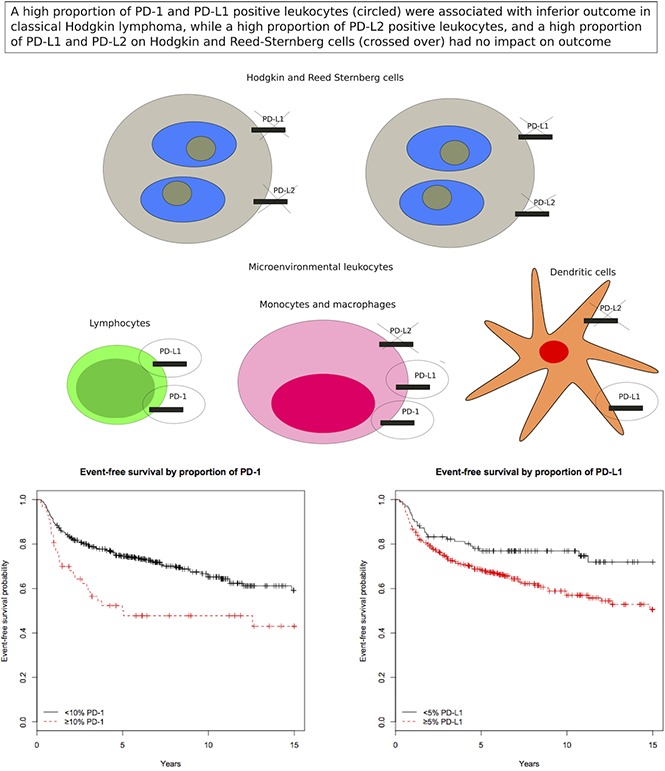
Key Points
High proportions of PD-1+ and PD-L1+ leukocytes in the Hodgkin lymphoma microenvironment are associated with inferior outcome.
Expression of PD-L1 and PD-L2 on Hodgkin and Reed-Sternberg cells has no impact on outcome.
Abstract
Immune checkpoint inhibition targeting the programmed death receptor (PD)-1 pathway is a novel treatment approach in relapsed and refractory classical Hodgkin lymphoma (cHL). Identifying patients with a high risk of treatment failure could support the use of PD-1 inhibitors as front-line treatment. Our aim was to investigate the prognostic impact of PD-1, programmed death-ligand 1 (PD-L1), and PD-L2 in the tumor microenvironment in diagnostic biopsies of patients with cHL. Patients from Denmark and Sweden, diagnosed between 1990 and 2007 and ages 15 to 86 years, were included. Tissue microarray samples were available from 387 patients. Immunohistochemistry was used to detect PD-1, PD-L1, and PD-L2, and the proportions of positive cells were calculated. Event-free survival (EFS; time to treatment failure) and overall survival (OS) were analyzed using Cox proportional hazards regression. High proportions of both PD-1+ (hazard ratio [HR], 1.77; 95% confidence interval [CI], 1.10-2.86) and PD-L1+ (HR = 1.89; 95% CI, 1.08-3.30) leukocytes in the microenvironment were associated with inferior EFS in a multivariate analysis (adjusted for white blood cell count >15 × 109/L, hemoglobin <105 g/L, albumin <40 g/L, B symptoms, extranodal involvement, stage, bulky tumor, nodular sclerosis subtype, Epstein-Barr virus status, lymphocyte count <0.6 × 109/L, sex, and country). A high proportion of PD-L1+ leukocytes was also associated with inferior OS in a multivariate analysis (HR, 3.46; 95% CI, 1.15-10.37). This is the first study to show a correlation after multivariate analysis between inferior outcome in cHL and a high proportion of both PD-1+ and PD-L1+ leukocytes in the tumor microenvironment.
Visual Abstract
Introduction
The tumor milieu in classical Hodgkin lymphoma (cHL) contains only sparse malignant Hodgkin and Reed-Sternberg (HRS) cells, whereas the bulk of the tumor comprises a complex microenvironment containing a variety of leukocytes and other stromal cells.1 Relapsed and refractory cHL have a poor prognosis after conventional therapy.2 Recently, immune checkpoint inhibitors targeting the programmed death receptor 1 (PD-1) pathway have shown encouraging results for treating such patients.3,4 These drugs herald a new therapeutic era in which the microenvironment is the primary target. If patients at especially high risk of treatment failure could be identified at the time of diagnosis, they might benefit from more intensive therapy, including immune checkpoint inhibitors as front-line treatment. Identification of microenvironment-associated risk factors in cHL might allow for more accurate prediction of outcome compared with established prognostic factors such as the International Prognostic Score (IPS).5,6
PD-1 is expressed by B and T lymphocytes: natural killer (NK) cells and monocytes. PD-1 has 2 ligands, programmed death-ligand 1 (PD-L1) and PD-L2 that bind to PD-1 and induce downregulation and apoptosis in most PD-1+ leukocytes.7 PD-L1 and PD-L2 are expressed by HRS cells as a result of upregulation of 9p24.1,8 and by nonmalignant microenvironmental leukocytes.9 Expression of PD-1 by lymphocytes generally indicates an anergic immune response in which the lymphocytes are unable to kill the malignant cells.10
Previous studies have indicated that high leukocyte PD-1 expression may be associated with inferior overall survival (OS).11-14 However, in only 1 of these did PD-1 remain associated with poorer OS after adjustment for parameters included in the IPS.11 In 1 study, high PD-L1 expression (on either HRS cells or leukocytes) was associated with inferior OS when coexpressed with high PD-1.13 9p24.1 gene amplifications and expression of PD-1 ligands on HRS cells were associated with inferior prognosis in 1 study.8 In contrast, 2 other studies found PD-1 ligand expression on HRS cells to have no prognostic impact.11,15
We evaluated expression of PD-1, PD-L1, and PD-L2 in tumor-infiltrating leukocytes and HRS cells in primary diagnostic biopsies from a large cHL cohort to determine the prognostic importance of this expression. Our hypothesis was that high microenvironmental expression of PD-1, PD-L1, and PD-L2 would be associated with inferior prognosis.
Materials and methods
Patients
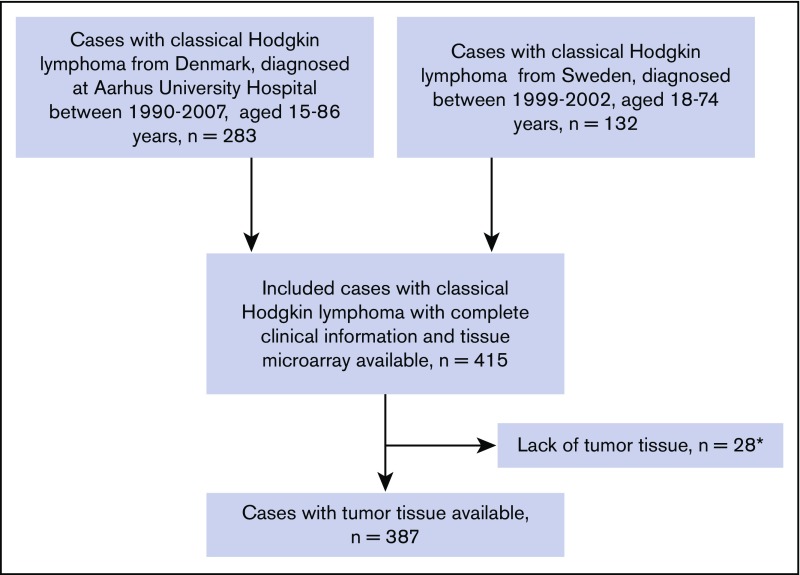
The study cohort consisted of cHL patients from Denmark (n = 259) and Sweden (n = 128) with representative tumor tissue available. Patients from Denmark were diagnosed at Aarhus University Hospital between 1990 and 2007 and were between ages 15 and 86 years.16 Patients from Sweden were diagnosed between 1999 and 2002 at ages 18 to 74 years.17 Clinical information was obtained from medical records and was available for the entire cohort (Figure 1).
