Abstract
Candida africana as a species recovered from female genital specimens is highly close to C. albicans. The present study was conducted to discriminate C. africana from presumptive vaginal C. albicans strains by molecular assay and evaluate their hemolysin activity, biofilm formation, and cohemolytic effect (CAMP) with vaginal bacterial flora. A total of 110 stock vaginal C. albicans isolates were examined by HWP1 gene amplification. Hemolysin activity and the ability of biofilm formation were evaluated by blood plate assay and visual detection methods, respectively. Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus agalactiae were used to evaluate the CAMP-like effects in Sabouraud blood agar media. Based on the size of the amplicons (941 bp), all isolates were identified as C. albicans. All samples were able to produce beta-hemolysin. Moreover, 69 out of 110 of the isolates (62.7%) were biofilm-positive, 54 out of 110 Candida isolates (49%) demonstrated cohemolytic effects with S. agalactiae, and 48 out of 110 showed this effect with S. aureus (43.6%). All isolates were CAMP-negative with S. epidermidis. We detected all isolates as Candida albicans and almost half of the isolates were CAMP-positive with S. aureus and S. agalactiae, suggesting that these bacteria increase the pathogenicity of Candida in vaginal candidiasis.
1. Introduction
Candida albicans is a normal constituent of the human flora present in vaginal mucosa and gastrointestinal tract. This species is the most common fungal pathogen isolated from patients with vulvovaginal candidiasis (VVC) [1]. More recently, atypical strains of C. albicans such as Candida africana, closely related to C. albicans and Candida dubliniensis, have been reported as the cause of vaginitis [2]. Candida africana has been isolated for the first time from patients in Africa and Germany and studied by Tietz et al. [3]. It also has been reported as a cause of VVC in many countries [4–6]. Romeo and Criseo described a specific molecular method for differentiating C. albicans, C. africana, and C. dubliniensis, which involves the use of a single pair of primers targeting the hyphal wall protein 1 (HWP1) gene and its homologs in a polymerase chain reaction- (PCR-) based assay [7]. Hyphal wall protein 1 is a gene that is required for virulence in systemic candidiasis [8]. Several factors such as adhesion, the formation of biofilm and germ tube, phenotypic switching, and synergy with bacteria and production of hydrolases have been proposed to trigger Candida spp. virulence [9].
Biofilms play an important role as reservoirs for pathogenic agents, allow coinfection with other pathogens, promote persistence of infection, and increase mortality rate. The ability of Candida species to form biofilms is an important factor in pathogenesis [10, 11]. Hemolysins play an important role in the infectious process in fungal and bacterial infections and have been reported to have cytotoxic, pore-forming, and lysis effects on eukaryotic cells [11]. The hemolytic factor of Candida species has been described by many researchers and a hemolysis test was developed to determine hemolytic factor [12, 13]. Correlation between fungi and bacteria sometimes leads to synergism in their pathogenicity. The cohemolytic effect, also known as “CAMP reaction,” was first described by Christie, Atkins, and Munch-Peterson in 1944. The cooperative (CAMP-like) lytic processes result from the interaction of at least two membrane-active agents of bacteria, with biological membranes [14]. The vaginal microbiome plays an important role in female health and consists of yeasts and different bacteria species.
Group B streptococci (GBS) are bacterial species that colonize the vagina in pregnant women. Streptococcus agalactiae is a commensal bacterium of human gastrointestinal and genital tracts. Recent studies have shown asymptomatic colonization rates of up to 36% in healthy women [15, 16]. Staphylococcus epidermidis and Staphylococcus aureus are two other bacteria that could be found in general flora of the female genital tract [17, 18].
To the best of our knowledge, there are no data on the cohemolytic effect of bacteria species that are generally part of normal vaginal flora with Candida albicans species causing vaginal candidiasis. In our previous studies, by using conventional and PCR-RFLP methods, no Candida africana was identified as the causative agent of vulvovaginal candidiasis and less data were found about the pathogenicity of the isolates [19, 20]. In the present study, we reexamine vaginal Candida spp isolates presumptively identified as Candida albicans by primary screening tests (germ tube positive and green color colony on CHROMagar media) for the presence of C. africana based on HWP1 gene amplification. Moreover, the ability of biofilm formation and hemolysin activity of the isolates are measured and, to determine synergism with bacteria, the cohemolytic effect (CAMP-like phenomenon) of the isolates with Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus agalactiae were evaluated.
