Abstract
Purpose of review:
Extracranial (EC) and intracranial (IC) large vessel atherosclerosis account for about 20% of ischemic stroke cases. In recent years, new treatments have emerged for treatment of both EC and IC disease.
Recent findings:
The stroke rate in patients with carotid stenosis is decreasing with modern medical therapy. For patients with asymptomatic stenosis, the stroke rate is likely <1% per year. Some subsets of patients with symptomatic carotid disease benefit less from revascularization, and medical management can be considered in these patients. A second clinical trial has confirmed that aggressive medical management is the treatment of choice for IC atherosclerotic disease. Vessel wall imaging may be useful to define pathophysiology in patients with IC stenosis and could ultimately help tailor therapy, but further studies are needed. Medical therapy is preferred to stenting for patients with vertebral artery–origin stenosis.
Summary:
Recent data and emerging concepts regarding large vessel atherosclerosis are provided.
Large vessel atherosclerosis accounts for more than 100,000 strokes per year in the United States. Although many clinicians are comfortable with evaluation for carotid stenosis as part of the “standard stroke workup,” it should be recognized that risk stratification is important to make sure that therapies with potential risk, such as carotid endarterectomy (CEA) or carotid artery stenting (CAS), are provided to the right patient in the right setting. Ensuring that carotid revascularization is provided in an evidence-based fashion is also important with regard to patient safety.
Intracranial atherosclerosis is sometimes overlooked as part of the stroke evaluation. However, it is an important cause of stroke, especially in African Americans, Asians, and Hispanics. We provide information on emerging concepts in evaluation and management of stroke due to large vessel atherosclerosis.
Asymptomatic carotid stenosis
In previous landmark trials such as the Asymptomatic Carotid Atherosclerosis Study (ACAS), CEA was associated with a 55% relative risk reduction for ischemic stroke compared to medical therapy.1 However, the absolute reduction was small, only 1.2% per year.2 The volume of carotid revascularization procedures increased sharply after the ACAS trial publication in 1995.
However, we should keep in mind that ACAS enrolled patients between 1987 and 1993, which was before the era of high-potency statins. Multimodality treatment with high-potency statins, antiplatelet therapy, targeted blood pressure lowering, and lifestyle modification now constitutes optimal medical therapy (OMT) and is recommended in stroke prevention guidelines.3 If we use OMT in patients with 70%–99% asymptomatic internal carotid artery (ICA) stenosis, would CEA still be beneficial?
Population-based studies have shown that the risk of stroke with asymptomatic ICA stenosis is likely <1% per year with modern medical therapy.4,5 Risk stratification techniques such as use of transcranial Doppler (TCD) for emboli detection and assessment of intracranial vascular reserve can identify patients at higher and lower stroke risk.6,7
The Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Study (CREST 2) is an NIH-sponsored study that is currently enrolling asymptomatic patients with 70%–99% ICA stenosis (http://www.crest2trial.org). There are 2 arms in the study, one comparing CEA + OMT vs OMT alone and one comparing CAS + OMT vs OMT alone. Neurologists should strongly consider referral of asymptomatic patients for this clinical trial because it will enhance our clinical decision-making abilities in the future.
Symptomatic carotid stenosis
Earlier clinical trials such as the North American Symptomatic Carotid Endarterectomy Trial (NASCET) found that CEA was beneficial for patients with severe (70%–99%) ICA stenosis and preceding stroke or TIA.8 However, this study was also done largely in the prestatin era and the initial results were published 25 years ago. As with asymptomatic carotid disease, the applicability of clinical trial data from before the era of high-potency statins and OMT has been called into question.
