Abstract
Long non-coding RNA HOX transcript antisense RNA (HOTAIR) has been demonstrated to exhibit oncogenic activity in several types of cancer, including hepatocellular carcinoma (HCC). However, the association between HOTAIR and HCC multidrug resistance remains uncertain. The present study aimed to investigate the role of HOTAIR in HCC chemoresistance; it was found that knockdown of HOTAIR expression in HCC Huh7 cells resulted in decreased cell proliferation and increased chemosensitivity to cisplatin. Furthermore, expression levels of ATP binding cassette subfamily B member 1 (ABCB1) mRNA and protein were decreased in Huh7 cells upon HOTAIR-knockdown. In addition, HOTAIR-knockdown reduced the levels of phosphorylated signal transducer and activator of transcription 3 (STAT3), and inhibition of STAT3 phosphorylation reduced HOTAIR-mediated ABCB1 expression. Together, these findings indicated that knockdown of HOTAIR in Huh7 cells decreased STAT3 activity and ABCB1 expression, and increased chemosensitivity to cisplatin. Thus HOTAIR could serve as a novel potential therapeutic target to reverse multidrug resistance in HCC.
Keywords: hepatocellular carcinoma, HOX transcript antisense RNA, cisplatin, multidrug resistance, ATP binding cassette subfamily B member 1, signal transducer and activator of transcription 3 signaling
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumor and the third leading cause of cancer-associated mortality worldwide (1,2). There are a variety of HCC treatment modalities, and chemotherapy is an important and irreplaceable therapeutic strategy for the majority of patients with HCC (3). Cisplatin is a common chemotherapeutic agent used for HCC treatment. However, cancer cells may develop multidrug resistance (MDR) against chemotherapeutic agents, including cisplatin, which leads to the failure of chemotherapy (4). There are a number of mechanisms involved in MDR. One form of MDR is caused by the overexpression of ATP-binding cassette sub-family B member 1 (ABCB1), which pumps anticancer agents out of cells, contributing to a reduced intracellular drug concentration and cytotoxicity (5,6). Inhibition of ABCB1 expression is able to reduce the resistance of HCC cells to anticancer agents (6). Therefore, identification of the mechanism of action and identification of novel targets for inhibiting ABCB1 may aid the reversal of MDR in HCC.
Long non-coding RNAs (lncRNAs) are non-protein-coding transcripts >200 nucleotides. Within the past 10–15 years, lncRNAs have gained the widespread attention of researchers, as they are frequently dysregulated in a variety of human diseases, including cancer, and have critical roles in regulating various biological processes (7,8). HOX transcript antisense RNA (HOTAIR), is located within the homeobox C (HOXC) gene cluster on chromosome 12, which interacts with polycomb repressive complex (PRC2) to enhance the trimethylation of lysine 27 of histone H3 (H3K27), leading to the repression of multiple genes (8,9). HOTAIR was initially identified to be upregulated, and to promote invasiveness and metastasis in breast cancer (8). In addition, HOTAIR was demonstrated to promote proliferation and reduce apoptosis in pancreatic cancer, colorectal cancer, and HCC (10–12). Overexpression of HOTAIR was positively associated with poor prognosis, tumor progression and recurrence in HCC (13,14). A recent study reported that HOTAIR also promoted the malignant growth of human liver cancer stem cells (15). A number of studies implicated that HOTAIR has important roles in the tumorigenesis of HCC (12–15). However, the association between HOTAIR and HCC MDR remains unclear.
The present study investigated the potential role of HOTAIR in the regulation of MDR in HCC. HOTAIR small interfering RNA (siRNA) was used to silence HOTAIR expression in human Huh7 HCC cells and then the chemosensitivity of Huh7 cells to cisplatin was examined. Knockdown of HOTAIR resulted in decreased cell proliferation and increased chemosensitivity to cisplatin. Furthermore, the increased chemosensitivity of Huh7 cells to cisplatin was associated with decreased expression levels of ABCB1 mRNA and protein when HOTAIR was knocked down. In addition, inhibition of signal transducer and activator of transcription 3 (STAT3) reduced HOTAIR-mediated ABCB1 expression. These results indicate that HOTAIR may have a particular important role in regulation of the chemosensitivity of human HCC cells. The findings of the present study provide a novel insight into the mechanism of HOTAIR in regulating MDR of HCC.
