERK2 siRNA. The p-ERK1/2, Snail1, E-cadherin and Vimentin protein level was detected by western blotting. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; DDR2, discoidin domain receptor tyrosine kinase 2; si, small interfering; TGF, transforming growth factor; p, phosphorylated; ERK, extracellular signal-regulated kinase.
These data suggested that DDR2 upregulation activated ERK2, which increased the Snail1 protein level and thus promoted EMT in PTC cells.
Discussion
In the present study, it was observed that DDR2 was upregulated in the PTC tumor tissues with local metastasis and human PTC cell lines when compared with non-tumor tissue samples, and a normal follicular cell line. The upregulation of DDR2 induced EMT in PTC cells, and promoted cell migration and invasion. The downregulation of DDR2 reversed TGF-β- and collagen I-induced EMT. The underlying mechanism beneath DDR2-induced EMT in PTC cells was also investigated in the present study. It was revealed that DDR2 overexpression significantly increased Snail1 protein expression without affecting its mRNA expression. The inhibition of Snail1 was sufficient to abrogate DDR2 overexpression-induced PTC cell EMT. In addition, the activation of ERK2 was revealed to increase Snail1 mRNA expression level.
As a RTK, DDR2 has been identified to be upregulated in a series of cancer cells and associated with poor prognosis in numerous cancer types such as breast cancer (15), HCC (18), head and neck squamous cell carcinoma (19) and gastric cancer (20). Zhang et al (15) reported that DDR2 was expressed in human invasive ductal breast cancer and correlated with a worse survival rate. In addition, Xie et al (18) demonstrated that DDR2 was highly expressed in HCC tumor tissues compared with in non-cancerous tissues. The DDR2 overexpression was predictive of short overall survival and disease-free survival in patients with HCC (18). In head and neck squamous cell carcinoma, DDR2 has been identified as upregulated in tumor tissues (19). The frequency and expression intensity of DDR2 were associated with the tumor pathological stage and lymph node metastasis (19). In a newly published study by Wang et al (20), high DDR2 expression in gastric cancer was identified to be associated with multiple tumor locations and intestinal-type gastric cancer. To the best of our knowledge, there are no previous studies regarding the expression profile of DDR2 in tumor tissues of PTC with local metastasis. A previous study by Rodrigues et al (21) demonstrated that DDR2 was overexpressed in aneuploidy PTC, and was associated with disease-associated mortality and distant metastasis. In the current study, it was revealed that DDR2 overexpression was a frequent event in tumor tissues of PTC with local metastasis and human PTC cell lines. However, the correlation between DDR2 overexpression and prognosis was not analyzed, due to lack of long-term follow-up data, which should be further investigated.
DDR2 was an essential mediator of TGF-β1- and collagen-induced EMT (23,24). In tumor tissues, DDR2 overexpression is often positively correlated with Vimentin and negatively with E-cadherin (19,20). DDR2 overexpression in tumor cells has previously been demonstrated to be sufficient in inducing morphological changes, and promoting enhanced migration and invasion, which are essential biological processes in tumor metastasis (23,25). The increased migration and invasion capacity are dependent on the secretion of matrix metalloproteinases (MMP) (26,27). MMP-2 and MMP-9 are two important subsets of downstream target genes of DDR2 signaling (16). Increased DDR2 may promote the generation and secretion of MMP-2, and MMP-9 in HCC and head and neck squamous cell carcinoma (18,19). In the present study, it was observed that the overexpression of DDR2 decreased E-cadherin and increased Vimentin expression levels, and promoted the migration and invasion of TPC-1 cells. It was speculated that the induced EMT, and enhanced migration and invasion were also the results of elevated MMP secretion.
The induced EMT following DDR2 overexpression observed in this study occurred partially due to the activation of ERK2 and the subsequent increase in Snail1 (23). DDR2 overexpression was identified to be associated with ERK activation and increased Snail1 protein level in tumor tissues. The present results suggest that the interaction between DDR2 and collagen I stimulated ERK2 activity, which then directly phosphorylated Snail1, leading to nuclear accumulation, and increased the Snail1 protein half-life. ERK2 activation is essential for Snail1 stabilization and EMT induction (28). DDR2 stabilizes Snail1 protein expression post-transcriptionally without affecting the transcription of Snail1 (15). This was consistent with the findings in the current study whereby overexpression of DDR2 did not change the mRNA expression of Snail1, but increased its protein expression.
