Abstract
Liver X receptors (LXRs) are important members of the nuclear receptor family that were originally determined to function in cholesterol transport and the regulation of immune responses. Synthetic LXR ligands have been developed to treat various diseases including diabetes, Alzheimer's disease and atherosclerosis. Previous studies have suggested that LXRs are also involved in numerous types of cancer and are therefore potential targets for cancer therapeutics. The present review summarizes LXR ligands and their mechanisms of action, the effects of LXRs in different types of cancer and their potential applications in clinical treatment. Together, the studies discussed in the present review indicate that LXRs may be potential targets for cancer therapeutics.
Keywords: liver X receptors, cancer, cancer therapeutics, clinical application
1. Introduction
The nuclear receptor (NR) superfamily consists of important ligand-inducible transcription factors that are involved in various physiological processes, including cell differentiation, embryonic development and metabolism (1,2). NR signaling serves important roles in a number of diseases including cancer, diabetes and metabolic diseases (1–3). Liver X receptors (LXRs) are important members of the NR family that were originally identified as major sensors of dietary cholesterol (4). Two LXRs, LXRα (NR1H3) and LXRβ (NR1H2), were initially identified in a human liver cDNA library screen as orphan receptors (5–7). LXRs are involved in cholesterol synthesis and transport, glucose homeostasis, and the modulation of inflammatory and immune responses (8–10). LXRs exhibit the typical structure of the NR superfamily. LXRα and LXRβ harbor four distinct domains: i) An N-terminal activation domain (AF-1); ii) a DNA-binding domain with two zinc fingers; iii) a hinge domain that binds co-repressors in the absence of ligand; and iv) a C-terminal domain that contains a hydrophobic ligand-binding domain and a transactivation domain (11,12).
2. LXR ligands and mechanism of action
LXRs were initially identified as orphan receptors. However, later studies identified oxysterols, or oxidized metabolites of cholesterol, as the endogenous ligands of LXRα and LXRβ (13,14). Furthermore, 22(R)-hydroxycholesterol, 20(S)-hydroxycholesterol, 24-hydroxycholesterol, 7α-hydroxycholesterol and 27-hydroxycholesterol also activate LXRs (13,15). Additionally, microbial and plant-derived sitosterol, sitostanol and acanthoic acid from Rollinia function as LXR agonists (16,17). Since the initial identification of endogenous LXR ligands, synthetic ligands have been developed by numerous pharmaceutical companies. T0901317 was the first synthetic LXR agonist to be developed, and is the most widely used in basic research. However, this compound may also activate two other NRs: Farnesoid X receptor and pregnane X receptor (18,19). A more selective synthetic agonist, GW3965, was identified in a screen of GlaxoSmithKline (Brentford, UK) compounds (20). However, neither of these agonists is used therapeutically owing to their temporary hypertriglyceridemic effects (20,21). More recently, promising synthetic ligands including 22(E)-ergost-22-ene-1α, 2β-diol and N,N-dimethyl-3β-hydroxycholenamide have been developed with fewer side effects and greater potential for use in clinical testing (22,23).
In the absence of a ligand, LXRs form obligate heterodimers with the retinoid X receptor. These heterodimers bind to target gene promoters harboring the conserved LXR-response element, which consists of two direct repeats of AGGTCA separated by four nucleotides or one nucleotide. The heterodimer forms a complex with NR co-repressor (NCoR) to block transcription (24). Upon ligand binding to the heterodimer, NCoR is released from the complex and co-activators are recruited, leading to chromatin remodeling and target gene transcription (12).
