Abstract
Lung cancer is a type of malignant tumor derived from the respiratory system, which is the leading cause of cancer-associated mortality worldwide, of which ~80% of cases are attributable to non-small cell lung cancer (NSCLC). A previous study demonstrated that 1α,25-Dihydroxyvitamin D3 (1α,25(OH)2D3), derived from the vitamin D metabolic pathway contributes an antitumor effect. Aberrant expression of the essential enzyme encoding genes, Cytochrome P450 Family 27 Subfamily A Member 1 (CYP27A1), Cytochrome P450 Family 27 Subfamily B Member 1 (CYP27B1), and Cytochrome P450 Family 24 Subfamily A Member 1 (CYP24A1) may be associated with lung cancer. However, a lack of evidence exists concerning the association between CYP27A1, CYP27B1, CYP24A1 expression and NSCLC. The aim of the present study was to investigate the functions of CYP27A1, CYP27B1 and CYP24A1 expression in NSCLC. Lung cancer tissue and para-carcinoma control tissue were collected from patients with NSCLC. Reverse transcription-quantitative polymerase chain reaction was applied to analyze CYP27A1, CYP27B1 and CYP24A1 mRNA expression in lung cancer tissues. An association analysis was performed between the aforementioned metabolic enzymes and patients with NSCLC age, gender, tumor node metastasis (TNM) stage, pathological type, differentiation and prognosis. CYP27B1 and CYP24A1 mRNA were upregulated in NSCLC compared with controls (P<0.05). However, no significant differences in CYP27A1 expression were observed between NSCLC and control. In addition, CYP24A1 expression was not associated with age, sex, smoking or TNM stage, but was associated with pathological type, differentiation and prognosis (P<0.05). CYP27B1 expression was significantly associated with TNM stage, differentiation, and prognosis, but not age, sex, smoking or pathological type. In conclusion, CYP27B1 and CYP24A1 may be considered as independent prognostic factors of NSCLC and may be novel therapeutic targets to assist clinical diagnosis, treatment and prognosis of the disease.
Keywords: vitamin D, Cytochrome P450 Family 27 Subfamily A Member 1, Cytochrome P450 Family 27 Subfamily B Member 1, Cytochrome P450 Family 24 Subfamily A Member 1, non-small cell lung cancer, prognosis
Introduction
Lung cancer is a type of malignant tumor derived from the respiratory system that is further classified into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC accounts for ~80% of lung cancer cases (1). NSCLC includes squamous cell carcinoma, adenocarcinoma and large cell carcinoma; of which adenocarcinoma presents with an increased onset. Adenocarcinoma demonstrates a reduced degree of malignancy compared with SCLC. The clinical symptoms of NSCLC differ depending on tumor size, location and metastatic range (2), and early NSCLC is often insidious, producing no visible symptoms until the disease is at an advanced stage. Due to the high degree of malignancy and rapid progression, the majority of patients are already in the mid-late stage of disease by the time of diagnosis. At this advanced stage of disease, surgical procedures are of no benefit to patients and therefore lung cancer is the principal cause of cancer-associated mortality worldwide (3,4). Due to the changes of lifestyle and environmental pollution aggravation, lung cancer presents an increasing rate of mortality, and younger trend (5,6). As the occurrence and development of lung cancer is multi-factorial, polygenic and multi-level process, there is still a requirement for an accurate diagnosis method in the early stages. However, the effects of treatments for advanced lung cancer are not favorable, with a poor prognosis (7,8). Thus, early detection, early diagnosis, and early treatment are essential to improve the quality of life and survival of patients with lung cancer.
In previous years, vitamin D has been demonstrated to exert a range of biological functions, which include promoting calcium absorption, maintaining bone formation and reducing tumor occurrence and development (9,10). Ultraviolet irradiation and appropriate vitamin D intake help reduce tumor incidence, including breast cancer, prostate cancer, lung cancer and colon cancer (11,12). Conversely, Vitamin D deficiency is associated with an increased incidence of cancer, suggesting that vitamin D content is negatively associated with tumor progression. The primary sources of vitamin D are nutritional or from sunlight. 1α,25-Dihydroxyvitamin D3 (1α,25(OH)2D3) is the main active metabolite of vitamin D which can inhibit lung cancer cell proliferation, activation and progression through promoting apoptosis (13,14). However, 1α,25(OH)2D3 requires activation by the vitamin D metabo1ic pathway (15). Therefore, it is postulated that the aberrant expression of the essential enzymes CYP27A1, CYP27B1, and CYP24A1 may be associated with lung cancer. However, no previous research has been conducted to clarify the association between CYP27A1, CYP27B1 and CYP24A1 expression with NSCLC. The aim of the present study was to investigate CYP27A1, CYP27B1, and CYP24A1 expression in NSCLC.