Although CEA was beneficial as a whole for patients with symptomatic stenosis, there was considerable heterogeneity relating to timing of the operation and patient sex. In a pooled analysis of the prior symptomatic trials and patients with 50%–99% stenosis, the number needed to treat (NNT) to prevent 1 stroke was 5 for patients operated on within 2 weeks of study entry and 125 for patients operated on more than 12 weeks after the prior vascular event.9 In terms of sex, the NNT for men was 9 and 36 for women. Therefore, we need to study modern medical therapy for symptomatic as well as asymptomatic ICA stenosis. It is notable that many would hesitate to use OMT alone in symptomatic disease instead of revascularization. It would make sense to initially study OMT in patient subgroups that derive less benefit from CEA, such as women and those with their last TIA/stroke more than 2 weeks previously.10,11 In addition, patients with retinal symptoms (as opposed to hemispheric ischemic events) and patients with 50%–69% stenosis (as opposed to severe stenosis) have a lower stroke risk and aggressive medical therapy could be tested in these subgroups as well.2
Another insight with regard to symptomatic ICA stenosis concerns the choice of revascularization method. A recent analysis found that CEA was associated with a lower complication rate than CAS (2.8% vs 9.4%) in patients who underwent a revascularization procedure within 7 days of a cerebral ischemic event.12 Given a more recent shift toward earlier revascularization, CEA appears to be the preferred treatment for patients with symptomatic stenosis.
Intracranial atherosclerotic disease
Intracranial atherosclerotic disease (ICAD)-associated strokes comprise at least 10% of ischemic strokes worldwide. Major racial differences exist: ICAD comprises 33%–54% of strokes in Asians, 9% in whites, 12% in African Americans, and 15% in Hispanics.13,14 Studies quote variable rates of stroke recurrence in different populations,15–18 but notably these studies predate use of what is now considered standard of care medical management.
The Stenting and Aggressive Medical Management for Preventing Stroke in Intracranial Stenosis (SAMMPRIS) trial has shown that medical management for symptomatic ICAD with dual antiplatelet therapy for 3 months, aggressive control of blood pressure and hyperlipidemia, and smoking cessation, diet, and exercise was superior to intracranial stenting and established the standard of care for medical management.19 The primary endpoint of SAMMPRIS was any periprocedural stroke or death within 30 days or stroke in the territory of the symptomatic vessel beyond 30 days or within 30 days of an interventional procedure. Long-term results of SAMMPRIS found that aggressive medical management was associated with a lower endpoint rate than medical therapy + intracranial stenting (15% vs 23%, p = 0.03).20 A second clinical trial confirmed the findings of SAMMPRIS and reported an unacceptably high complication rate with intracranial stenting.21
ICAD remains a common cause of stroke worldwide. Rate of recurrent stroke due to intracranial atherosclerosis has not been well established outside of a clinical trial setting or specifically in the post aggressive medical therapy era. More research is needed to determine factors contributing to disease progression and risk of recurrence in order to better target therapies. MyRIAD (Mechanisms of Early Recurrence in Intracranial Atherosclerotic Disease) is currently recruiting and will focus on mechanisms of recurrence in recently symptomatic patients with high-grade stenosis (www.clinicaltrials.gov; NCT 02121028).
Advanced imaging may aid in diagnosis and optimization of treatment
The underlying mechanisms of stroke due to ICAD are varied and include artery-to-artery emboli, in situ thromboembolism, hemodynamic impairment, and branch occlusive disease (BOD). Lesions can be lacunar, subcortical, cortical, or a combination of these.22 These variable mechanisms allude to the idea that nonstenotic plaques can also cause disease. Modalities commonly used for evaluation of ICAD, including noninvasive evaluation with TCD (with or without emboli detection), magnetic resonance angiography (MRA), or CT angiography or invasive imaging with digital subtraction angiography, can assess degree of stenosis and collateral flow but do not provide information about arterial wall structure or plaque characterization.
