Key Points
In unmanipulated haplo-HSCT, antigenic HLA-DRB1 match, stem cell source, conditioning, and donor sex are associated with GVHD.
The role of HLA-matching status and other factors influencing alloreactivity is more prominent with PTCy compared to ATG GVHD prophylaxis.
Abstract
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) with unmanipulated grafts is increasingly adopted for high-risk acute leukemia, with acute graft-versus-host disease (aGVHD) prophylaxis based on antithymocyte globulin (ATG) or posttransplant cyclophosphamide (PTCy) as main platforms. No consensus exists on selection criteria over several haploidentical donors. We evaluated the impact of donor-recipient antigenic and allelic HLA-A, -B, -C, and -DRB1 mismatches on mismatched haplotype on outcomes of 509 unmanipulated haplo-HSCTs performed for acute leukemia under a PTCy (N = 313) or ATG (N = 196) regimen. An antigenic but not allelic mismatch at the HLA-DRB1 locus was an independent risk factor for grade ≥2 aGVHD in PTCy (hazard ratio [HR], 2.0; 95% confidence interval [CI], 1.2-4.0; P = .02) but not in ATG regimens (HR, 1.3; 95% CI, 0.4-3.4; P = .6). Moreover, the hazards of aGVHD were significantly associated with other factors influencing alloreactivity, including peripheral blood as stem cell source (HR, 2.2; 95% CI, 1.4-3; P < .01), reduced-intensity conditioning (HR, 0.6; 95% CI, 0.4-0.9; P = .04), and female donors (HR, 1.8; 95% CI, 1-3.2; P = .05), in PTCy but not ATG regimens. No significant associations were found between cumulative number of HLA mismatches and GVHD, or between HLA-matching status and other study end points including transplant-related mortality, disease-free survival, and relapse. Based on these data, the role of HLA mismatching on unshared haplotype appears not to be sufficiently prominent to justify its consideration in haploidentical donor selection. However, the role of HLA matching in haploidentical HSCT might be modulated by GVHD prophylaxis, calling for further investigations in this increasingly relevant field.
Visual Abstract
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a central component of treatment algorithms for patients with high-risk acute leukemia.1,2 Donor-recipient HLA compatibility plays a critical role for successful HSCT. The increasing experience with unrelated donors and cord blood transplantation highlights the importance of ascertaining matching at least at HLA-A, -B, -C, and -DRB1 loci between patient and donor, in order to minimize the number of mismatches.3-10 Moreover, when only HLA-mismatched donors are available, clinical data suggest that risks associated with HLA mismatching are not equivalent across all HLA loci.11-19
HSCT from haploidentical donors, namely family-related donors who share a 1-haplotype only genotypical identity with the patient, is a valid option for the sizeable population of patients who lack a matched related or unrelated donor.20 Haploidentical HSCT (haplo-HSCT) presents a major immunological barrier because recognition of the recipient’s mismatched HLA by the donor’s cells provides a potent stimulus for graft-versus-host disease (GVHD), whereas recognition of the donor’s mismatched HLA by the recipient’s immune system leads to graft rejection. Despite this, over recent years, clinical protocols for unmanipulated T-cell–replete haplo-HSCT have been successfully developed, using either posttransplant cyclophosphamide (PTCy) or antithymocyte globulin (ATG) as alternative platforms to harness alloreactivity to the mismatched HLA haplotype.21-27
Although haploidentical donors are referred to as HLA-haplotype-mismatched, some recipients who share an HLA haplotype with their donor are also matched for 1 or more HLA antigens on the unshared haplotype. Until now, scarce evidence existed on the relative role of optimal HLA mismatching on the unshared haplotype in haplo-HSCT. Kasamon et al reported no association between the degree of HLA disparity on the mismatched haplotype and survival after nonmyeloablative transplants with PTCy.28 Huo et al reported that a HLA-B mismatch is associated with an increased risk of acute GVHD (aGVHD) and transplant-related mortality (TRM), as well as worse disease-free survival and overall survival (OS) after T-repleted haplo-HSCT.29 These results were, however, not confirmed in a more recent study by the same group.30
In order to overcome some limitations of previous single-center experiences and heterogeneity of included diseases, we performed a registry-based retrospective study on adult acute leukemia patients who received an unmanipulated haplo-HSCT, and who were reported to the Acute Leukemia Working Party (ALWP) registry of the European Society for Blood and Marrow Transplantation (EBMT).
The aim of the study was to evaluate whether HLA mismatches at specific loci on the unshared haplotype defined at the antigenic or the allelic level are associated with divergent clinical risks after unmanipulated haplo-HSCT.
Methods
Collection of data and inclusion criteria
We included adult patients with de novo acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) who underwent an unmanipulated haploidentical bone marrow (BM) or peripheral blood (PB) transplantation as their first HSCT between January 2007 and December 2014. EBMT centers were asked to provide HLA typing results for patients and donors at the best available resolution level, and to provide an update of transplantation outcomes through specific clinical forms. We selected 509 donor-recipient pairs for whom we had molecular HLA-A, -B, -C, and -DRB1 typing at an antigen (ie, first field; N = 335) or allelic (ie, second field; N = 174) resolution level.31 Haploidentical donors were defined as first-degree relatives who shared 1 HLA haplotype and were mismatched at 1 or more loci on the unshared haplotype.
The research was approved and conducted within the ALWP of EBMT. Patients gave informed consent for data entry into the EBMT registry and for its analysis in accordance with the Declaration of Helsinki.
HLA-matching status
High-resolution HLA allele typing was available for 174 of 509 pairs (34%), while it was missing in the remaining 335 of 509 pairs (66%).
In order to impute high-resolution matching in these pairs, we performed an in silico simulation for all donors and patients with the list of possible diplotypes generated using the German National Bone Marrow Donor Registry haplotype frequency data set. For 10 pairs, 1 or both of those diplotype lists were empty. For the rest, the 2 lists were compared (checking up to 800 000 combinations per pair) to find all possible haploidentical combinations and to calculate the conditional probability of each haplotype triplet according to an established algorithm.32,33 Mismatches at low resolution were directly observable and counted as allele mismatches. For each HLA match defined only at low resolution, we used the respective probability to be matched at high resolution: if it was ≥95%, we classified the respective locus to be matched, if it was ≤5%, we classified the locus to be mismatched. Donor-recipient pairs for whom the probability of each locus to be matched at high resolution was between 6% and 94% were excluded from the analysis, for a total of 418 pairs included to explore the role of high-resolution matching on outcomes.