3. Effects of LXRs in different types of cancer
LXRs and colon cancer
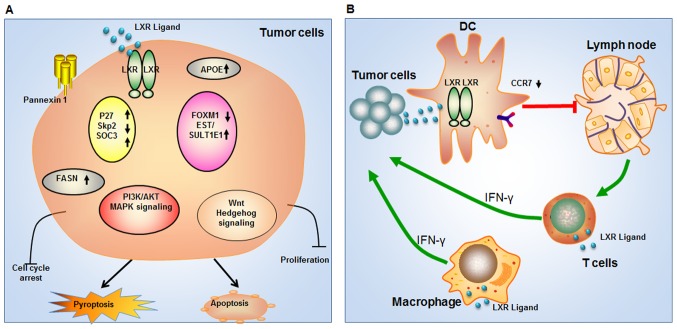
LXR agonists suppress colon carcinogenesis by several mechanisms (Fig. 1A). Uno et al (25) demonstrated that LXR agonists block the activation of Wnt signaling by suppressing the transactivation activity of β-catenin. In HCT116 cells, LXR agonists decrease the mRNA expression of β-catenin target genes, including MYC proto-oncogene, matrix metalloproteinase 7 and bone morphogenetic protein 4. Notably, in colon cancer cells, LXR agonists inhibit endogenous β-catenin activity and cell proliferation without inducing apoptosis. Consistent with this result, LXR ligands induce pyroptosis, rather than apoptosis, in human and murine colon cancer cells. Pyroptosis is dependent on caspase-1 activation. More importantly, LXRs also serve non-genomic roles due to the differential cytoplasmic localization of LXRβ. LXRβ is predominantly located in the cytoplasm of colon cancer cells and induces pyroptosis when bound by ligand (26,27). Previously, a study revealed that LXR activation leads to cell cycle arrest in colon cancer cell lines. Furthermore, proliferation markers in the colon are significantly increased in LXRαβ(−/−) mice compared with wild-type mice (28). Together, inhibition of the Wnt signaling pathway and cell cycle arrest serve important roles during LXR ligand-dependent inhibition of colon cancer cell proliferation (Table I). Ligand-mediated activation of LXR may suppress the growth of colon cancer (29).
Figure 1.
(A and B) Model of LXR agonist activation in various types of cancer and tumor immunity. LXR, liver X receptor; APOE, apolipoprotein E; p27, cyclin-dependent kinase inhibitor 1B; Skp2, S-phase kinase-associated protein 2; SOC3, suppressor of cytokine signaling 3; FOXM1, forkhead box M1; EST, estrogen sulfotransferase; SULT1E1, sulfotransferase family 1E member 1; FASN, fatty acid synthase; PI3K, phosphoinositide 3-kinase; MAPK, mitogen-activated protein kinase; IFN-γ, interferon-γ; AKT, protein kinase B; CCR7, C-C chemokine receptor type 7; DC, dendritic cell.
Table I.
Effects of LXR in different types of cancer.
| Ligand | Tumor type | Molecular mechanism | (Refs.) |
|---|---|---|---|
| LXRα and LXRβ | Colon | Wnt signaling pathway, cell cycle arrest and pyroptosis | (25–29) |
| LXRα and LXRβ | Prostate | AKT survival signaling, MAPK transduction pathways | (29–34) |
| LXRα and LXRβ | Breast | EST, E2F family members | (35–39) |
| LXRβ | Pancreatic | Inhibits cell proliferation, cell cycle arrest | (40,41) |
LXR, liver X receptor; AKT, protein kinase B; MAPK, mitogen-activated protein kinase; EST, estrogen sulfotransferase; E2F, E2 factor.
LXRs and prostate cancer
Liao and colleagues were the first to report that the LXR agonist T0901317 inhibits the proliferation of human prostate LNCaP cells (30). T0901317 decreases the number of cells in S-phase and increases the expression of the cyclindependent kinase inhibitor p27 (Fig. 1A). LXRα expression levels are associated with the sensitivity to T0901317-mediated growth inhibition. Additionally, T0901317-treated nude mice exhibited decreased growth of xenograft LNCaP tumors. Additional studies revealed that protein kinase B survival signaling is downregulated following T0901317 treatment, and that T0901317 induces the apoptosis of LNCaP cells in a lipid raft-dependent manner in vitro and in vivo (31). LXR agonists were demonstrated to inhibit cell proliferation and induce G1/S arrest through lipogenic activity (32). Furthermore, a prior study identified cross-talk between the androgen receptor and LXR, and determined that this interaction influences cellular cholesterol levels (33). The suppressor of cytokine signaling 3 pathway is also involved in LXR agonist-mediated prostate carcinogenesis (34) (Table I). Taken together, these results suggest that LXRs may be promising pharmacological targets for the treatment of prostate cancer.