Materials and methods
Patient selection
A total of 180 patients with NSCLC identified by pathological diagnosis were enrolled in the present study from the Affiliated Hospital of Qingdao University (Qingdao, China) between October 2013 and October 2015. All the selected patients with NSCLC received surgical treatment and included 92 males and 88 females. The age of the patients ranged between 45–87 years, with an average of 62.2±6.1 years of age. A total of 62 cases were diagnosed at stage I, 57 cases at stage II, 34 cases at stage III and 27 cases at stage IV of the disease. Pathologically, 51 patients presented with squamous cell carcinoma, 82 patients with adenocarcinoma and 47 patients with large cell carcinoma. There were 42 cases of poor differentiation, 71 cases of moderate differentiation, and 67 cases of well differentiation. Inclusion criteria for the study were as follows: All subjects were newly diagnosed with lung cancer; all subjects received surgery for the first time; no chemotherapy or radiotherapy was conducted prior to surgery; all subjects had signed an informed consent prior to the study. Exclusion criteria were as follows: Recurrent lung cancer; previously received surgery; previously received chemotherapy or radiotherapy; combined with other disease, including infectious disease, malignant tumor, severe diabetes, severe renal and liver disease, pulmonary fibrosis, bone metabolic disease and systemic autoimmune disease. Cancer tissues and para-carcinoma tissues (defined as controls) were collected and stored at −80°C. The experimental protocols for the present study were pre-approved by the Ethical Committee of the Affiliated Hospital of Qingdao University and written informed consent was obtained from all patients prior to the study.
Chemicals and reagents
The RNA extraction kit and reverse transcription kit were from R & D systems, Inc. (Minneapolis, MN, USA). Other common reagents were from Sangon Biotech Co., Ltd., (Shanghai, China). Real time PCR kit was from Thermo Fisher Scientific, Inc., (Waltham, MA, USA). The real time PCR amplifier was from ABI (Applied Biosystems; Thermo Fisher Scientific, Inc.). DNA amplifier was the PE Gene Amp PCR System 2400 (Applied.Biosystems; Thermo Fisher Scientific, Inc.).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from patient lung tissues using TRIzol® reagent, according to the manufacturer's protocol. Total RNA was reverse transcribed to cDNA using Superscript III reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.). qPCR was performed to detect CYP24A1, CYP27A1 or CYP27B1 relative gene expression using a qPCR kit (Power SYBR™ Green PCR Master Mix; Thermo Fisher Scientific, Inc.), run on the Perkin Elmer Gene Amp PCR System 2400 thermal cycler. The reaction conditions were as follows; 92°C for 1 min, followed by 35 cycles of 90°C for 30 sec, 58°C for 50 sec and 72°C for 35 sec. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was selected as an internal reference gene. Melting curve analysis was performed to verify the target specificity of primers. Relative gene expression levels were quantified using the 2−ΔΔCq method.
Clinical data collection
Patients' age, sex, clinical stage, pathological type, differentiation status and prognosis were recorded and followed up.
Western blot
Total proteins were extracted from tissues by the addition of RIPA lysis buffer (Thermo Fisher Scientific, Inc.) and incubated at 4°C for 30 min, followed by centrifugation at 12,000 × g for 10 min at 4°C. Supernatants were transferred to new tubes for quantification of extracted protein concentrations using a BCA assay. Total proteins (20 µg) were separated on 8–12% SDS-PAGE gel, and transferred to a PVDF membrane. Membranes were blocked by incubation with 5% skimmed milk for 1 h at 25°C. The membranes were incubated with primary antibodies, rabbit polyclonal to CYP24A1 (cat. no. ab96691, Abcam, Cambridge, UK), Rabbit polyclonal to CYP27A1 (cat. no. ab64889, Abcam) or Rabbit monoclonal to CYP27B1 (cat. no. EPR20271, Abcam), (1:1,000) at 4°C overnight. The following day, membranes were further incubated in horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no. ab6721, Abcam; 1:1,000) at 37°C for 1 h. Proteins were detected using enhanced chemiluminescence reagents (Thermo Fisher Scientific, Inc.).
Statistical analysis
Statistical analyses were performed using SPSS v.16.0 software (SPSS; Inc., Chicago, IL, USA) and data were presented as the mean ± standard deviation. Multiple group comparisons were performed using one-way analysis of variance and Fishers's least significant difference test. Enumerate data were presented as percentage and compared by χ2 test. Survival was assessed by Kaplan-Meier analysis. A Cox proportional hazards regression model was used for univariate and multivariate analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
CYP24A1, CYP27A1 and CYP27B1 mRNA expression in lung cancer
qPCR was conducted to analyze CYP24A1 mRNA expression in cancer tissue compared with para-carcinoma control tissue. The results demonstrated that CYP24A1 mRNA expression was significantly upregulated in cancer tissues compared with controls (P<0.05; Fig. 1A). Conversely, CYP27A1 mRNA levels were marginally elevated in cancer tissues compared with controls, but no significant differences were observed (Fig. 1B). However, relative CYP27B1 mRNA expression levels were significantly upregulated in cancer tissues compared with para-carcinoma control tissues (P<0.05; Fig. 1C).
Figure 1.