Among the 509 donor-recipient pairs for whom we considered antigenic HLA matching, 16% were matched at HLA locus A, 11% at locus B, 18% at locus C, and 18% at locus DRB1 (supplemental Table 1). The subgroup of 418 donor-recipient pairs for whom we could impute high-resolution HLA matching reflected the overall characteristics of the entire population (supplemental Table 1). Of them, among pairs with <4 allelic mismatches, 10% were matched at HLA locus A, 9% at locus B, 11% at locus C, and 9% at locus DRB1 (supplemental Table 1). The percentages of antigenic and allelic mismatches at each specific HLA locus in the 2 groups of adopted GVHD-prophylaxis regimens are shown in Table 1.
Table 1.
Donor-recipient HLA-matching status
| PTCy regimen, n (%) | ATG regimen, n (%) | P | |
|---|---|---|---|
| HLA-matching status in the entire population | N = 313 | N = 196 | |
| HLA-A | |||
| Antigen mismatch | 260 (83) | 170 (87) | .26 |
| Antigen match | 53 (17) | 26 (13) | |
| HLA-B | |||
| Antigen mismatch | 277 (88) | 177 (90) | .52 |
| Antigen match | 36 (12) | 19 (10) | |
| HLA-C | |||
| Antigen mismatch | 260 (83) | 157 (80) | .40 |
| Antigen match | 53 (17) | 39 (20) | |
| HLA-DRB1 | |||
| Antigen mismatch | 260 (83) | 158 (81) | .48 |
| Antigen match | 53 (17) | 38 (19) | |
| HLA-matching status in subgroup with HLA high-resolution imputation | N = 258 | N = 160 | |
| HLA-A | |||
| Antigen* mismatch | 222 (86) | 149 (93) | .03 |
| Antigen match | 36 (14) | 11 (7) | |
| Allele† mismatch | 229 (88) | 151 (94) | .07 |
| Allele match | 29 (12) | 9 (6) | |
| HLA-B | |||
| Antigen mismatch | 235 (91) | 146 (91) | .70 |
| Antigen match | 23 (9) | 14 (9) | |
| Allele mismatch | 239 (93) | 145 (91) | .46 |
| Allele match | 19 (7) | 15 (9) | |
| HLA-C | |||
| Antigen mismatch | 226 (88) | 133 (83) | .19 |
| Antigen match | 32 (12) | 27 (17) | |
| Allele mismatch | 236 (91) | 139 (87) | .13 |
| Allele match | 22 (9) | 21 (13) | |
| HLA-DRB1 | |||
| Antigen mismatch | 221 (86) | 140 (87) | .96 |
| Antigen match | 37 (14) | 20 (13) | |
| Allele mismatch | 236 (91) | 148 (92) | .71 |
| Allele match | 22 (9) | 12 (8) | |
| NK alloreactivity in GVL vector | |||
| Yes | 97 (38) | 67 (42) | .38 |
| No | 161 (62) | 93 (58) |
First field HLA typing as reported by the transplant centers.
Second field HLA typing was reported by the transplant centers in 174 pairs (134 PTCy, 40 ATG). For the remaining 244 pairs, allele level matching was imputed by in silico simulation as described in “Methods.”
In order to evaluate the possible differences on haplo-HSCT outcomes arising from the direction of HLA mismatches, we defined HLA mismatches as bidirectional, as host-versus-graft (HVG) direction only (that means that donor’s alleles were not shared by the recipient) or graft-versus-host (GVH) direction only (that means that recipient’s alleles were not shared by the donor). The numbers of bidirectional, HVG-, or GVH-mismatched pairs for each HLA locus, either in the group with antigenic HLA matching, and in the group with allelic HLA matching, are shown in supplemental Tables 3 and 4.
Clinical end points and statistical methods
Neutrophil engraftment was defined as achievement of an absolute polymorphonuclear leukocyte count >500 cells/mm3 for 3 consecutive days. aGVHD and chronic GVHD (cGVHD) were defined and scored according to the Glucksberg and Seattle criteria, respectively.34,35 TRM was defined as death from any cause while in continuous remission of the primary disease. Relapse was defined as hematological relapse with over 5% morphological blast counts in PB or BM. Leukemia-free survival (LFS) was defined as the interval from HSCT to either relapse or death in remission. OS was defined as the interval from HSCT to death from any cause.
In order to analyze the association between HLA matching and transplant end points taking into account the 2 main currently used GVHD-prophylaxis platforms (ie, high-dose PTCy and anti-human thymocyte immunoglobulin), we conducted our analyses separately for each of these platforms.
The χ2 test was adopted for the comparison of categorical variables between the 2 GVHD prophylaxis regimens; the Kruskal-Wallis test was adopted to compare median values of continuous variables between the 2 groups.
The probabilities of LFS and OS were estimated using the Kaplan-Meier estimator.36 Cumulative incidences were estimated for engraftment, GVHD, TRM, and relapse to accommodate competing risks.37 Relapse was a competing risk for TRM; death from any cause was a competing risk for engraftment and relapse. Both relapse and death from any causes were competing risks for GVHD.
Univariate comparisons of survival curves were made using the log-rank test38; the Gray test was used for univariate comparisons of cumulative incidence functions.39 Multivariate Cox proportional hazard models40 were built to adjust for clinical factors associated with a univariate analysis P value <.15. Covariates included in each model were: patient age (continuous) and sex, diagnosis (ALL vs AML), disease status at HSCT, donor sex, host-donor cytomegalovirus (CMV) status, intensity of conditioning regimen, stem cell source (PB vs BM), and transplant center. Interactions between each covariate and match at each HLA locus were tested and were not found.
Affirmation of the proportional hazard assumption was met for all variables. All tests were 2-sided. The type I error rate was fixed at 0.05 for determination of factors associated with time to event. Adjustments for multiple comparisons were performed. Statistical analyses were performed with SPSS (SPSS Inc/IBM, Armonk, NY) and R (R Development Core Team, Vienna, Austria) software.
Results
Patient population and transplant procedures
The main population characteristics are summarized in Table 2. AML was the main indication for haplo-HSCT in both GVHD-prophylaxis platforms. At the time of transplant, more than half of the patients were in complete hematological remission.
Table 2.
