Abstract
Background:
We sought to determine the usefulness of sonography in the detection of nerve involvement in patients with vasculitic neuropathy.
Methods:
We enrolled 16 consecutive patients with vasculitic neuropathy (11 systemic vasculitis and 5 single organ peripheral nerve vasculitis), who met the diagnostic criteria of the Peripheral Nerve Society, and 16 disease controls with noninflammatory axonal polyneuropathy (10 cryptogenic, 4 metabolic, 2 hereditary). Patients underwent standardized nerve conduction studies and assessment of muscle strength (Medical Research Council scale), in addition to sonography of large arm and leg nerves, and brachial plexus. Nerves were evaluated bilaterally at predetermined sites for nerve size (cross-sectional area) and presence of hypervascularization.
Results:
We found enlarged nerves at common sites of nerve compression in all vasculitic and control patients. Multifocal enlargement in arm nerves, proximal to common sites of nerve compression, was sensitive (94%) and specific (88%) for vasculitic neuropathy. Sonography showed nerve enlargement in 51% of clinically or electrodiagnostically unaffected nerves. Sonography of the brachial plexus was normal. We found hypervascularization in 3 patients with systemic vasculitis.
Conclusions:
Sonographic enlargement of arm nerves proximal to sites of nerve compression with sparing of the brachial plexus may indicate a pattern characteristic of patients with vasculitic neuropathy. Sonography may represent a sensitive and specific technique for the detection of inflammatory neuropathy.
Classification of evidence:
This study provides Class III evidence that sonographic enlargement of arm nerves proximal to sites of nerve compression accurately identifies patients with vasculitic neuropathy.
Vasculitic neuropathy is characterized by an acute or subacute and painful onset of sensory and motor deficits of multiple nerves.1–3 Peripheral nerve involvement may be a complication of large, medium-sized, or small vessel vasculitis syndromes, but may also be caused by so-called single organ peripheral nerve vasculitis or nonsystemic vasculitic neuropathy that is confined to nerve-associated vessels.1,2 Although clinical presentation is often typical, diagnosis of vasculitic neuropathy may not be straightforward even after extensive laboratory screening for systemic disease, nerve conduction studies (NCS), and nerve biopsy.4–8 Immunosuppressive treatment is often required to prevent accumulation of nerve damage.5,7,8
Sensitive, specific, and preferably noninvasive tools for the diagnostic workup of vasculitic neuropathies to shorten time to diagnosis and to facilitate treatment decisions are needed. High-resolution ultrasound evaluation of the peripheral nerves is an emerging technique for the diagnosis of mononeuropathies and polyneuropathies.9–13 A few published case reports have reported sonographic nerve enlargement in patients with vasculitic neuropathy,14,15 but few studies have documented nerve size in multiple nerves.16 Sonographic studies of patients with nonsystemic vasculitic neuropathy are lacking.13,16
In this study, we sought to determine the usefulness of sonography in the detection of nerve involvement in patients with vasculitic neuropathy. We assessed multiple sonographic parameters using an extensive protocol in 16 consecutive patients with systemic and nonsystemic vasculitic neuropathy and 16 disease controls with noninflammatory axonal polyneuropathy.
METHODS
Study population
Sixteen consecutive patients with vasculitic neuropathy were enrolled at the outpatient clinic for peripheral neuropathies at the University Medical Centre Utrecht, a tertiary center for neuromuscular disorders, between January 2013 and January 2015. The inclusion criterion was a diagnosis of pathologically definite, probable, or possible or clinically probable vasculitic neuropathy according to the diagnostic consensus criteria of the Peripheral Nerve Society (PNS), i.e., based on the combination of results from clinical history, clinical findings, NCS, laboratory screening for abnormalities associated with systemic vasculitis, and nerve biopsy.7 We also enrolled 16 disease controls with noninflammatory axonal polyneuropathy (10 cryptogenic, 4 metabolic, and 2 hereditary axonal polyneuropathies).17
Standard protocol approvals, registrations, and patient consent
The local medical ethical committee approved the study protocol. Written and informed consent was obtained from all participants.
Clinical assessment
We evaluated patients with a predetermined set of clinical and ancillary investigations. One of the authors (H.S.G.) assessed muscle strength of 13 muscle groups bilaterally (finger flexors, extensors, interossei, wrist extensors and flexors, biceps and triceps, deltoid, iliopsoas, hamstrings and quadriceps, foot extensors and flexors) using the Medical Research Council (MRC) scale. MRC data were used to calculate an MRC sumscore (range 0–130) and sumscores of sets of 2 muscles per fibular, ulnar, and median nerve.
Nerve conduction studies
We performed NCS of the median, ulnar, radial, tibial, fibular, and sural nerves. A Nicolet VIKING IV EMG machine (CareFusion Japan, Tokyo, Japan) was deployed after warming arms and legs in water at 37°C for 45 minutes.18 We assessed distal compound muscle action potentials (CMAP), distal motor latencies (DML), motor conduction velocities (MCV), and F-waves of median, ulnar, fibular, and tibial nerves unilaterally, sensory nerve action potentials (SNAP) of median, ulnar, and radial nerve on the same side as motor responses, and sural nerve bilaterally. Contralateral nerves were investigated if there were signs of weakness or sensory deficits. A decreased or absent distal CMAP or SNAP was considered electrodiagnostic proof of nerve involvement.