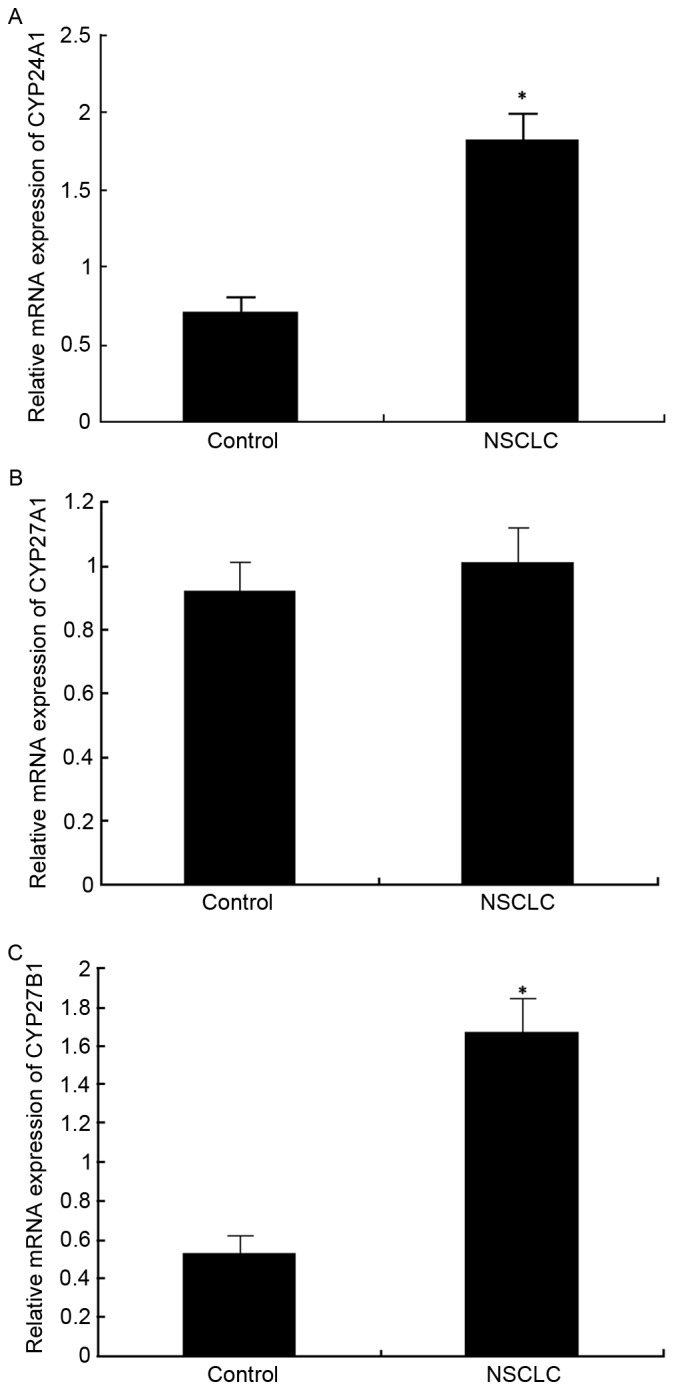
Gene expression levels of (A) CYP24A1, (B) CYP27A1 and (C) CYP27B1 in NSCLC and para-carcinoma tissues. *P<0.05 vs. control. CYP24A1, Cytochrome P450 Family 24 Subfamily A Member 1; CYP27A1, Cytochrome P450 Family 27 Subfamily A Member 1; CYP27B1, Cytochrome P450 Family 27 Subfamily B Member 1; NSCLC, non-small cell lung carcinoma.
CYP24A1, CYP27A1 and CYP27B1 protein expression in lung cancer
Comparable to the mRNA expression profiles, the protein expression of CYP24A1, CYP27A1 and CYP27B1 was elevated in cancer tissues compared with controls (Fig. 2).
Figure 2.
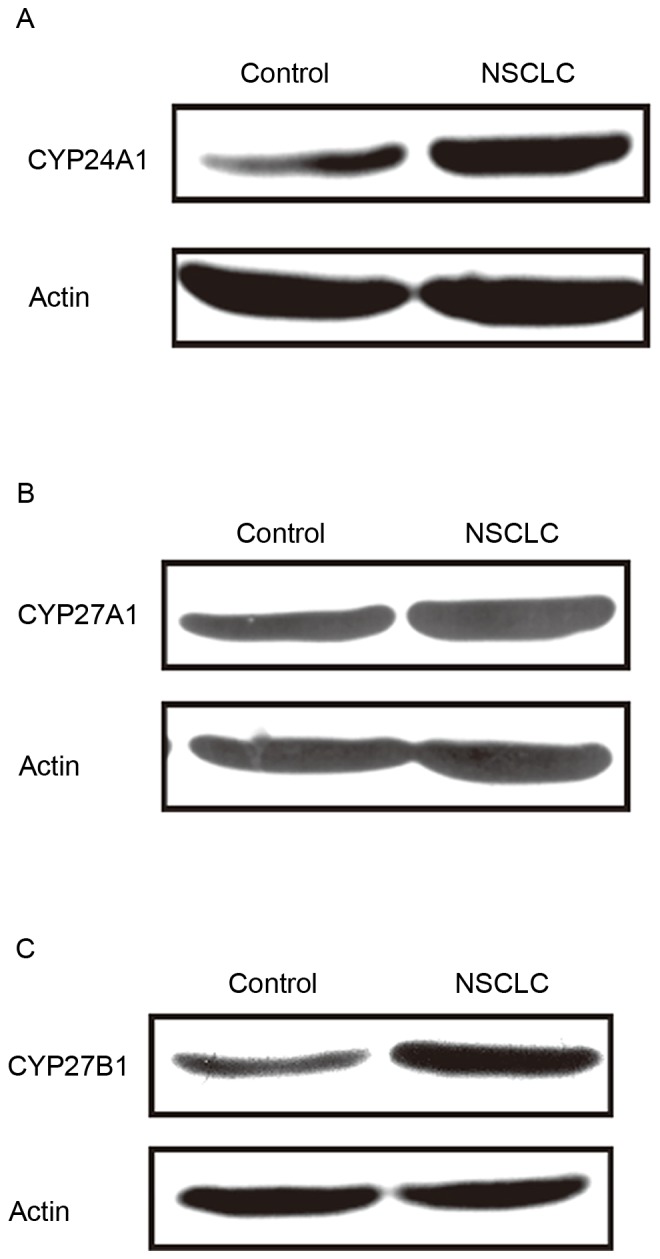
Western blot analysis of CYP24A1, CYP27A1 and CYP27B1 protein expression in NSCLC and para-carcinoma tissues. CYP24A1, Cytochrome P450 Family 24 Subfamily A Member 1; CYP27A1, Cytochrome P450 Family 27 Subfamily A Member 1; CYP27B1, Cytochrome P450 Family 27 Subfamily B Member 1; NSCLC, non-small cell lung carcinoma.