Figure 1.
Flowchart of patients included in the study. cHL patients diagnosed between 1990 and 2007 in Sweden and Denmark. Numbers of patients included in the cohort aged 15 to 86, with complete clinical follow-up information and with available tumor tissue. *Resulting from a lack of material on the available tissue microarray section, widespread fibrosis, or absence of HRS cells.
Treatment
Patients were treated according to national guidelines, as described.2,16 Limited-stage patients received 2 to 4 courses of chemotherapy and subsequent radiotherapy (Table 1). Advanced-stage patients were treated with 6 to 8 courses of chemotherapy with additional radiotherapy in the case of bulky tumor, slow tumor regression, or localized residual mass.2,16 Chemotherapy regimens used were mainly ABVD or BEACOPP. A few patients (n = 8) received MOPP/ABV, whereas some patients >65 years of age (n = 7) received CHOP. Between 1990 and 1997, 22 patients, mainly with stage I disease without B symptoms or bulky tumor, were treated with radiotherapy alone.16 Patients with relapsed and refractory cHL were mainly treated with high-dose chemotherapy and autologous stem cell transplantation.4
Table 1.
Clinical and pathological parameters and their distribution in the entire cohort of cHL patients and according to high and low expression of PD-1 and PD-L1 in the tumor microenvironment
| Entire cohort, (%) | High PD-1, ≥10%* | Low PD-1, <10%* | P value† | High PD-L1, ≥5%* | Low PD-L1, <5%* | P value† | |
|---|---|---|---|---|---|---|---|
| All patients | 387 (100) | 57 (100) | 330 (100) | 280 (100) | 102 (100) | ||
| Age, y | .07 | .27 | |||||
| ≥45 | 136 (35) | 26 (46) | 110 (33) | 102 (36) | 31 (30) | ||
| <45 | 251 (65) | 31 (54) | 220 (67) | 178 (64) | 71 (70) | ||
| Stage | .17 | .34 | |||||
| IA-IIA | 178 (46) | 31 (54) | 147 (45) | 124 (44) | 51 (50) | ||
| IIB-IVB | 208 (54) | 26 (46) | 182 (55) | 155 (56) | 51 (50) | ||
| Missing | 1 (0) | 0 (0) | 1 (0) | 1 (0) | 0 (0) | ||
| Sex | .55 | .21 | |||||
| Male | 214 (55) | 31 (54) | 183 (55) | 160 (57) | 51 (50) | ||
| Female | 173 (45) | 26 (46) | 147 (45) | 120 (43) | 51 (50) | ||
| Histologic subtype | .70 | .03 | |||||
| Nodular sclerosis | 313 (81) | 47 (82) | 266 (81) | 219 (78) | 90 (88) | ||
| Mixed cellularity | 65 (17) | 8 (14) | 57 (17) | 55 (20) | 9 (9) | ||
| Lymphocyte rich + lymphocyte depleted + unclassifiable cHL | 9 (2) | 2 (4) | 7 (2) | 6 (2) | 3 (3) | ||
| EBV+ | .55 | .007 | |||||
| Yes | 116 (30) | 15 (26) | 101 (31) | 94 (34) | 20 (20) | ||
| No | 269 (69) | 41 (72) | 228 (69) | 184 (66) | 82 (80) | ||
| Missing | 2 (1) | 1 (2) | 1 (0) | 2 (0) | 0 (0) | ||
| WBC count >15 × 109/L | .27 | .99 | |||||
| Yes | 46 (12) | 4 (7) | 42 (13) | 34 (12) | 12 (12) | ||
| No | 332 (86) | 52 (91) | 280 (85) | 242 (86) | 85 (83) | ||
| Missing | 9 (2) | 1 (2) | 8 (2) | 4 (2) | 5 (5) | ||
| Hemoglobin <105 g/L | .61 | <.001 | |||||
| Yes | 46 (12) | 8 (14) | 38 (12) | 41 (15) | 4 (4) | ||
| No | 331 (86) | 48 (84) | 283 (86) | 234 (84) | 93 (91) | ||
| Missing | 10 (2) | 1 (2) | 9 (2) | 5 (1) | 5 (5) | ||
| Albumin <40 g/L | .64 | .03 | |||||
| Yes | 126 (33) | 17 (30) | 109 (33) | 101 (36) | 23 (23) | ||
| No | 215 (56) | 33 (58) | 182 (55) | 150 (54) | 63 (61) | ||
| Missing | 46 (12) | 7 (12) | 39 (12) | 29 (10) | 16 (16) | ||
| ESR ≥50 mm/h | .07 | .37 | |||||
| Yes | 113 (29) | 11 (19) | 102 (31) | 86 (31) | 25 (25) | ||
| No | 205 (53) | 35 (62) | 170 (52) | 148 (53) | 55 (53) | ||
| Missing | 69 (18) | 11 (19) | 58 (18) | 46 (16) | 22 (22) | ||
| Lymphocyte count <0.6 × 109/L | 1.00 | .02 | |||||
| Yes | 17 (4) | 2 (4) | 15 (5) | 16 (6) | 1 (1) | ||
| No | 333 (86) | 51 (89) | 282 (85) | 239 (85) | 89 (87) | ||
| Missing | 37 (10) | 4 (7) | 33 (10) | 25 (9) | 12 (12) | ||
| Bulky tumor, diameter ≥10 cm | .76 | .007 | |||||
| Yes | 105 (28) | 14 (25) | 91 (28) | 86 (31) | 17 (17) | ||
| No | 261 (67) | 38 (66) | 223 (67) | 180 (64) | 78 (76) | ||
| Missing | 21 (5) | 5 (9) | 16 (5) | 14 (5) | 7 (7) | ||
| B symptoms | .11 | .02 | |||||
| Yes | 186 (48) | 22 (39) | 164 (50) | 145 (52) | 39 (38) | ||
| No | 199 (51) | 35 (61) | 164 (50) | 134 (48) | 62 (61) | ||
| Missing | 2 (1) | 0 (0) | 2 (0) | 1 (0) | 1 (1) | ||
| Extranodal involvement | 1.00 | .95 | |||||
| Yes | 23 (6) | 3 (5) | 20 (6) | 16 (6) | 6 (6) | ||
| No | 361 (93) | 54 (95) | 307 (93) | 262 (94) | 95 (93) | ||
| Missing | 3 (1) | 0 (0) | 3 (1) | 2 (0) | 1 (1) | ||
| Bone marrow involvement | .09 | .56 | |||||
| Yes | 6 (2) | 2 (4) | 4 (1) | 6 (2) | 0 (0) | ||
| No | 103 (27) | 8 (14) | 95 (29) | 83 (30) | 19 (19) | ||
| Missing | 278 (71) | 47 (82) | 231 (70) | 191 (68) | 83 (81) | ||
| Country | .07 | .02 | |||||
| Denmark | 259 (69) | 44 (77) | 215 (70) | 178 (64) | 78 (76) | ||
| Sweden | 128 (31) | 13 (23) | 115 (30) | 102 (36) | 24 (24) | ||
| IPS | .55 | .006 | |||||
| ≥3 | 91 (24) | 13 (23) | 78 (24) | 75 (27) | 15 (15) | ||
| <3 | 223 (58) | 38 (67) | 185 (56) | 149 (53) | 70 (69) | ||
| Missing | 73 (18) | 6 (10) | 67 (20) | 56 (20) | 17 (16) | ||
| IPS‡ | .25 | .03 | |||||