Ultrasound studies
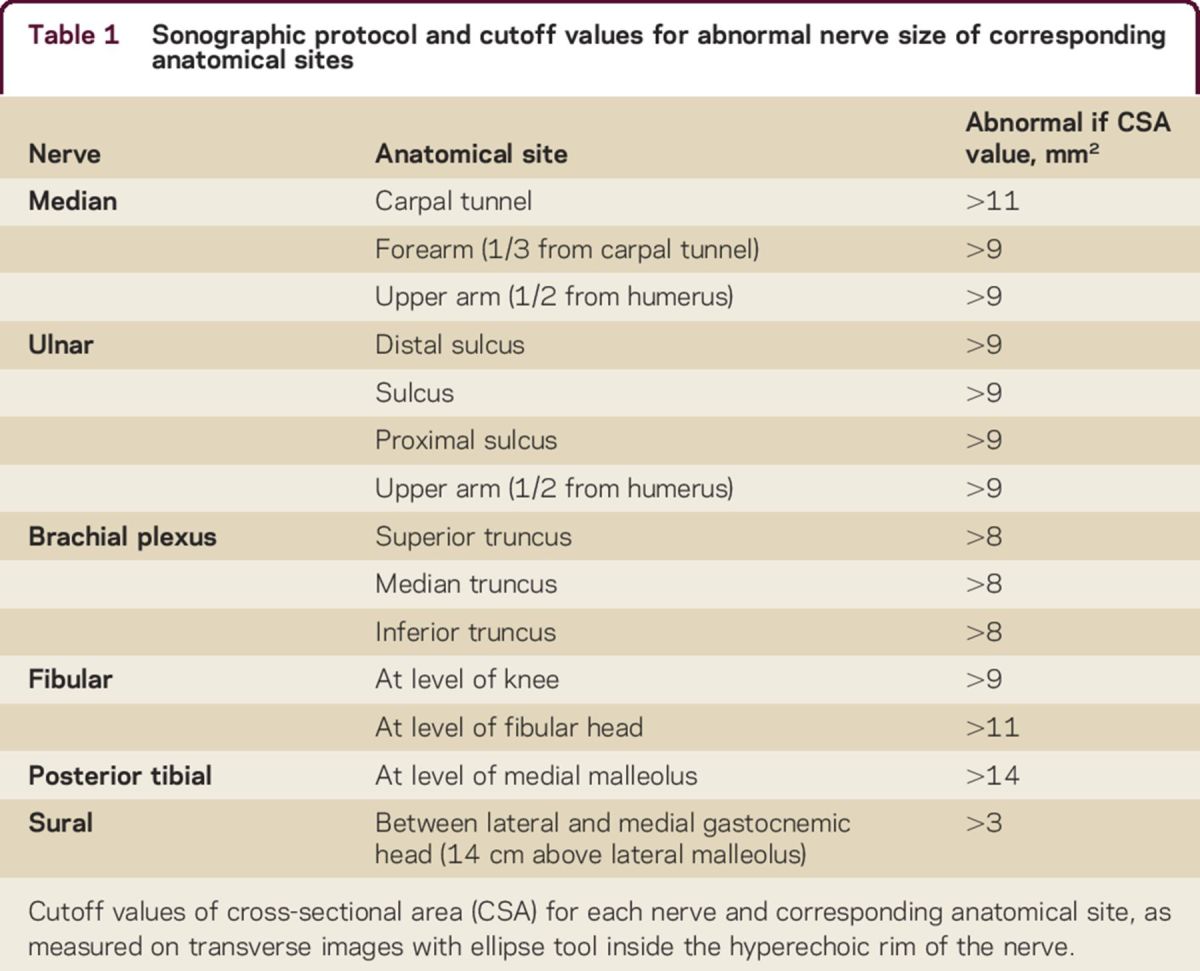
A Philips iU22 (Philips Medical Instruments, Bothell, WA) with a 5–17 MHz linear array transducer was deployed to evaluate the peripheral nerves. Nerve size and vascularization were determined bilaterally of median nerve (carpal tunnel, forearm, upper arm), ulnar nerve (cubital sulcus, distal and proximal cubital sulcus, upper arm), fibular nerve (fibular head, popliteal fossa), tibial nerve (medial malleolus), and sural nerve (14 cm above lateral malleolus).19,20 Nerve size of the trunks in the brachial plexus was also assessed bilaterally.19,20 Nerve size was measured on transverse images with the ellipse tool defining the area within the hyperechoic rim of the nerve.19 In order to evaluate nerve enlargement outside the predetermined sites, we also scanned median and ulnar nerve from axilla to wrist.19 We used previously reported cutoff values for nerve enlargement (table 1).19,20 Nerve enlargement was further categorized into (1) enlargement at common sites of nerve entrapment and (2) enlargement proximal to these sites: forearm and upper arm for median nerve, proximal and distal cubital sulcus, upper arm for ulnar nerve, brachial plexus, popliteal fossa for fibular nerve, tibial and sural nerve. Power Doppler was deployed to screen for presence of increased nerve vascularization at each site, with the exception of the brachial plexus.19–21 The normal vascularization of nerves cannot be visualized with contemporary ultrasound machines; therefore any presence of vascularization on power Doppler was considered abnormal or increased vascularization.19 Echogenicity of the nerve was also measured at these sites; fascicle size was only assessed in median, ulnar, and fibular nerves. Nerve echogenicity and fascicle size were assessed quantitatively, using the ImageJ software (NIH, Bethesda, MD).19 Nerve echogenicity was determined offline with automatic thresholding techniques, applying the MaxEntropy, RenyiEntropy, and Yen thresholding methods.19,22 Blinded to the NCS and independent from the authors diagnosing vasculitic neuropathy (A.F.J.E.V., N.C.N., W.L.v.d.P., L.H.v.d.B.), one of the authors (H.S.G.) performed the sonographic examinations and successively a second author (L.H.V.) rated all sonographic measurements.
