Abstract
Formation of chondrocyte clusters is not only a morphological sign of osteoarthritis but it is also observed in cell culture. Active locomotion of chondrocytes is controlled by integrins in vitro. Integrins bind to Laminin-A4 (LAMA4), a protein that is highly expressed in vivo in clusters of hypertrophic chondrocytes. We tested if LAMA4 is relevant for cluster formation. Human chondrocytes were cultured in a 2D matrigel model and treated with different concentrations of a monoclonal inhibitory anti-LAMA4-antibody. Migration and cluster formation was analysed using live cell imaging technique. Full genome gene expression analysis was performed to assess the effect of LAMA4 inhibition. The data set were screened for genes relevant to cell motility. F-actin staining was performed to document cytoskeletal changes. Anti-LAMA4 treatment significantly reduced the rate of cluster formation in human chondrocytes. Cells changed their surface morphology and exhibited fewer protrusions. Expression of genes associated with cellular motility and migration was affected by anti-LAMA4 treatment. LAMA4-integrin signalling affects chondrocyte morphology and gene expression in vitro, thereby contributing to cluster formation in human osteoarthritic chondrocytes.
Keywords: chondrocytes, migration, LAMA4, cluster, osteoarthritis
Motility of chondrocytes is essential during growth, development, wound healing, and in the manufacturing of scaffolded cartilage implants.1,2
It was hypothesized that in osteoarthritis (OA) movement of chondrocytes within the cartilage matrix occurs during the formation of chondrocyte clusters.3,4 These clusters are a typical histological feature in OA.5,6 Chondrocyte clusters adjacent to severe cartilage degeneration have specific progenitor and proliferative potential.7 Yet the aggregation of hypertrophic chondrocytes could also be caused by locomotory activity, which has been shown to occur in cell culture models using e.g. collagen enriched hydrogel matrices.7–9
Locomotion of cells is dependent on adhesion molecules and integrin expression. Integrins regulate the proliferation, migration, survival, and differentiation of chondrocytes. It has been recognized that the ligand for integrins α1-, α2-, α3-, and α10β1 is collagen, while for integrin α6β1, the ligand is laminin in particular, integrins α6β1 and α7β1 bind to laminin A4 (LAMA4), which has been implicated in cell migration of endothelial cells, tumor cells and transendothelial migration of leukocytes.10–13 We have previously described an increased expression of LAMA4 in osteoarthritic cartilage—especially in clusters of chondrocytes in high grade OA.14 We were interested, if blocking LAMA4 has an effect on chondrocyte clustering. Here we report our observations on the role of LAMA4 in human chondrocyte migration and cluster formation in vitro.
METHODS
Patient Samples
We obtained cartilage samples from fifteen female patients undergoing total knee arthroplasty due to severe primary OA as described before.14,15 Informed consent was given by patients and the protocol was approved by the ethical committee at the Medical University of Graz. The age of the patients ranged from 56 to 72 (mean 63, 5 years, body mass index varied from 29 to 37 mean 31).
Grading of OA and Immunofluoresence for LAMA4
Cartilage specimens were fixed in 4% formaldehyde solution for 24 h and then embedded in paraffin. For histological grading sections of 3 μm were stained with 0.1% safranin O solution, 0.001% fast green solution and Weigerts iron hematoxylin solution.16 Grading was performed according to the OARSI cartilage histopathology grading system.17 For confirmation of LAMA4 expression, human chondrocytes were isolated after matrix digestion.14 The cells were grown on a chamber slide (Beckton Dickinson, Franklin, New Jersey) over night and fixed in 3, 7% formaldehyde. Binding of an anti-LAMA4 antibody (Abcam, Cambridge, UK) was detected immunohistochemically by staining with FITC-conjugated goat anti-rabbit IgG antibody (Abcam, Cambridge, UK). Nuclei were counterstained with Hoechst 33342 (Life Technologies, Carlsbad, California, US).