2. Material and Methods
2.1. The Isolates
A total of 110 stock strains of Candida albicans species recovered from patients with vaginal candidiasis were included in this study. All species were identified as Candida albicans, mostly based on initial screening through use of germ tube test and a green color colony on chromogenic medium (CHROMagar Candida, France). The isolates were subcultured on Sabouraud dextrose agar (Merck, Germany) and incubated at 30°C 24 h before use.
2.2. HWP1 Gene Amplification
Genomic DNA from Candida isolates was extracted through the boiling method described by Makimura et al. [21] with small modifications. Briefly, a small amount of yeast colony was boiled for 15 minutes in lysis buffer containing 30 mM EDTA, 0.5% SDS, and 100 mM Tris-HCl. A solution of potassium acetate (2.5 M) was then added to the lysate and held on ice for 1 hour and then centrifuged at 12000 rpm for 5 min and the supernatant was transferred to a new tube. Yeast DNA in the supernatant was precipitated with isopropanol, washed twice with ethanol, air-dried, and resuspended in 50 μl of distilled water prior to use for PCR.
The PCR primers (CR-f 5′-GCTACCACTTCAGAATCATCATC-3′ and CR-r 5′-GCACCTTCAGTCGTAGAGACG-3′) were used for HWP1 gene amplification to distinguish among C. albicans, C. dubliniensis, and C. africana based on the distinct amplicon size, 700 bp for C. africana, 569 bp for C. dubliniensis, and approximately 941 bp for C. albicans. Amplification was carried out in a final volume of 25 μl using Ampliqon master mix kit (Denmark). The PCR reaction conditions for 35 cycles were as follows: initial denaturation at 94°C for 5 min, denaturation step 30 s at 94°C, annealing at 62°C for 45 sec, and extension for 45 sec at 72°C, followed by a final extension at 72°C for 7 min. PCR products were separated in a 1.3% (wt/vol) agarose after gel electrophoresis and visualized by staining with ethidium bromide (0.5 μg/ml). C. albicans (ATCC 10261), C. dubliniensis (CBS 8501), and C. africana (IFRC 707) were used as controls.
2.3. Assessment of Hemolytic and CAMP-Like Effect
Hemolytic activity was evaluated using blood plate assay described by Luo et al. [22]. Sabouraud dextrose agar of sheep blood was prepared by adding 7% v/v of fresh sheep blood and 3% w/v glucose. A yeast suspension equivalent to 2 McFarland turbidity standards was prepared using the pure yeast cultures. Then, 10 μL of inoculation was spotted on sheep blood SDA plates and incubated at 37°C for 48 h. The presence of a distinctive translucent halo around the inoculum site indicated positive hemolytic activity. The results were expressed as beta (complete), gamma (incomplete), and alpha (no hemolysis). Evaluation of cohemolytic effect was performed using standard strains of bacteria including Staphylococcus aureus (ATCC25923), Staphylococcus saprophyticus (PTCC1440), and Streptococcus agalactiae (PTCC1768). After 2 days of incubation for the evaluation of hemolytic activity, a loop was used to streak each bacterium in straight lines across the plate at a distance of 10 mm from the edge of yeast colony border. The plates were then incubated at 37°C and inspected daily for 2 days. A distinct arrowhead of hemolysis at the intersection of the tester strain and the Candida colony streaks was considered as CAMP positive [21]. C. albicans (ATCC10261) was used as a positive control.