Table 1.
Sonographic protocol and cutoff values for abnormal nerve size of corresponding anatomical sites
Statistical analysis
We used SPSS 22.0 software (SPSS, IBM, Armonk, NY) for statistical analysis. Nonparametric tests were deployed, Spearman rho for associations between variables (age, disease duration, MRC sumscore, number of enlarged nerves, nerve and fascicle size, echogenicity) and Mann-Whitney for comparing ordinal (sex, clinical presentation, any weakness in arm or leg, any sensory disturbance in arm or leg, clinical involvement of median and ulnar nerve, treatment at time of sonographic interrogation and hypervascularization, distal CMAP, DML, SNAP, MCV) with continuous variables. We used Benjamini-Hochberg correction for multiple testing where appropriate and set the level of significance at a p value of 0.05.
RESULTS
Clinical characteristics
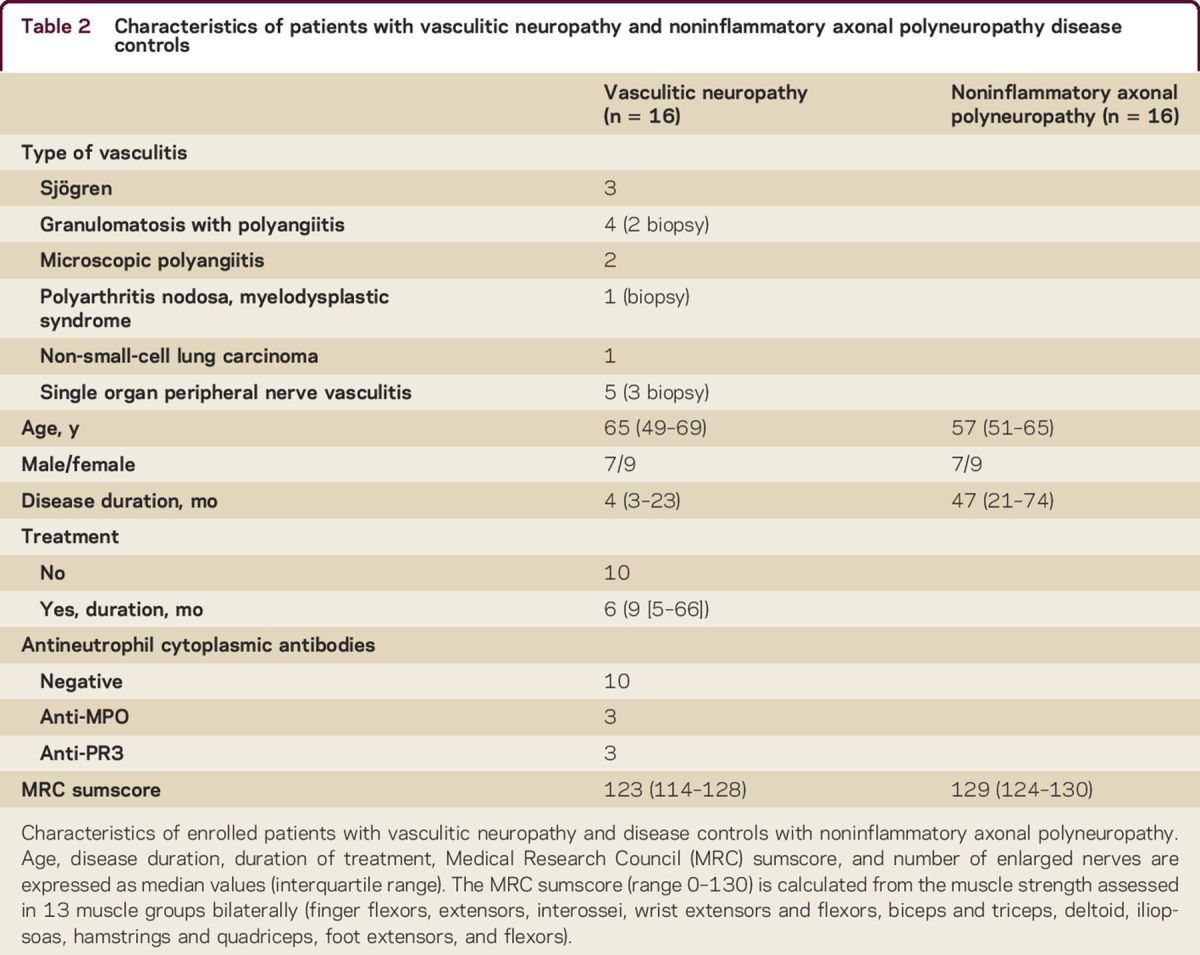
We enrolled 16 patients with vasculitic neuropathy and 16 noninflammatory axonal polyneuropathy disease controls (table 2). Six patients underwent a sural nerve biopsy. Five fulfilled the PNS diagnostic criteria for pathologically definite, 1 pathologically probable, and the remaining 11 clinically probable vasculitic neuropathy (table e-1 at Neurology.org/cp).7 Seven patients were already under treatment at the time of referral (and enrollment), with median treatment duration of 9 months (interquartile range 5–66). There was no difference in age, sex, disease duration, or MRC sumscore between treated and untreated patients in the vasculitic neuropathy group. There was no correlation between age, sex, or disease duration and MRC sumscore or weakness in median, ulnar, or fibular nerve innervated muscles.
