Abstract
Convolvulus austroaegyptiacus Abdallah & Sa'ad (CA) and Convolvulus pilosellifolius Desr. (CP) are commonly used in the Saudi Arabia folk medicine. They are potent in treating the ulcers and skin diseases. The lack of information about their biological activities led us to investigate the possible biological activities by determination of antibacterial and antioxidant activities of total ethanolic extracts and various fractions. Total flavonoid contents of the plants were determined by colorimetric method while total phenols were determined by using Folin-Ciocalteu method. In vitro antibacterial activity was studied against E. coli, P. aeruginosa, and B. subtilis, and the total antioxidant capacity was evaluated by radical scavenging method. IC50 were found to be 21.81, 17.62, and 3.31 μg/mL for CA, CP, and vitamin C, respectively, while the lowest MIC value of 0.25 mg/mL was recorded with CP extract against B. subtilis. Around 21 compounds are tentatively elucidated from both plants using rapid, simple, and high-resolution analytical technique for chemical profiling of natural compounds by direct analysis in real-time of flight-mass spectrometry, of which 17 were not isolated or reported previously.
1. Introduction
Traditional medicine from plant extracts has proved to be clinically effective and relatively less toxic than the existing drugs [1]. Successful determination of biologically active compounds from plant material is largely dependent on the type of solvent used in the extraction procedure [2]. Many solvents have been used to extract active materials from plants such as alcohols (ethanol or methanol), diethyl ether, chloroform, ethyl acetate, n-butanol, and water [3].
Phytochemicals (secondary metabolites) are bioactive chemicals of plant origin. They are naturally synthesized in all parts of the plant body: bark, leaves, stems, roots, flowers, fruits, seeds, and so on [2]. They have been recognized as the basis for traditional herbal medicine practiced in the past and now [4]. All plant parts are usually screened for phytochemicals that may be present; the presence of a phytochemical of interest may lead to its further isolation, purification, and characterization. Then it can be used as the basis for a new pharmaceutical product.
Free radicals which are delivered as a consequence of typical biochemical responses in the body are involved in cancer, ischemic heart disease, inflammation, diabetes, aging, atherosclerosis, immunosuppression, and neurodegenerative disorders [5]. The human body has characteristic barrier system to counter free radical as proteins, for example, catalase, superoxide dismutase, and glutathione peroxidase. Selenium, vitamin C, β-carotene, vitamin E, lycopene, lutein, and different carotenoids have been utilized as supplementary antioxidants. Hence, the secondary metabolites of the plant as flavonoids and terpenoids act an important role in the defense against free radicals [6].
Recently, researchers have focused on increasing human infections caused by bacteria and fungi. Medicinal plants represent a rich source of antimicrobial agents. Because microorganisms have developed resistance to many antibiotics [7], the use of plant extracts and their isolated compounds as a resistant against microorganisms has been increased [8].
Antibacterial activity of different plant extracts was documented in the literature using various organic solvents such as ethanol, methanol, and petroleum ether. Muslim and his coauthor [9] studied the antibacterial activity of Convolvulus arvensis and the results demonstrated a promising effect. A significant cytotoxic activity for essential oil of Convolvulus althaeoides L. was reported [10]. Our search was carried out with the total extract (ethanol) and other solvent soluble fractions (diethyl ether, chloroform, ethyl acetate, and n-butanol) to found out the ability of traditional medicinal plants CA and CP collected from Riyadh, KSA, as an antibacterial agents.
Convolvulaceae (bindweed family) is one of the largest plant families; it includes mostly twining herbs or shrubs, comprising about 85 genera and 2800 species that are further characterized by having the flowers solitary or in terminal [11]. Convolvulaceae family is well known and distinctive as a whole; however its division into species and genera might be difficult. In order to make the taxonomic relationship clear, seed morphology has been studied [12]. This family contains a large number of important plants which are used in treatment of various diseases such as headache, constipation, rheumatism, diabetes, and skin infection [13]. Members of this family mainly contain phenolics, tropane alkaloids, flavonoids, and coumarins [14].
Convolvulus is the largest genus of family Convolvulaceae; it contains 250 species present as herbs, trees, or shrubs [15]. Many Convolvulus species are valuable ornamentals, medicinal and food crops [16]. They possess cytotoxic [17], antioxidant, anti-inflammatory, antiasthma, antijaundice [18], anticancer, and antiulcer activities [19]. In addition, they can be used in treatment of coughs and asthma [13]. They contain many phytochemical groups such as phenolics, tropane alkaloids, sterols, resins, and sugars [14].
Convolvulus austroaegyptiacus Abdallah & Sa'ad (CA) and Convolvulus pilosellifolius Desr. (CP) are some of Convolvulus species. These plants are creeping or twining herbs or shrubs of various appearances and habits. Leaves are undivided or lobed, if parted mostly 1 of glabrous calyx, relatively big, not crowded, funnel-shaped, with entire, 5-angled or 5-plaited limb, and no regular colored stripes like those of Ipomoea [15]. These plants are mainly used in Saudi Arabia as folk medicine for treatment of ulcer diseases. Our research group studied the antiulcerogenic activity of CA and the results showed a promising activity [20]. Although the antiulcerogenic activity of CP is still under study, two compounds (quercetin and kaempferol) were characterized in its composition using HPLC [21].