Patients, donors, and transplant characteristics
| PTCy-based GVHD prophylaxis, N = 313 | ATG-based GVHD prophylaxis, N = 196 | P | |
|---|---|---|---|
| Patient age, median (range), y | 46 (18-77) | 44 (18-76) | .52 |
| Patient sex, male (%) | 182 (58) | 110 (56) | .62 |
| Time from diagnosis to transplant, median (range), mo | 9 (2-121) | 9 (2-192) | .77 |
| AML/ALL, n (%) | 233 (74)/80 (26) | 140 (71)/56 (29) | .45 |
| Disease status at transplant, n (%) | |||
| CR1 | 117 (37) | 65 (33) | |
| CR ≥2 | 90 (29) | 47 (24) | .12 |
| Advanced (primary induction failure, relapse) | 106 (34) | 84 (43) | |
| Cytogenetic risk stratification, n (%)* | |||
| AML | |||
| Favorable | 22 (13) | 12 (10) | .78 |
| t(8;21)(q22;q22); RUNX1-RUNX1T1 | |||
| inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11 | |||
| Mutated NPM1 without FLT3-ITD (normal karyotype) | |||
| Mutated CEBPA (normal karyotype) | |||
| Intermediate I | 82 (48) | 56 (46) | |
| Mutated NPM1 and FLT3-ITD (normal karyotype) | |||
| Wild-type NPM1 and FLT3-ITD (normal karyotype) | |||
| Wild-type NPM1 without FLT3-ITD (normal karyotype) | |||
| Intermediate II | 30 (18) | 22 (18) | |
| t(9;11)(p22;q23); MLLT3-MLL | |||
| Cytogenetic abnormalities not classified as favorable or adverse | |||
| Adverse | 37 (21) | 31 (26) | |
| inv(3)(q21q26.2) or t(3;3)(q21;q26.2); RPN1-EVI1 | |||
| t(6;9)(p23;q34); DEK-NUP214 | |||
| t(v;11)(v;q23); MLL rearranged | |||
| 5 or del(5q); −7; abnl(17p); complex karyotype | |||
| ALL | |||
| Standard | 18 (41) | 20 (43) | .80 |
| Poor | 26 (59) | 26 (57) | |
| Complex (>5 abn), t(9;22), t(4;11), t(8;14), hypodiploid | |||
| Donor age, median (range), y | 38 (13-70) | 39 (12-74) | .17 |
| Donor sex, male (%) | 170 (54) | 101 (52) | .54 |
| Female donor/male recipient, n (%) | 79 (25) | 55 (28) | .49 |
| CMV serostatus, donor/patient, n (%) | |||
| Negative/negative | 29 (9) | 28 (14) | .07 |
| Other combinations | 284 (91) | 168 (86) | |
| Intensity of conditioning regimen, n (%) | |||
| RIC | 140 (59) | 70 (36) | |
| MAC | 173 (41) | 126 (64) | <.01 |
| Donor stem cell source, n (%) | |||
| PB | 167 (53) | 142 (72) | |
| BM | 146 (47) | 54 (28) | <.01 |
Sixty-one percent of patients received high-dose PTCy as selective alloreactive T-cell depletion, majorly associated with calcineurin inhibitors and mycophenolate mofetil. Thirty-nine percent of patients received an ATG-based GVHD prophylaxis (median dose, 30 mg/kg; range, 2.5-80 mg/kg), mostly combining calcineurin inhibitors or sirolimus and mycophenolate mofetil.
A RIC regimen was more likely adopted in PTCy-based than ATG-based regimens (59% vs 36%, respectively; P < .01). The applied regimen showed a broad variety, with busulfan- and fludarabine-based protocols being the most frequently used. Total-body irradiation was part of the conditioning in 11% of patients treated with a RIC and 19% of patients treated with a myeloablative regimen in the PTCy group, whereas it was used in 5% of patients treated with a RIC and 17% of patients treated with a myeloablative regimen in the ATG group. The stem cell source was BM for 47% of patients in the PTCy-based regimens, whereas in the ATG-based regimens only 28% of patients received BM-derived stem cells (P < .01).
Overall outcomes
The end points in this study were engraftment, 100-day aGVHD, and 2-year cGVHD, TRM, relapse, LFS, and OS. The cumulative incidence of neutrophil engraftment at 60 days was 95% ± 2%, with a median time to engraftment of 17 (range, 3-94) days. The overall cumulative incidences of grade ≥2 aGVHD and grade ≥3 aGVHD at 100 days were 31% ± 5% and 12% ± 3%, respectively, in PTCy-based regimens, whereas they were 34% ± 7% and 14% ± 4%, respectively, in ATG-based regimens.
The 2-year cumulative incidence of cGVHD was 27% ± 5% and 32% ± 7% in PTCy and ATG-based regimens, respectively. The 2-year cumulative incidence of extensive cGVHD was 11% ± 4% and 13% ± 4% in PTCy and ATG-based regimens, respectively.
The cumulative incidence of 2-year TRM was 27% ± 5% in PTCy regimens and 29% ± 6% in ATG regimens. Death was mainly due to infections (53% of patients: bacterial in 54% of cases, viral and fungal in 28% and 18% of cases, respectively), followed by GVHD (30%, in half of the cases GVHD was associated with infections). In the remaining 17% of patients, death was due to other causes including pulmonary toxicity, sinusoidal obstruction syndrome, hemorrhage, graft failure, secondary malignancies, and cardiac toxicity in decreasing order of frequency.
The 2-year cumulative incidence of hematological relapse was 32% ± 5% in PTCy regimens and 38% ± 7% in ATG regimens.
Median follow-up for survivors was 23 (range, 1.5-61) months in patients receiving PTCy regimens, and 36 (range, 1.3-93) months in those receiving ATG. The probabilities of OS and LFS at 2 years after transplantation were 44% ± 6% and 39% ± 6%, respectively, in the PTCy group, and 40% ± 7% and 33% ± 7%, respectively, in the ATG group.
Effect of HLA disparities on clinical outcome
Association between matching at each of the 4 HLA loci (A, B, C, DRB1) and clinical outcome was analyzed separately in patients receiving GVHD prophylaxis with PTCy and ATG.