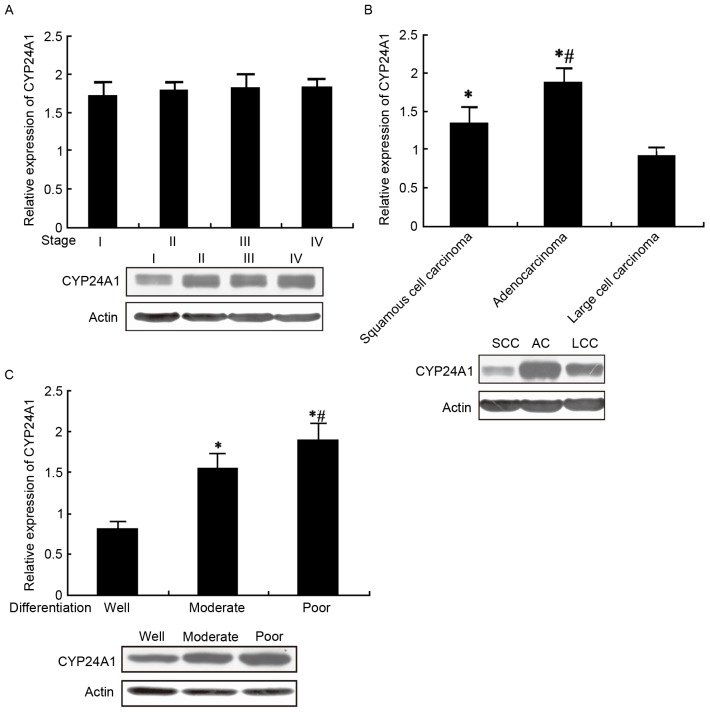
CYP24A1 expression in patients with NSCLC with different pathological characteristics
qPCR and western blot analysis were used to detect CYP24A1 mRNA and protein expression in patients with NSCLC with different TNM stage, pathological type and differentiation status. The results from the present study suggested that CYP24A1 mRNA and protein levels increased with increasing TNM stages, although no statistical differences were observed. CYP24A1 levels were markedly elevated in adenocarcinoma compared with squamous cell carcinoma and large cell carcinoma (P<0.05), but there was no significant difference between squamous and large cell carcinoma. CYP24A1 demonstrated increased expression in the poorly differentiated group, followed by the moderately differentiated compared with well-differentiated groups (P<0.05; Fig. 3A-C).
Figure 3.
CYP24A1 mRNA and protein expression in patients with NSCLC with different pathological characteristics. (A) CYP24A1 mRNA and protein expression in patients with NSCLC with different TNM stages. (B) CYP24A1 mRNA and protein expression in patients with NSCLC with different pathological types *P<0.05 vs. large cell carcinoma; #P<0.05 vs. squamous cell carcinoma; (C) CYP24A1 mRNA and protein expression in NSCLC patients with different differentiation, *P<0.05 vs. well differentiated; #P<0.05 vs. moderately differentiated. CYP24A1, Cytochrome P450 Family 24 Subfamily A Member 1; NSCLC, non-small cell lung carcinoma.
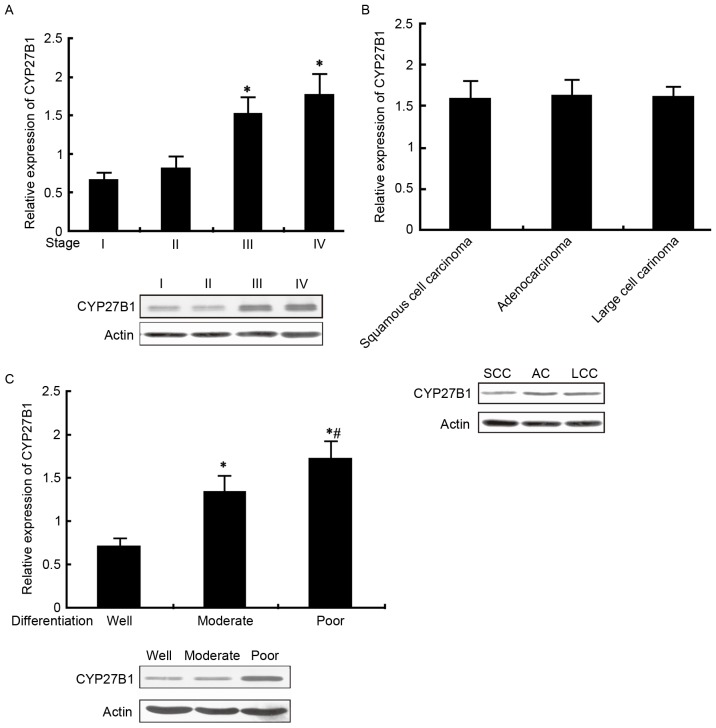
CYP27B1 expression in patients with NSCLC with different pathological characteristics
Analysis by qPCR and western blot was applied to measure CYP27B1 mRNA and protein expression in patients with NSCLC with different TNM stage, pathological type and differentiation status. The results from the present study revealed that CYP27B1 mRNA and protein levels were significantly elevated with increasing TNM stage (III and IV vs. I and II, P<0.05). However, the levels of CYP27B1 were not significantly different between different pathological types. Furthermore, CYP24A1 levels were increased in the poorly differentiated group, followed by moderately differentiated and well differentiated (P<0.05; Fig. 4A-C).