Direct analysis in real-time of flight-mass spectrometry (DART) is a relatively recent ion source. Its atmospheric pressure ion source instantaneously ionizes any physical state samples (gases, liquids, or solids) in their native state under ambient conditions [22]. The power of DART ion source appears when it is combined with high-resolution mass analyzer as it gives exact molecular weight of ionized compounds from the samples and therefore provides matching molecular formula [23]. Recently, DART-ToF-MS is a strong candidate as a rapid and powerful technique for profiling of several types of samples including the major constituents of total plant species with high resolution mass number directly in real time. Many qualitative analyses of different organic compounds including phytochemicals, metabolites, pharmaceuticals, and synthetic organic molecules have been approved by DART-MS [24]. Since sample preparation or vacuum ionization steps can be omitted in DART-MS analysis, high throughput fingerprinting study of natural samples is possible and this feature is considered one of the most advantageous characteristics of DART ion source [23]. In addition to that, DART ion source ionizes moderately polar to highly nonpolar compounds [25]. Therefore, phenolic compounds are easily ionized and suitable for detection.
To our best knowledge, CA and CP plants are used in folk medicine for treatment of various diseases; however, they have never been studied as antioxidant and antibacterial. In addition, this is the first study using a unique DART-ToF-MS for identification of chemical constituents of the two plants in which new compounds have been identified for the first time in these plants. Consequently, this work has been intended to explore the phytochemical screening and to assess the flavonoids content, total phenol, antimicrobial, antioxidant potentials and DART profiling of the total extracts and different fractions obtained from the aerial parts of CA and CP. Figure 1 shows the plants under investigation.
Figure 1.
The two plants under investigation were (a) CA and (b) CP plants.
2. Materials and Methods
2.1. General
Polyethylene glycol (PEG) with average relative molecular weight of 600 was purchased from Sigma-Aldrich, (Steinheim, Germany). All other solvents and chemicals used were purchased at analytical grade from Merck (Darmstadt, Germany). UV spectra were recorded on UV-1800 Shimadzu spectrophotometer (Tokyo, Japan) (λmax in nm).
2.2. Collection and Preparation of Samples
Aerial parts of CA and CP were collected during flowering stage from the Alrawda, Riyadh district, Saudi Arabia. Samples of the aerial parts were air dried in shade, reduced to fine powder, and kept for total phenols, flavonoids content, and phytochemical investigation.
2.3. Extraction and Fractionation Procedure
100 g of air dried powder from each plant under investigation (CA and CP) was separately extracted by refluxing in 200 mL ethanol 70% three times for 3 hours, filtered off, and concentered using rotary evaporator to obtain 50 g from each plants. The residue obtained from each plant was separately suspended in 200 mL water followed by filtering over a piece of cotton. The filtered aqueous layers were successively fractionated using several solvents such as diethyl ether, chloroform, ethyl acetate, and n-butanol, and the extracts were dried using anhydrous sodium sulfate, followed by concentrations to obtain 5, 3, 10, and 30 g for diethyl ether, chloroform, ethyl acetate, and n-butanol, respectively, from CA and 3, 2, 9 and 31 g for diethyl ether, chloroform, ethyl acetate, and n-butanol, respectively, from CP.
2.4. Preliminary Phytochemical Screening
Preliminary phytochemical screening was carried out for the ethanolic plant extracts and the four fractions from each plant (diethyl ether, chloroform, ethyl acetate, and n-butanol) for their usual plant secondary metabolites using the standard methods [26]. The screening was performed for coumarins, flavonoids, saponins, tannins, triterpenes/steroids, alkaloids, phenolic compounds, and anthraquinones. The precipitate formation or the color intensity was used as analytical responses to these tests.
2.5. Determination of Total Phenolic Compounds Contents
Total phenolic content in the plant ethanolic extract and fractions was determined using spectrophotometric method [27]. Briefly, 0.5 mL of Folin-Ciocalteu's reagent and 1 mg from total extract and fractions was mixed separately with 10 mL of distilled water for 3 min, followed by adding of 1 mL of saturated Na2CO3; then the volume was made up to 25 mL. The samples were kept for 1 h in the dark place and the absorbance was measured at 750 nm wavelength against a blank. The samples were prepared in triplicate for each analysis and the mean value of absorbance was obtained. Different concentrations of gallic acid (5, 10, 20, 40, 60, 80, 100, and 150 mg mL−1) were prepared to obtain a calibration curve. The total phenolic content was expressed in milligrams of gallic acid equivalent per gram of extract [28].
2.6. Determination of Total Flavonoids Contents
Total flavonoids content was determined by a colorimetric method. 0.25 mg from total extract and fractions was separately diluted with 5 mL of distilled water in a 10 mL measuring flask. Then 0.3 mL of a 5% NaNO2 solution was added to the mixture. The test tubes were left in the dark for 6 min, then 0.6 mL of a 10% AlCl3·6H2O solution was added, and the tubes were returned to the dark place for complete reaction. Consequently, 2 mL of 1 M NaOH was added to the mixture, and the total volume was made up to 10 mL with distilled water. After mixing of the solution, the absorbance was measured at 510 nm wavelength. The samples were prepared in triplicate for each analysis and the mean value of absorbance was obtained. The flavonoid content was calculated using a quercetin calibration curve and the results were expressed as mg of quercetin equivalent per gram of extract [29].
2.7. Evaluation of Antioxidant Activity
The antioxidant activity of the two plant crude extracts against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was determined by UV spectrophotometry at 518 nm. The activity was measured according to the method previously described [30]. Different concentrations of the plant extracts were prepared using analytical methanol (1, 3, 7, 10, 20, 30, 40, 50, 80, and 100 μg mL−1). Vitamin C was used as an antioxidant standard. 1 mL from each extract and 3 mL of methanol were mixed by 0.5 mL of 1.0 mM DPPH in methanol and allowed to react at room temperature for 30 minutes. Same amount of methanol and DPPH was mixed to prepare the blank solution. The samples were prepared in triplicate for each analysis and the mean value of absorbance was obtained. The radical scavenging activity was calculated using the following formula:
| (1) |
in which Ab is absorption of the blank sample and Aa is absorption of the extract.
Extract concentration providing 50% inhibition (IC50) was calculated from the plot of inhibition percentage against extract concentration.