| ≥3 | 71 (34) | 12 (46) | 59 (32) | 59 (38) | 11 (22) | ||
| <3 | 118 (57) | 13 (50) | 105 (58) | 82 (53) | 35 (69) | ||
| Missing | 19 (9) | 1 (4) | 18 (10) | 14 (9) | 5 (9) | ||
| Treatment | .15 | .18 | |||||
| ABVD | 311 (80) | 45 (79) | 266 (81) | 217 (78) | 90 (88) | ||
| BEACOPP | 33 (8) | 3 (5) | 30 (9) | 27 (10) | 5 (5) | ||
| MOPP/ABV | 8 (2) | 0 (0) | 8 (2) | 7 (3) | 1 (1) | ||
| CHOP | 7 (2) | 1 (2) | 6 (2) | 6 (2) | 1 (1) | ||
| Radiotherapy only | 22 (6) | 7 (12) | 15 (5) | 17 (5) | 5 (5) | ||
| Other or unknown chemotherapy | 6 (2) | 1 (2) | 5 (1) | 6 (2) | 0 (0) |
Boldface font indicates statistical significance (P < .05).
ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisolone; MOPP/ABV, mechlorethamine, vincristine, procarbazine, and prednisolone/adriamycin, bleomycin, and vinblastine.
Proportion of positive leukocytes.
χ2 or Fisher's exact test for low vs high PD-1 or PD-L1; missing cases not included.
Only patients with advanced stage disease.
Tissue samples
Formalin-fixed, paraffin-embedded tumor tissue blocks were collected from the archives of the participating pathology departments. Danish cases were reviewed by S.H.-D.; Swedish cases were reviewed by a group of expert hematopathologists, as described previously.17 The tumor biopsies were reclassified as nodular sclerosis, mixed cellularity, lymphocyte depleted, lymphocyte rich, or cHL not otherwise specified.16,17 Tissue microarrays (TMAs) were constructed using standard techniques, containing either 2 or 3 tumor cores per Swedish or Danish case, respectively. Each core was 1 mm in diameter. A total of 387 cHL samples with sufficient tumor tissue were included in the study. Twenty-eight cases were excluded from the initial cohort (n = 415) because of inadequate tumor material in the selected cores (Figure 1). A close correlation has been found comparing the distribution of tumor-infiltrating leukocytes in TMA cores and whole tissue sections.18
Immunohistochemistry for PD-1
Immunohistochemical stains for PD-1 were performed at Aarhus University Hospital using 4 µm paraffin TMA sections with the Ventana BenchMark XT automated staining system (Ventana Medical Systems, Tucson, AZ). PD-1 expression was identified using mouse monoclonal antibody (mAb) NAT105/MRQ-22 (Cell Marque, Rocklin, CA), diluted 1:100. Antigen was retrieved in Ventana Cell Conditioning 1 (pH8.5) and positive signals were detected using ultraView Universal Detection Kit (Ventana). The sections were counterstained with Mayer hematoxylin.
Immunohistochemistry for PD-L1 and PD-L2
Immunohistochemical double stains for PD-L1/paired box protein 5 (PAX-5), and PD-L2/PAX-5 were performed at Uppsala University Hospital using 4-µm paraffin TMA sections with the Intellipath FLX automated staining system (Biocare Medical, Pacheco, CA). PD-L1 expression was identified using rabbit mAb E1L3N/13684 (Cell Signaling Technology, Danvers, MA), diluted 1:50. PD-L2 expression was identified using rabbit mAb D7U8C/82723 (Cell Signaling Technology), diluted 1:100. PAX-5 was identified using mouse mAb M7307/DAK-Pax5 (Dako, Santa Clara, CA), diluted 1:50. Antigen was retrieved in Tris-EDTA buffer (pH9.0) in a pressure cooker, PD-L1 and PD-L2 were detected using the Betazoid DAB detection kit (brown) (Biocare Medical), and PAX-5 was detected using Warp Red chromogen (Biocare Medical). The sections were counterstained with Intellipath FLX hematoxylin.
Evaluation of PD-L1 and PD-L2 on tumor cells
The proportions of HRS cells expressing PD-L1 and PD-L2 were manually evaluated. HRS cells with brown membranous PD-L1 or PD-L2 staining and weak red nuclear PAX-5 staining were designated as positive. Proportions of PD-L1+ and PD-L2+ HRS cells were calculated by dividing the number of positive HRS cells by the combined number of positive and negative HRS cells.
Evaluation of PD-1, PD-L1, and PD-L2 in the tumor microenvironment
The proportions of PD-1+, PD-L1+, and PD-L2+ leukocytes were calculated with image analysis software (Visiomorph, Visiopharm, Hørsholm, Denmark). Lymphocytes and monocytes were included in the estimates. HRS cells, granulocytes, and large activated macrophages were excluded in the PD-1 analysis, whereas all cells were included in the PD-L1 and PD-L2 analyses. The software was fitted to designate cells with brown immunohistochemical membranous staining as positive. Proportions of PD-1+, PD-L1+, and PD-L2+ cells were calculated dividing the number of positive cells by the combined number of positive and negative cells. Proportions of PD-L1+ and PD-L2+ leukocytes were determined by subtracting the positive and negative HRS cells (from the manual estimation) from the overall cellularity (calculated by the software). Areas with fibrosis or reactive germinal centers were excluded. The proportion of each marker was calculated as the average count of the 2 or 3 cores from that case. Tumor sections were also manually reviewed to determine if PD-1+ lymphocytes formed rosettes around the HRS cells. Cases with at least 1 HRS cells with PD-1+rosettes were considered as positive.