Figure 4.
CYP27B1 mRNA and protein expression in patients with NSCLC with different pathological characteristics. (A) CYP27B1 mRNA and protein expression in patients with NSCLC with different TNM stages. (B) CYP27B1 mRNA and protein expression in patients with NSCLC with different pathological types, *P<0.05, vs. large cell carcinoma; #P<0.05 vs. squamous cell carcinoma; (C) CYP27B1 mRNA and protein expression in patients with NSCLC with different differentiation status, *P<0.05 vs. well differentiated; #P<0.05 vs. moderately differentiated. CYP27B1, Cytochrome P450 Family 27 Subfamily B Member 1; NSCLC, non-small cell lung carcinoma.
CYP24A1 and CYP27B1 mRNA expression levels in patients with NSCLC
The differences in CYP24A1 and CYP27B1 mRNA expression in patients with NSCLC in association with age, sex, smoking and prognosis were compared. The results from the present study demonstrated that comparisons of CYP24A1 mRNA levels in patients with different age, sex and smoking presented no significant differences (Table II). In the prognosis analysis, patients with a survival time of <5 years had increased CYP24A1 mRNA levels compared with patients with a survival time ≥5 years (P<0.05). Similar with CYP24A1, CYP27B1 mRNA levels demonstrated no significant differences in patients with different sex, gender or smoking status. In the prognosis analysis, CYP27B1 mRNA levels were increased in patients with a survival time of <5 years compared with a survival time of ≥5 years (P<0.05; Table II). To compare the differences in survival time between patients with varying CYP24A1 or CYP27B1 expression levels, Kaplan-Meier curve analysis was performed. Patients with increased CYP24A1 or CYP27B1 expression demonstrated a significantly shorter survival time compared with patients with reduced CYP24A1 or CYP27B1 expression (Fig. 5A and B; P<0.05).
Table II.
Comparison of CYP24A1 and CYP27B1 mRNA expression in patients with NSCLC in association with age (60 years-cut off value), sex, smoking and survival time (5 years-cut off value).
| Variable | CYP24A1 mRNA levels | CYP27B1 mRNA levels |
|---|---|---|
| Age, years | ||
| <60 | 1.78±0.31 | 1.61±0.25 |
| ≥60 | 1.82±0.19 | 1.68±0.91 |
| Sex | ||
| Male | 1.81±0.55 | 1.71±0.61 |
| Female | 1.79±0.13 | 1.65±0.53 |
| Smoking | ||
| No | 1.80±0.17 | 1.62±0.47 |
| Yes | 1.83±0.22 | 1.70±0.74 |
| Survival time, years | ||
| <5 | 1.87±0.91 | 1.79±0.78 |
| ≥5 | 1.32±0.26a | 1.21±0.15a |
P<0.05. CYP24A1, Cytochrome P450 Family 24 Subfamily A Member 1; CYP27B1, Cytochrome P450 Family 27 Subfamily B Member 1.
Figure 5.
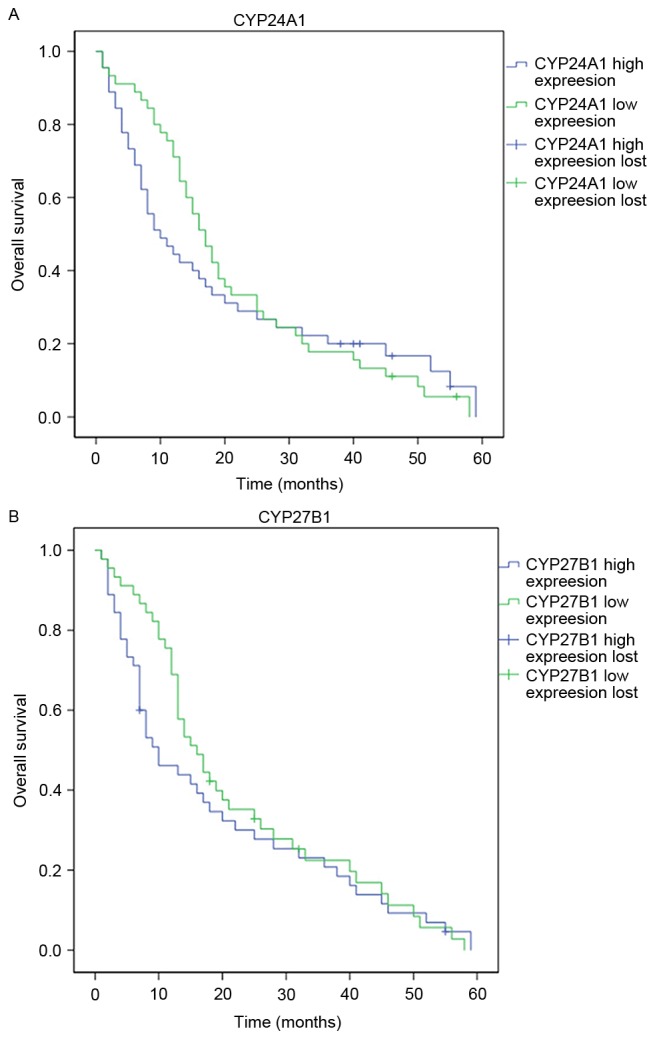
Kaplan-Meier curve analysis of overall survival in patients with high or low expression of (A) CYP24A1 or (B) CYP27B1. CYP24A1, Cytochrome P450 Family 24 Subfamily A Member 1; CYP27B1, Cytochrome P450 Family 27 Subfamily B Member.