2.8. Evaluation of Antibacterial Activity
The crude plant extracts and their successive fractions were subjected to antibacterial evaluation. Susceptibility of the bacterial strains to the two plant extracts and fractions was investigated using disk diffusion method [31]. The concentration of the cell suspension was adjusted to the 2.0 McFarland standard [30] and 50 μL of each microorganism's suspension was spread on a Mueller-Hinton agar plate. Filter paper disks of 5 mm diameter impregnated with 5 mg of each extract and fraction (50 μL of stock solutions) were placed onto the surface of the agar and incubated at 37°C for 24 h. The antibacterial activity of the plant extracts and fractions was tested against three microorganisms, B. subtilis, P. aeruginosa, and E. coli. Doxycycline (20 μg mL−1) was used as positive control and ethanol solvent was used as negative control. The diameters of the inhibition zones were measured to the nearest millimeter.
2.9. Determination of Minimum Inhibitory Concentration (MIC)
MIC represents the lowest extract concentration which prevents the microorganism visible growth. It was carried out for the bacterial strains to the ethanolic extracts of CA and CP. A 100 μL of the inoculum was spread onto 20 mL Mueller–Hinton broth (MHB) supplemented with the extracts at concentrations ranging from (0.0–100 mg mL−1) in Petri dishes, with 5% dimethyl sulfoxide (DMSO). These serially cultures were then incubated at 37°C for 48 h. 10% DMSO was used as a control [31].
2.10. DART-ToF-MS Conditions
High-resolution mass measurements were carried out using a Jeol the AccuTOF LC-plus JMS-T100 LP atmospheric pressure ionization ToF-MS (Jeol, Tokyo, Japan) equipped with a DART ion source (IonSense, Saugus, MA, USA). The mass spectrometer was operated in +ve-ion mode. The orifice 1 and 2 potentials were set to 20 and 5 V. The ring lens potential was set to 13 V. Orifice 1 was set to a temperature of 80°C. The RF ion guide potential was 300 V. The DART ion source was operated with He gas at 4.0 L min−1 flow. The gas heater was set to 200°C. The potential on the discharge needle electrode of the DART source was set to 3.0 kV; perforated and grid electrode potentials were at 100 and 250 V, respectively.
Data acquisition was monitored in the range of 10 to 1000 m/z at acquisition rate 5 spectra min−1. The distance between the DART gun exit and mass spectrometer inlet was 10 mm. In order to perform mass drift compensation for accurate mass measurements, a PEG with 200 μg mL−1 solution in methanol was applied before each analysis run. The elemental composition has been determined on selected peaks using the MassCentre software, version 1.3.m.
2.11. Statistical Analysis
All experiments were done in triplicate and the data were expressed as the mean ± SD. IC50 values were determined by interpolation.
3. Results and Discussion
3.1. Phytochemical Screening
The two plant extracts were defatted with water followed by extracted with different organic solvents according to increasing the polarities; diethyl ether, chloroform, ethyl acetate, and n-butanol. Results obtained from qualitative analysis of phytochemicals of the total ethanolic extracts and their successive fractions of both plants under investigation (CA and CP) are presented in Table 1.
Table 1.
Phytochemical screening of ethanolic extract (total extract) and fractions from CA and CP.
| Group | CA | CP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diethyl ether | CHCl3 | EtOAc | n-Butanol | Total extract | Diethyl ether | CHCl3 | EtOAc | n-Butanol | Total extract | |
| Alkaloids | − | − | − | − | − | − | − | − | − | − |
| Flavonoids | − | − | ± | + | + | − | − | ± | + | + |
| Saponins | − | − | + | + | + | − | − | + | + | + |
| Steroids | + | + | − | − | + | + | + | − | − | + |
| Tannins | − | − | − | + | + | − | − | − | + | + |
| Triterpenoids | + | + | − | − | + | + | + | − | − | + |
| Carbohydrate | − | − | ± | + | + | − | − | ± | + | + |
| Amino acids & proteins | − | − | + | + | + | − | − | + | + | + |
| Anthraquinones | − | − | − | − | − | − | − | − | − | − |
| Coumarins | − | − | + | + | + | − | − | + | + | + |
+: present, −: absent, ±: trace, CHCl3: chloroform, and EtOAc: ethyl acetate.
Phytochemical analysis revealed the presence of coumarins, steroids, flavonoids, saponins, tannins, triterpenoids, carbohydrates, amino acids, and proteins, while both alkaloids and anthraquinones were absent in the crude extracts for both plants. Only steroids and triterpenoids were present in both diethyl ether and chloroform fractions. The chemical contents in the different fractions are considered as secondary metabolite components which are known to be biologically active ingredients. They are directly responsible for different activities such as antimicrobial, antioxidant, antifungal, and anticancer [32]. Normally these secondary metabolites components were isolated from the polar extract [33]. The most important bioactive compounds, which are flavonoids, were found in ethanol (total extract) and n-butanol fraction for both plants under investigation, while traces of flavonoids were present in ethyl acetate fraction. Many researchers reported that flavonoids and phenolic compounds in plants proved to possess multiple biological effects including antioxidant, anti-inflammatory, antimicrobial, antiangiogenic, anticancer, and antiallergic activities. In general, phenolic compounds and their derivatives are also considered as primary antioxidants or free radical scavengers [34].
3.2. Total Phenolic Compounds and Total Flavonoid Contents
The total phenolic contents of the ethanolic extract of CA and CP and their successive fractions were determined using the Folin-Ciocalteu reagent in comparison with standard gallic acid, and the result was expressed in terms of mg gallic acid g−1 extract. The results for both plants in Table 2 indicated that the total phenolic of the total extract (ethanolic extract) for CA (125.44 mg g−1) is lower than for CP (129.97 mg g−1). Total phenolic in ethyl acetate fraction of both plants (146.83 and 158.14 mg g−1 for CA and CP, resp.) was higher than those which found with n-butanol fraction (92.00 and 95.05 mg g−1 for CA and CP, resp.) followed by chloroform then diethyl ether fractions.