Definition of cutoff for each group
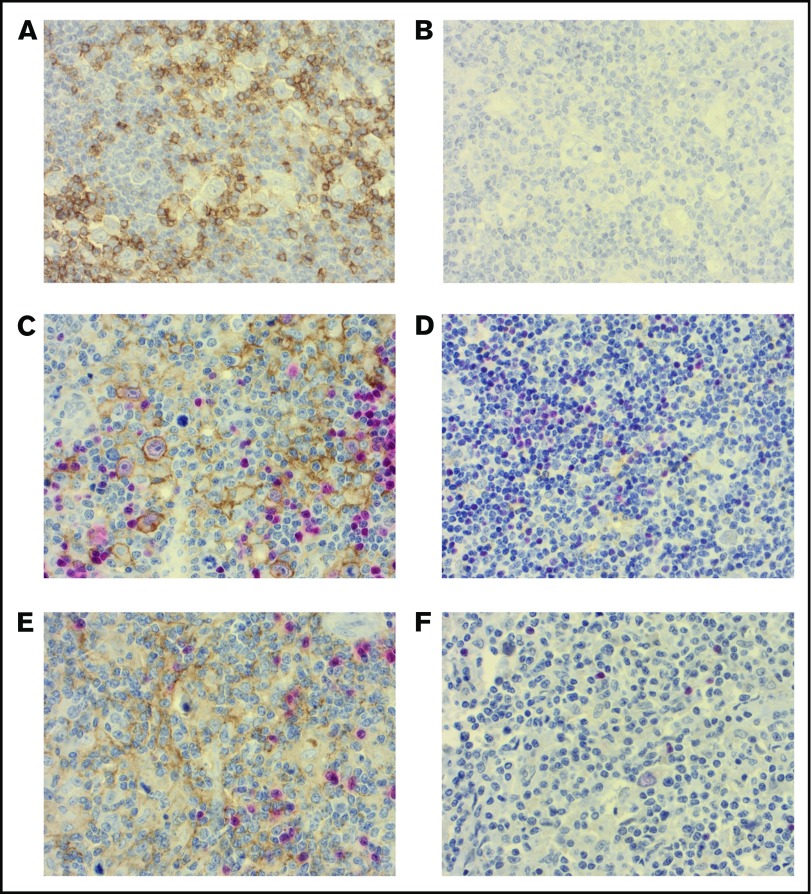
Different cutoffs for proportions of PD-1+, PD-L1+, and PD-L2+ leukocytes and for HRS PD-L1 and PD-L2 cell expression were tested in univariate analyses for event-free survival (EFS) and OS with Cox proportional hazards regression (see supplemental Table 1). The strongest discrimination between the groups regarding EFS and OS was chosen. High vs low proportion of PD-1+ leukocytes was defined as ≥10% vs <10% PD-1+ positive leukocytes (Figure 2A-B). High vs low proportion of PD-L1+ leukocytes was defined as ≥5% vs <5% PD-L1+ leukocytes (Figure 2C-D). High vs low proportion of PD-L2+ leukocytes was defined as ≥5% vs <5% PD-L2+ leukocytes (Figure 2E-F). High vs low proportion of PD-L1+ HRS cells was defined as ≥90% vs <90% PD-L1 HRS cells. High vs low proportion of PD-L2+ HRS cells was defined as ≥50% vs <50% PD-L2 HRS cells.
Figure 2.
Immunohistochemical stains in the study. High (A) and low (B) proportion of PD-1+ (brown) leukocytes. High (C) and low (D) proportion of PD-L1+ (brown) leukocytes. High (E) and low (F) proportion of PD-L2+ (brown) leukocytes. Red, PAX5. Original magnification ×400 for all panels.
Statistical methods
Tabulated values were compared using the χ2 or Fisher's exact tests. Survival outcomes were EFS and OS. EFS was defined as the time from diagnosis to treatment failure, including progression or relapse of cHL, discontinuation of treatment, or death from any cause. OS was defined as the time from diagnosis to death from any cause. Survival outcomes were estimated by the Kaplan-Meier method and compared using the log-rank test and Cox proportional hazards regression. A simple multivariate model included adjustment for age only, whereas a full multivariate model for EFS also included adjustment for white blood cell count (WBC) ≤/>15 × 109/L, hemoglobin ≥/<105 g/L, albumin ≥/<40 g/L, B symptoms (yes/no), extranodal involvement (yes/no), stage (I-IIA/IIB-IV), bulky tumor (yes/no), nodular sclerosis histology (yes/no), Epstein-Barr virus (EBV) positivity (yes/no), lymphocyte count ≥/<0.6 × 109/L, sex, and country. The fully adjusted multivariate model for OS also included adjustment for erythrocyte sedimentation rate (ESRs) ≥/<50 mm/h. Cases with missing information on some variable were omitted from the multivariate analysis. Year of diagnosis (1990-1998 vs 1999-2007) was added to each fully adjusted multivariate Cox regression model. The proportional hazards assumption was tested and was not violated. If not otherwise specified, age was treated as a continuous variable. Analyses were also stratified based on country (Sweden and Denmark), stage (I-IIA vs IIB-IV), and age (≥45 vs <45 years) (supplemental Figures 1 and 2; supplemental Tables 2 and 3). Pairwise tests for interaction between independent significant variables were performed. Statistical analyses were performed using R software. All statistical tests were 2-sided and P values <.05 were considered statistically significant.
Ethics
The study was approved by the Danish Data Protection Agency and by the relevant regional ethical committees according to the Declaration of Helsinki in both countries (reference number 99-154 for Swedish cases; reference number s1-16-02-668-14 [Data Protection Agency] and 20070067 [Regional Ethical Committee] for Danish cases).
Results
Descriptive data
In the entire cohort, 54% of the patients presented with advanced stage (IIB-IV) and 38% of these had 3 or more IPS risk factors (Table 1). Histologically, 81% had nodular sclerosis, 17% mixed cellularity, and 2% other or unclassifiable cHL. Median follow-up time was 11.0 years (range, 0.2-18.6 years). In the entire cohort, 133 patients (34%) suffered from treatment failure and 79 patients (20%) died.
The distribution of PD-1+, PD-L1+, and PD-L2+ leukocytes are shown in supplemental Figure 3. Fifteen percent of all patients (n = 57) had a high proportion (≥10%) of PD-1+ leukocytes, 73% (n = 280) had a high proportion (≥5%) of PD-L1+ leukocytes, and 11% (n = 41) had a high proportion (≥5%) of PD-L2+ leukocytes. PD-1+ leukocytes forming rosettes around HRS cells were seen in a few cases (n = 24). All HRS cells were negative for PD-1, 9% of cases had ≥90% HRS cells positive for PD-L1, and 9% of cases had ≥50% HRS cells positive for PD-L2. There was a weak correlation between PD-L1 and PD-L2 regarding both proportion of positive leukocytes and HRS cell expression, whereas PD-1 was not correlated with any of the other markers (supplemental Table 4).
There were no statistically significant differences in the distribution of clinical and pathological parameters among patients with tumors expressing high or low PD-1 (Table 1). In patients with a high proportion of PD-L1+ leukocytes, this distribution was more prevalent with other histological subtypes than nodular sclerosis, EBV+ tumors, hemoglobin <105 g/L, albumin <40 g/L, lymphocyte count < 0.6 × 109/L, bulky tumor, B symptoms, ≥3 IPS criteria, and Swedish patients (Table 1).
Event-free survival
PD-1, PD-L1, and PD-L2 on leukocytes.