Table 2.
Characteristics of patients with vasculitic neuropathy and noninflammatory axonal polyneuropathy disease controls
Sonographic studies
We detected nerve enlargement at multiple common sites of nerve compression (i.e., cubital sulcus, carpal tunnel, and fibular head) in all patients with vasculitic neuropathy and disease controls (figure 1, table 3). Multifocal enlargement of at least 1 arm nerve proximal to common sites of nerve compression was seen in 15 of 16 (94%) patients with vasculitic neuropathy and only in 2 of 16 (12%) disease controls (table 3, p < 0.001). Enlargement of arm nerves proximal to common sites of nerve compression was symmetric in 56% of the patients with vasculitic neuropathy but in none of the disease controls (table 3, table e-2). Ulnar (69%) and median (62%) nerves were the most frequently affected nerves (table 3). None of the patients with vasculitic neuropathy had enlargement of the brachial plexus (table 3). Proximal nerve segments were more frequently enlarged in the 5 patients with nonsystemic vasculitic neuropathy (28 sites) than in the 11 patients with systemic vasculitic neuropathy (33 sites; p = 0.02). Nerve size did not correlate with age, sex, or disease duration. Hypervascularization was present in 3 patients (19%) with systemic vasculitis: 3 nerves in one patient (left median nerve at carpal tunnel and left ulnar nerve at sulcus, right fibular nerve at fibular head) and 1 nerve in 2 patients (left ulnar, right sural nerve, respectively [figure 2, table e-3]); 2 of these patients were treatment-naive and 1 had undergone a longstanding immune-modulating (prednisone, azathioprine, and cyclophosphamide) treatment.
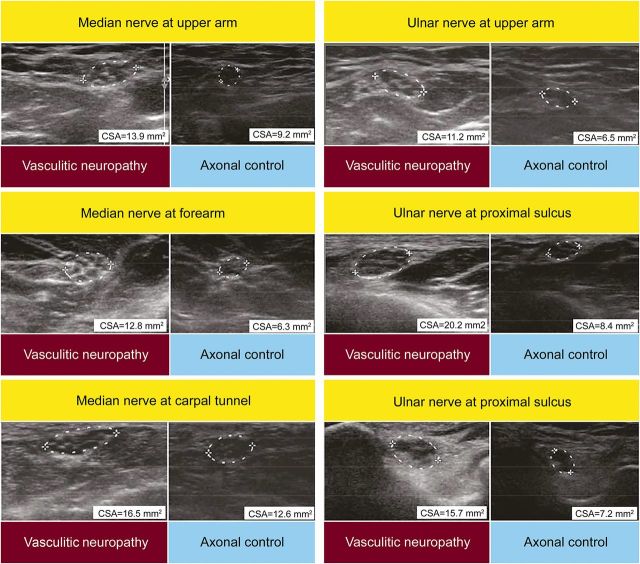
Figure 1. Sonography findings in a representative patient with vasculitic neuropathy and a noninflammatory axonal disease control.