LXRs and breast cancer
Estrogen metabolism serves an important role in breast cancer and LXR controls estrogen homeostasis (35) (Table I). Estrogen sulfotransferase (EST) is a transcriptional target of LXR. In in vitro and in vivo breast cancer models, LXR inhibits estrogen-dependent cancer cell proliferation by regulating the hepatic expression of EST. These results suggest that LXR inhibits breast cancer growth through a novel mechanism involving the regulation of estrogen homeostasis (35). However, LXR agonists were demonstrated to decrease the proliferation of several breast cancer cell lines independent of lipid biosynthesis (36). LXR agonists decrease the proliferation of cells in S-phase and induce G1 arrest and apoptosis in MCF-7 cells (37,38). Microarray analysis of gene expression revealed that LXR ligands target a set of common responsive genes, including those regulated by E2 factor family members (39).
LXRs and pancreatic cancer
Pancreatic cancer is one of the most fatal types of cancer; the 5-year survival rate of patients with pancreatic ductal adenocarcinoma (PDAC) is only 5%. LXR agonists inhibit cell proliferation, cell-cycle arrest and colony formation in pancreatic cancer cell lines and regulate multiple gene networks involved in cell-cycle arrest and growth factor signaling (40) (Fig. 1A). However, LXR agonists do not induce apoptosis in PDAC cells (40). Further study is required to clarify the types of cell death induced by LXR agonists. Notably, a prior study revealed that the LXR agonist 22(R)-dihydroxycholesterol (Oxy16) inhibits pancreatic cancer cell-induced paracrine Hedgehog signaling (Table I). However, this inhibition is independent of LXR activation (41).
4. LXRs and tumor immunity
It has been reported that LXRs are involved in innate and adaptive immune responses in various diseases (42,43). LXRαβ(−/−) mice are highly susceptible to intracellular bacterial infection, suggesting that LXR-dependent gene expression serves an important role in innate immunity (42). A-Gonzalez et al (44) revealed that LXR signaling promotes apoptotic cell clearance by macrophages and the maintenance of immune tolerance. LXR signaling is also involved in the regulation of other types of immune cell (Fig. 1B). LXR activation inhibits lymphocyte proliferation, and loss of LXR expression confers a proliferative advantage on lymphocytes (45). LXR agonists induce interferon-γ expression in macrophages and T-cells, and increase the survival rate and the tumor-free population of mice inoculated with tumor cells (46,47). However, further studies are required to clarify whether these effects are conserved in other model systems. In Th17 cells, ectopic expression of LXR negatively regulates differentiation, whereas loss of LXR expression promotes differentiation (48). The role of LXR in dendritic cells (DCs) and tumor immunity is controversial. Villablanca et al (49) reported that LXR ligands released from tumor cells inhibit C-C chemokine receptor type 7 (CCR7) expression on maturing DCs. In mice, abrogating LXR agonist release from tumor cells controls tumor growth by recovering DC migration to the tumor area (49). However, another study revealed that treatment with LXR agonists increased CCR7 mRNA expression in an immature DC line (50). Additional investigations are required to determine the underlying molecular mechanism of the potentially opposing roles of LXR.
The antitumor effects of LXR agonists are also evident in the tumor microenvironment. LXR agonists impair the compartmentation of vascular endothelial growth factor receptor-2 in lipid rafts and decrease tumor growth by inhibiting angiogenesis (51). Furthermore, LXRβ agonist treatment induces apolipoprotein E secretion by stromal and tumor cells and blocks tumor growth, angiogenesis and metastasis (52). However, further studies are required in order to investigate whether other types of cell in the tumor microenvironment are influenced by LXRβ agonists.