Materials and methods
Cell line and culture
The Human Huh7 HCC cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in RPMI-1,640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humid atmosphere with 5% CO2. The culture medium was changed every 24 h. AG490 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used as an inhibitor of STAT3 (16), at the final concentrations of 25 µM for 1 h at room temperature prior to siRNA transfection.
HOTAIR siRNA transfection
Knockdown of human HOTAIR (National Center for Biotechnology Information gene ID, 100,124,700) was achieved using transfection with siRNAs. The siRNA sequences were as follows: HOTAIR, 5′-UAACAAGACCAGAGAGCUGUU-3′; negative control (NC), 5′-TTCTCCGAACGTGTCACGT-3′. The siRNA were synthesized by GenePharma (GenePharma Co, Ltd, Shanghai, China). Cells were transfected with 100 nM siRNA duplexes using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) following manufacturer's protocols. The mock transfected cells were transfected with Lipofectamine 2000 without siRNA (17). Following transfection, cells were incubated in a humidified chamber supplemented with 5% CO2 at 37°C. At 48 h after transfection, cells were harvested for RT-qPCR and Western blotting assay.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to determine expression of lncRNA HOTAIR (12), and mRNA expression of ABCB1 (16). Briefly, at 48 h after transfection, cells were harvested in TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's protocols. Total RNA was reverse transcribed into cDNA using PrimeScript™ RT Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). RT-qPCR amplification reactions were performed with SYBR® Premix Ex Taq™ II (Takara Biotechnology Co., Ltd.). RT-qPCR was performed on CFX96 Real-Time PCR Detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Thermocycling conditions were as follows: Initial denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 20 sec, annealing at 58°C for 30 sec and elongation at 72°C for 30 sec. All fold changes were calculated using the comparative Cq (2−ΔΔCq) method using GAPDH for normalization (18). Primer sequences used in this study were as follows: ABCB1 forward, 5′-CTTCAGGGTTTCACATTTGGC-3′ and reverse, 5′-GGTAGTCAATGCTCCAGTGG-3′; GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′.
Western blotting assay
At 48 h after transfection, cells and supernatant of each transfection group were collected. The cells were lysed with radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Nantong, China) in the presence of protease/phosphatase inhibitor cocktail (Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C for 30 min and the lysate was centrifuged at 12,000 × g, 4°C for 20 min. The concentration of protein was measured using a Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific, Inc.). Equal amounts (30 µg per lane) of extracted protein samples were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Following blocking with 5% non-fat milk for 1 h at room temperature, the membranes were incubated with the following primary antibodies at 4°C under gentle agitation overnight: ABCB1 (1:1,000; ab155421; Abcam, Cambridge, UK), pSTAT3 (1:1,000; #9145; Cell Signaling Technology, Inc.), STAT3 (1:1,000; #9,139; Cell Signaling Technology, Inc.) and GAPDH (1:5,000; sc-47,724; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Following washing with TBST, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000; #1,662,408; Bio-Rad Laboratories, Inc.) for 1 h at room temperature. The Supersignal® West Pico Chemiluminescent substrate (Thermo Fisher Scientific, Inc.) was used to visualize protein bands on X-ray films, and ImageJ software version 1.5 (National Institutes of Health, Bethesda, MD, USA) gel analysis was used to quantify the protein bands from western blotting films.
Cell migration assay
Cell migration was tested using a Transwell chamber assay. In total, 1.0×105 cells were seeded on an 8-µm pore size Transwell (Merck KGaA) in serum-free RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.), while RPMI-1,640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) was placed in the lower well. After incubation at 37°C for 48 h, non-migrating cells in the upper chamber surface were removed, migration cells were stained with the Three Step Stain Set kit (Thermo Fisher Scientific, Inc.) for 30 min at room temperature. The number of migrated cells was counted in 5 randomly selected fields using a light microscope at a magnification of ×200.