Nerve enlargement (i.e., cross-sectional area [CSA] >9) of median nerve and ulnar nerve at multiple sites outside common sites of nerve compression in the patient with vasculitic neuropathy (left panels), but only in the median nerve at the carpal tunnel in the noninflammatory axonal disease control (right panels), i.e., CSA >11 mm2.
Table 3.
Sites and frequencies at which nerve sonography reveals focal enlargement in nerves
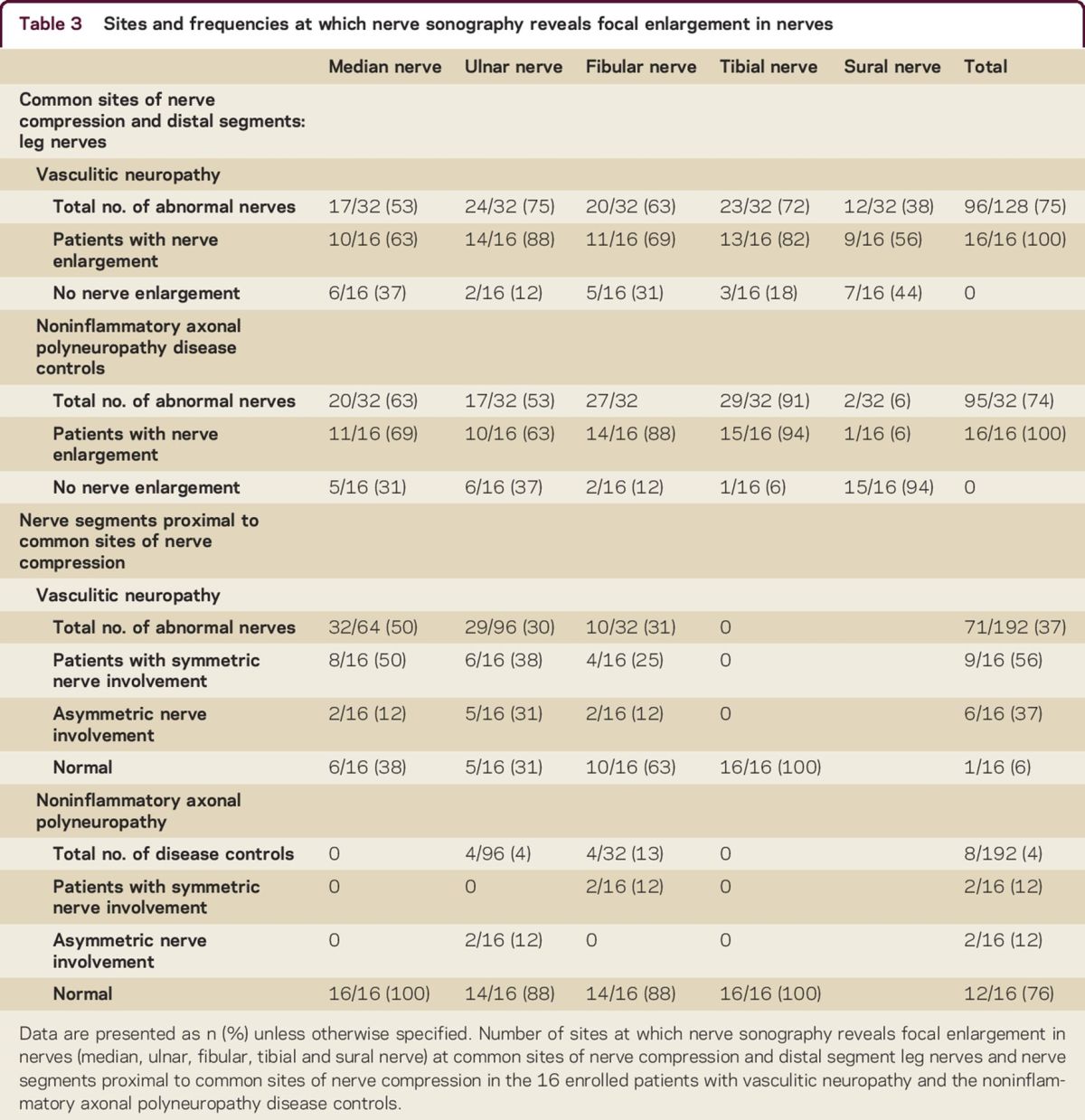
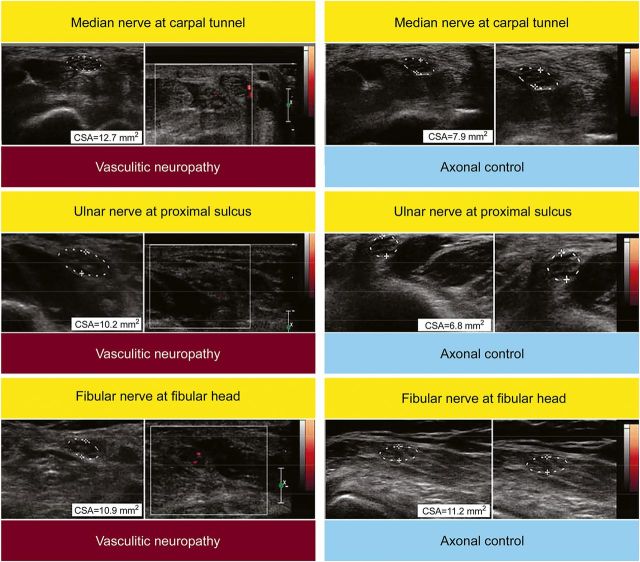
Figure 2. Doppler ultrasound findings in a representative patient with vasculitic neuropathy and a noninflammatory axonal disease control.
Increased nerve vascularization in the patient with vasculitic neuropathy (left panels) in the median, ulnar, and fibular nerve, but not in the noninflammatory axonal disease control (right panels). CSA = cross-sectional area.
Correlation with clinical findings
Sonography abnormalities were not only found in weak limbs of patients with vasculitic neuropathy. We found nerve enlargement in 16/19 (84%) arms and 11/12 (92%) legs with normal muscle strength, and in 13/15 (87%) arms and a single leg with normal sensory function. The number of enlarged nerves, echogenicity, and fascicle size did not correlate with MRC sumscore or the specific sumscore of muscle groups innervated by individual nerves.
Correlations with electrodiagnostic findings
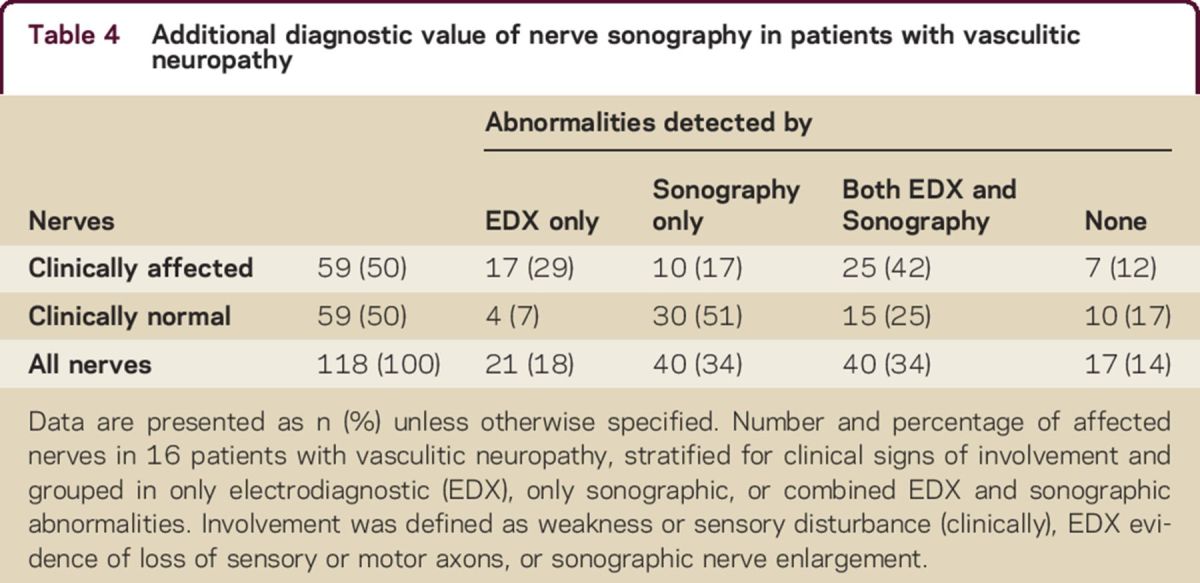
In patients with vasculitic neuropathy, we found nerve enlargement in 51% of the nerves with no signs of clinical or electrodiagnostic involvement (table 4). We found nerve enlargement in 12/14 (86%) ulnar, 6/9 (67%) tibial, 8/13 (62%) median, and all 3 fibular nerves without clinical and electrodiagnostic signs of involvement in the vasculitic neuropathy group. None of the recorded distal CMAP, DML, or SNAP of the investigated nerves correlated with age, sex, or disease duration. The number of enlarged nerves, echogenicity, and fascicle size did not correlate with decreased distal CMAP, prolongation of DML, decreased SNAP, or reduction in MCV.