In conclusion, the present study revealed that overexpression of DDR2 in tumor tissues of PTC with local metastasis and human PTC cell lines was a frequent event. Furthermore, the results suggest that the upregulation of DDR2 induced EMT, and promoted cell migration and invasion of PTC cells by activating the ERK2/Snail1 signaling pathway, making it a promising therapeutic target for reducing PTC local and distant metastasis.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 3.Jung CK, Little MP, Lubin JH, Brenner AV, Wells SA, Jr, Sigurdson AJ, Nikiforov YE. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014;99:E276–E285. doi: 10.1210/jc.2013-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sipos JA, Mazzaferri EL. Thyroid cancer epidemiology and prognostic variables. Clin Oncol (R Coll Radiol) 2010;22:395–404. doi: 10.1016/j.clon.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 6.Luster M, Weber T, Verburg FA. Differentiated thyroid cancer-personalized therapies to prevent overtreatment. Nat Rev Endocrinol. 2014;10:563–574. doi: 10.1038/nrendo.2014.100. [DOI] [PubMed] [Google Scholar]
- 7.Vasko V, Espinosa AV, Scouten W, He H, Auer H, Liyanarachchi S, Larin A, Savchenko V, Francis GL, de la Chapelle A, et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion; Proc Natl Acad Sci USA; 2007; pp. 2803–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 9.Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 10.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Lv N, Shan Z, Gao Y, Guan H, Fan C, Wang H, Teng W. Twist1 regulates the epithelial-mesenchymal transition via the NF-kappaB pathway in papillary thyroid carcinoma. Endocrine. 2016;51:469–477. doi: 10.1007/s12020-015-0714-7. [DOI] [PubMed] [Google Scholar]
- 12.Buehler D, Hardin H, Shan W, Montemayor-Garcia C, Rush PS, Asioli S, Chen H, Lloyd RV. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol. 2013;26:54–61. doi: 10.1038/modpathol.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leitinger B, Kwan AP. The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol. 2006;25:355–364. doi: 10.1016/j.matbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Carafoli F, Bihan D, Stathopoulos S, Konitsiotis AD, Kvansakul M, Farndale RW, Leitinger B, Hohenester E. Crystallographic insight into collagen recognition by discoidin domain receptor 2. Structure. 2009;17:1573–1581. doi: 10.1016/j.str.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, Keely PJ, Longmore GD. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol. 2013;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D, Ko P, You E, Rhee S. The intracellular juxtamembrane domain of discoidin domain receptor 2 (DDR2) is essential for receptor activation and DDR2-mediated cancer progression. Int J Cancer. 2014;135:2547–2557. doi: 10.1002/ijc.28901. [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Bihan D, Chang F, Huang PH, Farndale RW, Leitinger B. Discoidin domain receptors promote α1β1-and α2β1-integrin mediated cell adhesion to collagen by enhancing integrin activation. PLoS One. 2012;7:e52209. doi: 10.1371/journal.pone.0052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie B, Lin W, Ye J, Wang X, Zhang B, Xiong S, Li H, Tan G. DDR2 facilitates hepatocellular carcinoma invasion and metastasis via activating ERK signaling and stabilizing SNAIL1. J Exp Clin Cancer Res. 2015;34:101. doi: 10.1186/s13046-015-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Lu W, Zhang S, Zhu C, Ren T, Zhu T, Zhao H, Liu Y, Su J. Overexpression of DDR2 contributes to cell invasion and migration in head and neck squamous cell carcinoma. Cancer Biol Ther. 2014;15:612–622. doi: 10.4161/cbt.28181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YG, Xu L, Jia RR, Wu Q, Wang T, Wei J, Ma JL, Shi M, Li ZS. DDR2 induces gastric cancer cell activities via activating mTORC2 signaling and is associated with clinicopathological characteristics of gastric cancer. Dig Dis Sci. 2016;61:2272–2283. doi: 10.1007/s10620-016-4116-3. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues R, Roque L, Espadinha C, Pinto A, Domingues R, Dinis J, Catarino A, Pereira T, Leite V. Comparative genomic hybridization, BRAF, RAS, RET, and oligo-array analysis in aneuploid papillary thyroid carcinomas. Oncol Rep. 2007;18:917–926. [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Walsh LA, Nawshad A, Medici D. Discoidin domain receptor 2 is a critical regulator of epithelial-mesenchymal transition. Matrix Biol. 2011;30:243–247. doi: 10.1016/j.matbio.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang ZL, Fan ZQ, Jiang HD, Qu JM. Selective Cox-2 inhibitor celecoxib induces epithelial-mesenchymal transition in human lung cancer cells via activating MEK-ERK signaling. Carcinogenesis. 2013;34:638–646. doi: 10.1093/carcin/bgs367. [DOI] [PubMed] [Google Scholar]
- 26.Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, Friedman SL. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest. 2001;108:1369–1378. doi: 10.1172/JCI200112373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Mañes S, Brückner K, Goergen JL, Lemke G, Yancopoulos G, et al. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep. 2001;2:446–452. doi: 10.1093/embo-reports/kve094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strippoli R, Benedicto I, Pérez Lozano ML, Cerezo A, López-Cabrera M, del Pozo MA. Epithelial-to-mesenchymal transition of peritoneal mesothelial cells is regulated by an ERK/NF-kappaB/Snail1 pathway. Dis Model Mech. 2008;1:264–274. doi: 10.1242/dmm.001321. [DOI] [PMC free article] [PubMed] [Google Scholar]