Discussion
Lung cancer is the most common type of cancer and recurrent respiratory tract malignant tumor worldwide, and NSCLC accounts for the majority of lung cancer cases with poor prognosis (1). The average survival period for patients with NSCLC is short even following surgery, chemotherapy and radiotherapy (16). Following an in-depth investigation of lung cancer biology and pathogenesis over previous years, there have been notable advances in the diagnosis and treatment of lung cancer. However, the identification of specific biomarkers for the early diagnosis and prognosis of lung cancer is still required (17). The most bioactive metabolite of vitamin D, 1α,25(OH)2D3 has been confirmed to contribute various non-endocrine functions in addition to maintaining calcium stability; including anti-proliferative activity, induction of cell differentiation, promotion of cell cycle stagnation and apoptosis, inhibiting cytopoiesis and regulating tumor occurrence and development (18,19). Vitamin D generates 1α,25(OH)2D3 through the metabolic pathway. CYP27A1, mainly expressed in the liver and intestinal tract, encodes for 25-hydroxylase which metabolizes vitamin D to 1,25(OH)D (20). CYP27B1, widely expressed in multiple organs, encodes 25-hydroxy vitamin D 1-α hydroxylase to catalyze 1,25(OH)D to active 1α,25(OH)2D3 (21). CYP24A1 encodes 25-hydroxy vitamin D3-24-hydroxylase that converts active metabolites to inactive 24,25-dihydroxy vitamin D (21). Thus, CYP27A1, CYP27B1, and CYP24A1 are three key enzymes in the vitamin D metabolic pathway. However, the association between CYP27A1, CYP27B1, and CYP24A1 and NSCLC have not been fully elucidated.
CYP27A1, CYP27B1, and CYP24A1 belong to the cytochrome P450 (CYP450) family. CYP450 is an important microsomal multi-functional oxidase family of hemoglobin enzymes. CYP450 is expressed in various tissues and organs, and has a variety of biological functions, including oxidation and metabolism of endogenous and exogenous substances. Previous studies have demonstrated that CYP24A1 is overexpressed in skin (22), colon (23), ovarian (24), prostate (25) and lung cancer (26). It promotes tumorigenesis through the stimulation of signaling pathways and is associated with poor prognosis (27,28). However, there is still a lack of evidence to support the association of CYP27A1 and CYP27B1 with lung cancer. The present study confirmed that CYP27B1 and CYP24A1 mRNA expression were significantly elevated in patients with NSCLC. CYP24A1 expression was not associated with age, sex, smoking or TNM stage, but with pathological type, differentiation and prognosis. CYP27B1 expression was significantly associated with TNM stage, differentiation and prognosis, as opposed to age, sex, smoking status and pathological type. However, a previous study revealed that increased CYP27B1 expression in tumors was significantly associated with improved overall survival (29). However, this association was attenuated when covariates and confounding variables were adjusted in the regression model. The discrepancies between the present study and the Kong et al (29) study may be due to the clinical characteristics of enrolled patients, including age, sex and disease stage.
It has been suggested that the effect of 1α,25(OH)2D3 on tumor cell development was dependent on CYP27B1 and CYP24A1 activity. In addition, the polymorphism of CYP24A1 and CYP27B1 has been demonstrated to be involved in the modulation of vitamin D metabolism in colon cancer, evidenced by marked changes in enzymatic activity in colon cancer cells, resulting from the polymorphism of CYP24A1 and CYP27B1 (30). These alterations may possibly cause differential 1,25D exposure in colonic cells, which may render individuals more susceptible to the development of colon cancer.
In conclusion, CYP27B1 and CYP24A1 may be treated as independent prognostic factors of NSCLC, and may be new therapeutic targets to assist clinical diagnosis, treatment and prognosis of disease. However, the exact mechanism by how CYP27B1 and CYP24A1 are involved in the pathogenesis of NSCLC remains unclear and requires further investigation.
Table I.
Primer sequences for reverse transcription-quantitative polymerase chain reaction.
| Gene | (Forward 5′-3′) | (Reverse 5′-3′) |
|---|---|---|
| GADPH | AGTGCCAGCCTCGTCTCATAG | CGTTGAACTTGCCGTGGGTAG |
| CYP24A1 | GCCGTATTTAAAAGCCTGTCTGAA | ACCTGGGTATTTAGCATGAGCACTG |
| CYP27A1 | CGTATTGCAATGTCTGAGCCA | ACCTGAATACCTGAATGTACCT |
| CYP27B1 | TATTGCCGTAGCCAATGTCTG | GGTACCTGAATGACCTGAATGCA |
Acknowledgements
The present study was funded by an Academic Fund for Young Scientist Award, The Affiliated Hospital of Qingdao University, Qingdao, China (grant no. 2208).