Table 4.
Additional diagnostic value of nerve sonography in patients with vasculitic neuropathy
DISCUSSION
We found multiple focally enlarged nerves outside common sites of nerve compression in all patients with vasculitic neuropathy. Symmetric enlargement of peripheral nerves was more common than asymmetric involvement and we did not find enlargement of the brachial plexus in any patient with vasculitic neuropathy. Nerve enlargement was prominent in both arms and legs, even in the absence of clinical weakness, sensory deficits, and nerve conduction abnormalities. This pattern of nerve involvement, i.e., of at least one proximal segment of median or ulnar nerve and sparing of the brachial plexus, was both sensitive and specific for vasculitic neuropathy. Our data indicate that ultrasound may represent a valuable tool for the detection of peripheral nerve involvement in vasculitic neuropathies.
Two previous studies (a case report and case series) documented nerve enlargement in patients with vasculitic neuropathies.14,15 The standardized and extensive ultrasound protocol that we used to investigate nerve enlargement in patients with both systemic and nonsystemic vasculitic neuropathy and a relevant disease control group allowed us to assess patterns of nerve enlargement and usefulness of sonography during the clinical workup. The finding of multiple, focally enlarged nerves outside common sites of nerve compression in arms and legs, but not in the brachial plexus, may represent a characteristic pattern of sonographic nerve involvement in vasculitic neuropathy. Our data suggest that ultrasound sensitivity may be slightly higher in leg nerves, which may reflect their relatively low endoneurial capillary density,23 while specificity is higher in arm nerves. We found frequent nerve enlargement in proximal nerve segments, in line with observations that nerve infarction commonly occurs here.24 Normal findings in the brachial plexus, which may reflect its relatively high endoneurial capillary density and thus a potentially higher resistance to ischemic injury,23–25 can help to distinguish vasculitic neuropathy from demyelinating asymmetric neuropathies such as multifocal motor neuropathy and Lewis-Sumner syndrome.26,27 The finding of symmetric nerve enlargement would further support the diagnosis of vasculitic neuropathy. Systematic assessment of the presence of fascicle size and echogenicity revealed that these parameters probably do not have added value. Taken together, our data indicate that ultrasound investigation of the large arm nerves on both sides (and, if normal, the leg nerves) in combination with the brachial plexus can be used to discriminate vasculitic neuropathies from other axonal and asymmetric neuropathies. This sonographic protocol takes only a small time effort, in general less than 15 minutes.
We found hypervascularization, which may reflect inflammation28,29 or angiogenesis,30,31 in 3 vasculitic neuropathy patients, but in none of the disease controls. We previously reported hypervascularization in chronic inflammatory demyelinating neuropathy, but all these patients had a clinically symmetric neuropathy.20 These findings suggest that increased neural vascularization might be specific for vasculitic neuropathy in the setting of a subacute progressive asymmetric neuropathy. With the recognition that it was seen only in 3 patients, this hypervascularization indicates focal disease activity and may represent inflammatory cell infiltration of neural vessels in vasculitic neuropathy. Other sonography parameters, including fascicle size and echogenicity, did not correlate with any clinical and nerve conduction study findings. It is not known how the central pathogenetic processes of vasculitis neuropathy, i.e., vessel wall inflammation, destruction of the vessel wall, occlusion of the vessel lumen, and nerve ischemia, eventually lead to focal enlargement of nerves.5,8 Although the usefulness of other sonography parameters than cross-sectional area may useful for the dissection of pathogenetic properties of vasculitis neuropathies, they do not have diagnostic added value.
Our study has some limitations. Our patient group was heterogeneous with respect to disease duration and treatment (duration). Treatment effects and incremental sonomorphologic alterations with longer disease duration cannot be completely ruled out at this stage. It is unlikely, however, that they affect the presence of nerve enlargement as we found this in patients with both short and long disease durations, and before and after the start of treatment.
Both ultrasound and nerve conduction abnormalities were common in nerves that seemed clinically unaffected.3,32–34 Sonography is more time-efficient and has better patient compliance than NCS, which may be poorly tolerated in patients with vasculitic neuropathies due to pain. It may have higher sensitivity for detecting inflammatory neuropathy than nerve biopsy, which suffers from obvious limitations of sampling from the sural or superficial fibular nerve and has limited sensitivity. In this context, nerve sonography may represent a sensitive and patient-friendly technique for detection of peripheral nerve involvement in vasculitic neuropathies.