Table 2.
Total phenols (mg gallic acid/g extract) and total flavonoids (mg quercetin/g extract) of CA and CP.
| Plant extract | CA | CP | ||
|---|---|---|---|---|
| Total phenols | Total flavonoids | Total phenols | Total flavonoids | |
| Diethyl ether | 30.31 ± 0.053 | 9.15 ± 0.026 | 27.70 ± 0.020 | 8.14 ± 0.026 |
| Chloroform | 39.00 ± 0.182 | 20.23 ± 0.026 | 45.09 ± 0.061 | 22.33 ± 0.026 |
| Ethyl acetate | 146.83 ± 0.089 | 123.88 ± 0.026 | 158.14 ± 0.078 | 125.92 ± 0.035 |
| n-Butanol | 92.00 ± 0.178 | 45.40 ± 0.020 | 95.05 ± 0.046 | 46.37 ± 0.036 |
| Ethanolic extract | 125.44 ± 0.044 | 91.61 ± 0.062 | 129.97 ± 0.087 | 92.58 ± 0.036 |
Values are mean ± SD of 3 replicates.
Colorimetric estimation of the total flavonoids of ethanolic extract of both plants and their successive fractions calculated based on quercetin proved that the total flavonoid contents of the total extract (ethanolic extract) for CA (91.61 mg g−1) was lower than that of CP (92.58 mg g−1). The total flavonoids in ethyl acetate fraction of both plants (123.88 and 125.92 mg g−1 for CA and CP, resp.) were higher than those of n-butanol fraction (45.40 and 46.37 mg g−1 CA and CP, resp.) followed by chloroform and diethyl ether fractions.
According to the total phenolic and total flavonoids assay, the obtained results proved that CP extract has the highest total phenol and flavonoid contents. This demonstrated that the phenolic compounds of these plants might consist mainly of flavonoids, which are mainly in the form of glycosides, as they are more concentrated in the polar solvents, which is more effective in extracting phenolic compounds from plant materials than the less polar solvents [35]. Previous studies recorded that phenolic compounds including flavonoids are associated with strong antioxidant activity and they possess healthy benefits [36].
3.3. Antioxidant Activity
The antioxidant activity of ethanolic extract of CA and CP was measured by the ability to scavenge DPPH free radicals comparing with vitamin C. The scavenging effects of both plant extracts and the standard on the DPPH radical were expressed as half maximal inhibitory concentration (IC50) values; the results are reported in Table 3. Lower IC50 value reflects higher DPPH radical scavenging activity. According to the results obtained, the ethanolic extract of CP showed significant DPPH activity with the IC50 value of 17.62 μg mL−1, while IC50 of vitamin C as standard was 3.31 μg mL−1.
Table 3.
Percentage of inhibition of DPPH and IC50 for ethanolic extract of two plants at different concentrations (μg/mL) compared with vitamin C.
| Concentration (μg/mL) | % inhibition by plant | % inhibition by vitamin C | |
|---|---|---|---|
| CA | CP | ||
| 1 | 27.22 ± 0.75 | 31.84 ± 1.07 | 43.03 ± 1.10 |
| 3 | 35.90 ± 0.63 | 39.56 ± 1.10 | 45.78 ± 0.98 |
| 7 | 38.87 ± 1.51 | 41.09 ± 0.92 | 51.32 ± 1.11 |
| 10 | 41.58 ± 1.38 | 43.18 ± 1.26 | 56.38 ± 1.21 |
| 20 | 59.20 ± 0.61 | 60.37 ± 1.58 | 65.89 ± 1.43 |
| 30 | 63.91 ± 1.23 | 64.58 ± 1.42 | 69.40 ± 0.89 |
| 40 | 65.56 ± 1.14 | 66.21 ± 0.85 | 71.35 ± 1.32 |
| 50 | 67.11 ± 1.53 | 69.67 ± 1.45 | 76.71 ± 1.04 |
| 80 | 83.34 ± 1.19 | 87.48 ± 1.12 | 99.54 ± 1.22 |
| 100 | 86.11 ± 1.15 | 90.17 ± 0.96 | 100.96 ± 1.06 |
| IC50 (μg/mL) | 21.81 | 17.62 | 3.31 |
Values are mean ± SD of 3 replicates.
Different experiments have been performed to recognize the property of plant extracts to scavenge the free radicals [37]. DPPH is a free radical compound which has scavenging ability for antioxidants samples and shows good absorbance at 517 nm [38]. Vitamin C is usually used as a standard antioxidant and it has a strong DPPH scavenging property [39]. Several studies demonstrated a linear correlation between antioxidant activity and phenolic content of plant extracts [40]. Our findings were not an exceptional case, as they indicated that CP extract consists of high quantity of phenolic compounds and showed promising antioxidant activity with 17.62 μg mL−1 IC50 value.
3.4. Antibacterial Activity
To our knowledge, no previous publications have reported the antibacterial activity of our plants. Therefore, antibacterial activity of total extract and successive fractions of CA and CP against the tested bacteria strains was assessed by the presence and absence of inhibition zones using disk diffusion method. The inhibition zone produced by the crude extracts and fractions for both plants on different bacterial strains was between 4 mm and 20 mm. The antimicrobial studies revealed that the ethanolic extract and fractions of CP showed inhibitory effects on B. subtilis, P. aeruginosa, and E. coli, while the crude extract and fractions of CA exhibited less inhibitory effects on these microorganisms as shown in Table 4. The MIC value was lowest for the ethanolic extract of CP (0.25 mg mL−1) against B. subtilis followed by the ethanolic extract of CA (0.78 mg mL−1) against B. subtilis (Table 5).