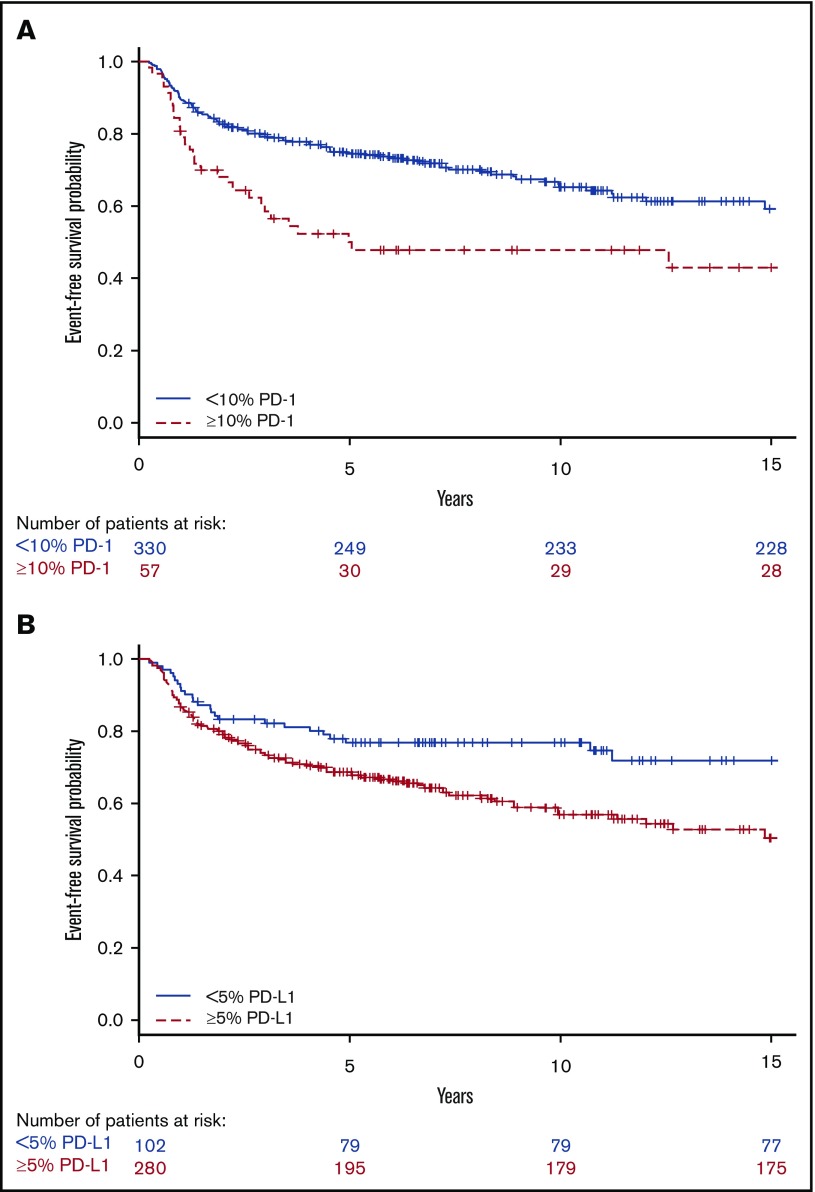
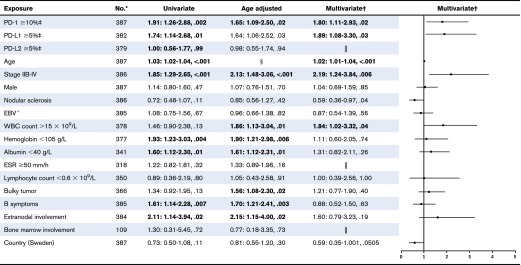
Patients with tumors containing high vs low proportions of PD-1+ leukocytes had 29 (51%) and 104 (32%) events, respectively. Patients with tumors containing high vs low proportions of PD-L1+ leukocytes had 106 (38%) and 26 (25%) events, respectively. Patients with tumors with a high proportion of PD-1+ leukocytes had shorter EFS compared with patients with a low proportion of PD-1+ leukocytes (Figure 3), representing a univariate hazard ratio (HR) of 1.91 (95% confidence interval [CI], 1.26-2.88) (Table 2). This association was especially observed in patients with a high proportion of PD-1 in univariate analyses, in patients aged ≥45 years, and in patients with advanced stage (IIB-IV) disease (supplemental Table 2). Patients with tumors with a high proportion of PD-L1+ leukocytes had shorter EFS (Figure 3), represented by a univariate HR of 1.74 (95% CI, 1.14-2.68) (Table 2). This association was especially observed in patients with a high proportion of PD-L1 in univariate analyses in patients with advanced stage (IIB-IV) disease (supplemental Table 2). Several clinical parameters were associated with shorter EFS in univariate and/or age-adjusted analyses, including WBC >15 × 109/L, hemoglobin <105 g/L, B symptoms, extranodal involvement, age, advanced stage (IIB-IV), bulky tumor, and albumin <40 g/L (Table 2). All significant parameters, together with nodular sclerosis histology, EBV+ tumors, lymphocyte count <0.6 × 109/L, sex, and country, were included in the multivariate model. In the full multivariate analysis, a high proportion of PD-1+ leukocytes, as well as a high proportion of PD-L1+ leukocytes remained as independent significant prognostic factors, along with WBC >15 × 109/L, age, and advanced stage (IIB-IV) (Table 2). There were no formal statistically significant interactions between independent statistically significant factors (supplemental data). A high proportion of PD-1+ leukocytes was associated with inferior EFS in patients with advanced stage when adjusted for the IPS factors (supplemental Table 5). A high proportion of PD-L2+ leukocytes (supplemental Table 2) and tumors containing PD-1+ rosettes around the HRS cells (HR, 1.23 [95% CI, 0.64-2.34]) did not affect EFS in univariate analyses.
Figure 3.
Univariate analyses for EFS. Kaplan-Meier estimates according to (A) ≥10% (red, dashed line) PD-1 and <10% (blue, solid line) PD-1 expressing leukocytes (log-rank P = .002) and (B) ≥5% (red, dashed line) PD-L1 and <5% (blue, solid line) PD-L1 expressing leukocytes (log-rank P = .01).
Table 2.
Relative risk of an event (progression, relapse, or death from any cause) estimated as HRs with 95% CIs and P values among cHL patients by putative prognostic factors
 |
Boldface font indicates statistical significance (P < .05).
Number of cases with information enabling evaluation of EFS.
For the multivariate model, all factors were included that were of significance (P < .05) or borderline significance (P < .10) in univariate or age-adjusted analyses (PD-1, PD-L1, age, stage, WBC count, hemoglobin, albumin, bulky tumor, B symptoms, and extranodal involvement). Also, nodular sclerosis histology, EBV status, lymphocyte count, male sex, and country (Denmark/Sweden) were included because the distribution of these variables differed between high and low PD-L1 (Table 1). Male sex was also added to the multivariate model.
Proportion of positive leukocytes.
Not applicable.
Not tested.
PD-L1 and PD-L2 in tumor cells.
Expression on a high proportion of HRS cells of neither PD-L1 nor PD-L2 (supplemental Table 2) affected EFS in univariate analyses.
Overall survival
PD-1, PD-L1, and PD-L2 in leukocytes.