F-actin Staining
Cells were seeded on glass coverslips in 6-well plates for 24 h and cultivated in serum-free media DMEM with 2A3 antibody (10 μg/ml), a monoclonal inhibitory anti-LAMA4-antibody18 or unspecific mouse immunoglobulin IgM (Invitrogen, Camarillo, CA). Thereafter, fixed cells were permeabilized with 0.2% Triton X-100 in PBS and stained for F-actin (filamentous-actin) Alexa Fluor 488 Phalloidin (Life Technologies, Carlsbad, California). Nuclei were stained using Hoechst-33342. Digital morphometry with a confocal laser scanning microscope was performed focusing on cytoskeletal observations including superficial protrusions (LSM 510meta, Zeiss, Oberkochen, Germany). Each experiment was repeated three times in ten samples. An experienced pathologist (K.B.) and rheumatologist (F.MF) performed the microscopical semiquantitative evaluation.
Matrigel Cell Culture and Live Cell Imaging
Human chondrocytes were prepared with matrigel matrix (Matrigel Matrix Basement Membrane, BD). 10.000 cells were overlayed on the surface of a matrigel matrix layer in a 96-well plate (Brand) and cultivated in 5% CO2 in DMEM/F-12 1:1 supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin/amphotericin B. Viability was tested using a viability assay (Viability Kit L3224, Invitrogen, CA) according to the manufacturer’s protocol. For inhibition of LAMA4 five (0, 10, 20, 30, 40 μg/ml) different concentrations of the 2A3 antibody (0 μg/ml to 40 μg/ml) and of unspecific immunoglobuline as negative control where added to the medium. Images were acquired at 50× fold magnification every hour for 24 h using the cell observer system (Zeiss, Jena, Germany) and assembled to movies (Supplemental information 1_ movie). Hela cells were used as controls. Digital pictures were then processed and analyzed using the public domain computer-assisted image analysis software ImageJ19 powered by specifically developed macroroutines. The main steps of the procedure are illustrated in Supplement information 2. The experiments were repeated three times on four samples.
cDNA Microarray Analysis
Samples from four human chondrocyte donors were cultured in monolayers.14 Chondrocytes were treated with 10 ng/ml IL-1, 10 ng/ml unspecific immunoglobuline M (IgM) +10 ng/ml IL-1 and 10 ng/ml anti-LAMA4-antibody+ 10 ng/ml IL-1. Untreated chondrocytes served as a negative control. The resulting sixteen chondrocyte samples were processed using the Affimetrix human 2.0 ST Array. Detailed information can be found in the supplemental Material and Methods section. (Supplemental information 3, Material and Methods).
Reverse Transcription qPCR (RT-qPCR)
RT-qPCR was performed to validate candidate genes identified by the Affimetrix array. We selected genes involved in motility (CDLN-1), migration (ARHGAP11A), matrixmetallo-proteinases (MMP-3, MMP-16), anti-inflammatory features in arthritis (NR4A3) and chondrogenesis (SPRY) for validation of the array data. (Supplemental information 3, Material and Methods).
Statistical Analyses
Live cell imaging
As illustrated in Supplement information 1, the rate of clustering was the main outcome parameter estimated to characterize the cell dynamics in each sample during the 24 h of observation. The results obtained for each sample were then grouped according to culture conditions, and the groups were compared using one-way ANOVA followed by Dunnet’s test for multiple comparisons vs. the control (untreated) group. All the statistical analyses were performed using SPSS 13.0 statistical package (SPSS Inc., Chicago, IL). A value of p =0.05 was considered the limit for statistical significance.
Affimetrix Microarray
Statistical tests were performed by 2-way ANOVA and multiple testing corrections (FDR5%) using Partek Genomic Suite v6.6 software (Partek Inc., St Louis, MO). If a FDR5% wasn‘t possible, p-values less than or equal to 0.005 or 0.05 were defined. The FDR is the expected proportion of false positives among all significant hypotheses; for example, if 1000 observations were experimentally predicted to be different, and a maximum FDR for these observations was 0.05, then 50 of these observations would be expected to be false positives. Filtering by deregulation of genes factor of 2 (fold change) with a limit of FC 1, 5 for deregulation was defined.
RT-qPCR
Groups were compared using one-way ANOVA followed by Bonferoni’s post hoc test for multiple comparisons vs. the control (untreated) group. All the statistical analyses were performed using SPSS 13.0 statistical package (SPSS Inc., Chicago, IL). A value of p =0.05 was considered the limit for statistical significance.