2.4. Determination of Biofilm Formation
The evaluation of biofilm formation was carried out through the visual detection (tube method) [10]. Briefly, 10 mL of Sabouraud dextrose broth medium supplemented with glucose (final concentration 8% w/v) in a screw-capped conical polystyrene tube was inoculated with loopful of yeast colonies and incubated without agitation at 35°C for 48 h. After incubation, the broth in the tube was aspirated gently using a sterile Pasteur pipette. Tubes were washed twice with sterile distilled water and then stained with 1% (w/v) safranine. Next, the tubes were decanted after 10 min and examined for the presence of an adherent visible biofilm layer at the bottom and on the wall of the tubes. The test was run in duplicate and the results were expressed as negative (−), weak (+), moderate (++), and strong (+++). Staphylococcus epidermidis (PTCC 1435) and C. albicans (ATCC10261) were used as positive controls.
2.5. Statistical Analysis
The Chi-square and Fisher's exact tests were used for data statistical analysis. A P value ≤ 0.05 was considered as significant.
3. Results
In this study, 110 presumptive strains of vaginal Candida albicans were examined for the presence of Candida africana by HWP1 gene amplification. All the isolates produced a DNA fragment of 941 bp, indicating that these strains belong to C. albicans. Also, none of the isolates produced a DNA fragment of 700 bp, corresponding to C. africana, or 569 bp fragment corresponding to C. dubliniensis (Figure 1).
Figure 1.
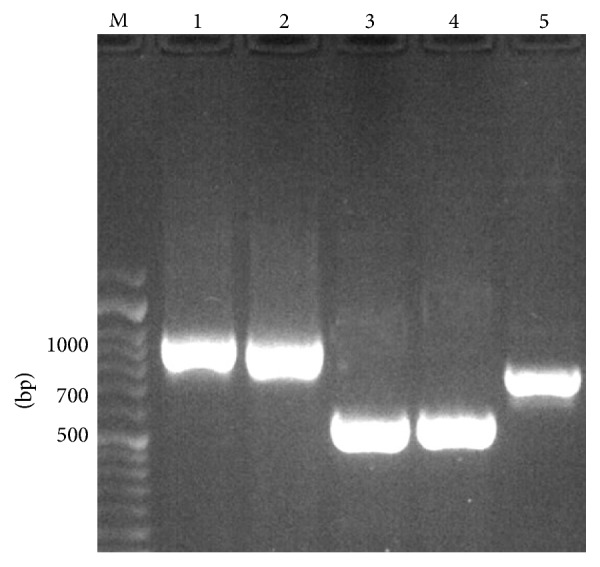
Agarose gel electrophoresis of HWP1 gene amplification (PCR products): C. albicans (ATCC10261) lane 1, C. albicans isolate (lanes 2), C. dubliniensis (CBS 8501) lanes 3 and 4, C. africana (IFRC707) lane 5, and molecular size markers lane M.
Blood plate assay reveals that all the isolates had hemolytic activity and exhibited the zone of complete hemolysis (beta) on sheep blood SDA at 48 h after inoculation: 41 (37.3%) C. albicans strains were biofilm-negative, 22 (20%) of the strains were strongly positive, 19 (17.3%) strains were moderately positive, and 28 strains (25.4%) were weakly positive (Table 1).
Table 1.
Distribution profile of enzymatic activity, biofilm production, and CAMP-like effect on vaginal Candida albicans species isolates. S. aureus (aur.), S. saprophyticus (sap.), and S. agalactiae (aga.).
| Species | Tests | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PCR (HWP1) | Hemolysin | CAMP-like effect | Biofilm | ||||||
| beta | sap. | aur. | aga. | Neg. | 1+ | 2+ | 3+ | ||
| Candida albicans | 110 | 110 | 0 | 48 | 54 | 41 | 28 | 19 | 22 |
| Percent | 100% | 100% | 0% | 43.6% | 49% | 37.3% | 25.4% | 17.3% | 20% |
The CAMP phenomenon was detected using Staphylococcus aureus and Streptococcus agalactiae. The results show that 54 out of 110 (49%) and 48 out of 110 (43.6%) of the isolates produced a zone of complete half-moon-shaped hemolysis below the border of fungal colony and colony streak of S. aureus and S. agalactiae, respectively (Figure 2). The remaining strains failed to produce a positive result.