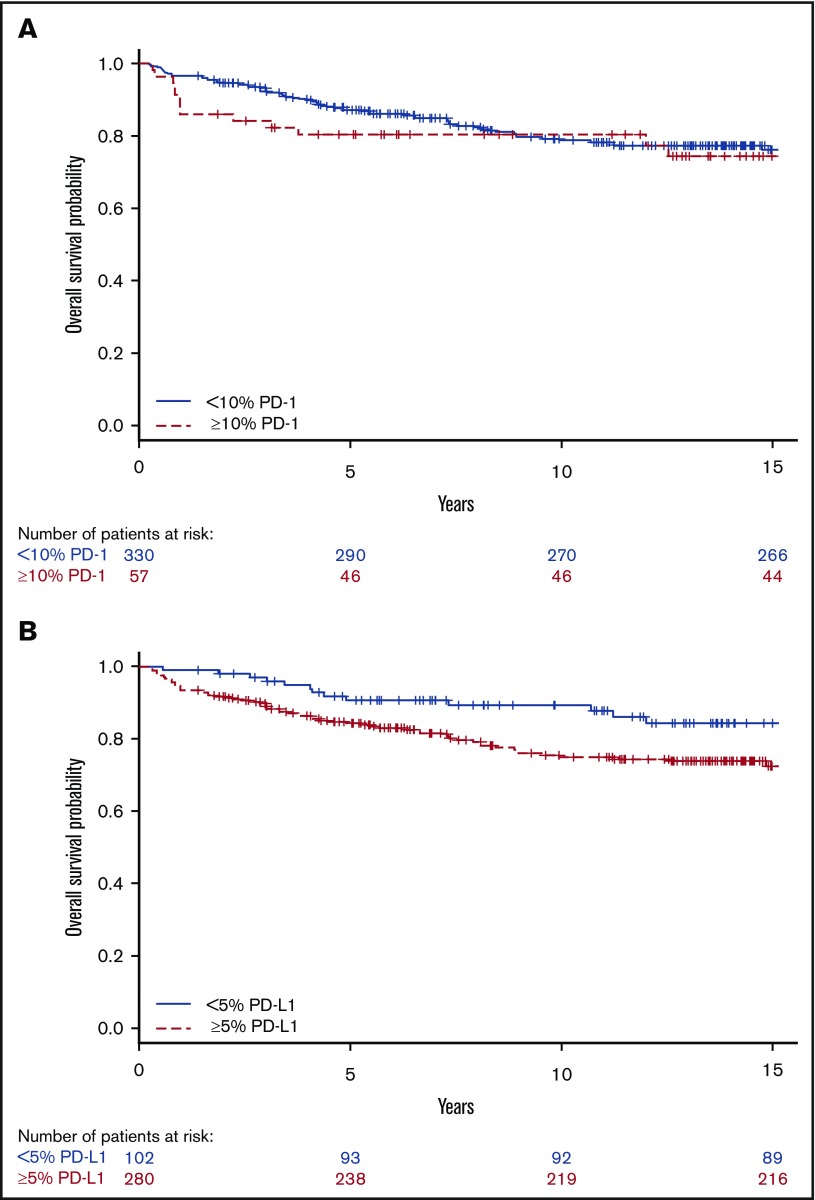
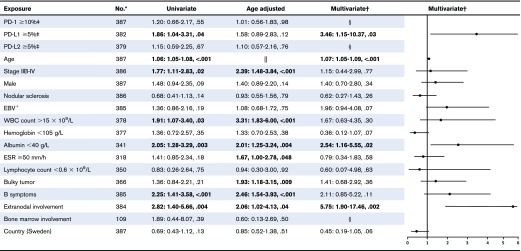
Thirteen patients (23%) with a high proportion of PD-1+ leukocytes died compared with 66 patients (20%) with a low proportion of PD-1+. Sixty-five patients (23%) with a high proportion of PD-L1+ leukocytes died compared with 14 patients (14%) with a low proportion. Patients with tumors containing a high proportion of PD-L1+ leukocytes had shorter OS compared with those with a low proportion (Figure 4), represented by a univariate HR of 1.86 (95% CI, 1.04-3.31) (Table 3; supplemental Table 3). The well-known negative prognostic factors of age, advanced stage (IIB-IV), albumin <40 g/L, WBC >15 × 109/L, B symptoms, extranodal involvement, ESR ≥50 mm/h, and bulky tumor were all associated with inferior OS in univariate and/or age-adjusted analyses (Table 3). All significant parameters, together with nodular sclerosis histology, EBV+ tumor, lymphocyte count <0.6 × 109/L, sex, and country, were included in the multivariate model. In the fully adjusted multivariate analysis, a high proportion of PD-L1+ leukocytes remained as an independent statistically significant prognostic factor, along with age, albumin <40 g/L, and extranodal involvement (Table 3). There were no formal statistically significant interactions between independent significant factors (supplemental data). A high proportion of PD-1+ and PD-L2+ leukocytes, and tumors containing PD-1+ rosettes around the HRS cells (HR, 0.34 [95% CI, 0.08-1.38]), did not affect OS in univariate analyses.
Figure 4.
Univariate analyses for OS. Kaplan-Meier estimates according to (A) ≥10% (red, dashed line) PD-1 and <10% (blue, solid line) PD-1 expressing leukocytes (log-rank P = .55) and (B) ≥5% (red, dashed line) PD-L1 and <5% (blue, solid line) PD-L1 expressing leukocytes (log-rank P = .03).
Table 3.
Relative risk of death from any cause estimated as HRs with 95% CIs, and P values among cHL patients by putative prognostic factors
 |
Boldface font indicates statistical significance (P < .05).
Number of cases with information enabling evaluation of overall survival
For the multivariate model, all factors were included that were of significance (P < 0.05), or borderline significance (P < 0.10) in univariate or age adjusted analyses (PD-L1, age, stage, male sex, WBC count, albumin, ESR, bulky tumor, B symptoms, and extranodal involvement). Also, nodular sclerosis histology, EBV status, hemoglobin, lymphocyte count, and country (Denmark/Sweden) were added to the multivariate model because the distribution of these variables differed between high and low PD-L1 (Table 1).
Proportion of positive leukocytes
Not tested.
Not applicable.
PD-L1 and PD-L2 in tumor cells.
Neither PD-L1 nor PD-L2 expression on high proportions of HRS cells (supplemental Table 3) affected OS in univariate analyses.
Discussion
In this population-based study in cHL, patients with primary tumors containing high proportions of PD-1 and PD-L1+ leukocytes had a poorer EFS compared with patients with low proportions of PD-1+ and PD-L1+ leukocytes in the fully adjusted multivariate model. Patients with tumors with a high proportion of PD-L1+ leukocytes had a markedly inferior OS in a fully adjusted multivariate analysis. The negative prognostic association was found in both study cohorts (Sweden and Denmark; supplemental data).