RESULTS
Chondrocytes Form Clusters in a 2D Matrigel Model
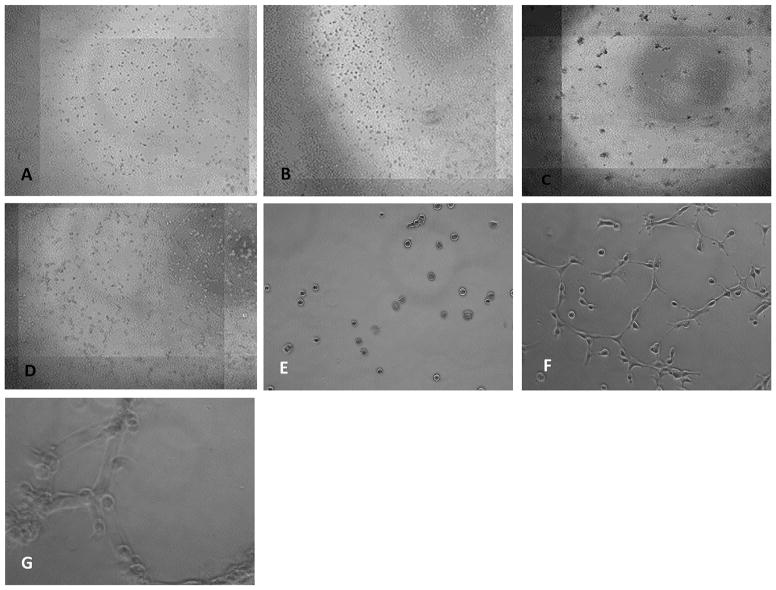
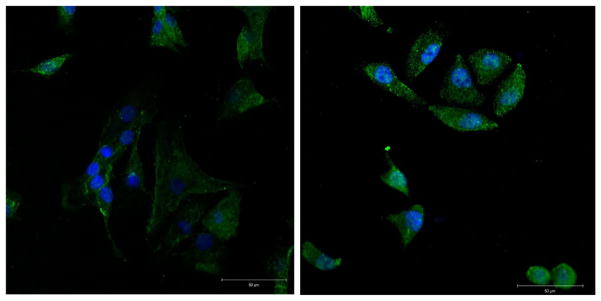
Using live cell imaging technique over 24 h of culture, we documented the migration of first passage chondrocytes on the surface of a matrigel matrix. Cells did not enter the matrixgel itself. The cells moved towards each other and formed clusters of approximately 30–50 cells. Cellular communication was suggested by the formation of intercellular extensions. Repeatedly, single chondrocytes without protrusions were repulsed from clusters and moved further away (Fig. 1). Hela cells did not form such clusters under the same circumstances. LAMA4 protein was detected by immunofluorescence staining of cultured chondrocytes and Hela cells (Fig. 2).
Figure 1.
Representative live cell images taken at two time points. Human chondrocytes (A) and Hela cells (B) are randomly distributed after 1 h of cell culture. After 24 h human chondrocytes have clustered (C), whereas Hela cells (D) do not cluster. Magnification 20× Human chondrocytes in culture after 1 h (E) and after six hours they start forming clusters and develop filopodia/cell surface protrusions to get in contact to each other. These filopodia pull the cells to each other’s. Interestingly there are several cells not being involved in this process not migrating towards each other’s. (F). After 24 h human chondrocytes have formed clusters of several cells. (G) Magnification 40×.
Figure 2.
Cytoplasmatic LAMA4 Expression in human chondrocytes and Hela cells by immunofluoresence (green). Chondrocytes nuclei are stained with HOECHST 33342 (blue). Magnification 40×.