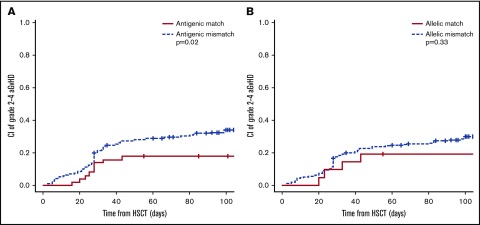
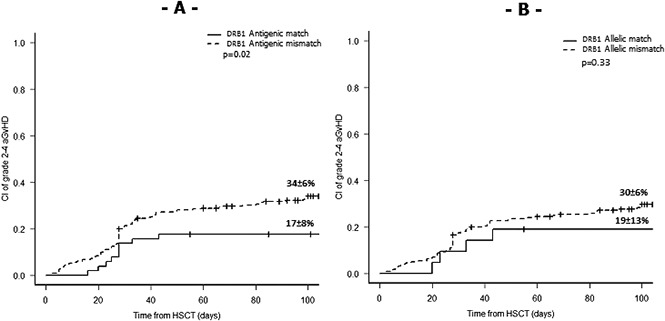
In univariate models, the only significant association found was for aGVHD, which was significantly higher in the presence of an antigenic (P = .02) but not an allelic (P = .33) HLA-DRB1 mismatch in PTCy regimens (Figure 1; supplemental Table 2). This was reflected also examining the cumulative incidence of aGVHD selectively on the cohort of patients with high-resolution HLA imputation, where donor-recipient pairs with an antigen mismatch at the HLA-DRB1 locus had a trend to higher incidence of grade 2-4 aGVHD compared with HLA-DRB1 antigen-matched pairs (33% ± 7% vs 19% ± 10%, respectively; P = .1).
Figure 1.
Influence of HLA-DRB1 matching status on aGVHD 2-4 in PTCy regimens. (A) Stratification according to antigenic HLA-DRB1 matching (N = 313). (B) Stratification according to allelic HLA-DRB1 matching (N = 258).
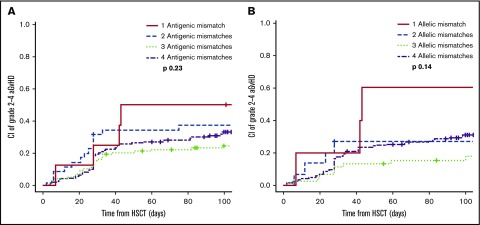
Consistently, this association was dependent on the directionality of the mismatch, with significantly higher incidence of aGVHD in bidirectional or GVH-directed antigenic HLA-DRB1 mismatches compared with others (P = .05; supplemental Table 4). In contrast, the association between HLA-DRB1 mismatches and aGVHD could not be appreciated in ATG-based transplants (antigenic mismatches, P = .54; allelic mismatches, P = .08) (supplemental Table 2). The association with HLA-DRB1 locus matching was not reflective of the total number of HLA mismatches because no association was found between the incidence of aGVHD and the cumulative number of antigenic or allelic HLA mismatches on the unshared haplotype (Figure 2).
Figure 2.
Influence of the number of HLA-A, B, C, DRB1 mismatches on aGVHD 2-4 in PTCy regimens. (A) Stratification according to the number of antigenic mismatches on the unshared haplotype (N = 313). (B) Stratification according to the number of allelic mismatches on the unshared haplotype (N = 258).
For the other clinical end points including engraftment, severe aGVHD, cGVHD, TRM, relapse, LFS, and OS, no associations with mismatching at any of the HLA loci could be found, neither in PTCy nor in ATG regimen (supplemental Table 2). Likewise, no associations with any outcome end point were found with the cumulative number of HLA locus mismatches on the unshared haplotype (supplemental Table 5).
The association between antigenic HLA-DRB1 mismatching and aGVHD in the PTCy but not in the ATG regimen was confirmed after adjusting for the significant clinical factors including diagnosis, disease status at transplant, stem cell source, donor sex, conditioning regimen, and recipient age (Table 3). Antigenic HLA-DRB1 mismatching was an independent predictive factor for aGVHD after haplo-HSCT with PTCy GVHD prophylaxis, with significantly higher adjusted hazards compared with HLA-DRB1 matching (hazard ratio [HR], 2.0; 95% confidence interval [CI], 1.2-4.0; P = .03). In contrast, allele level mismatches had no effect.
Table 3.
Multivariate regression models for associations between HLA-matching status and clinical outcome in PTCy regimens
| aGVHD ≥2 | cGVHD | TRM | Relapse | OS | LFS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| HLA-matching status at the antigenic level | ||||||||||||
| HLA-A, mismatch vs match | 0.7 (0.4-1.1) | .14 | 0.9 (0.5-1.7) | .80 | 0.7 (0.4-1.1) | .14 | 1.3 (0.7-2.3) | .38 | 0.9 (0.6-1.3) | .48 | 0.9 (0.6-1.4) | .65 |
| HLA-B, mismatch vs match | 0.8 (0.4-1.5) | .51 | 1.4 (0.6-3.4) | .45 | 1.9 (0.7-4.9) | .20 | 0.7 (0.4-1.5) | .39 | 1.2 (0.6-2.1) | .62 | 1.1 (0.6-1.9) | .79 |
| HLA-C, mismatch vs match | 1.1 (0.6-2) | .70 | 1.6 (0.8-3.4) | .21 | 0.9 (0.5-1.9) | .87 | 1.5 (0.8-2.8) | .25 | 1.2 (0.7-2) | .49 | 1.2 (0.7-1.9) | .47 |
| HLA-DRB1, mismatch vs match | 2 (1.2-4) | .03* | 0.8 (0.5-1.4) | .40 | 0.7 (0.4-1.2) | .18 | 1.4 (0.8-2.6) | .25 | 1 (0.6-1.5) | .89 | 1 (0.7-1.6) | .84 |
| Leukemia diagnosis, ALL vs AML | — | — | — | — | 1.7 (1-2.8) | .06 | 1.5 (0.9-2.5) | .10 | 1.8 (1.2-2.6) | <10−2* | 1.5 (1.1-2.2) | .03* |
| Disease status, advanced vs CR1 | — | — | — | — | 1.7 (1.1-2.9) | .05* | 5.2 (3-9.1) | <10−5* | 3.4 (2.3-5.1) | <10−5* | 3 (2-4.4) | <10−5* |
| Stem cell source, PB vs BM | 2.2 (1.4-3) | <10−2* | — | — | — | — | — | — | — | — | — | — |
| Donor sex, female vs male | 1.7 (1.1-2.8) | .04* | — | — | — | — | 0.6 (0.4-0.9) | .04* | 0.7 (0.5-0.9) | .04* | 0.7 (0.5-0.9) | .03* |
| CMV status, neg/neg vs other | — | — | — | — | — | — | — | — | — | — | — | — |
| Conditioning intensity, RIC vs MAC | 0.6 (0.4-0.9) | .04* | — | — | — | — | — | — | — | — | — | — |
| Recipient age, 10-y intervals | — | — | — | — | 1.2 (1-1.4) | .05 | — | — | — | — | — | — |
| HLA-matching status at the allelic level | ||||||||||||
| HLA-A, mismatch vs match | 0.7 (0.4-1.5) | .42 | 0.9 (0.4-2.2) | .87 | 1.3 (0.5-3.2) | .54 | 1.3 (0.6-3) | .48 | 1.4 (0.7-2.7) | .31 | 1.3 (0.7-2.3) | .45 |