A high proportion of both PD-1+ lymphocytes and PD-L1+ leukocytes in the cHL tumor microenvironment are biomarkers for an anergic tumor milieu.1 In our study cohort, 15% of the patients had a high proportion of PD-1+ leukocytes and 51% of those experienced an event. Seventy-three percent of the patients had a high proportion of PD-L1+ leukocytes, and 38% of those experienced an event. Our results indicate that conventional front-line treatment is insufficient for these patients.19,20
Comparison with previous studies regarding PD-1
The prognostic relevance of the PD-1 pathway in cHL has previously been shown. Four studies11-14 reported an inferior OS in patients with tumors with a high proportion of PD-1+ leukocytes, although high PD-1 remained an independent statistically significant factor for OS in only 1 of the series.11 Unlike our study, Koh et al11 considered only a high (≥3) vs low (<3) number of IPS criteria on the whole cohort. IPS is used in clinical settings to risk-stratify patients with advanced stage only.6 Novel methodological strengths of our study include consideration of each IPS criterion as a separate risk factor in patients with advanced stages only. Two other studies found no prognostic importance of microenvironmental PD-1 expression21,22; however, these studies contained fewer cases (n = 88 and n = 144). The varying results might partly be due to different cutoffs used by different studies, including 0.5%,14 10%,22 and 20%11,21,22 of PD-1+ leukocytes. In addition, 1 study used 23 PD-1+ cells/mm2 as the cutoff.12 We considered using different cutoffs to predict outcome, including 20% as used in most previous studies, which was also associated with inferior EFS in our series (supplemental Table 1). One recent study found that high proportions of PD-1– (in a rosetting or diffuse pattern) and CD68-expressing cells in the tumor microenvironment, in combination with STAT1 – HRS cells, were associated with inferior outcome in fluorodeoxyglucose positron emission tomography (PET)– patients. This is an intriguing finding, suggesting a possible future use for microenvironment-derived risk factors to identify fluorodeoxyglucose positron emission tomography–negative patients at high risk of treatment failure.23 However, we found no prognostic impact of PD-1+ lymphocytes forming rosettes around the HRS cells, which might partly be due to the low number of cases that expressed PD-1+ rosettes in our material (n = 24).
Comparison with previous studies regarding PD-L1 and PD-L2
High PD-L1 (expressed by HRS cells or leukocytes) was associated with inferior OS when coexpressed with high PD-1 in 1 study, although this finding was not significant in multivariate analysis.13 We found that a high proportion of PD-L1+ leukocytes was an independent prognostic marker of inferior OS, a novel finding compared with previous studies. We considered different cutoffs for microenvironmental expression of PD-L1, including 20%, as used by Paydas et al,13 which was also associated with inferior OS in univariate analysis. Expression of PD-L1 and PD-L2 on the HRS cells had no prognostic impact in our series. A recent publication reported that expression of PD-L1 and PD-L2 on HRS cells was upregulated in association with genetic alterations at the 9p24.1/PD-L1/PD-L2 locus.8 The authors found that 9p24.1 copy number alterations were a defining feature of cHL, and patients with tumors harboring 9p24.1 amplifications had a shorter progression-free survival. In contrast, 2 other studies reported no association between expression of PD-L1,15 and both PD-L1 and PD-L211 on HRS cells and outcome; however, these studies only investigated the expression of PD-1 ligands on the HRS cells and not on the microenvironmental leukocytes, as we did in our study. The cutoff for a high proportion of PD-1 ligands on HRS cells varies between studies, including both 5%13,15 and 20% of patient tumors.11 We also considered these cutoffs, but they were not associated with outcome. Our findings suggest that it is inadequate to solely investigate PD-1 ligand expression on the tumor cells and that analysis of expression in the microenvironment is also needed.
Mechanisms
Microenvironmental expression of PD-L2 was not associated with outcome in our cohort. Expression of PD-L1 is restricted to antigen-presenting cells, but may also be expressed by B and T lymphocytes, NK cells, monocytes, dendritic cells, and mast cells following exposure to proinflammatory cytokines.24,25 PD-L2 expression is mainly restricted to dendritic cells and macrophages and is not induced by inflammatory cytokines.24 PD-L1 is probably the main inducer of immunosuppression in malignant conditions because of its inducible capacity,24 something that might also explain why PD-L2 was not associated with outcome in our cohort. In addition, this might partly be due to weaker and less distinct staining of PD-L2 compared with PD-L1 (Figure 2). HRS cells with genetic alterations at the 9p24.1 locus show constitutive activation of both PD-L1 and PD-L2, promoting the expression of PD-1 in microenvironmental T lymphocytes.26 However, we found no correlation between the expression of PD-1 and PD-L1 or PD-L2 on leukocytes or HRS cells.
Macrophages have been associated with inferior prognosis in cHL in several studies,5,27 whereas other studies found no association with outcome.28,29 Macrophages are able to express PD-L1.25 Our findings, showing a dismal outcome for patients with a high proportion of PD-L1+ leukocytes, are possibly in part attributable to the expression of PD-L1 by macrophages. In addition, the conflicting results regarding macrophages and their prognostic impact in previous studies may be due to a variable expression of PD-L1 in different subsets of macrophages, where PD-L1 is mostly expressed by M2 macrophages.25 However, other leukocytes are also able to express PD-L1,24 and this probably contributes to creating an immunologically crippled tumor milieu in cases with a high proportion of PD-L1+ leukocytes.
PD-1 is expressed by regulatory T lymphocytes (Tregs) and induces signals for proliferation rather than apoptosis30 in these cells. Tregs are able to downregulate the actions of different leukocytes (including cytotoxic T lymphocytes, NK cells, and B lymphocytes) that may aid in tumor cell eradication.30 In line with this, blockade of Tregs might contribute to some extent to the success of treatment with PD-1–inhibiting drugs in various malignancies31,32 by unblocking leukocytes with tumor eradicating capabilities.
Methods
Immunohistochemistry is an appropriate method to evaluate the microenvironment in cHL. By performing microscopic evaluation of tumor tissue sections, it is possible to assess whether it is malignant or nonmalignant cells that express PD-L1 and PD-L2 and exclude germinal centers containing abundant PD-1 expressing follicular T helper lymphocytes.33 Using sequencing34 and other methods based on analysis of molecules extracted from tumor tissues may potentially be uninterpretable, because neoplastic cells are mixed with abundant non-neoplastic tissue. Manual estimations proved to correlate with the calculations by the software in this study; hence, PD-1 and PD-L1 might be immunohistochemical markers to implement in routine pathologic diagnostic settings. However, there are also limitations of immunohistochemistry, including inconsistent results (both staining intensity and number of positive cells) depending on the primary antibody and staining method used, staining reproducibility, and subjective interpretation of results.35
Strengths and weaknesses
Strengths of our study include the population-based setting with histopathological review of the cases, the large and homogeneously treated cohort, similar results in the 2 patient populations, and our digital image analysis approach. A weaknesses includes multiple testing, which might have generated statistical significance in some settings by chance alone. There are known limitations with multivariate analysis when incorporating several variables with limited number of events; such analyses should be interpreted with care.36 However, we found no relevant interactions between independent statistically significant factors for EFS and OS. In addition, this is a retrospective study, so we were not able to adjust for potential unknown residual confounders.