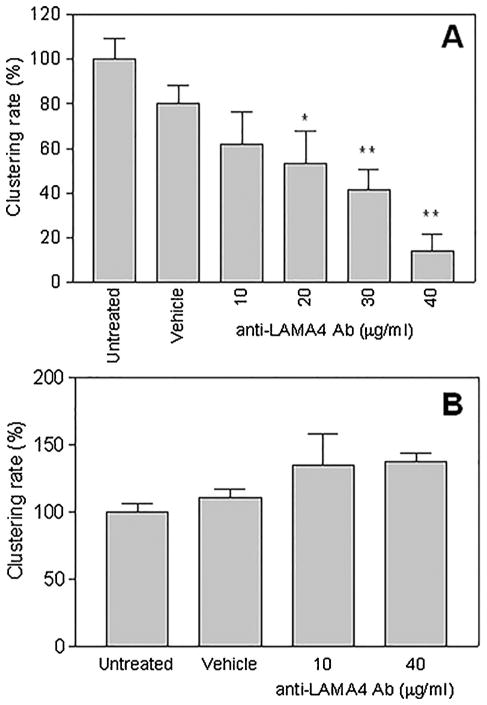
LAMA4 Blockade Decreases Cluster Size in a Dose Dependent Manner
Exposure to the anti-LAMA4 antibody 2A3 decreased the formation of cellular protrusions and the formation rate of cell clusters observed in live cell imaging. 2A3 used in different concentrations (10 ug/ml, 20 ug/ml, 30 ug/ml, and 40 ug/ml) leads to a significant and dose-dependent reduction of cluster size in OA chondrocytes (p <0, 05). The unspecific immunoglobuline had no effect on the rate of cluster formation (Fig. 3). The live/dead assay was used to prove that all the cells in culture were alive during live cell imaging experiments. Anti-LAMA4 2A3 antibody was not toxic in vitro. Human chondrocytes were viable over 24 h regardless of the dosage of 2A3 antibody treatment (Fig. S1).
Figure 3.
Cell clustering rate following LAMA4 blockade by 2A3 antibody. Values are the mean of three experiments each performed in triplicate and standard deviation. They are expressed as percent of the value observed in the untreated (control) group. In chondrocyte cultures (A) the cell clustering rate was reduced in a dose-dependent manner. This effect was not seen in Hela cells after LAMA4 blockade (B). * p <0.05, ** p <0.01 (Dunnet’s test for multiple comparisons vs. the untreated group).
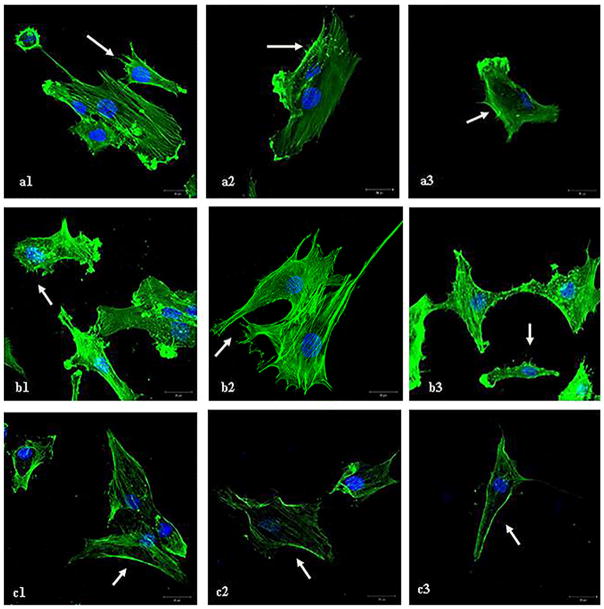
LAMA4 Blockade Reduces the Number of Cellular Protrusions in Cultured Human Chondrocytes
Cytoskeletal F-actin staining showed multiple protrusions on the cellular surface. Some were small, of hair-like appearance (microspikes), other filopodia established cell-cell contact to chondrocytes. After LAMA4 blockade, microspikes were reduced. The cell surface appeared smooth and plain. In comparison, the chondrocytes treated with unspecific immunoglobuline did not change appearance of the cell surface (Fig. 4).
Figure 4.
F-actin staining on human chondrocytes samples from three patients. After treatment with the 2A3 antibody human chondrocytes show a decrease in the appearance of small cellular protrusions on the cell surface (c) compared to untreated human chondrocytes (a) and human chondrocytes treated with a human immunoglobuline a control (b). Microspikes and the changed cell surface are marked with an arrow (→). The reduction was described semiquantitativly (+, ++, +++) from two blinded investigators.