Table 4.
Antibacterial activity of CA and CP extracts and fractions.
| Plant | Inhibition zone (mm) | Microorganisms tested | Doxycycline (control) | ||||
|---|---|---|---|---|---|---|---|
| EtOH (crude) | Diethyl ether | CHCl3 | EtOAc | n-Butanol | |||
| CA | 7 | 6 | 8 | 6.3 | 4 | B. subtilis | 13 |
| 16 | 11 | 12 | 10 | 4.3 | P. aeruginosa | 18 | |
| 7 | 6.3 | 7 | 9 | 5 | E. coli | 10 | |
|
| |||||||
| CP | 9 | 6.2 | 10 | 8.2 | 5.7 | B. subtilis | 12 |
| 20 | 12.7 | 14 | 16.1 | 8.9 | P. aeruginosa | 20 | |
| 9.8 | 6.5 | 8 | 10 | 7.7 | E. coli | 11 | |
IZ: inhibition zone, EtOH: ethanol, CHCl3: chloroform, and EtOAc: ethyl acetate.
Table 5.
MIC against different strains of bacteria for ethanolic extracts of CA and CP.
| Plant | MIC (mg/mL) | Bacterial strains |
|---|---|---|
| CA | 0.78 | B. subtilis |
| 1.54 | P. aeruginosa | |
| 1.20 | E. coli | |
|
| ||
| CP | 0.25 | B. subtilis |
| 1.06 | P. aeruginosa | |
| 0.93 | E. coli | |
Many previous studies revealed that the plants play an important role in development of new therapeutic sources. The first step in order to achieve this target is the in vitro antibacterial activity test [41]. Reviewing the literature, the presence of the secondary metabolites in the plant extract like saponins, tannins, flavonoids, coumarin, phenol, and triterpenoids has a promising activity against pathogens and helps the antibacterial activities of plants [42]. According to previous study, the ability of plant extracts against bacteria depends on the solubility of the bioactive constituents [43]. Our results clarified that the crude extract of CP proved its efficiency to be used as source for antibacterial compounds compared with some standard antibiotics due to its inhibitory effects on B. subtilis, P. aeruginosa, and E. coli with lowest MIC value (0.25 mg mL−1) against B. subtilis.
3.5. DART-ToF-MS of the Plants Extract
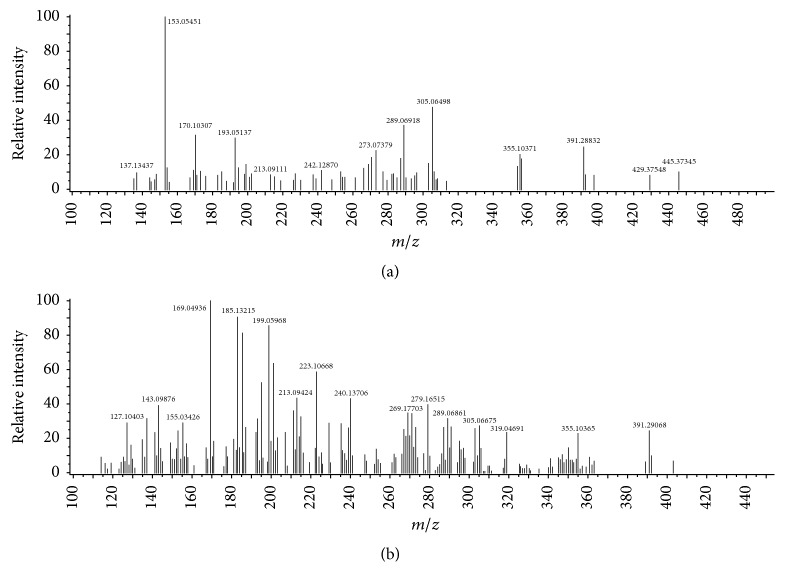
Spectrometry analysis is one of the most powerful analytical methods available for exact structural identification of organic compounds. In this study, DART-ToF-MS profiles of the total CA and CP plants extract were obtained. Both plants were subjected to DART-ToF-MS analysis under the same conditions. As shown in Figure 2 and reported in Table 6, peaks corresponding to the molecular species of phellandrenes (m/z 137), p-hydroxyphenylacetic acid (m/z 153), scopoletin (m/z 193), ferulic acid (m/z 195), syringic acid (m/z 199), pinosylvin (m/z 213), apigenin/galangin (m/z 271), naringenin (m/z 273), kaempferol/luteolin/fisetin (m/z 287), eriodictyol/aromadendrin (m/z 289), quercetin (m/z 303), taxifolin (m/z 305), and scopolin (m/z 355) were observed in the DART mass spectra in both plants. All selected peaks of the major components were on the type of protonated molecular ions [M + H]+ related to the highest peak intensity of the same compound. In addition, the unsaturated degree of each compound is reported in Table 6.
Figure 2.
DART mass spectra of (a) CA and (b) CP plants.
Table 6.