5. Conclusion
LXR ligands have been developed to treat various diseases, including diabetes, Alzheimer's disease, atherosclerosis and cancer. In spite of progress, a number of fundamental questions remain with respect to cancer therapeutics. A number of natural and synthetic LXR ligands have been identified. T0901317 and GW3965 are the most widely used agonists for mechanistic and functional studies of LXR activity. However, T0901317 has been reported to increase triacylglycerol levels in the plasma and liver and was therefore suggested to be a poor candidate for clinical application (29). New synthetic agonists, including LXR-623, were developed to minimize the side effects on plasma triacylglycerol levels for clinical testing. The development of novel agonists, particularly for cancer treatment, may accelerate basic and clinical applications. The expression profiles of the two LXRs in clinical samples are unknown, and whether these expression profiles are associated with disease subtype, pathological parameters or disease outcomes requires clarification. Endogenous ligands in tumor and stromal cells must also be identified. Taken together, these results suggest that LXRs may be potential targets for cancer therapeutics.
Acknowledgements
The present review was supported by the National Natural Science Foundation of China (grant nos. 81502088, 81502621 and 81300287), Special Postdoctoral Science Foundation of China (no. 2017T100335), the Natural Science Foundation of Jiangsu Province (grant no. BK20140539) and the Start-Up Research Funding of Jiangsu University for Distinguished Scholars (grant nos. 14JDG065 and 15JDG021).
References
- 1.Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 2.Nuclear Receptors Nomenclature Committee, corp-author. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/S0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 3.Evans RM, Mangelsdorf DJ. Nuclear receptors, RXR, and the big bang. Cell. 2014;157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/S0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 5.Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol Cell Biol. 1994;14:7025–7035. doi: 10.1128/MCB.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 7.Shinar DM, Endo N, Rutledge SJ, Vogel R, Rodan GA, Schmidt A. NER, a new member of the gene family encoding the human steroid hormone nuclear receptor. Gene. 1994;147:273–276. doi: 10.1016/0378-1119(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Rogers PM, Stayrook KR, Su C, Varga G, Shen Q, Nagpal S, Burris TP. The selective Alzheimer's disease indicator-1 gene (Seladin-1/DHCR24) is a liver X receptor target gene. Mol Pharmacol. 2008;74:1716–1721. doi: 10.1124/mol.108.048538. [DOI] [PubMed] [Google Scholar]
- 9.Cha JY, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282:743–751. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 10.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viennois E, Pommier AJ, Mouzat K, Oumeddour A, El Hajjaji FZ, Dufour J, Caira F, Volle DH, Baron S, Lobaccaro JM. Targeting liver X receptors in human health: Deadlock or promising trail? Expert Opin Ther Targets. 2011;15:219–232. doi: 10.1517/14728222.2011.547853. [DOI] [PubMed] [Google Scholar]
- 12.Viennois E, Mouzat K, Dufour J, Morel L, Lobaccaro JM, Baron S. Selective liver X receptor modulators (SLiMs): What use in human health? Mol Cell Endocrinol. 2012;351:129–141. doi: 10.1016/j.mce.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 13.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 14.Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, Mangelsdorf DJ. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta; Proc Natl Acad Sci USA; 1999; pp. 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 16.Plat J, Nichols JA, Mensink RP. Plant sterols and stanols: Effects on mixed micellar composition and LXR (target gene) activation. J Lipid Res. 2005;46:2468–2476. doi: 10.1194/jlr.M500272-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Jayasuriya H, Herath KB, Ondeyka JG, Guan Z, Borris RP, Tiwari S, de Jong W, Chavez F, Moss J, Stevenson DW, et al. Diterpenoid, steroid, and triterpenoid agonists of liver X receptors from diversified terrestrial plants and marine sources. J Nat Prod. 2005;68:1247–1252. doi: 10.1021/np050182g. [DOI] [PubMed] [Google Scholar]