Cell viability assay
Cell viability assay was performed by using the Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) method. Briefly, cells were seeded in 96-well plates (Corning Incorporated, Corning, NY, USA). Following overnight culture, Huh7 cells were transfected with siHOTAIR or control siRNA for 24 h, then exposed to cisplatin (Selleck Chemicals, Houston, TX, USA) at different final concentrations (0.1, 0.25, 0.5, 1.0, 2.5, 5 µmol/l) for 48 h in a CO2 incubator, after which the viability was accessed. The CCK-8 assay was used to detect the chemosensitivity of cells according to the manufacturer's protocol. The absorbance at 450 nm was measured using a microplate reader. A total of six replicate wells were used for each group.
Statistical analysis
Results are presented as mean of three independent experiments ± standard deviation. Statistical analyses were performed using one-way analysis of variance and LSD t-test to compare between multiple groups and Student's t-test to compare between two groups using GraphPad Prism (version 4.0; GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Knockdown of HOTAIR by specific siRNA
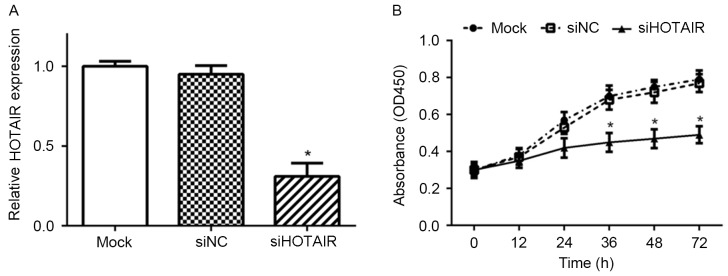
To investigate the role of HOTAIR in HCC cells, specific HOTAIR siRNA (siHOTAIR) was transfected into the Huh7 cell line using Lipofectamine 2000. As presented in Fig. 1, transfection of Huh7 cells with siHOTAIR resulted in significant knockdown of HOTAIR expression. Furtheremore, NC siRNA (siNC) had no significant impact on HOTAIR expression level compared with mock cells.
Figure 1.
Knockdown of HOTAIR inhibited Huh7 cell proliferation. (A) HOTAIR expression in Huh7 cells transfected with siHOTAIR or siNC, or mock cells, were detected by reverse transcription-quantitative polymerase chain reaction (*P<0.05 vs. siNC); (B) Cell proliferation of Huh7 cells was tested by Cell Counting kit-8 assay (*P<0.05 vs. siNC). siHOTAIR, small interfering RNA targeted at HOX transcript antisense RNA; NC, negative control.
Knockdown of HOTAIR inhibits the proliferation of Huh7 cells
To examine the role of HOTAIR on prolferation of Huh7 cells, the effect of siHOTAIR on cell growth was assessed. As presented in Fig. 1, the proliferative capacity of Huh7 cells transfected with siHOTAIR was significantly decreased, as compared with siNC and mock cells (P<0.05).
Knockdown of HOTAIR inhibits the migration of Huh7 cells
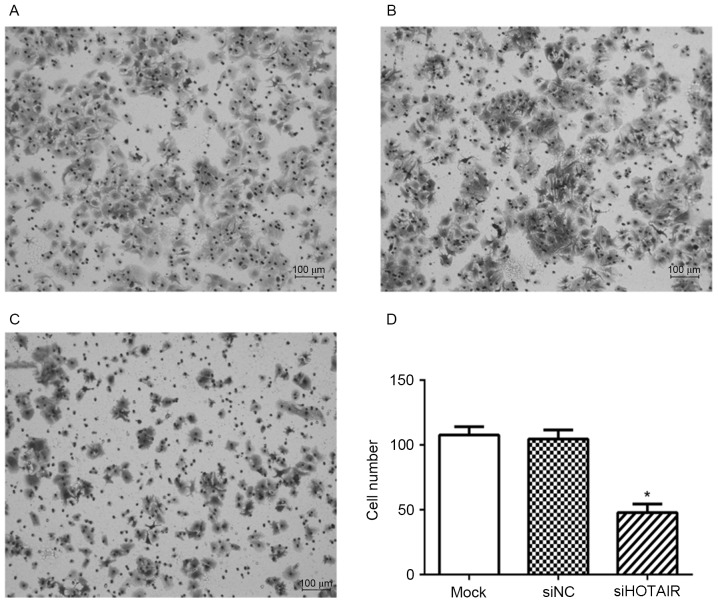
The results of Transwell cell migration assay are presented in Fig. 2. Knockdown of HOTAIR significantly inhibited the migration ability of Huh7 cells with 47.83±6.55 cells invaded, whereas 107.7±6.32 and 104.6±6.93 migrating cells were observed in mock, and siNC cells, respectively (P<0.05, Fig. 2).