| HLA-B, mismatch vs match | 0.6 (0.2-1.5) | .26 | 2.2 (0.5-9.3) | .26 | 1.1 (0.3-4) | .89 | 1.1 (0.4-3.5) | .80 | 0.9 (0.4-2.2) | .86 | 1.2 (0.5-2.7) | .73 |
| HLA-C, mismatch vs match | 2.8 (0.9-8.8) | .06 | 1.4 (0.4-4.3) | .58 | 1.8 (0.5-6) | .35 | 1.4 (0.6-3.6) | .44 | 1.6 (0.8-3.5) | .22 | 1.6 (0.8-3.2) | .22 |
| HLA-DRB1, mismatch vs match | 1.3 (0.4-3.4) | .60 | 1.4 (0.5-3.8) | .54 | 0.6 (0.3-1.2) | .13 | 1.2 (0.5-3.1) | .68 | 0.7 (0.4-1.4) | .35 | 0.9 (0.5-1.5) | .54 |
| Leukemia diagnosis, ALL vs AML | — | — | — | — | 1.9 (1-3.4) | .05* | 1.5 (0.8-3.1) | .13 | 1.9 (1.2-3) | <10−2* | 1.7 (1.1-2.5) | .02* |
| Disease status, advanced vs CR1 | — | — | — | — | 1.3 (0.7-2.4) | .36 | 5.2 (2.7-9.7) | <10−4* | 2.9 (1.9-4.5) | <10−4* | 2.6 (1.7-4) | <10−4* |
| Stem cell source, PB vs BM | 2.8 (1.7-4.7) | <10−2* | — | — | — | — | — | — | — | — | — | — |
| Donor sex, female vs male | 1.8 (1-3.2) | .05* | — | — | — | — | 0.6 (0.4-0.9) | .03* | 0.6 (0.4-0.8) | .02* | 0.6 (0.4-0.8) | .02* |
| CMV status, neg/neg vs other | — | — | — | — | — | — | — | — | — | — | — | — |
| Conditioning intensity, RIC vs MAC | 0.6 (0.3-0.9) | .04* | — | — | — | — | — | — | — | — | — | — |
| Recipient age, 10-y intervals | — | — | — | — | 1.3 (1-1.4) | .05 | — | — | — | — | — | — |
Results for HLA matching were adjusted for the covariates listed.
—, Specific clinical variable was not significant in the multivariate model for the examined endpoint.
Statistically significant values.
Consistent with the univariate results, this association was not observed in patients receiving ATG (Table 4). Similarly, antigenic or allelic HLA mismatches at the other loci were not independently associated with any of the other outcome end points regardless of the GVHD prophylaxis (Tables 3 and 4).
Table 4.
Multivariate regression models for associations between HLA-matching status and clinical outcome in ATG regimens
| aGVHD ≥2 | cGVHD | TRM | Relapse | OS | LFS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| HLA-matching status at the antigenic level | ||||||||||||
| HLA-A, mismatch vs match | 1.2 (0.5-2.7) | .70 | 1.7 (0.8-4) | .20 | 1 (0.4-2.4) | .99 | 0.8 (0.4-1.8) | .62 | 0.9 (0.5-1.7) | .80 | 0.9 (0.5-1.6) | .72 |
| HLA-B, mismatch vs match | 1.2 (0.4-3.5) | .72 | 0.8 (0.3-2.4) | .74 | 1 (0.3-2.9) | .97 | 0.6 (0.2-1.8) | .39 | 0.9 (0.4-2) | .78 | 0.8 (0.4-1.7) | .53 |
| HLA-C, mismatch vs match | 1.2 (0.6-2.5) | .66 | 1.2 (0.6-2.4) | .69 | 1.4 (0.6-3.1) | .41 | 1.3 (0.5-3.2) | .55 | 1.4 (0.7-2.5) | .34 | 1.3 (0.7-2.4) | .35 |
| HLA-DRB1, mismatch vs match | 1.4 (0.7-2.8) | .36 | 1.2 (0.6-2.5) | .55 | 2.3 (0.9-6) | .08 | 0.7 (0.0.4-1.2) | .18 | 1.2 (0.7-1.9) | .55 | 1 (0.6-1.6) | .95 |
| Leukemia diagnosis, ALL vs AML | — | — | — | — | 1.9 (1.1-3.3) | .03* | 1.7 (1-3.1) | .06 | 2 (1.4-3.1) | <10−2* | 1.8 (1.2-2.7) | .04* |
| Disease status, Advanced vs CR1 | 2 (1.1-3.7) | .03* | — | — | 2.2 (1.2-4.1) | .01* | 8.6 (4.2-17) | <10−5* | 3.9 (2.4-6.3) | <10−5* | 4.3 (2.8-6.9) | <10−5* |
| Stem cell source, PB vs BM | — | — | — | — | — | — | — | — | — | — | — | — |
| Donor sex, female vs male | — | — | — | — | — | — | — | — | — | — | — | — |
| CMV status, neg/neg vs other | — | — | — | — | — | — | — | — | — | — | — | — |
| Conditioning intensity, RIC vs MAC | — | — | — | — | — | — | — | — | — | — | — | — |
| Recipient age, each 10 y | — | — | — | — | — | — | — | — | — | — | — | — |
| HLA-matching status at the allelic level | ||||||||||||
| HLA-A, mismatch vs match | 2.3 (0.5-11) | .29 | 2 (0.4-9.1) | .38 | 0.6 (0.2-1.7) | .31 | 0.5 (0.2-1.7) | .29 | 0.7 (0.3-1.7) | .46 | 0.5 (0.2-1.2) | .11 |
| HLA-B, mismatch vs match | 3.3 (0.7-16) | .13 | 1.8 (0.5-6.7) | .39 | 1.4 (0.4-4.7) | .59 | 0.5 (0.1-1.6) | .21 | 1.1 (0.5-2.8) | .78 | 0.9 (0.4-2) | .74 |
| HLA-C, mismatch vs match | 0.7 (0.3-1.9) | .49 | 1.3 (0.5-3.5) | .61 | 1.1 (0.4-2.9) | .89 | 1.8 (0.5-6.3) | .34 | 1.1 (0.5-2.4) | .87 | 1.3 (0.6-2.7) | .56 |
| HLA-DRB1, mismatch vs match | 0.4 (0.2-1.1) | .06 | 0.9 (0.3-2.6) | .78 | 1.1 (0.4-3.3) | .89 | 0.8 (0.3-2.4) | .74 | 0.9 (0.4-2) | .81 | 0.9 (0.4-2) | .89 |
| Leukemia diagnosis, ALL vs AML | — | — | — | — | 1.8 (1-3.3) | .05* | 1.9 (1-3.7) | .05* | 1.9 (1.2-3) | <10−2* | 1.7 (1.1-2.5) | .02* |
| Disease status, advanced vs CR1 | 2 (1-4.1) | .05* | — | — | 2.6 (1.3-5) | <10−2* | 6.5 (2.9-14) | <10−4* | 3.4 (2-5.7) | <10−4* | 1.8 (1.2-2.7) | <10−2* |
| Stem cell source, PB vs BM | — | — | — | — | — | — | — | — | — | — | — | — |
| Donor sex, female vs male | — | — | — | — | — | — | — | — | — | — | — | — |
| CMV status, neg/neg vs other | — | — | — | — | — | — | — | — | — | — | — | — |
| Conditioning intensity, RIC vs MAC | — | — | — | — | — | — | — | — | — | — | — | — |
| Recipient age, each 10 y | — | — | — | — | — | — | — | — | — | — | — | — |
Results for HLA matching were adjusted for the covariates listed.