Conclusions
Our study is the hitherto largest to investigate the prognostic impact of PD-1, PD-L1, and PD-L2 expression in the cHL microenvironment. We also are the first to report that a high proportion of PD-1+ and PD-L1+ leukocytes are associated with inferior EFS in multivariate analysis and that a high proportion of PD-L1+ leukocytes is associated with inferior OS in multivariate analysis. These markers thus identify subgroups of patients with increased risks of treatment failure and death, perhaps from an exhausted immune system that is unable to kill the tumor cells. We believe that these patients would benefit from more aggressive chemotherapy regimens and, possibly, from PD-1 inhibitors as front-line treatment. A rational next step would be to examine the expression of PD-1 and PD-1 ligands in the cHL microenvironment in patients treated with PD-1–inhibiting drugs to determine whether expression of PD-1 and/or PD-1 ligands are also predictive markers of treatment response.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by the Swedish Cancer Society (CAN 2016/440) and the Gullstrand Foundation (I.G.), Uppsala County, Sweden, and the Karen Elise Jensens Foundation and the Danish Research Council (F.d.).
Authorship
Contribution: F.d., P.K., M.L., and S.H.-D. collected tumor material and clinical data on the Danish study subjects; S.H.-D. reviewed the histopathology of the Danish samples; J.M. collected clinical data on the Danish study subjects; I.G. collected clinical data on the Swedish study subjects; P.H. coordinated the statistical analysis; D.M. collected the tumor material from the Swedish pathology departments; R.-M.A. and D.M. analyzed Epstein-Barr virus status on the Swedish tumor material; P.H. evaluated PD-1, PD-L1, and PD-L2 in the tumor material; P.H., D.M., and I.G. wrote the paper; and all authors analyzed data, contributed to the critical revision of the manuscript, and have read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Hollander, Department of Immunology, Genetics and Pathology, Uppsala University, Dag Hammarskjölds väg 20, 75185 Uppsala, Sweden; e-mail: peter.hollander@igp.uu.se.
References
- 1.Vardhana S, Younes A. The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica. 2016;101(7):794-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glimelius I, Ekberg S, Jerkeman M, et al. . Long-term survival in young and middle-aged Hodgkin lymphoma patients in Sweden 1992-2009-trends in cure proportions by clinical characteristics. Am J Hematol. 2015;90(12):1128-1134. [DOI] [PubMed] [Google Scholar]
- 3.Ansell SM, Lesokhin AM, Borrello I, et al. . PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glimelius I, Diepstra A. Novel treatment concepts in Hodgkin lymphoma. J Intern Med. 2017;281(3):247-260. [DOI] [PubMed] [Google Scholar]
- 5.Steidl C, Lee T, Shah SP, et al. . Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362(10):875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339(21):1506-1514. [DOI] [PubMed] [Google Scholar]
- 7.Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12(10):2575-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roemer MG, Advani RH, Ligon AH, et al. . PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34(23):2690-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145-152. [DOI] [PubMed] [Google Scholar]
- 10.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh YW, Jeon YK, Yoon DH, Suh C, Huh J. Programmed death 1 expression in the peritumoral microenvironment is associated with a poorer prognosis in classical Hodgkin lymphoma. Tumour Biol. 2016;37(6):7507-7514. [DOI] [PubMed] [Google Scholar]
- 12.Muenst S, Hoeller S, Dirnhofer S, Tzankov A. Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol. 2009;40(12):1715-1722. [DOI] [PubMed] [Google Scholar]
- 13.Paydas S, Bağır E, Seydaoglu G, Ercolak V, Ergin M. Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol. 2015;94(9):1545-1552. [DOI] [PubMed] [Google Scholar]
- 14.Greaves P, Clear A, Owen A, et al. . Defining characteristics of classical Hodgkin lymphoma microenvironment T-helper cells. Blood. 2013;122(16):2856-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menter T, Bodmer-Haecki A, Dirnhofer S, Tzankov A. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum Pathol. 2016;54:17-24. [DOI] [PubMed] [Google Scholar]
- 16.Andersen MD, Kamper P, Nielsen PS, et al. . Tumour-associated mast cells in classical Hodgkin’s lymphoma: correlation with histological subtype, other tumour-infiltrating inflammatory cell subsets and outcome. Eur J Haematol. 2016;96(3):252-259. [DOI] [PubMed] [Google Scholar]
- 17.Smedby KE, Hjalgrim H, Melbye M, et al. . Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;97(3):199-209. [DOI] [PubMed] [Google Scholar]
- 18.Glimelius I, Qvarnström F, Simonsson M, et al. . Tissue microarray and digital image analysis: a methodological study with special reference to the microenvironment in Hodgkin lymphoma. Histopathology. 2012;61(1):26-32. [DOI] [PubMed] [Google Scholar]
- 19.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25(2):214-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo M, Fu L. The effect of chemotherapy on programmed cell death 1/programmed cell death 1 ligand axis: some chemotherapeutical drugs may finally work through immune response. Oncotarget. 2016;7(20):29794-29803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen TT, Frater JL, Klein J, et al. . Expression of TIA1 and PAX5 in classical Hodgkin lymphoma at initial diagnosis may predict clinical outcome. Appl Immunohistochem Mol Morphol. 2016;24(6):383-391. [DOI] [PubMed] [Google Scholar]
- 22.Chetaille B, Bertucci F, Finetti P, et al. . Molecular profiling of classical Hodgkin lymphoma tissues uncovers variations in the tumor microenvironment and correlations with EBV infection and outcome. Blood. 2009;113(12):2765-3775. [DOI] [PubMed] [Google Scholar]
- 23.Agostinelli C, Gallamini A, Stracqualursi L, et al. . The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin’s lymphoma: a retrospective, European, multicentre cohort study. Lancet Haematol. 2016;3(10):e467-e479. [DOI] [PubMed] [Google Scholar]
- 24.Yao S, Chen L. PD-1 as an immune modulatory receptor. Cancer J. 2014;20(4):262-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016;7:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green MR, Monti S, Rodig SJ, et al. . Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamper P, Bendix K, Hamilton-Dutoit S, Honoré B, Nyengaard JR, d’Amore F. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma. Haematologica. 2011;96(2):269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azambuja D, Natkunam Y, Biasoli I, et al. . Lack of association of tumor-associated macrophages with clinical outcome in patients with classical Hodgkin’s lymphoma. Ann Oncol. 2012;23(3):736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kayal S, Mathur S, Karak AK, et al. . CD68 tumor-associated macrophage marker is not prognostic of clinical outcome in classical Hodgkin lymphoma. Leuk Lymphoma. 2014;55(5):1031-1037. [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez-Perea AL, Arcia ED, Rueda CM, Velilla PA. Phenotypical characterization of regulatory T cells in humans and rodents. Clin Exp Immunol. 2016;185(3):281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaudhary B, Elkord E, Regulatory T. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel). 2016;4(3):E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621-663. [DOI] [PubMed] [Google Scholar]
- 34.Scott DW, Steidl C. The classical Hodgkin lymphoma tumor microenvironment: macrophages and gene expression-based modeling. Hematology Am Soc Hematol Educ Program. 2014;2014:144-150. [DOI] [PubMed] [Google Scholar]
- 35.Matos LL, Trufelli DC, de Matos MG, da Silva Pinhal MA. Immunohistochemistry as an important tool in biomarkers detection and clinical practice. Biomark Insights. 2010;5:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Concato J, Feinstein AR, Holford TR. The risk of determining risk with multivariable models. Ann Intern Med. 1993;118(3):201-210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.