Changes in Gene transcription After LAMA4 Blockade
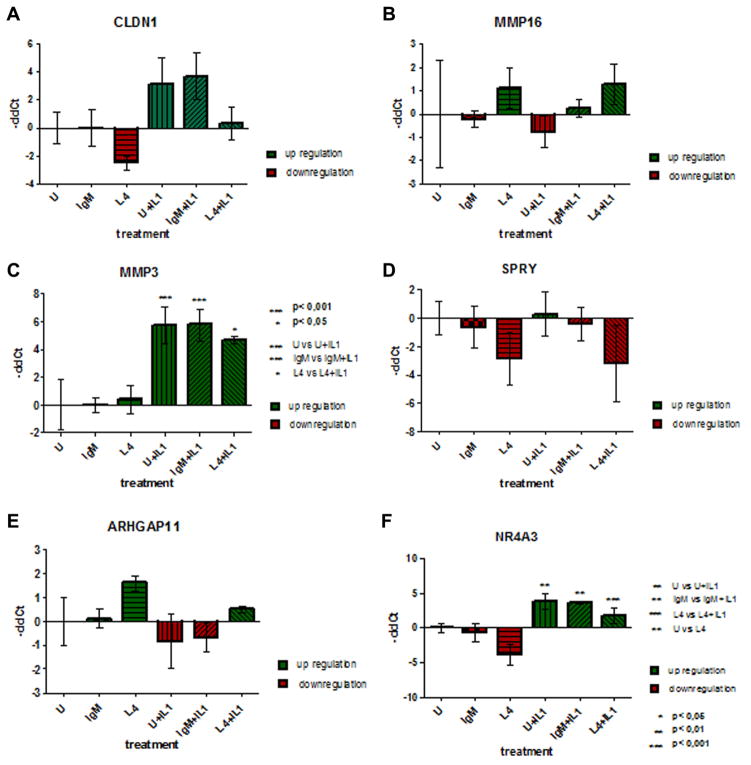
Signals for binding of a total of 53617 targeted probes were detected. Full annotation was possible for 43550 target probes, whereas 1078 were unknown. A total of 593 genes were significantly differentially expressed after LAMA4 blockade. 320 genes were upregulated, 273 genes were downregulated (Fig. S2). The Affimetrix analyses including all affected genes are attached in Supplement information 3. Raw data was uploaded to NCBI gene expression omnibus (GEO) and can be accessed at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE68428. RT-qPCR of selected genes was done to validate the results of the cDNA microarray. Changes in gene expression detected by RT-qPCR were in accordance with the cDNA microarray results. LAMA4 blockade reduced MMP3 expression, supporting our previous data.14 Figure 5 shows the changes in gene transcription validated by qPCR.
Figure 5.
Quantitative polymerase chain reaction results. Validation of the microarray results using RT-qPCR. The ΔCq method was computed to determine differences in concentrations between samples based on normalization on the reference genes. ΔΔCq was calculated based on the ‘untreated group’. Statistical analysis was computed with GraphPad Prism 5 using One-way Anova und Bonferoni-post test to detect significant differences between the groups.