Figure 2.
Knockdown of HOTAIR inhibited Huh7 cell migration. Cell migration of Huh7 cells transfected with (A) mock cells, (B) siNC or (C) siHOTAIR were detected by Transwell assay. (D) The number of migrating cells was compared (*P<0.05). siHOTAIR, small interfering RNA targeted at HOX transcript antisense RNA; NC, negative control.
Knockdown of HOTAIR sensitizes Huh7 cells to cisplatin
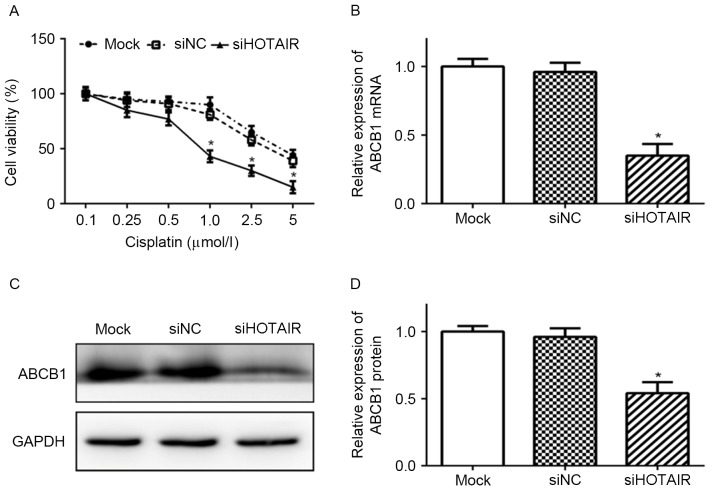
To evaluate the effect of HOTAIR on chemosensitivity of Huh7 cells, the viability of Huh7 cells transfected with siHOTAIR and then exposed to cisplatin were assessed by CCK-8 assay. As presented in Fig. 3, Huh7 cells transfected with siHOTAIR were more sensitive to cisplatin than siNC and mock cells, indicating that knockdown of HOTAIR enhanced the sensitivity of Huh7 cells to cisplatin.
Figure 3.
Knockdown of HOTAIR sensitized Huh7 cells to cisplatin and reduced ABCB1 expression. (A) Huh7 cells transfected with siHOTAIR or siNC were treated with cisplatin (0.1, 0.25, 0.5, 1.0, 2.5 or 5.0 µmol/l) for 48 h. Cell viability was assessed by Cell Counting kit-8 assay (*P<0.05 vs. siNC). Expression levels of ABCB1 (B) mRNA and (C, D) protein in Huh7 cells transfected with siHOTAIR or siNC, or mock cells was detected by reverse transcription-quantitative polymerase chain reaction and western blot analysis, respectively (*P<0.05 vs. siNC). siHOTAIR, small interfering RNA targeted at HOX transcript antisense RNA; NC, negative control; ABCB1, ATP binding cassette subfamily B member 1.