Figure 2.
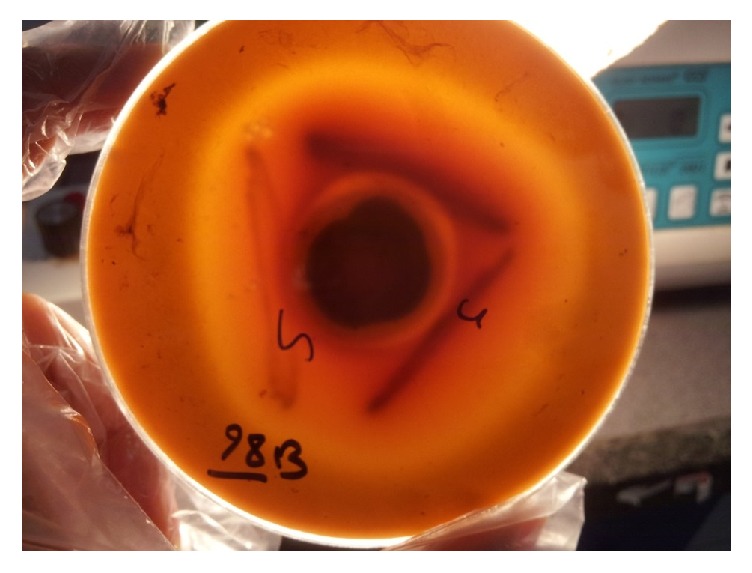
Cohemolytic effect: CAMP-positive (zone of half-moon shaped hemolysis) with S. aureus and S. agalactiae. CAMP-negative (left) with S. saprophyticus.
No cohemolytic effect was detected using Staphylococcus saprophyticus (Table 1).
4. Discussion
Vulvovaginal candidiasis (VVC) is one of the most common forms of Candida infections, mostly caused by Candida albicans, which affect up to 75% of women at least once in their lifetime [23]. Candida africana is an opportunistic yeast pathogen that has been linked to vaginal candidiasis. This yeast was first described in 1995 as an atypical chlamydospore-negative Candida albicans strain [24].
For the past 15 years, several reports have described “atypical” isolates of C. albicans and demonstrated that C. africana and C. albicans isolates are too closely together. Also, Candida africana has been considered as a new species based on ribosomal DNA sequences data in phylogenetic studies. It is often difficult to identify such atypical Candida strains at the species level by routine tests [2, 3]. Romeo and Criseo [7] described a specific molecular method for differentiating C. africana from C. albicans, using a single pair of primers targeting the HWP1 gene. By this method, Yazdanparast et al. could identify five isolates (4.38%) as C. africana from Iranian patients with vulvovaginal candidiasis [6]. Similar to our results, Gumral et al. [25] in Turkey could not identify any C. africana species among vaginal C. albicans isolates.
Shan et al. in China among 1014 isolates presumptively identified as C. albicans could identify 15 (1.5%) to be C. africana isolates [1]. In their study, none of the isolates was verified to be C. dubliniensis, which is in line with our study. Mucci et al. in Argentina [26] by amplification of the HWP1 gene on 42 C. albicans isolates could not identify any isolates of C. africana. In another work, Hazirolan et al. [27] in Turkey, among 375 vulvovaginal C. albicans complex species, could discriminate only three isolates (0.8%) of C. africana by different molecular methods (HWP1, ITS, and D1/D2).
These data indicated the low prevalence of C. africana in vulvovaginal samples. This low rate is mostly because C. africana have an atypical carbohydrate assimilation profile and cannot be identified from C. albicans complex species by routine laboratory tests [27]. Moreover, since molecular methods are not used for diagnosis of this species in common laboratories, the results are often misdiagnosed. Despite worldwide distribution of C. africana, due to the low incidence rate, it is necessary to study more samples. Biofilm formation is one of the major factors contributing to the virulence of Candida. Candida cells exhibit distinct properties in a biofilm such as resistance to several antimicrobial drugs [9]. The range of positivity in biofilm formation of C. albicans species had variation in different levels in many study reports [9, 10, 28]. The results of the present study reveal that 62.7% of isolates were biofilm-positive and only 20% of those demonstrate strong biofilm formation based on our categories. Yigit et al. in Turkey [9] reported that 88.0% of C. albicans strains were biofilm positive and, in Mukhia et al. study [28], 52.3% of C. albicans isolates were biofilm-positive. Overall, we could consider that this factor has a potential for pathogenicity.