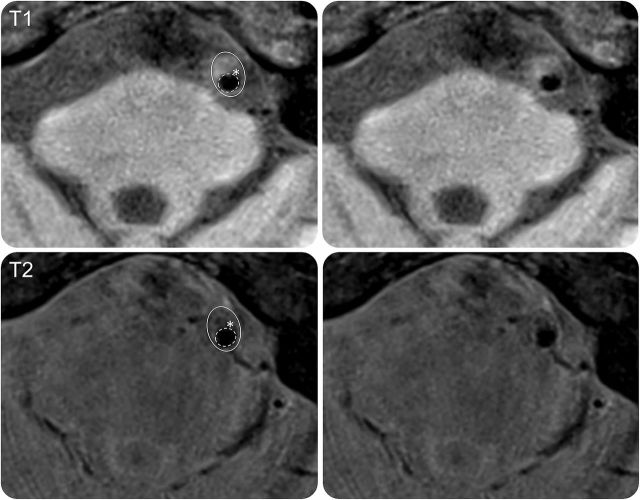
Given that there are varying mechanisms of cerebral ischemia due to ICAD, there is a newer focus on the use of advanced imaging modalities to better visualize the intracranial arterial wall and determine subset of disease, with the goal of identifying patients at high risk of recurrent ischemia and distinguishing ICAD from other types of intracranial vessel pathology. One current modality being used is high-resolution MRI (HR-MRI), which provides detailed plaque characterization and can determine high-risk plaque components, including lipid-rich necrotic core, intraplaque hemorrhage, calcium, and fibrous cap (figure).23 Atherosclerotic plaques tend to be eccentric and have enhancement and intraplaque hemorrhage, which may help distinguish them from other causes of intracranial vessel disease, including dissection, vasculitis, and reversible cerebral vasoconstriction syndrome.23 Intraplaque hemorrhage, appearing as a high-intensity signal, has been associated with ipsilateral stroke.24 A recent study compared HR-MRI characteristics of BOD (parent artery occluding perforator origin) and non- BOD (artery-to-artery) ICAD. BOD had milder stenosis than non-BOD (p < 0.001), less remodeling (p = 0.005), and less plaque enhancement.25
Figure. Proximal basilar artery symptomatic stenosis.
Image shows a cross-section of a symptomatic basilar artery plaque. Top row T1 and bottom row T2 images show heterogeneous plaque with lipid (isointense on T1, hypointense on T2) and irregularity of the lumen surface (dashed line) over the plaque consistent with ruptured fibrous cap (white asterisk). Figure courtesy of Dr. Tanya Turan.
Although advanced imaging modalities are promising, many limitations to routine use in clinical practice exist. There is lack of pathologic correlation with imaging findings, especially for intracranial vessels. There is no widely accepted standard protocol for imaging acquisition or for interpretation of these images. Given that advanced imaging is not standard of care, cost may be prohibitive outside of the research setting.26 Studies involving advanced imaging have been single-center studies and broader replication is required. The feasibility of adopting advanced imaging modalities and the standardization of these modalities for use in clinical practice are unclear. However, they may be useful for patient risk stratification in the future.
Vertebral artery stenting is not more effective than medical therapy
The suggestion that risk of recurrent stroke in patients with vertebrobasilar (VB) stenosis is high has led to the occasional use of stenting in patients with extracranial vertebral artery–origin stenosis. The Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke Trial (VERiTAS) used quantitative MRA (Q MRA) to assess flow in the VB territory of patients on standard medical therapy in order to assess hemodynamic compromise and risk of stroke.27 Those classified as low flow on Q MRA had 78% VB stroke–free survival at 1 year compared with 96% VB stroke–free survival in the normal flow group, demonstrating that distal flow status is an independent predictor of stroke risk.28 Although SAMMPRIS found that best medical treatment was better and safer than stenting for intracranial atherosclerosis, is there value in extracranial vertebral artery procedures?
A recently published study focused on comparison of medical therapy vs stenting of the vertebral arteries. The Vertebral Artery Stenting Trial (VAST) was a randomized open-label trial that enrolled 115 patients with VB TIA or minor stroke in the previous 6 months with vertebral stenosis of ≥50% on 2 imaging modalities.29 Patients were randomized 1:1 to stenting plus best medical treatment (n = 57) or best medical treatment alone (n = 58). Both medical therapy and choice of stent were left up to the treating physician. All patients in the stenting arm received clopidogrel 75 mg daily in addition to aspirin (or vitamin K antagonist) at least 5 days before the procedure and for at least 30 days after. In the stenting arm, 16% had intracranial (V4) disease and in the medical arm 17% had intracranial disease. Primary outcome was a composite of stroke, vascular death, or myocardial infarction within 30 days of treatment.