- 18.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houck KA, Borchert KM, Hepler CD, Thomas JS, Bramlett KS, Michael LF, Burris TP. T0901317 is a dual LXR/FXR agonist. Mol Genet Metab. 2004;83:184–187. doi: 10.1016/j.ymgme.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Collins JL, Fivush AM, Watson MA, Galardi CM, Lewis MC, Moore LB, Parks DJ, Wilson JG, Tippin TK, Binz JG, et al. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem. 2002;45:1963–1966. doi: 10.1021/jm0255116. [DOI] [PubMed] [Google Scholar]
- 21.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice; Proc Natl Acad Sci USA; 2002; pp. 7604–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko E, Matsuda M, Yamada Y, Tachibana Y, Shimomura I, Makishima M. Induction of intestinal ATP-binding cassette transporters by a phytosterol-derived liver X receptor agonist. J Biol Chem. 2003;278:36091–36098. doi: 10.1074/jbc.M304153200. [DOI] [PubMed] [Google Scholar]
- 23.Quinet EM, Savio DA, Halpern AR, Chen L, Miller CP, Nambi P. Gene-selective modulation by a synthetic oxysterol ligand of the liver X receptor. J Lipid Res. 2004;45:1929–1942. doi: 10.1194/jlr.M400257-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Hu X, Li S, Wu J, Xia C, Lala DS. Liver X receptors interact with corepressors to regulate gene expression. Mol Endocrinol. 2003;17:1019–1026. doi: 10.1210/me.2002-0399. [DOI] [PubMed] [Google Scholar]
- 25.Uno S, Endo K, Jeong Y, Kawana K, Miyachi H, Hashimoto Y, Makishima M. Suppression of beta-catenin signaling by liver X receptor ligands. Biochem Pharmacol. 2009;77:186–195. doi: 10.1016/j.bcp.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Derangère V, Chevriaux A, Courtaut F, Bruchard M, Berger H, Chalmin F, Causse SZ, Limagne E, Végran F, Ladoire S, et al. Liver X receptor β activation induces pyroptosis of human and murine colon cancer cells. Cell Death Differ. 2014;21:1914–1924. doi: 10.1038/cdd.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Courtaut F, Derangère V, Chevriaux A, Ladoire S, Cotte AK, Arnould L, Boidot R, Rialland M, Ghiringhelli F, Rébé C. Liver X receptor ligand cytotoxicity in colon cancer cells and not in normal colon epithelial cells depends on LXRβ subcellular localization. Oncotarget. 2015;6:26651–26662. doi: 10.18632/oncotarget.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vedin LL, Gustafsson JA, Steffensen KR. The oxysterol receptors LXRα and LXRβ suppress proliferation in the colon. Mol Carcinog. 2013;52:835–844. doi: 10.1002/mc.21924. [DOI] [PubMed] [Google Scholar]
- 29.Chuu CP. Modulation of liver X receptor signaling as a prevention and therapy for colon cancer. Med Hypotheses. 2011;76:697–699. doi: 10.1016/j.mehy.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 30.Fukuchi J, Kokontis JM, Hiipakka RA, Chuu CP, Liao S. Antiproliferative effect of liver X receptor agonists on LNCaP human prostate cancer cells. Cancer Res. 2004;64:7686–7689. doi: 10.1158/0008-5472.CAN-04-2332. [DOI] [PubMed] [Google Scholar]
- 31.Pommier AJ, Alves G, Viennois E, Bernard S, Communal Y, Sion B, Marceau G, Damon C, Mouzat K, Caira F, et al. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–2723. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 32.Kim KH, Lee GY, Kim JI, Ham M, Won Lee J, Kim JB. Inhibitory effect of LXR activation on cell proliferation and cell cycle progression through lipogenic activity. J Lipid Res. 2010;51:3425–3433. doi: 10.1194/jlr.M007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krycer JR, Brown AJ. Cross-talk between the androgen receptor and the liver X receptor: Implications for cholesterol homeostasis. J Biol Chem. 2011;286:20637–20647. doi: 10.1074/jbc.M111.227082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu W, Yao J, Huang Y, Li Q, Li W, Chen Z, He F, Zhou Z, Yan J. LXR agonist regulates the carcinogenesis of PCa via the SOCS3 pathway. Cell Physiol Biochem. 2014;33:195–204. doi: 10.1159/000356662. [DOI] [PubMed] [Google Scholar]