The capacity of Candida spp. to colonize host tissue and cause tissue invasion has been associated with its ability to produce extracellular enzymes [9]. The hemolytic factor of Candida species as a probable virulence factor of pathogenesis was described by many investigators and, in most studies, C. albicans exhibit beta hemolytic activity [11–13, 29]. All the isolates in our study were able to produce beta-hemolysin. Thus, we could suggest that this factor has a role in pathogenesis on the isolates. The first step in the pathogenesis of vaginal infections is the interaction of the microbial agents with the normal vaginal microflora.
Cooperative (CAMP-like) lytic processes are the result of the interaction of at least two membrane-active factors of bacteria with biological membranes.
There are limited data on CAMP-like activities on dermatophyte pathogenesis, but not on Candida spp. [30, 31]. It is therefore important to investigate the possibility of cooperative hemolytic activities between Candida and vaginal bacterial microflora. The vaginal microflora, which is composed of a different type of bacteria, presents one of the most important defense mechanisms for preventing the proliferation of microorganisms' foreign to the vagina. Staphylococcus aureus, Staphylococcus saprophyticus, and Streptococcus agalactiae are bacteria that could be found occasionally or permanently as vaginal microflora [17, 18]. Our data indicate that almost half of the vaginal Candida albicans isolates in this study had synergy with S. aureus and S. agalactiae, but not with S. saprophyticus. Our data show that existence of these bacteria in the vaginal area and close to Candida spp can increase the pathogenesis of Candida spp.
5. Conclusion
Despite global and broad geographical distribution of C. africana, we could not isolate any species of this organism from the vaginal presumptive isolates of Candida albicans. By amplification of HWP1 gene, we could easily identify and confirm these isolates as Candida albicans. Based on our data, the activity rate of hemolysin and the ability to produce biofilm among the isolates play a role in the pathogenicity of the Candida. In this study, for the first time, the cohemolytic effect between Candida and vaginal microflora was evaluated. S. aureus and S. agalactiae were CAMP-positive and showed synergy with almost half of the isolates. Overall, these bacteria were found to have a role in increasing pathogenicity of Candida in vaginal candidiasis.
Acknowledgments
This study was funded by Deputy of Research and Technology of Shiraz University of Medical Sciences, Shiraz, Iran (Grant no. 12513).
Disclosure
This study was extracted from an M.S. thesis by Mahboubeh Bordbar.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Keyvan Pakshir contributed to study concept and design, guided in the laboratory investigations, and drafted and revised the manuscript. Mahbobeh bordbar contributed to laboratory examination and interpreted the data. Kamiar Zomorodian contributed to study concept and design and drafted and revised the manuscript. Hasti Nouraei contributed to molecular setting and laboratory examination. Hossein Khodadadi contributed to the laboratory procedures and participated in data management and analysis. All authors read the manuscript and approved it before submission.