Exact mass data from the DART mass spectra of CA and CP plant constituents.
| Plant | Experimental mass (m/z) | Calculated mass (m/z) | Mass error (mmu) | Formula | Remarks | Unsaturation degree |
|---|---|---|---|---|---|---|
| CA | 137.13437 | 137.13303 | 1.34 | C10H17 | Phellandrenes | 2.5 |
| 153.05451 | 153.05516 | −0.65 | C8H9O3 | p-Hydroxyphenylacetic acid | 4.5 | |
| 193.05137 | 193.05007 | 1.30 | C10H9O4 | Scopoletin | 6.5 | |
| 195.06742 | 195.06572 | 1.70 | C10H11O4 | Ferulic acid | 5.5 | |
| 199.05796 | 199.06063 | −2.67 | C9H11O5 | Syringic acid | 4.5 | |
| 213.09111 | 213.09155 | −0.44 | C14H13O2 | Pinosylvin | 8.5 | |
| 271.06318 | 271.06063 | 2.55 | C15H11O5 | Apigenin/galangin | 10.5 | |
| 273.07379 | 273.07628 | −2.49 | C15H13O5 | Naringenin | 9.5 | |
| 287.05627 | 287.05554 | 0.73 | C15H11O6 | Kaempferol/luteolin/fisetin | 10.5 | |
| 289.06918 | 289.07119 | −2.01 | C15H13O6 | Eriodictyol/aromadendrin | 9.5 | |
| 303.05202 | 303.05045 | 1.57 | C15H11O7 | Quercetin | 10.5 | |
| 305.06498 | 305.06610 | −1.12 | C15H13O7 | Taxifolin | 9.5 | |
| 355.10371 | 355.10287 | 0.84 | C16H19O9 | Scopolin | 7.5 | |
|
| ||||||
| CP | 137.13172 | 137.13303 | −1.31 | C10H17 | Phellandrenes | 2.5 |
| 153.05573 | 153.05516 | 0.57 | C8H9O3 | p-Hydroxyphenylacetic acid | 4.5 | |
| 155.03426 | 155.03442 | −0.16 | C7H7O4 | Protocatechuic acid/ gentisic acid |
4.5 | |
| 169.04936 | 169.05007 | −0.71 | C8H9O4 | Vanillic acid | 4.5 | |
| 193.05084 | 193.05007 | 0.77 | C10H9O4 | Scopoletin | 6.5 | |
| 195.06620 | 195.06572 | 0.48 | C10H11O4 | Ferulic acid | 5.5 | |
| 199.05968 | 199.06063 | −0.95 | C9H11O5 | Syringic acid | 4.5 | |
| 213.09424 | 213.09155 | 2.69 | C14H13O2 | Pinosylvin | 8.5 | |
| 271.06165 | 271.06063 | 1.02 | C15H11O5 | Apigenin/galangin | 10.5 | |
| 273.07482 | 273.07628 | −1.46 | C15H13O5 | Naringenin | 9.5 | |
| 287.05517 | 287.05554 | −0.37 | C15H11O6 | Kaempferol/luteolin/fisetin | 10.5 | |
| 289.06861 | 289.07119 | −2.58 | C15H13O6 | Eriodictyol/aromadendrin | 9.5 | |
| 303.05172 | 303.05045 | 1.27 | C15H11O7 | Quercetin | 10.5 | |
| 305.06675 | 305.06610 | 0.65 | C15H13O7 | Taxifolin | 9.5 | |
| 319.04691 | 319.04536 | 1.55 | C15H11O8 | Myricetin | 11 | |
| 355.10365 | 355.10287 | 0.78 | C16H19O9 | Scopolin | 7.5 | |
Furthermore, three compounds, protocatechuic acid/gentisic acid, vanillic acid, and myricetin corresponding to m/z 155, 169, and 319, respectively, have been observed in CP plant but not detected in CA plant.
Since its soft ionization technique, DART ion source provides relatively simple mass spectra with minimal fragmentations, consisting of the major constituents of the protonated molecules in the case of positive ion mode. All the results prove that DART-ToF-MS is an appropriate method to differentiate between the two studied plants and for rapid identification of their major components, which mainly consist of flavonoids and phenols as mentioned in the phytochemical screening section.
On the other hand, the unsaturated degree of each compound helps to predict the chemical structures by determination how many double bond and rings are present in the compound, which also confirms the expected flavonoids and phenols compounds. It should be noted that some compounds such as apigenin and galangin have the same molecular formula and exact mass. Therefore, they cannot be distinguished on the basis of high-resolution mass spectrometry using DART-ToF-MS technique.
4. Conclusion
We demonstrate that DART-ToF-MS combined by multivariate analysis allows for rapid screening and metabolic characterization of different compounds from plant extracts without complex metabolic preparation steps such as phenol and flavonoid compounds. With this method, 21 phenol and flavonoid compounds are tentatively detected and identified in CA and CP; 17 of them are reported from these plants for the first time. The obtained results confirmed that DART-ToF-MS data were specific and valuable since it can directly ionize various organic molecules. Therefore, it would be a technique of choice for simple and rapid screening of natural products and a wide variety of materials with very simple or no sample preparation. The newly discovered phenol and flavonoid compounds expand our understanding on the chemical constituents of CA and CP and can be targets for further phytochemical studies. The literature survey indicated that there is no research that has been carried out on antioxidant potent and antibacterial activity of these plant species; therefore, this study can be a guideline for further biological activities investigations.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no. RGP-1438-0007.
Contributor Information
Asma'a Al-Rifai, Email: asma-alrifai@hotmail.com.