Footnotes
Supplemental data at Neurology.org/cp
STUDY FUNDING
Supported by the Prinses Beatrix Spierfonds (grant W.OR14-08).
DISCLOSURES
H.S. Goedee receives research support from the Prinses Beatrix Spierfonds. W.L. van der Pol serves on a scientific advisory board for SMA Europe and receives research support from the Prinses Beatrix Fonds, The Netherlands ALS Foundation, and Stichting Spieren voor Spieren. J.-T. H. van Asseldonk, A.F.J.E. Vrancken, and N.C. Notermans report no disclosures. L.H. Visser receives research support from the Prinses Beatrix Spierfonds and Netherlands Organisation for Health Research and Development. L.H. van den Berg serves on scientific advisory boards for Biogen Idec, Baxtalta, Prinses Beatrix Spierfonds, Thierry Latran Foundation, and Cytokinetics; has received funding for travel or speaker honoraria from Baxalta; received an educational grant from Baxter International Inc.; serves on the editorial boards of Amyotrophic Lateral Sclerosis, Frontotemporal Degeneration, and Journal of Neurology, Neurosurgery and Psychiatry; and receives research support from The Netherlands ALS Foundation and Netherlands Organisation for Health Research and Development (Vici scheme), the Prinses Beatrix Spierfonds, and the European Community's Health Seventh Framework Programme (grant agreement no. 259867). Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
AUTHOR CONTRIBUTIONS
H.S. Goedee: patient enrollment and evaluation according to the study protocol, acquisition of data, contribution to study design, analysis and interpretation of data, statistical analysis, drafting/revision of the manuscript, obtaining funding. W.L. van der Pol and L.H. van den Berg: patient enrollment, contribution to study design, analysis and interpretation of data, revision of the manuscript, obtaining funding. A.F.J.E. Vrancken and NC Notermans: patient enrollment and revision of the manuscript. J.-T.H. van Asseldonk and L.H. Visser: analysis and interpretation of data, revision of the manuscript, obtaining funding.
REFERENCES
- 1.Bouche P, Leger JM, Travers MA, Cathala HP, Castaigne P. Peripheral neuropathy in systemic vasculitis: clinical and electrophysiologic study of 22 patients. Neurology 1986;36:1598–1602. [DOI] [PubMed] [Google Scholar]
- 2.Collins MP, Periquet MI, Mendell JR, Sahenk Z, Nagaraja HN, Kissel JT. Nonsystemic vasculitic neuropathy: insights from a clinical cohort. Neurology 2003;61:623–630. [DOI] [PubMed] [Google Scholar]
- 3.Seo JH, Ryan HF, Claussen GC, Thomas TD, Oh SJ. Sensory neuropathy in vasculitis: a clinical, pathologic, and electrophysiologic study. Neurology 2004;63:874–878. [DOI] [PubMed] [Google Scholar]
- 4.Collins MP, Mendell JR, Periquet MI, et al. . Superficial peroneal nerve/peroneus brevis muscle biopsy in vasculitic neuropathy. Neurology 2000;55:636–643. [DOI] [PubMed] [Google Scholar]
- 5.Gwathmey KG, Burns TM, Collins MP, Dyck PJ. Vasculitic neuropathies. Lancet Neurol 2014;13:67–82. [DOI] [PubMed] [Google Scholar]
- 6.Vrancken AF, Gathier CS, Cats EA, Notermans NC, Collins MP. The additional yield of combined nerve/muscle biopsy in vasculitic neuropathy. Eur J Neurol 2011;18:49–58. [DOI] [PubMed] [Google Scholar]
- 7.Collins MP, Dyck PJ, Gronseth GS, et al. . Peripheral Nerve Society Guideline on the classification, diagnosis, investigation, and immunosuppressive therapy of non-systemic vasculitic neuropathy: executive summary. J Peripher Nerv Syst 2010;15:176–184. [DOI] [PubMed] [Google Scholar]
- 8.Vrancken AF, Said G. Vasculitic neuropathy. Handb Clin Neurol 2013;115:463–483. [DOI] [PubMed] [Google Scholar]
- 9.Beekman R, Schoemaker MC, Van Der Plas JP, et al. . Diagnostic value of high-resolution sonography in ulnar neuropathy at the elbow. Neurology 2004;62:767–773. [DOI] [PubMed] [Google Scholar]
- 10.Visser LH, Smidt MH, Lee ML. High-resolution sonography versus EMG in the diagnosis of carpal tunnel syndrome. J Neurol Neurosurg Psychiatry 2008;79:63–67. [DOI] [PubMed] [Google Scholar]
- 11.Beekman R, Wokke JH, Schoemaker MC, Lee ML, Visser LH. Ulnar neuropathy at the elbow: follow-up and prognostic factors determining outcome. Neurology 2004;63:1675–1680. [DOI] [PubMed] [Google Scholar]
- 12.Visser LH, Hens V, Soethout M, De Deugd-Maria V, Pijnenburg J, Brekelmans GJ. Diagnostic value of high-resolution sonography in common fibular neuropathy at the fibular head. Muscle Nerve 2013;48:171–178. [DOI] [PubMed] [Google Scholar]
- 13.Goedee HS, Brekelmans GJ, van Asseldonk JT, Beekman R, Mess WH, Visser LH. High resolution sonography in the evaluation of the peripheral nervous system in polyneuropathy: a review of the literature. Eur J Neurol 2013;20:1342–1351. [DOI] [PubMed] [Google Scholar]