References
- 1.Gudala S, Khan U, Kanungo N, Bandaru S, Hussain T, Parihar M, Nayarisseri A, Mundluru HP. Identification and pharmacological analysis of high efficacy small molecule inhibitors of EGF-EGFR interactions in clinical treatment of non-small cell lung carcinoma: A computational approach. Asian Pac J Cancer Prev. 2015;16:8191–8196. doi: 10.7314/APJCP.2015.16.18.8191. [DOI] [PubMed] [Google Scholar]
- 2.Xia M, Duan ML, Tong JH, Xu JG. MiR-26b suppresses tumor cell proliferation, migration and invasion by directly targeting COX-2 in lung cancer. Eur Rev Med Pharmacol Sci. 2015;19:4728–4737. [PubMed] [Google Scholar]
- 3.Paz-Ares L, Hirsh V, Zhang L, de Marinis F, Yang JC, Wakelee HA, Seto T, Wu YL, Novello S, Juhász E, et al. Monotherapy administration of sorafenib in patients with non-small cell lung cancer (MISSION) trial: A phase III, multicenter, placebo-controlled trial of sorafenib in patients with relapsed or refractory predominantly nonsquamous non-small-cell lung cancer after 2 or 3 previous treatment regimens. J Thorac Oncol. 2015;10:1745–1753. doi: 10.1097/JTO.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 4.Guerrera F, Tabbò F, Bessone L, Maletta F, Gaudiano M, Ercole E, Annaratone L, Todaro M, Boita M, Filosso PL, et al. The Influence of tissue ischemia time on RNA integrity and patient-derived xenografts (PDX) engraftment rate in a non-small cell lung cancer (NSCLC) biobank. PLoS One. 2016;11:e0145100. doi: 10.1371/journal.pone.0145100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tai CJ, Wang CK, Tai CJ, Tzao C, Lien YC, Hsieh CC, Hsieh CI, Wu HC, Wu CH, Chang CC, et al. Evaluation of safety and efficacy of salvage therapy with sunitinib, docetaxel (Tyxane) and cisplatinum followed by maintenance vinorelbine for unresectable/metastatic nonsmall cell lung cancer: Stage 1 of a simon 2 stage clinical trial [Corrected] Medicine (Baltimore) 2015;94:e2303. doi: 10.1097/MD.0000000000002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson AG, Young K, Balchin K, Owen T, Ashworth A. Reasons for palliative treatments in stage III non-small-cell lung cancer: What contribution is made by time-dependent changes in tumour or patient status? Curr Oncol. 2015;22:399–404. doi: 10.3747/co.22.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki S, Yoshioka Y, Ko R, Katsura Y, Namba Y, Shukuya T, Kido K, Iwakami S, Tominaga S, Takahashi K. Diagnostic significance of cerebrospinal fluid EGFR mutation analysis for leptomeningeal metastasis in non-small-cell lung cancer patients harboring an active EGFR mutation following gefitinib therapy failure. Respir Investig. 2016;54:14–19. doi: 10.1016/j.resinv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Kepka L, Socha J. PET-CT limitations in early stage non-small cell lung cancer: To whom more aggressive approach in radiotherapy and surgery should be directed? J Thorac Dis. 2015;7:1887–1890. doi: 10.3978/j.issn.2072-1439.2015.11.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piotrowska A, Wierzbicka J, Nadkarni S, Brown G, Kutner A, Żmijewski MA. Antiproliferative activity of double point modified analogs of 1,25-dihydroxyvitamin D2 against human malignant melanoma cell lines. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17010076. pii: E76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguirre M, Manzano N, Salas Y, Angel M, Díaz-Couselo FA, Zylberman M. Vitamin D deficiency in patients admitted to the general ward with breast, lung, and colorectal cancer in buenos aires, argentina. Arch Osteoporos. 2016;11:4. doi: 10.1007/s11657-015-0256-x. [DOI] [PubMed] [Google Scholar]
- 11.Wen Y, Da M, Zhang Y, Peng L, Yao J, Duan Y. Alterations in vitamin D signaling pathway in gastric cancer progression: A study of vitamin D receptor expression in human normal, premalignant, and malignant gastric tissue. Int J Clin Exp Pathol. 2015;8:13176–13184. [PMC free article] [PubMed] [Google Scholar]
- 12.Vashi PG, Edwin P, Popiel B, Gupta D. The relationship between circulating 25-hydroxyvitamin D and survival in newly diagnosed advanced non-small-cell lung cancer. BMC Cancer. 2015;15:1012. doi: 10.1186/s12885-015-2043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khayatzadeh S, Feizi A, Saneei P, Esmaillzadeh A. Vitamin D intake, serum Vitamin D levels, and risk of gastric cancer: A systematic review and meta-analysis. J Res Med Sci. 2015;20:790–796. doi: 10.4103/1735-1995.168404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibler EA, Molmenti Sardo CL, Dai Q, Kohler LN, Anderson Warren S, Jurutka PW, Jacobs ET. Physical activity, sedentary behavior, and vitamin D metabolites. Bone. 2016;83:248–255. doi: 10.1016/j.bone.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Z, Yi X, Luo B, Zhu J, Wu Y, Jiang W, Chu H, Yang Z, Li S, Zhu H, et al. Induced sputum deposition improves diagnostic yields of pulmonary alveolar proteinosis: A clinicopathological and methodological study of 17 cases. Ultrastruct Pathol. 2016;40:7–13. doi: 10.3109/01913123.2015.1104404. [DOI] [PubMed] [Google Scholar]