Knockdown of HOTAIR reduces the expression of ABCB1 in Huh7 cells
To evaluate the effect of HOTAIR on cisplatin sensitivity of Huh7 cells further, the expression of ABCB1, an important mediator of MDR, was detected. As presented in Fig. 3, ABCB1 expression was positively associated with HOTAIR expression. Compared with siNC and mock cells, the expression levels of ABCB1 mRNA and protein were significantly lower in siHOTAIR cells (P<0.05).
Knockdown of HOTAIR inhibits the expression of phosphorylated (p)-STAT3 in Huh7 cells
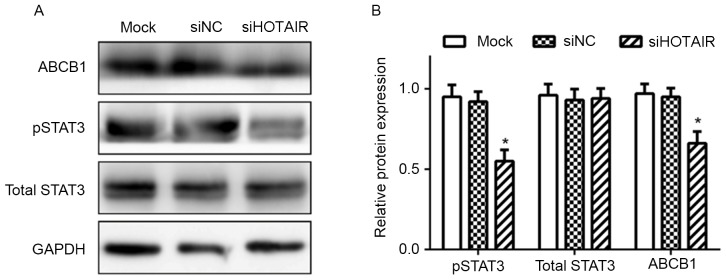
Since STAT3 has been reported to be involved in the regulation of ABCB1 expression (19), whether HOTAIR regulated ABCB1 expression via the STAT3 signaling was investigated. As demonstrated in Fig. 4, levels of p-STAT3 was significantly decreased in siHOTAIR cells (P<0.05); in addition, the expression of total STAT3 was not significantly affected in siHOTAIR cells, compared with siNC and mock cells.
Figure 4.
Knockdown of HOTAIR inhibited expression of pSTAT3 in Huh7 cells. (A) Representative image and (B) quantification of expression levels of phosphorylated STAT3, total STAT3 and ABCB1 protein in Huh7 cells transfected with siHOTAIR or siNC, or mock cells were detected by western blot analysis (*P<0.05 vs. siNC). siHOTAIR, small interfering RNA targeted at HOX transcript antisense RNA; NC, negative control; ABCB1, ATP binding cassette subfamily B member 1; pSTAT3, phosphorylated signal transducer and activator of transcription 3.
Inhibition of STAT3 reduces HOTAIR-mediated ABCB1 expression
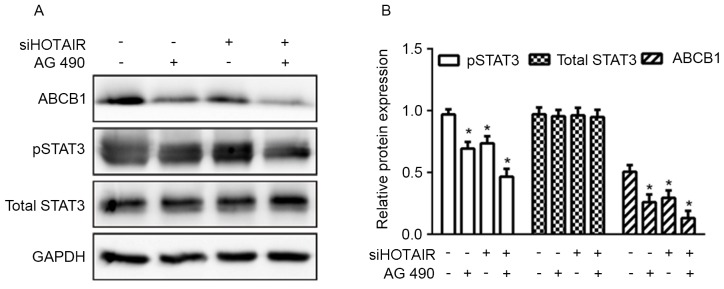
To further investigate the effect of STAT3 signaling on HOTAIR-mediated ABCB1 expression, Huh7 cells were treated with AG490, a STAT3 inhibitor (16), for 1 h prior to siRNA transfection. As presented in Fig. 5, levels of ABCB1 and pSTAT3 were significantly reduced in the AG490-treated group (transfected with either with siHOTAIR or siNC), but the suppresion was more evident in the siHOTAIR group. Overall, the data indicated that HOTAIR mediated ABCB1 expression and chemoresistance to cisplatin through the STAT3 signaling pathway in HCC.
Figure 5.
Inhibition of STAT3 reduced HOTAIR-mediated ABCB1 expression in Huh7 cells. (A) Representative image and (B) quantification of Huh7 cells treated with the STAT3 inhibitor AG490 for 1 h before siHOTAIR or siNC transfection, expression levels of phosphorylated STAT3, total STAT3 and ABCB1 protein in Huh7 cells were detected by western blot analysis (*P<0.05). siHOTAIR, small interfering RNA targeted at HOX transcript antisense RNA; NC, negative control; ABCB1, ATP binding cassette subfamily B member 1; pSTAT3, phosphorylated signal transducer and activator of transcription 3.