- 35.Gong H, Guo P, Zhai Y, Zhou J, Uppal H, Jarzynka MJ, Song WC, Cheng SY, Xie W. Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Mol Endocrinol. 2007;21:1781–1790. doi: 10.1210/me.2007-0187. [DOI] [PubMed] [Google Scholar]
- 36.Vedin LL, Lewandowski SA, Parini P, Gustafsson JA, Steffensen KR. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis. 2009;30:575–579. doi: 10.1093/carcin/bgp029. [DOI] [PubMed] [Google Scholar]
- 37.Chuu CP, Lin HP. Antiproliferative effect of LXR agonists T0901317 and 22(R)-hydroxycholesterol on multiple human cancer cell lines. Anticancer Res. 2010;30:3643–3648. [PubMed] [Google Scholar]
- 38.El Roz A, Bard JM, Huvelin JM, Nazih H. LXR agonists and ABCG1-dependent cholesterol efflux in MCF-7 breast cancer cells: Relation to proliferation and apoptosis. Anticancer Res. 2012;32:3007–3013. [PubMed] [Google Scholar]
- 39.Nguyen-Vu T, Vedin LL, Liu K, Jonsson P, Lin JZ, Candelaria NR, Candelaria LP, Addanki S, Williams C, Gustafsson JÅ, et al. Liver × receptor ligands disrupt breast cancer cell proliferation through an E2F-mediated mechanism. Breast Cancer Res. 2013;15:R51. doi: 10.1186/bcr3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Candelaria NR, Addanki S, Zheng J, Nguyen-Vu T, Karaboga H, Dey P, Gabbi C, Vedin LL, Liu K, Wu W, et al. Antiproliferative effects and mechanisms of liver X receptor ligands in pancreatic ductal adenocarcinoma cells. PLoS One. 2014;9:e106289. doi: 10.1371/journal.pone.0106289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, Stappenbeck F, Matsui W, Parhami F. Inhibition of pancreatic cancer cell-induced paracrine hedgehog signaling by liver X receptor agonists and Oxy16, a naturally occurring oxysterol. J Cell Biochem. 2017;118:499–509. doi: 10.1002/jcb.25668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O'connell RM, Cheng G, Saez E, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 43.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 44.A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Díaz M, Gallardo G, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Q, Ma X, Chen Y, Zhang L, Jiang M, Li X, Xiang R, Miao R, Hajjar DP, Duan Y, Han J. Identification of interferon-γ as a new molecular target of liver X receptor. Biochem J. 2014;459:345–354. doi: 10.1042/BJ20131442. [DOI] [PubMed] [Google Scholar]
- 47.Wang Q, Sun L, Yang X, Ma X, Li Q, Chen Y, Liu Y, Zhang D, Li X, Xiang R, et al. Activation of liver X receptor inhibits the development of pulmonary carcinomas induced by 3-methylcholanthrene and butylated hydroxytoluene in BALB/c mice. Sci Rep. 2016;6:27295. doi: 10.1038/srep27295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui G, Qin X, Wu L, Zhang Y, Sheng X, Yu Q, Sheng H, Xi B, Zhang JZ, Zang YQ. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Invest. 2011;121:658–670. doi: 10.1172/JCI42974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, Sanvito F, Ponzoni M, Valentinis B, Bregni M, et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16:98–105. doi: 10.1038/nm.2074. [DOI] [PubMed] [Google Scholar]
- 50.Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, Gautier EL, Rubinstein D, Hong C, Liu J, et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noghero A, Perino A, Seano G, Saglio E, Lo Sasso G, Veglio F, Primo L, Hirsch E, Bussolino F, Morello F. Liver X receptor activation reduces angiogenesis by impairing lipid raft localization and signaling of vascular endothelial growth factor receptor-2. Arterioscler Thromb Vasc Biol. 2012;32:2280–2288. doi: 10.1161/ATVBAHA.112.250621. [DOI] [PubMed] [Google Scholar]
- 52.Pencheva N, Buss CG, Posada J, Merghoub T, Tavazoie SF. Broad-spectrum therapeutic suppression of metastatic melanoma through nuclear hormone receptor activation. Cell. 2014;156:986–1001. doi: 10.1016/j.cell.2014.01.038. [DOI] [PubMed] [Google Scholar]