The primary outcome occurred in 5% (3 of 57) in the stenting group (2 with intracranial stenosis) and in 2% (1 of 58) in the medical group. One-year event rates of stroke in territory supplied by the symptomatic vessel were 9% in the stenting group and 7% in the medical treatment group. At 12 months, 4 of 47 patients with a stent had an occlusion and 3 of 53 medically treated patients had an occlusion. Cumulative incidence of recurrent stroke on medical therapy was 7% during a 3-year median follow-up period. This is considered a relatively low risk of recurrent stroke in the medical management arm. This number is in the setting of nonstandardized medical therapy and brings up the idea that perhaps medical management of extracranial disease could be optimized even further by implementation of SAMMPRIS medical management with a short course of dual antiplatelets and aggressive risk factor control.
On the basis of SAMMPRIS and VAST, both showing superiority of medical treatment over stenting, the recommendation is for aggressive medical therapy for intracranial VB disease as well as for extracranial vertebral artery–origin stenosis.
DISCUSSION
Patients with both extracranial and intracranial large vessel disease require aggressive medical therapy. The neurologist can be the “captain of the ship” in management of these patients. Targeted carotid revascularization can be useful for select patients. Ongoing trials will assess whether improvements in medical therapy have erased the gap with CEA. More research is needed to determine factors contributing to disease progression and risk of recurrent stroke in patients with ICAD. Vessel wall imaging may be useful to define pathophysiology in patients with intracranial stenosis and could ultimately help tailor therapy. The VAST trial has added to the knowledge base of optimal treatment of vertebral disease and demonstrated that medical therapy is preferred to stenting for patients with vertebral artery–origin stenosis.
Stroke due to large vessel atherosclerosis: Five new things
The rate of stroke with asymptomatic carotid stenosis is falling, prompting new trials, such as CREST 2, comparing modern medical therapy with carotid endarterectomy or stenting.
In patients with symptomatic carotid stenosis, if revascularization is to be done within 2 weeks of the last symptomatic event, carotid endarterectomy has a lower periprocedural stroke rate than carotid stenting.
A second randomized trial has confirmed that aggressive medical management is the treatment of choice for patients with severe intracranial stenosis.
Vessel wall imaging may be useful to distinguish between intracranial atherosclerosis and other pathologies such as vasculitis and may aid in assessment of stroke mechanism and lead to targeted therapies. However, more standardization is needed before it is adopted for clinical use.
A recent trial did not show benefit for stenting of vertebral artery–origin stenosis, and aggressive medical management may benefit patients with extracranial disease as well as those with intracranial disease.