Discussion
MDR of cancer cells is one of primary reasons for the failure of HCC chemotherapy and its suppression may increase the efficacy of chemotherapy. A previous study confirmed that inhibition of homeobox transcription factor NANOG reduced ABCB1 expression, leading to the enhanced chemosensitivity to doxorubicin (17). The present study demonstrated the role of the lncRNA HOTAIR in mediating MDR of HCC cells by regulating ABCB1 expression, and revealed that the knockdown of HOTAIR increased chemosensitivity to cisplatin by suppressing STAT3/ABCB1 signaling.
lncRNAs have been identified as novel mediators of critical biological activities in hepatocarcinogenesis (7). lncRNA HOTAIR has been reported to be aberrantly upregulated in several types of cancer, including HCC, and has roles in regulating the proliferation, apoptosis, invasiveness and metastasis of HCC (12,13). The data of the present study validated that the knockdown of HOTAIR inhibited cell proliferation and migration of Huh7 cells, which was consistent with previous studies (12,20). However, the association between HOTAIR and HCC MDR has rarely been reported. The present study identified that the knockdown of HOTAIR increased the chemosensitivity of Huh7 cells to cisplatin by reducing ABCB1 expression, indicating the role of HOTAIR in mediating MDR in HCC. These findings revealed that the knockdown of HOTAIR combined with cisplatin chemotherapy may serve as a potential therapeutic strategy to reverse MDR for HCC treatment. However, the mechanism by which HOTAIR regulates ABCB1 expression is largely unknown. It has been confirmed that HOTAIR recruits PRC2 to regulate chromosome occupancy by enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2; a subset of PRC2), which leads to H3K27 trimethylation and the repression of multiple genes (7). It has been reported that inhibition of EZH2 may decrease the expression of ABCB1 as well as ABC subfamily C member 1 and ABC subfamily G member 2 (junior blood group) mRNA, and protein expression levels (21,22). Together with these findings, the present data indicated that HOTAIR may regulate ABCB1 expression in an EZH2-dependent manner. HOTAIR represses target gene expression, including Wnt inhibitory factor 1, by promoting histone H3K27 methylation in the promoter region via EZH2, and then activation of Wnt/β-catenin signaling (23). However, data from the present and previous studies revealed that HOTAIR-knockdown or EZH2 inhibition may decrease ABCB1 expression (21,22), indicating that HOTAIR regulated ABCB1 expression not via the EZH2-mediated H3K27 methylation of the ABCB1 promoter, but possibly through another mechanism.
Although the mechanism for transcriptional regulation of ABCB1 is not fully understood, a number of transcription factors, including Ras GTPase, SP1 transcription factor, nuclear factor-κB and STAT3 have been reported to be involved. STAT3, a transcription factor that participates in numerous cytokine signaling, has been demonstrated to be constitutively activated by tyrosine phosphorylation in HCC (24). Activation of STAT3 is associated with resistance of tumor cells to chemotherapeutic agents (25), and the downregulation of STAT3 is able to overcome chemoresistance (16). The present study identified that STAT3 activation had a mechanistic role in HOTAIR-mediated ABCB1 expression and MDR of HCC. Knockdown of HOTAIR reduced levels of p-STAT3 and increased chemosensitivity to cisplatin. Inhibition of STAT3 phosphorylation reduced HOTAIR-mediated ABCB1 expression in the present study. Furthermore, it has been reported that pSTAT3 directly mediates ABCB1 transcription, and regulates multidrug efflux in breast and ovarian cancer cells (19). Thus, the data from the present study may explain the association between HOTAIR and MDR of HCC, and also revealed the possible underlying mechanism that HOTAIR mediated chemoresistance to cisplatin in HCC through activating the STAT3/ABCB1 signaling pathway. In addition, the findings of the present study added HOTAIR to the increasing list of upstream pathway leading to STAT3 activation, including interleukin-6, leukemia inhibitory factor, cytoplasmic tyrosine-protein kinase BMX and various receptor tyrosine kinases (23,25). However, the molecular mechanism by which HOTAIR regulates STAT3 activation remains unclear. It has been reported that HOTAIR in laryngeal squamous cell cancer promoted the methylation of phosphatase and tensin homolog (PTEN), a negative regulator for phosphoinositide-3 kinase (PI3K)/RAC serine/threonine protein kinase (AKT) signaling pathway (26). HOTAIR-mediated PTEN methylation resulted in downregulation of PTEN mRNA and protein, leading to the activation of PI3K/AKT signaling. There are a number of negative regulators for STAT3 pathway, including suppressor of cytokine signaling 3 (SOCS3) (27); thus, further studies are required to confirm whether HOTAIR is able to regulate STAT3 activation through SOCS3 methylation.