Ahmad Aqel, Email: aifseisi@ksu.edu.sa.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Awaad A. S., El-Meligy R. M., Qenawy S. A., Atta A. H., Soliman G. A. Anti-inflammatory, antinociceptive and antipyretic effects of some desert plants. Journal of Saudi Chemical Society. 2011;15(4):367–373. doi: 10.1016/j.jscs.2011.02.004. [DOI] [Google Scholar]
- 2.Lalitha T. P., Jayanthi P. Preliminary studies on phytochemicals and antimicrobial activity of solvent extracts of Eichhornia crassipes (Mart.) Solms. Asian Journal of Plant Science & Research. 2012;2:115–122. [Google Scholar]
- 3.Poulson H., Preime Loft S. Role of oxidative DNA damage in cancer initiation and promotion. European Journal of Cancer Preventive. 1998;7:9–16. doi: 10.1097/00008469-199710000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Govindarajan R., Vijayakumar M., Pushpangadan P. Antioxidant approach to disease management and the role of “Rasayana” herbs of Ayurveda. Journal of Ethnopharmacology. 2005;99(2):165–178. doi: 10.1016/j.jep.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Chanda S., Kaneria M., Nair R. Antibacterial activity of Psoralea corylifolia L. seed and aerial parts with various extraction methods. Research Journal of Microbiology. 2011;6(2):124–131. doi: 10.3923/jm.2011.124.131. [DOI] [Google Scholar]
- 6.Adeshina G. O., Onaolapo J. A., Ehinmidu J. O., Odama L. E. Phytochemical and antimicrobial studies of the ethyl acetate extract of Alchornea cordifolia leaf found in Abuja, Nigeria. Journal of Medicinal Plants Research. 2010;4(8):649–658. [Google Scholar]
- 7.Muslim R., Fozia R., Ali, et al. Comparative antibacterial study of Convolvulus arvensis collected from different areas of Khyber Pakhtunkhwa, Pakistan. IRJP. 2012;3:220–222. [Google Scholar]
- 8.Hassine M., Zardi-Berguaoui A., Znati M., Flamini G., Ben Jannet H., Hamza M. A. Chemical composition, antibacterial and cytotoxic activities of the essential oil from the flowers of Tunisian Convolvulus althaeoides L. Natural Product Research (Formerly Natural Product Letters) 2014;28(11):769–775. doi: 10.1080/14786419.2013.879476. [DOI] [PubMed] [Google Scholar]
- 9.Robertson K. R. Odonellia, a New Genus of Convolvulaceae from Tropical America. Brittonia. 1982;34(4):p. 417. doi: 10.2307/2806498. [DOI] [Google Scholar]
- 10.Abdel Khalik K. N., Osman A. K. Seed morphology of some species of Convolvulaceae from Egypt (Identification of species and systematic significance) Feddes Repertorium. 2007;118(1-2):24–37. doi: 10.1002/fedr.200711123. [DOI] [Google Scholar]
- 11.Donia A. M., Alqasoumi S. I., Awaad A. S., Cracker L. Antioxidant activity of Convolvulus hystrix Vahl and its chemical constituents. Pakistan Journal of Pharmaceutical Sciences. 2011;24:143–147. [PubMed] [Google Scholar]
- 12.Nacef S., Jannet H. B., Abreu P., Mighri Z. Phenolic constituents of Convolvulus dorycnium L. flowers. Phytochemistry Letters. 2010;3(2):66–69. doi: 10.1016/j.phytol.2009.12.001. [DOI] [Google Scholar]
- 13.Migahid A. M. Flora of Saudi Arabia. 3rd. Riyadh: KSA. University Libraries, King Saud University; 1988. [Google Scholar]
- 14.Stefanović S., Krueger L., Olmstead R. G. Monophyly of the convolvulaceae and circumscription of their major lineages based on DNA sequences of multiple chloroplast loci. American Journal of Botany. 2002;89(9):1510–1522. doi: 10.3732/ajb.89.9.1510. [DOI] [PubMed] [Google Scholar]
- 15.Arora M., Malhotra M. A review on macroscopical, phytochemical and biological studies on Convolvulus arvensis (field bindweed. Pharmacologyonline. 2011;3:p. 1296. [Google Scholar]
- 16.Griffin W. J., Lin G. D. Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry. 2000;53(6):623–637. doi: 10.1016/S0031-9422(99)00475-6. [DOI] [PubMed] [Google Scholar]
- 17.Todd F. G., Stermitz F. R., Schultheis P., Knight A. P., Traub-Dargatz J. Tropane alkaloids and toxicity of Convolvulus arvensis. Phytochemistry. 1995;39(2):301–303. doi: 10.1016/0031-9422(94)00969-Z. [DOI] [PubMed] [Google Scholar]
- 18.Awaad A. S., Al-Rifai A. A., El-Meligy R. M., Alafeefy A. M., Zain M. E. New Activities for Isolated Compounds from Convolvulus austro-aegyptiacus as Anti-ulcerogenic, Anti-Helicobacter pylori and Their Mimic Synthesis Using Bio-guided Fractionation. Phytotherapy Research. 2015;29(9):1311–1316. doi: 10.1002/ptr.5379. [DOI] [PubMed] [Google Scholar]
- 19.Al-Rifai A., Aqel A., Awaad A., ALOthman Z. A. Analysis of Quercetin and Kaempferol in an Alcoholic Extract of Convolvulus pilosellifolius using HPLC. Communications in Soil Science and Plant Analysis. 2015;46(11):1411–1418. doi: 10.1080/00103624.2015.1043454. [DOI] [Google Scholar]
- 20.Bajpai V., Sharma D., Kumar B., Madhusudanan K. P. Profiling of Piper betle Linn. Cultivars by direct analysis in real time mass spectrometric technique. Biomedical Chromatography. 2010;24(12):1283–1286. doi: 10.1002/bmc.1437. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee S., Madhusudanan K. P., Khanuja S. P. S., Chattopadhyay S. K. Analysis of cell cultures of Taxus wallichiana using direct analysis in real-time mass spectrometric technique. Biomedical Chromatography. 2008;22(3):250–253. doi: 10.1002/bmc.919. [DOI] [PubMed] [Google Scholar]