—, Specific clinical variable was not significant in the multivariate model for the examined endpoint.
Statistically significant values.
Predicted natural killer (NK) alloreactivity in the graft-versus-leukemia (GVL) vector according to the “ligand-ligand incompatibility” model was tested both in PTCy and in ATG regimens. The frequencies of pairs with predicted NK alloreactivity are shown in Table 1. Apparently, pairs with predicted NK alloreactivity in the PTCy setting seemed to suffer from a higher relapse rate in univariate analysis (supplemental Table 6); however, this did not translate into a worse progression-free survival or OS. A subanalysis performed only on AML patients did not differ from the above-mentioned results.
Other clinical factors and transplant outcomes
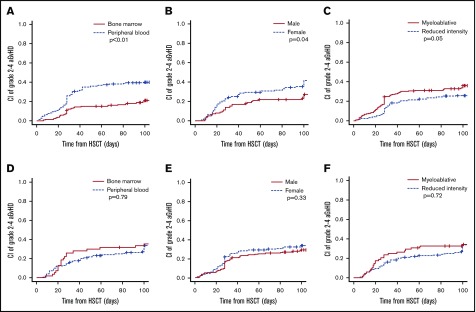
The GVHD prophylaxis regimen modulated the influence of variables other than HLA-matching status as well. Of note, PB as stem cell source and myeloablative conditioning were both independently associated with a higher HR for grade 2-4 aGVHD in the PTCy but not in the ATG regimens (Tables 3 and 4). Moreover, patients receiving haploidentical transplantation from female donors (irrespective of the patients’ sex) had a higher incidence of grade 2-4 aGVHD in the PTCy but not in the ATG regimens, and experienced a lower incidence of relapse together with a better OS and LFS in this group (Figure 3).
Figure 3.
Influence of clinical variables on aGVHD 2-4 in unmanipulated HSCT. Stratification according to stem cell source (A,D), donor sex (B,E), and intensity of conditioning regimen (C,F) in PTCy regimens (A-C) or ATG regimens (D-F).
In contrast, advanced disease status was an independent factor for aGVHD in the ATG but not in the PTCy regimens.
Consistent with previous reports,20 advanced disease status at transplantation was correlated with worse LFS and OS, as well as with higher rates of relapse and TRM. Finally, a diagnosis of ALL was associated with worse LFS and OS. Patients suffering from ALL showed a higher risk of relapse and TRM especially with ATG-based regimens (Table 3).
Discussion
The present study investigates, for the first time, the role of antigen and allele HLA mismatches on the clinical outcome of haplo-HSCT in the context of the 2 majorly used platforms for GVHD prophylaxis, PTCy and ATG. We show that HLA matching on the unshared haplotype has a limited impact, with only antigenic HLA-DRB1 mismatches significantly associated with the single end point grade ≥2 aGVHD in transplants performed under the PTCy regimen, and no association between the cumulative number of HLA mismatches and outcome. This finding has important practical implications because several haploidentical donors are available for most patients and therefore selection based on optimal HLA mismatching on the unshared haplotype is a tempting concept. Our data do not provide support for this notion, but rather suggest that HLA mismatching on the unshared haplotype should not be used as a major factor in haploidentical donor selection. A possible explanation for this observation could be that the presence of mismatches at the other loci on the unshared haplotype might blur subtle effects from matches at individual loci. In line with this concept, the only significant association we observed in this study between HLA-DRB1 mismatching and aGVHD was present at the antigenic but not at the allelic level, further suggesting that subtle molecular differences in HLA polymorphism are probably too weak to make a difference in the clinical setting of haplo-HSCT. Further biological investigations regarding T-cell alloreactivity in the haploidentical context are warranted to clarify this point.
Our finding is in contrast with the report by Kasamon et al, in which no significantly increased risk of aGVHD was associated with a HLA-DRB1 antigen mismatch on the unshared haplotype in patients undergoing unmanipulated BM haplo-HSCT with a nonmyeloablative regimen and PTCy as GVHD prophylaxis. This difference may be dictated by differences in the intensity of the conditioning regimen and graft source in the study by Kasamon et al28: as shown in our current analysis, RIC regimens and BM transplants were both associated with a significant reduction of aGVHD incidence.
Perhaps the most important finding from our study is that the GVHD prophylaxis modulates the influence of different variables, including but not limited to HLA matching on outcome, in particular aGVHD. Indeed, our data suggest that the risk of aGVHD in PTCy but not ATG regimens is particularly sensitive to variables influencing T-cell alloreactivity including not only antigenic HLA-DRB1 matching status but also stem cell source (with peripheral blood stem cells likely containing a higher dose of administered T cells), conditioning intensity, and donor sex. This could reflect the different intrinsic mechanism by which T cells are eliminated globally or only after activation in the ATG and the PTCy regimen, respectively. It is interesting to note that of these variables, only donor sex also had an influence on relapse risk in PTCy regimen, which was not offset by a higher TRM and resulted in a disease-free survival advantage. This beneficial effect might imply peculiar biology of relapse after haploidentical transplantation which is frequently characterized by immune escape mechanisms including loss of the unshared HLA haplotype.41 This could suggest further investigation in the setting of PTCy platforms, where a globally reduced TRM could tilt the balance in favor of improved survival in patients grafted from female donors who could potentially exert a more potent GVL effect possibly being sensitized by previous pregnancies.