In conclusion, the present study identified that HOTAIR-knockdown decreased cellular proliferation and cisplatin resistance of human Huh7 HCC cells. In addition, HOTAIR-knockdown resulted in a decrease in STAT3 activity and ABCB1 expression, and increased chemosensitivity to cisplatin. Furthermore, inhibition of STAT3 phosphorylation reduced HOTAIR-mediated ABCB1 expression and chemoresistance. The findings of the present study indicate that HOTAIR may have a role in mediating MDR by regulating STAT3/ABCB1 signaling, and identify HOTAIR as a potential novel therapeutic target to reverse MDR in HCC.
Acknowledgements
The present study was supported by The National Natural Science Foundation of China (grant no. 81000889), The Science and Technology Planning Project of Guangdong Province, China (grant no. 2014A020212094), The Natural Science Foundation of Guangdong Province, China (grant no. 2016A030313218), The Project of Sun Yat-Sen Memorial Hospital (grant no. YXQH201704), The Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology [grant no. (2013) 163] and The Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes (grant no. KLB09001).
References
- 1.Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32(Suppl 1):S225–S237. doi: 10.1016/S0168-8278(00)80428-6. [DOI] [PubMed] [Google Scholar]
- 2.Chiba T, Iwama A, Yokosuka O. Cancer stem cells in hepatocellular carcinoma: Therapeutic implications based on stem cell biology. Hepatol Res. 2016;46:50–57. doi: 10.1111/hepr.12548. [DOI] [PubMed] [Google Scholar]
- 3.Warmann S, Hunger M, Teichmann B, Flemming P, Gratz KF, Fuchs J. The role of the MDR1 gene in the development of multidrug resistance in human hepatoblastoma: Clinical course and in vivo model. Cancer. 2002;95:1795–1801. doi: 10.1002/cncr.10858. [DOI] [PubMed] [Google Scholar]
- 4.Zhuo L, Liu J, Wang B, Gao M, Huang A. Differential miRNA expression profiles in hepatocellular carcinoma cells and drug-resistant sublines. Oncol Rep. 2013;29:555–562. doi: 10.3892/or.2012.2155. [DOI] [PubMed] [Google Scholar]
- 5.Roninson IB, Chin JE, Choi KG, Gros P, Housman DE, Fojo A, Shen DW, Gottesman MM, Pastan I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci USA. 1986;83:4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye CG, Yeung JH, Huang GL, Cui P, Wang J, Zou Y, Zhang XN, He ZW, Cho CH. Increased glutathione and mitogen-activated protein kinase phosphorylation are involved in the induction of doxorubicin resistance in hepatocellular carcinoma cells. Hepatol Res. 2013;43:289–299. doi: 10.1111/j.1872-034X.2012.01067.x. [DOI] [PubMed] [Google Scholar]
- 7.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K, Jutooru I, Chadalapaka G, Frank J, Burghardt R, Kim S, Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 12.Fu WM, Zhu X, Wang WM, Lu YF, Hu BG, Wang H, Liang WC, Wang SS, Ko CH, Waye MM, et al. HOTAIR mediates hepatocarcinogenesis through suppressing miRNA-218 expression and activating P14 and P16 signaling. J Hepatol. 2015;63:886–895. doi: 10.1016/j.jhep.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Ishibashi M, Kogo R, Shibata K, Sawada G, Takahashi Y, Kurashige J, Akiyoshi S, Sasaki S, Iwaya T, Sudo T, et al. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol Rep. 2013;29:946–950. doi: 10.3892/or.2012.2219. [DOI] [PubMed] [Google Scholar]