References
- 1.Shan Y., Fan S., Liu X., Li J. Prevalence of Candida albicans-closely related yeasts, Candida africana and Candida dubliniensis, in vulvovaginal candidiasis. Medical Mycology. 2014;52(6):636–640. doi: 10.1093/mmy/myu003. [DOI] [PubMed] [Google Scholar]
- 2.Tietz H.-J., Hopp M., Schmalreck A., Sterry W., Czaika V. Candida africana sp. nov., a new human pathogen or a variant of Candida albicans? Mycoses. 2001;44(11-12):437–445. doi: 10.1046/j.1439-0507.2001.00707.x. [DOI] [PubMed] [Google Scholar]
- 3.Tietz H. J., Küssner A., Thanos M., et al. Phenotypic and genotypic characterization of unusual vaginal isolates of Candida albicans from Africa. J Clin Microbiol. 1995;33:2462–2465. doi: 10.1128/jcm.33.9.2462-2465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romeo O., Criseo G. Molecular epidemiology of Candida albicans and its closely related yeasts Candida dubliniensis and Candida africana. Journal of Clinical Microbiology. 2009;47(1):212–214. doi: 10.1128/JCM.01540-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nnadi N. E., Ayanbimpe G. M., Scordino F., et al. Isolation and molecular characterization of Candida africana from Jos, Nigeria. Medical Mycology. 2012;50(7):765–767. doi: 10.3109/13693786.2012.662598. [DOI] [PubMed] [Google Scholar]
- 6.Yazdanparast S. A., Khodavaisy S., Fakhim H., et al. Molecular characterization of highly susceptible Candida africana from vulvovaginal candidiasis. Mycopathologia. 2015;180(5-6):317–323. doi: 10.1007/s11046-015-9924-z. [DOI] [PubMed] [Google Scholar]
- 7.Romeo O., Criseo G. First molecular method for discriminating between Candida africana, Candida albicans, and Candida dubliniensis by using hwp1 gene. Diagnostic Microbiology and Infectious Disease. 2008;62(2):230–233. doi: 10.1016/j.diagmicrobio.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Staab J. F., Bradway S. D., Fidel P. L., Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283(5407):1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- 9.Yigit N., Aktas E., Dagistan S., Ayyildiz A. Investigating biofilm production, coagulase and hemolytic activity in Candida species isolated from denture stomatitis patients. The Eurasian Journal of Medicine. 2011;43(11):27–32. doi: 10.5152/eajm.2011.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gokce G., Cerikcioglu N., Yagci A. Acid proteinase, phospholipase, and biofilm production of Candida species isolated from blood cultures. Mycopathologia. 2007;164(265) doi: 10.1007/s11046-007-9053-4. [DOI] [PubMed] [Google Scholar]
- 11.Elek S. D., Levy E. Distribution of hæmolysins in pathogenic and non-pathogenic staphylococci. The Journal of Pathology. 1950;62(4):541–554. doi: 10.1002/path.1700620405. [DOI] [PubMed] [Google Scholar]
- 12.Manns M. J., Mosser D. M., Bukley H. R. Production of a hemolytic factor by Candida albicans. Infect Immun. Infect Immun. 1994;62:5154–5156. doi: 10.1128/iai.62.11.5154-5156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Negri M. F., Guilhermetti E., Alves A. A., Paulac R., Svidzinski T. I. E. Hemolytic activity and production of germ tubes related to pathogenic potential of clinical isolates of Candida albicans. J Basic Appl Pharm Sci. 2010:31–89. [Google Scholar]
- 14.Christie K., Atkins N., Munch-Petersen E. A note on a lytic phenomenon shown by group B streptococci. Immunology & Cell Biology. 1944;22(3):197–200. doi: 10.1038/icb.1944.26. [DOI] [Google Scholar]
- 15.Ruíz F. O., Gerbaldo G., García M. J., Giordano W., Pascual L., Barberis I. L. Synergistic effect between two bacteriocin-like inhibitory substances produced by lactobacilli strains with inhibitory activity for Streptococcus agalactiae. Current Microbiology. 2012;64(4):349–356. doi: 10.1007/s00284-011-0077-0. [DOI] [PubMed] [Google Scholar]