It should be noted that the population included in this study partially overlaps with the recently published data by Ruggeri et al,27 in which haplo-HSCT using PTCy was compared with ATG platforms, showing significantly better LFS, lower incidence of GVHD, and nonrelapse mortality of the former regimen compared with the latter in patients with AML in complete remission. In the study by Ruggeri et al,27 the incidence of aGVHD was not influenced by the stem cell source or intensity of conditioning regimens in multivariate analysis adjusting for the adopted GVHD prophylaxis. We hypothesize that the inclusion of advanced status diseases and ALL patients in our cohort could partly explain these results. In addition, different statistical approaches were used in the 2 studies as we separately analyzed the outcomes according to the 2 GVHD platforms and focused on match status at each specific HLA locus as a main effect term in our multivariate model.
Our study has several limitations. In particular, although this is the largest number of haploidentical transplants in which the HLA effect and the GVHD prophylaxis effect has been studied so far, the study might still have limited power to detect subtle associations, in particular for the transplants with high-resolution allele typing which represented an 85% subgroup of the entire cohort.
For the same reasons, we were unable to analyze whether transplantation outcomes in our cohort could be influenced by the permissibility of HLA disparities depending on their immunogenicity. Larger cohorts and implementation with functional biological assays are needed to examine whether prioritization of haploidentical donors should be based on specific mismatches predicted to elicit differential T-cell allorecognition.
Moreover, we were unable to assess the impact of HLA-DQ and -DP because the relevant typing data were not available. Even if HLA-DQ is in strong linkage disequilibrium with other HLA class II loci, we cannot exclude that we missed some information potentially derived from this locus. For HLA-DP, where the existence of permissive mismatches based on functional data has been reported to be associated with clinical outcome of unrelated HSCT,42-44 we could not analyze whether this applied also to our haploidentical cohort. The role of noninherited maternal antigens45 could also not be investigated here because only a small minority of patients had a complete HLA familiar typing that could allow assignment of mismatched haplotypes, given that parents are not usually typed, particularly for adult patients.
The clinical success obtained with haploidentical transplantation both with the ATG and the PTCy conditioning regimen platform has allowed this potentially curative treatment modality to be offered over recent years to many patients who before were precluded from it. This has led to a dramatic increase in the clinical application and relevance of this therapeutic approach not only in Europe, with over 1500 such transplants performed in 2014 alone,46 but also worldwide, in particular in the United States and China. The finding from this study that the role of HLA mismatching on the unshared is not sufficiently prominent to justify its consideration in haploidentical donor selection, and that the role of HLA and other variables appears to be modulated by the adopted GVHD prophylaxis, will be of clinical relevance in this setting. Retrospective and prospective confirmation of these data in further homogeneous and well-sized cohorts are clearly warranted.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all investigators and data managers for their dedicated patient care. M.M. thanks J. V. Melo (University of Adelaide, Adelaide, Australia and Imperial College, London, United Kingdom) for critical reading of the manuscript.
M.M. acknowledges the support received from the Association for Training, Education and Research in Hematology, Immunology and Transplantation (ATERHIT). The Saint-Antoine hospital group was supported by several grants from the Hospital Clinical Research Program (PHRC) from the French National Cancer Institute (M.M.). K.F., M.M., and A.N. were supported by the European Commission Transcan JTC2012 (Cancer12-045-HLALOSS) grant for New Technologists.
Thanks to the EBMT centers for their participation in this study.
Authorship
Contribution: F.L. designed, performed, and coordinated the research, and collected, analyzed, and interpreted the data; F.L. and K.F. wrote the manuscript; M.L. designed the research and performed the statistical analyses; F.C., K.F., A.N., and M.M. designed the research, contributed to data, and commented on the manuscript; C.R.M. performed the high-resolution HLA imputation; A.R., A.B., and M.B. commented on the manuscript; and Z.G., Y.K., W.A., B.B., J.T., A.B., D.B., G.M., A.S., M.B., and D.W.B. contributed to data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesca Lorentino, Hematology and Bone Marrow Transplant Unit, San Raffaele Scientific Institute, Via Olgettina 60, 20132 Milan, Italy; e-mail: lorentino.francesca@hsr.it.
References
- 1.Thomas ED. Marrow transplantation for malignant disease. Am J Med Sci. 1987;294(2):75-79. [DOI] [PubMed] [Google Scholar]
- 2.Thomas E, Storb R, Clift RA, et al. . Bone-marrow transplantation (first of two parts). N Engl J Med. 1975;292(16):832-843. [DOI] [PubMed] [Google Scholar]
- 3.Anasetti C, Amos D, Beatty PG, et al. . Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320(4):197-204. [DOI] [PubMed] [Google Scholar]
- 4.Beatty PG, Clift RA, Mickelson EM, et al. . Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313(13):765-771. [DOI] [PubMed] [Google Scholar]
- 5.Loiseau P, Busson M, Balere ML, et al. . HLA Association with hematopoietic stem cell transplantation outcome: the number of mismatches at HLA-A, -B, -C, -DRB1, or -DQB1 is strongly associated with overall survival. Biol Blood Marrow Transplant. 2007;13(8):965-974. [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Klein J, Haagenson M, et al. . High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576-4583. [DOI] [PubMed] [Google Scholar]
- 7.Petersdorf EW, Gooley T, Malkki M, Horowitz M; International Histocompatibility Working Group in Hematopoietic Cell Transplantation. Clinical significance of donor-recipient HLA matching on survival after myeloablative hematopoietic cell transplantation from unrelated donors. Tissue Antigens. 2007;69(suppl 1):25-30. [DOI] [PubMed] [Google Scholar]
- 8.Eapen M, Rubinstein P, Zhang MJ, et al. . Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369(9577):1947-1954. [DOI] [PubMed] [Google Scholar]
- 9.Kamani N, Spellman S, Hurley CK, et al. ; National Marrow Donor Program. State of the art review: HLA matching and outcome of unrelated donor umbilical cord blood transplants. Biol Blood Marrow Transplant. 2008;14(1):1-6. [DOI] [PubMed] [Google Scholar]
- 10.Gluckman E, Rocha V, Arcese W, et al. ; Eurocord Group. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32(4):397-407. [DOI] [PubMed] [Google Scholar]