- 14.Gao JZ, Li J, DU JL, Li XL. Long non-coding RNA HOTAIR is a marker for hepatocellular carcinoma progression and tumor recurrence. Oncol Lett. 2016;11:1791–1798. doi: 10.3892/ol.2016.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, An J, Wu M, Zheng Q, Gui X, Li T, Pu H, Lu D. LncRNA HOTAIR promotes human liver cancer stem cell malignant growth through downregulation of SETD2. Oncotarget. 2015;6:27847–27864. doi: 10.18632/oncotarget.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Real PJ, Sierra A, De Juan A, Segovia JC, Lopez-Vega JM, Fernandez-Luna JL. Resistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene. 2002;21:7611–7618. doi: 10.1038/sj.onc.1206004. [DOI] [PubMed] [Google Scholar]
- 17.Zhou JJ, Deng XG, He XY, Zhou Y, Yu M, Gao WC, Zeng B, Zhou QB, Li ZH, Chen RF. Knockdown of NANOG enhances chemosensitivity of liver cancer cells to doxorubicin by reducing MDR1 expression. Int J Oncol. 2014;44:2034–2040. doi: 10.3892/ijo.2014.2347. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Bourguignon LY, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem. 2008;283:17635–17651. doi: 10.1074/jbc.M800109200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Zhang L, Zhang L, Wang Y, Li H, Ren X, Wei F, Yu W, Liu T, Wang X. Long non-coding RNA HOTAIR promotes tumor cell invasion and metastasis by recruiting EZH2 and repressing E-cadherin in oral squamous cell carcinoma. Int J Oncol. 2015;46:2586–2594. doi: 10.3892/ijo.2015.2976. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Liu G, Lin C, Liao G, Tang B. Silencing the EZH2 gene by RNA interference reverses the drug resistance of human hepatic multidrug-resistant cancer cells to 5-Fu. Life Sci. 2013;92:896–902. doi: 10.1016/j.lfs.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Fan TY, Wang H, Xiang P, Liu YW, Li HZ, Lei BX, Yu M, Qi ST. Inhibition of EZH2 reverses chemotherapeutic drug TMZ chemosensitivity in glioblastoma. Int J Clin Exp Pathol. 2014;7:6662–6670. [PMC free article] [PubMed] [Google Scholar]
- 23.Ge XS, Ma HJ, Zheng XH, Ruan HL, Liao XY, Xue WQ, Chen YB, Zhang Y, Jia WH. HOTAIR, a prognostic factor in esophageal squamous cell carcinoma, inhibits WIF-1 expression and activates Wnt pathway. Cancer Sci. 2013;104:1675–1682. doi: 10.1111/cas.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Yang Z, Ma A, Qu Y, Xia S, Xu D, Ge C, Qiu B, Xia Q, Li J, Liu Y. Growth arrest and DNA damage 45G down-regulation contributes to Janus kinase/signal transducer and activator of transcription 3 activation and cellular senescence evasion in hepatocellular carcinoma. Hepatology. 2014;59:178–189. doi: 10.1002/hep.26628. [DOI] [PubMed] [Google Scholar]
- 25.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, Aggarwal BB. Resveratrol inhibits proliferation, induces apoptosis and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Feng J, Wu T, Wang Y, Sun Y, Ren J, Liu M. Long intergenic noncoding RNA HOTAIR is overexpressed and regulates PTEN methylation in laryngeal squamous cell carcinoma. Am J Pathol. 2013;182:64–70. doi: 10.1016/j.ajpath.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 27.Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, Iida M, Kobayashi T, Yoshimura A. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene. 2006;25:2520–2530. doi: 10.1038/sj.onc.1209281. [DOI] [PubMed] [Google Scholar]