- 17.Ortakoylu MG, Iliaz S, Bahadir A, Aslan A, Iliaz R, Ozgul MA, Urer HN. Diagnostic value of endobronchial ultrasound-guided transbronchial needle aspiration in various lung diseases. J Bras Pneumol. 2015;41:410–414. doi: 10.1590/S1806-37132015000004493. (In English, Portuguese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin K, You Y, Swier V, Tang L, Radwan MM, Pandya AN, Agrawal DK. Vitamin D protects against atherosclerosis via regulation of cholesterol efflux and macrophage polarization in hypercholesterolemic swine. Arterioscler Thromb Vasc Biol. 2015;35:2432–2442. doi: 10.1161/ATVBAHA.115.306132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fedorova OV, Zernetkina VI, Shilova VY, Grigorova YN, Juhasz O, Wei W, Marshall CA, Lakatta EG, Bagrov AY. Synthesis of an endogenous steroidal na pump inhibitor marinobufagenin, implicated in human cardiovascular diseases, is initiated by CYP27A1 via bile acid pathway. Circ Cardiovasc Genet. 2015;8:736–745. doi: 10.1161/CIRCGENETICS.115.001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gascon-Barré M, Demers C, Ghrab O, Theodoropoulos C, Lapointe R, Jones G, Valiquette L, Ménard D. Expression of CYP27A, a gene encoding a vitamin D-25 hydroxylase in human liver and kidney. Clin Endocrinol (Oxf) 2001;54:107–115. doi: 10.1046/j.1365-2265.2001.01160.x. [DOI] [PubMed] [Google Scholar]
- 21.Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys. 2012;523:95–102. doi: 10.1016/j.abb.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mast N, Lin JB, Pikuleva IA. Marketed drugs can inhibit cytochrome P450 27A1, a potential new target for breast cancer adjuvant therapy. Mol Pharmacol. 2015;88:428–436. doi: 10.1124/mol.115.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brożyna AA, Jochymski C, Janjetovic Z, Jóźwicki W, Tuckey RC, Slominski AT. CYP24A1 expression inversely correlates with melanoma progression: Clinic-pathological studies. Int J Mol Sci. 2014;15:19000–19017. doi: 10.3390/ijms151019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horváth HC, Lakatos P, Kósa JP, Bácsi K, Borka K, Bises G, Nittke T, Hershberger PA, Speer G, Kállay E. The candidate oncogene CYP24A1: A potential biomarker for colorectal tumorigenesis. J Histochem Cytochem. 2010;58:277–285. doi: 10.1369/jhc.2009.954339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother Pharmacol. 2006;57:234–240. doi: 10.1007/s00280-005-0059-7. [DOI] [PubMed] [Google Scholar]
- 26.Whitlatch LW, Young MV, Schwartz GG, Flanagan JN, Burnstein KL, Lokeshwar BL, Rich ES, Holick MF, Chen TC. 25-Hydroxyvitamin D-1alpha-hydroxylase activity is diminished in human prostate cancer cells and is enhanced by gene transfer. J Steroid Biochem Mol Biol. 2002;81:135–140. doi: 10.1016/S0960-0760(02)00053-5. [DOI] [PubMed] [Google Scholar]
- 27.Chen G, Kim SH, King AN, Zhao L, Simpson RU, Christensen PJ, Wang Z, Thomas DG, Giordano TJ, Lin L, et al. CYP24A1 is an independent prognostic marker of survival in patients with lung adenocarcinoma. Clin Cancer Res. 2011;17:817–826. doi: 10.1158/1078-0432.CCR-10-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shui IM, Mondul AM, Lindström S, Tsilidis KK, Travis RC, Gerke T, Albanes D, Mucci LA, Giovannucci E, Kraft P. Breast and Prostate Cancer Cohort Consortium Group: Circulating vitamin D, vitamin D-related genetic variation, and risk of fatal prostate cancer in the national cancer institute breast and prostate cancer cohort consortium. Cancer. 2015;121:1949–1956. doi: 10.1002/cncr.29320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee WR, Ishikawa T, Umetani M. The interaction between metabolism, cancer and cardiovascular disease, connected by 27-hydroxycholesterol. Clin Lipidol. 2014;9:617–624. doi: 10.2217/clp.14.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong J, Xu F, Qu J, Wang Y, Gao M, Yu H, Qian B. Genetic polymorphisms in the vitamin D pathway in relation to lung cancer risk and survival. Oncotarget. 2014;6:2573–2782. doi: 10.18632/oncotarget.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs ET, Van Pelt C, Forster RE, Zaidi W, Hibler EA, Galligan MA, Haussler MR, Jurutka PW. CYP24A1 and CYP27B1 polymorphisms modulate vitamin D metabolism in colon cancer cells. Cancer Res. 2013;73:2563–2573. doi: 10.1158/0008-5472.CAN-12-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]