- 11.Morishima Y, Kashiwase K, Matsuo K, et al. ; Japan Marrow Donor Program. Biological significance of HLA locus matching in unrelated donor bone marrow transplantation. Blood. 2015;125(7):1189-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flomenberg N, Baxter-Lowe LA, Confer D, et al. . Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923-1930. [DOI] [PubMed] [Google Scholar]
- 13.Petersdorf EW. Optimal HLA matching in hematopoietic cell transplantation. Curr Opin Immunol. 2008;20(5):588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morishima Y, Sasazuki T, Inoko H, et al. . The clinical significance of human leukocyte antigen (HLA) allele compatibility in patients receiving a marrow transplant from serologically HLA-A, HLA-B, and HLA-DR matched unrelated donors. Blood. 2002;99(11):4200-4206. [DOI] [PubMed] [Google Scholar]
- 15.Woolfrey A, Klein JP, Haagenson M, et al. . HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(6):885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beatty PG, Anasetti C, Hansen JA, et al. . Marrow transplantation from unrelated donors for treatment of hematologic malignancies: effect of mismatching for one HLA locus. Blood. 1993;81(1):249-253. [PubMed] [Google Scholar]
- 17.Petersdorf EW, Gooley TA, Anasetti C, et al. . Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92(10):3515-3520. [PubMed] [Google Scholar]
- 18.Eapen M, Klein JP, Sanz GF, et al. ; Eurocord-European Group for Blood and Marrow Transplantation; Netcord; Center for International Blood and Marrow Transplant Research. Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. Lancet Oncol. 2011;12(13):1214-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kögler G, Enczmann J, Rocha V, Gluckman E, Wernet P. High-resolution HLA typing by sequencing for HLA-A, -B, -C, -DR, -DQ in 122 unrelated cord blood/patient pair transplants hardly improves long-term clinical outcome. Bone Marrow Transplant. 2005;36(12):1033-1041. [DOI] [PubMed] [Google Scholar]
- 20.Piemontese S, Ciceri F, Labopin M, et al. ; Acute Leukemia Working Party (ALWP) of the European Group for Blood and Marrow Transplantation (EBMT). A survey on unmanipulated haploidentical hematopoietic stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29(5):1069-1075. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Liu DH, Liu KY, et al. . Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer. 2013;119(5):978-985. [DOI] [PubMed] [Google Scholar]
- 22.Di Bartolomeo P, Santarone S, De Angelis G, et al. . Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121(5):849-857. [DOI] [PubMed] [Google Scholar]
- 23.Peccatori J, Forcina A, Clerici D, et al. . Sirolimus-based graft-versus-host disease prophylaxis promotes the in vivo expansion of regulatory T cells and permits peripheral blood stem cell transplantation from haploidentical donors. Leukemia. 2015;29(2):396-405. [DOI] [PubMed] [Google Scholar]
- 24.Luznik L, O’Donnell PV, Symons HJ, et al. . HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacigalupo A, Dominietto A, Ghiso A, et al. . Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplant. 2015;50(suppl 2):S37-S39. [DOI] [PubMed] [Google Scholar]
- 26.Cieri N, Greco R, Crucitti L, et al. . Post-transplantation cyclophosphamide and sirolimus after haploidentical hematopoietic stem cell transplantation using a treosulfan-based myeloablative conditioning and peripheral blood stem cells. Biol Blood Marrow Transplant. 2015;21(8):1506-1514. [DOI] [PubMed] [Google Scholar]
- 27.Ruggeri A, Sun Y, Labopin M, et al. . Post-transplant cyclophosphamide versus anti-thymocyte-globulin as graft- versus-host disease prophylaxis in haploidentical transplant. Haematologica. 2017;102(2):401-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasamon YL, Luznik L, Leffell MS, et al. . Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16(4):482-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huo MR, Xu LP, Li D, et al. . The effect of HLA disparity on clinical outcome after HLA-haploidentical blood and marrow transplantation. Clin Transplant. 2012;26(2):284-291. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Chang YJ, Xu LP, et al. . Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014;124(6):843-850. [DOI] [PubMed] [Google Scholar]
- 31.Marsh SG, Albert ED, Bodmer WF, et al. . An update to HLA nomenclature, 2010. Bone Marrow Transplant. 2010;45(5):846-848. [DOI] [PubMed] [Google Scholar]
- 32.Pauni V, Gragert L, Schneider J, et al. . Charting improvements in US Registry HLA typing ambiguity using a typing resolution score. Hum Immunol. 2016;77(7):542-549. [DOI] [PubMed] [Google Scholar]
- 33.Kollman C, Maiersb M, Gragertb L, et al. . Estimation of HLA-A, -B, -DRB1 haplotype frequencies using mixed resolution data from a national registry with selective retyping of volunteers. Hum Immunol. 2007;68(12):950-958. [DOI] [PubMed] [Google Scholar]
- 34.Glucksberg H, Storb R, Fefer A, et al. . Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295-304. [DOI] [PubMed] [Google Scholar]
- 35.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215-233. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481. [Google Scholar]
- 37.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695-706. [DOI] [PubMed] [Google Scholar]
- 38.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163-170. [PubMed] [Google Scholar]
- 39.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141-1154. [Google Scholar]
- 40.Cox DR. Regression models and life-tables. J R Stat Soc Ser B. 1972;34(2):187-220. [Google Scholar]
- 41.Vago L, Perna SK, Zanussi M, et al. . Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478-488. [DOI] [PubMed] [Google Scholar]
- 42.Fleischhauer K, Shaw BE, Gooley T, et al. ; International Histocompatibility Working Group in Hematopoietic Cell Transplantation. Effect of T-cell-epitope matching at HLA-DPB1 in recipients of unrelated-donor haemopoietic-cell transplantation: a retrospective study. Lancet Oncol. 2012;13(4):366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crocchiolo R, Zino E, Vago L, et al. ; Gruppo Italiano Trapianto di Midollo Osseo, Cellule Staminale Ematopoietiche (CSE) e Terapia Cellulare; Italian Bone Marrow Donor Registry. Nonpermissive HLA-DPB1 disparity is a significant independent risk factor for mortality after unrelated hematopoietic stem cell transplantation. Blood. 2009;114(7):1437-1444. [DOI] [PubMed] [Google Scholar]
- 44.Pidala J, Lee SJ, Ahn KW, et al. . Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood. 2014;124(16):2596-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Rood JJ, Loberiza FR Jr, Zhang MJ, et al. . Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99(5):1572-1577. [DOI] [PubMed] [Google Scholar]
- 46.Passweg JR, Baldomero H, Bader P, et al. . Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. 2016;51(6):786-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Döhner H, Estey EH, Amadori S, et al. ; European LeukemiaNet. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-474. [DOI] [PubMed] [Google Scholar]
- 48.Hunger SP, Mullighan CG. Redefining ALL classification: toward detecting high-risk ALL and implementing precision medicine. Blood. 2015;125(26):3977-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.