AUTHOR CONTRIBUTIONS
Dr. Marulanda-Londoño: wrote first draft, collected references. Dr. Chaturvedi: critical revision and added references, abstract, introduction, and summary box.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
E. Marulanda-Londono reports no disclosures. S. Chaturvedi serves as a consultant for Merck and is on the executive committee of the CREST 2 and ACT I trials; serves on the editorial boards of Neurology and Journal of Stroke & Cerebrovascular Disease, as Assistant Editor for Stroke and as a contributing editor for NEJM Journal Watch Neurology; receives research support from Pfizer, Boehringer-Ingelheim, and NIH/NINDS for serving on grant review committee; and has participated in medico-legal cases. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421–1428. [PubMed] [Google Scholar]
- 2.Chaturvedi S, Bruno A, Feasby T, et al. . Carotid endarterectomy—an evidence-based review: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005;65:794–801. [DOI] [PubMed] [Google Scholar]
- 3.Meschia JF, Bushnell C, Boden-Albala B, et al. . Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:3754–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marquardt L, Fairhead JF, Rothwell PM. Lower rates of intervention for symptomatic carotid stenosis in women than in men reflect differences in disease incidence: a population-based study. Stroke 2010;41:16–20. [DOI] [PubMed] [Google Scholar]
- 5.den Hartog AG, Achterberg S, Moll FL, et al. . SMART Study Group. Asymptomatic carotid artery stenosis and the risk of ischemic stroke according to subtype in patients with clinical manifest arterial disease. Stroke 2013;44:1002–1007. [DOI] [PubMed] [Google Scholar]
- 6.Spence JD, Coates V, Li H, et al. . Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010;67:180–186. [DOI] [PubMed] [Google Scholar]
- 7.Reinhard M, Schwarzer G, Briel M, et al. . Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology 2014;83:1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnett HJM, Taylor DW, Eliasziw M, et al. . Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415–1425. [DOI] [PubMed] [Google Scholar]
- 9.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004;363:915–924. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi S. Aggressive medical therapy alone is adequate in certain patients with severe symptomatic carotid stenosis. Stroke 2013;44:2957–2958. [DOI] [PubMed] [Google Scholar]
- 11.De Rango P, Brown MM, Leys D, et al. . Management of carotid stenosis in women: consensus document. Neurology 2013;80:2258–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rantner B, Goebel G, Bonati LH, Ringleb PA, Mas JL, Fraedrich G; Carotid Stenting Trialists' Collaboration. The risk of carotid artery stenting compared with carotid endarterectomy is greatest in patients treated within 7 days of symptoms. J Vasc Surg 2013;57:619–626.e2; discussion 625–626. [DOI] [PubMed] [Google Scholar]
- 13.White H, Boden-Albala B, Wang C, et al. . Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005;111:1327–1331. [DOI] [PubMed] [Google Scholar]
- 14.Battistella V, Elkind M. Intracranial atherosclerotic disease. Eur J Neurol 2014;21:956–962. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Zhao X, Liu L, et al. . Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 2014;45:663–669. [DOI] [PubMed] [Google Scholar]
- 16.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. . Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005;352:1305–1316. [DOI] [PubMed] [Google Scholar]
- 17.Mazighi M, Tanasescu R, Ducrocq X, et al. . Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology 2006;66:1187–1191. [DOI] [PubMed] [Google Scholar]
- 18.Kwon SU, Hong KS, Kang DW, et al. . Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke 2011;42:2883–2890. [DOI] [PubMed] [Google Scholar]
- 19.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. . SAMMPRIS Trial Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. . Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014;383:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaidat OO, Fitzsimmons B, Woodward B, et al. . VISSIT Trial Investigators. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 2015;313:1240–1248. [DOI] [PubMed] [Google Scholar]
- 22.Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet 2014;383:984–998. [DOI] [PubMed] [Google Scholar]
- 23.Choi YJ, Jung SC, Lee DH. Vessel wall imaging of the intracranial and cervical carotid arteries. J Stroke 2015;17:238–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu WH, Li ML, Gao S, et al. . Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol 2012;71:195–198. [DOI] [PubMed] [Google Scholar]
- 25.Ryoo S, Lee MJ, Cha J, Jeon P, Bang OY. Differential vascular pathophysiologic types of intracranial atherosclerotic stroke: a high-resolution wall magnetic resonance imaging study. Stroke 2015;46:2815–2821. [DOI] [PubMed] [Google Scholar]
- 26.Bodle JD, Feldmann E, Swartz RH, Rumboldt Z, Brown T, Turan TN. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke 2013;44:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin-Hanjani S, Du X, Rose-Finnell L, et al. . VERiTAS Study Group. Hemodynamic features of symptomatic vertebrobasilar disease. Stroke 2015;46:1850–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amin-Hanjani S, Pandey DK, Rose-Finnell L, et al. . Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA Neurol 2016;73:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Compter A, van der Worp HB, Schonewille WJ, et al. . Stenting versus medical treatment in patients with symptomatic vertebral artery stenosis: a randomised open-label phase 2 trial. Lancet Neurol 2015;14:606–614. [DOI] [PubMed] [Google Scholar]