- 22.Singh A., Bajpai V., Srivastava M., Arya K. R., Kumar B. Rapid screening and distribution of bioactive compounds in different parts of Berberis petiolaris using direct analysis in real time mass spectrometry. Journal of Pharmaceutical Analysis. 2015;5(5):332–335. doi: 10.1016/j.jpha.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hossain M. A., Nagooru M. R. Biochemical profiling and total flavonoids contents of leaves crude extract of endemic medicinal plant Corydyline terminalis L. Kunth. Pharmacognosy Journal. 2011;3(24):25–30. doi: 10.5530/pj.2011.24.5. [DOI] [Google Scholar]
- 24.Morlock G., Ueda Y. New coupling of planar chromatography with direct analysis in real time mass spectrometry. Journal of Chromatography A. 2007;1143(1-2):243–251. doi: 10.1016/j.chroma.2006.12.056. [DOI] [PubMed] [Google Scholar]
- 25.Hossain M. A., Shah M. D., Gnanaraj C., Iqbal M. In vitro total phenolics, flavonoids contents and antioxidant activity of essential oil, various organic extracts from the leaves of tropical medicinal plant Tetrastigma from Sabah. Asian Pacific Journal of Tropical Medicine. 2011;4(9):717–721. doi: 10.1016/S1995-7645(11)60180-6. [DOI] [PubMed] [Google Scholar]
- 26.Bao J., Cai Y., Sun M., Wang G., Corke H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. Journal of Agricultural and Food Chemistry. 2005;53(6):2327–2332. doi: 10.1021/jf048312z. [DOI] [PubMed] [Google Scholar]
- 27.Liu M., Li X. Q., Weber C., Lee C. Y., Brown J., Liu R. H. Antioxidant and antiproliferative activities of raspberries. Journal of Agricultural and Food Chemistry. 2002;50(10):2926–2930. doi: 10.1021/jf0111209. [DOI] [PubMed] [Google Scholar]
- 28.Ayoola G. A., Sofidiya T., Odukoya O., Coker H. A. B. Phytochemical screening and free radical scavenging activity of some Nigerian medicinal plants. Journal of Pharmaceutical Sciences and Pharmacy Practice. 2006;8:133–136. [Google Scholar]
- 29.Thitilertdecha N., Teerawutgulrag A., Rakariyatham N. Antioxidant and antibacterial activities of Nephelium lappaceum L. extracts. LWT - Food Science and Technology. 2008;41(10):2029–2035. doi: 10.1016/j.lwt.2008.01.017. [DOI] [Google Scholar]
- 30.Testing, Sixteenth informational supplement M100-S16 , Clinical and Laboratory Standards Institute. Wayne: Clinical and Laboratory Standards Institute, Performance Standards for Antimicrobial Susceptibility; 2006. [Google Scholar]
- 31.Khadidja M., Nassima B., Chahrazed B., Xavier F. Chemical composition and antimicrobial activity of essential oils isolated from Algerian Juniperus phoenicea L. and Cupressus sempervirens L. Journal of Medicinal Plants Research. 2010;4:959–964. [Google Scholar]
- 32.Anyasor G. N., Olusola Ogunwenmo K., Oyelana O. A., Akpofunure B. E. Phytochemical constituents and antioxidant activities of aqueous and methanol stem extracts of Costus afer Ker Gawl. (Costaceae) African Journal of Biotechnology. 2010;9(31):4880–4884. [Google Scholar]
- 33.Hatipoĝlu G., Sökmen M., Bektaş E., et al. Automated and standard extraction of antioxidant phenolic compounds of Hyssopus officinalis L. ssp. angustifolius. Industrial Crops and Products. 2013;43(1):427–433. doi: 10.1016/j.indcrop.2012.07.028. [DOI] [Google Scholar]
- 34.Liu J., Wang C., Wang Z., Zhang C., Lu S., Liu J. The antioxidant and free-radical scavenging activities of extract and fractions from corn silk (Zea mays L.) and related flavone glycosides. Food Chemistry. 2011;126(1):261–269. doi: 10.1016/j.foodchem.2010.11.014. [DOI] [Google Scholar]
- 35.Pattanaik C., Reddy C. S., Dhal N. K. Phytomedicinal study of coastal sand dune species of Orissa. Indian Journal of Traditional Knowledge. 2008;7(2):263–268. [Google Scholar]
- 36.Sakanaka S., Tachibana Y., Okada Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (Kakinoha-cha) Food Chemistry. 2005;89(4):569–575. doi: 10.1016/j.foodchem.2004.03.013. [DOI] [Google Scholar]
- 37.Pavithra K., Vadivukkarasi S. Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq.) Cogn. Food Science and Human Wellness. 2015;4(1):42–46. doi: 10.1016/j.fshw.2015.02.001. [DOI] [Google Scholar]
- 38.Juan M.-Y., Chou C.-C. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiology. 2010;27(5):586–591. doi: 10.1016/j.fm.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Tona L., Kambu K., Ngimbi N., Cimanga K., Vlietinck A. J. Antiamoebic and phytochemical screening of some Congolese medicinal plants. Journal of Ethnopharmacology. 1998;61(1):57–65. doi: 10.1016/S0378-8741(98)00015-4. [DOI] [PubMed] [Google Scholar]
- 40.Pensec F., Szakiel A., Pączkowski C., et al. Characterization of triterpenoid profiles and triterpene synthase expression in the leaves of eight Vitis vinifera cultivars grown in the Upper Rhine Valley. Journal of Plant Research. 2016;129(3):499–512. doi: 10.1007/s10265-016-0797-0. [DOI] [PubMed] [Google Scholar]
- 41.Nenaah E. G., Ahmed M. E. Antimicrobial activity of extracts and latex of calotropis procera (Ait.) and synergistic effect with reference antimicrobials. Research Journal of Medicinal Plant. 2011;5(6):706–716. doi: 10.3923/rjmp.2011.706.716. [DOI] [Google Scholar]
- 42.Khan F. A., Hussain I., Farooq S., Ahmad M., Arif M., Ur Rehman I. Phytochemical Screening of Some Pakistanian Medicinal Plants. Middle-East Journal of Scientific Research. 2011;8:575–578. [Google Scholar]
- 43.Waterhouse A. Determination of total phenolics. Current Protocols in Food Analytical Chemistry. 2003:I1.1.1–I1.1.8. [Google Scholar]