- 14.Nodera H, Sato K, Terasawa Y, Takamatsu N, Kaji R. High-resolution sonography detects inflammatory changes in vasculitic neuropathy. Muscle Nerve 2006;34:380–381. [DOI] [PubMed] [Google Scholar]
- 15.Ito T, Kijima M, Watanabe T, Sakuta M, Nishiyama K. Ultrasonography of the tibial nerve in vasculitic neuropathy. Muscle Nerve 2007;35:379–382. [DOI] [PubMed] [Google Scholar]
- 16.Grimm A, Decard BF, Bischof A, Axer H. Ultrasound of the peripheral nerves in systemic vasculitic neuropathies. J Neurol Sci 2014;347:44–49. [DOI] [PubMed] [Google Scholar]
- 17.Visser NA, Notermans NC, Linssen RS, van den Berg LH, Vrancken AF. Incidence of polyneuropathy in Utrecht, the Netherlands. Neurology 2015;84:259–264. [DOI] [PubMed] [Google Scholar]
- 18.Van Asseldonk JT, Van den Berg LH, Kalmijn S, Wokke JH, Franssen H. Criteria for demyelination based on the maximum slowing due to axonal degeneration, determined after warming in water at 37 degrees C: diagnostic yield in chronic inflammatory demyelinating polyneuropathy. Brain 2005;128:880–891. [DOI] [PubMed] [Google Scholar]
- 19.Goedee HS, Brekelmans GJ, van den Berg LH, Visser LH. Distinctive patterns of sonographic nerve enlargement in Charcot-Marie-Tooth type 1A and hereditary neuropathy with pressure palsies. Clin Neurophysiol 2015;126:1413–1420. [DOI] [PubMed] [Google Scholar]
- 20.Goedee HS, Brekelmans GJ, Visser LH. Multifocal enlargement and increased vascularization of peripheral nerves detected by sonography in CIDP: a pilot study. Clin Neurophysiol 2014;125:154–159. [DOI] [PubMed] [Google Scholar]
- 21.Frijlink DW, Brekelmans GJ, Visser LH. Increased nerve vascularization detected by color Doppler sonography in patients with ulnar neuropathy at the elbow indicates axonal damage. Muscle Nerve 2013;47:188–193. [DOI] [PubMed] [Google Scholar]
- 22.Boom J, Visser LH. Quantitative assessment of nerve echogenicity: comparison of methods for evaluating nerve echogenicity in ulnar neuropathy at the elbow. Clin Neurophysiol 2012;123:1446–1453. [DOI] [PubMed] [Google Scholar]
- 23.Kozu H, Tamura E, Parry GJ. Endoneurial blood supply to peripheral nerves is not uniform. J Neurol Sci 1992;111:204–208. [DOI] [PubMed] [Google Scholar]
- 24.Dyck PJ, Conn DL, Okazaki H. Necrotizing angiopathic neuropathy: three-dimensional morphology of fiber degeneration related to sites of occluded vessels. Mayo Clin Proc 1972;47:461–475. [PubMed] [Google Scholar]
- 25.Fujimura H, Lacroix C, Said G. Vulnerability of nerve fibres to ischaemia: a quantitative light and electron microscope study. Brain 1991;114:1929–1942. [DOI] [PubMed] [Google Scholar]
- 26.Beekman R, van den Berg LH, Franssen H, Visser LH, van Asseldonk JT, Wokke JH. Ultrasonography shows extensive nerve enlargements in multifocal motor neuropathy. Neurology 2005;65:305–307. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya K, Sugiyama A, Ito S, et al. . Reconstruction magnetic resonance neurography in chronic inflammatory demyelinating polyneuropathy. Ann Neurol 2015;77:333–337. [DOI] [PubMed] [Google Scholar]
- 28.Martinoli C, Derchi LE, Bertolotto M, et al. . US and MR imaging of peripheral nerves in leprosy. Skeletal Radiol 2000;29:142–150. [DOI] [PubMed] [Google Scholar]
- 29.Bathala L, Kumar K, Pathapati R, Jain S, Visser LH. Ulnar neuropathy in Hansen disease: clinical, high-resolution ultrasound and electrophysiologic correlations. J Clin Neurophysiol 2012;29:190–193. [DOI] [PubMed] [Google Scholar]
- 30.Yanik B, Conkbayir I, Keyik B, Yoldas TK. Sonographic findings in a case of polyneuropathy associated with POEMS syndrome. J Clin Ultrasound 2011;39:473–476. [DOI] [PubMed] [Google Scholar]
- 31.Gupta R, Gray M, Chao T, Bear D, Modafferi E, Mozaffar T. Schwann cells upregulate vascular endothelial growth factor secondary to chronic nerve compression injury. Muscle Nerve 2005;31:452–460. [DOI] [PubMed] [Google Scholar]
- 32.Cruz Martinez A, Barbado FJ, Ferrer MT, Vazquez JJ, Perez Conde MC, Gil Aquado A. Electrophysiological study in systemic necrotizing vasculitis of the polyarteritis nodosa group. Electromyogr Clin Neurophysiol 1988;28:167–173. [PubMed] [Google Scholar]
- 33.Nicolai A, Bonetti B, Lazzarino LG, Ferrari S, Monaco S, Rizzuto N. Peripheral nerve vasculitis: a clinico-pathological study. Clin Neuropathol 1995;14:137–141. [PubMed] [Google Scholar]
- 34.Bennett DL, Groves M, Blake J, et al. . The use of nerve and muscle biopsy in the diagnosis of vasculitis: a 5 year retrospective study. J Neurol Neurosurg Psychiatry 2008;79:1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]