- 16.Hensler M. E., Liu G. Y., Sobczak S., Benirschke K., Nizet V., Heldt G. P. Virulence role of group B Streptococcusβ-hemolysin/cytolysin in a neonatal rabbit model of early-onset pulmonary infection. The Journal of Infectious Diseases. 2005;191(8):1287–1291. doi: 10.1086/428946. [DOI] [PubMed] [Google Scholar]
- 17.Larsen B., Monif G. R. G. Understanding the bacterial flora of the female genital tract. Clinical Infectious Diseases. 2001;32(4):e69–e77. doi: 10.1086/318710. [DOI] [PubMed] [Google Scholar]
- 18.Corbishley C. M. Microbial flora of the vagina and cervix. Journal of Clinical Pathology. 1977;30(8):745–748. doi: 10.1136/jcp.30.8.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pakshir K., Yazdani M., Kimiaghalam R. Etiology of vaginal candidiasis in Shiraz, Southern Iran. Research Journal of Microbiology. 2007;2(9):696–700. doi: 10.3923/jm.2007.696.700. [DOI] [Google Scholar]
- 20.Jafari M., Salari S., Pakshir K., Zomorodian K. Exoenzyme activity and possibility identification of Candida dubliniensis among Candida albicans species isolated from vaginal candidiasis. Microbial Pathogenesis. 2017;110:73–77. doi: 10.1016/j.micpath.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Makimura K., Murayama S. Y., Yamaguchi H. Detection of a wide range of medically important fungi by the polymerase chain reaction. Journal of Medical Microbiology. 1994;40(5):358–364. doi: 10.1099/00222615-40-5-358. [DOI] [PubMed] [Google Scholar]
- 22.Luo G., Samaranayake L. P., Yau J. Y. Y. Candida species exhibit differential in vitro hemolytic activities. Journal of Clinical Microbiology. 2001;39(8):2971–2974. doi: 10.1128/JCM.39.8.2971-2974.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobel J. D. Vulvovaginal candidosis. The Lancet. 2007;369(9577):1961–1971. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 24.Romeo O., Criseo G. Candida africana and its closest relatives. Mycoses. 2011;54(6):475–486. doi: 10.1111/j.1439-0507.2010.01939.x. [DOI] [PubMed] [Google Scholar]
- 25.Gumral R., Sancak B., Guzel A. B., Saraçli M. A., Ilkit M. Lack of Candida africana and Candida dubliniensis in vaginal Candida albicans isolates in Turkey using HWP1 gene polymorphisms. Mycopathologia. 2011;172(1):73–76. doi: 10.1007/s11046-011-9401-2. [DOI] [PubMed] [Google Scholar]
- 26.Mucci M. J., Cuestas M. L., Landanburu M. F., Mujica M. T. Prevalence of Candida albicans, Candida dubliniensis and Candida africana in pregnant women suffering from vulvovaginal candidiasis in Argentina. Revista Iberoamericana de Micología. 2017;34(2):72–76. doi: 10.1016/j.riam.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Hazirolan G., Altun H., Gumral R., Gursoy N., Otlu B., Sancak B. Prevalence of Candida africana and Candida dubliniensis, in vulvovaginal candidiasis: first Turkish Candida africana isolates from vulvovaginal candidiasis. Journal de Mycologie Médicale. 2017;27(3):376–381. doi: 10.1016/j.mycmed.2017.04.106. [DOI] [PubMed] [Google Scholar]
- 28.Mukhia K. R., Urhekar A. D. Biofilm production by various Candida species isolated from various clinical specimens. I J S R. 2016;5(6):2388–2392. [Google Scholar]
- 29.Pakshir K., Zomorodian K., Karamitalab M., Jafari M., Taraz H., Ebrahimi H. Phospholipase, esterase and hemolytic activities of Candida spp. isolated from onychomycosis and oral lichen planus lesions. Journal de Mycologie Médicale. 2013;23(2):113–118. doi: 10.1016/j.mycmed.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Dogen A., Gumral R., Ilkit M. Haemolytic and co-haemolytic (CAMP-like) activity in dermatophytes. Mycoses. 2015;58(1):40–47. doi: 10.1111/myc.12269. [DOI] [PubMed] [Google Scholar]
- 31.Schaufuss P., Brasch J., Steller U. Dermatophytes can trigger cooperative (CAMP-like) haemolytic reactions. British Journal of Dermatology. 2005;153(3):584–590. doi: 10.1111/j.1365-2133.2005.06679.x. [DOI] [PubMed] [Google Scholar]