DISCUSSION
Chondrocyte cluster formation is a histological sign of late stage OA.17,20 In this study we documented live in vitro migration of human osteoarthritic chondrocytes into clusters and a significant reduction of cluster formation by blocking LAMA4. As indicated by filopodia formation, clustering might be an active process under these culture conditions. Unspecific effects of the culture matrix are unlikely, since endothelial cells showed a typical net-like formation and Hela cells formed undifferentiated proliferating cell clumps resulting in a monolayer instead of defined structures.21
It is known that chondrocytes have the ability to migrate in vivo by filopodia protrusion and collagen degradation and in vitro on enriched hydrogels.4,9 Kouri et al. documented different subpopulations of chondrocytes in OA cartilage. Subpopulation type 1 found in the superficial zone is able to form filopodia. Type 2a and type 2b were categorized as secretory cells. In these cells the amount of cell cilia was low and they were hardly present in cell aggregates. Type 3 cells were common in OA cartilage showing various stages of degeneration.3 Chondrocytes derived from traumatized or enzymatically treated cartilage have been described to contain clones with a heterogeneous ability to migrate in vivo.4,22 In accordance we found behaviourally different populations of chondrocytes in the cell culture, some are able to form protrusions on the surface and form aggregates, while others are immobile cells with no surface irregularities.4,20
Cell cilia and lamellopodia are important for cell motility in various cell types reacting to extracellular matrix proteins and mechanosensory stimuli.23–25 We show reduced cluster formation of chondrocytes in vitro by blocking LAMA4. We hypothesize that LAMA4 might be important to guide the cells to these cell formations. Microspikes are involved in directed migration and cells tend to move in the direction of microspike development.26,27 In our study chondrocytes showed less microspike protrusions on the cell surface after LAMA4 blockade. The cells did not loose the ability to migrate; instead it appeared, that they were not able to aggregate to cell clusters. This supports the hypothesis, that LAMA4 is involved in directed motility by mediating microspike development. Interestingly, developmental reports document, that the directed motility of cells is influenced by LAMA4.28,29
Recent publications report the importance of integrins and gap junction proteins for cartilage homeostasis.30 Interestingly, mRNA expression of 2 GAPases (ARHGAP11A and B) known to suppress filopodia and mikrospike protrusions, were upregulated by inhibition of LAMA4.26,27 Parvin A (PARVA) is another molecule involved in lamellopodia formation. A higher expression of PARVA leads to a round surface of the cell by reorganization of actin and less lamellopodia formation. PARVA and ILK bind to a complex that mediates integrin signaling to alpha actinin, which regulates actin polymerization.31,32 Our array data revealed a downregulation of Parvin A by interleukin-1 stimulation, whereas LAMA4 blockade lead to an upregulation of this protein.
Our data suggests that LAMA4 is important for targeted migration, without inhibiting movement, as described in other cell types.10,13,28 Claudin-1 (CLDN-1) enhances targeted migration and invasiveness of tumor cells in tissue.33,34 LAMA4 blockade down regulated CLDN-1 expression in chondrocytes, whereas IL-1 upregulated its expression. Thus, some of the effects of LAMA4 on migration could be mediated through CLDN-1.
Downregulation of the nuclear receptor subfamily group A number 1–3 is considered a protective sign in osteoarthritis and inflammatory diseases.35,36 Our microarray data revealed an induction of NR4A3 after Interleukin-1 treatment as shown previously37 and a downregulation of this gene by LAMA4 blockade. We observed a similar pattern of expression for Sprouty homolog 2 (SPRY2), a gene expressed in chondrogenesis acting as a functional inhibitor of fibroblast growth factor.38
Matrixmetalloproteinases
Cartilage degradation by matrix metalloproteinases (MMP) and aggrecanases is a hallmark of OA.39–42 We have previously reported, that MMP3 expression was down regulated by LAMA4 blockade.14 The results of our microarray supported these previous findings. Fragments of LAMA4 have been shown to bind to syndecans and thus may modulate MMP3 expression.43 Activation and translocation of MMP3 affects chondrocyte proliferation and ECM formation.44 Interestingly, MMP3 is important in gliomas for directed migration and metastasis.44,45 We searched for other MMPs differentially expressed by LAMA4 blockade. MMP16 was detected being upregulated by LAMA4 blockade. MMP16 expression led to invasiveness and migration of glioma cells.46 Additionally MMP16 is important in chondrogenesis and a possible regeneration candidate.47 Our data shows an increased expression of MMP16 after LAMA4 blockade, suggesting a more migration-friendly milieu or activating regeneration by “re-inducing” chondrogenesis programs.
Limitations of our study are that we are not able to perform the experiments on healthy, non-arthritic cartilage. Due to ethical reasons we were not able to collect tissue from young or healthy individuals. To limit chondrocyte dedifferentiation, we cultured chondrocytes over no more than 3 passages.48
In this work we could show that inhibition of LAMA4 leads to diminished cluster formation in an in vitro model. The formation of microspikes and directed migration seemed to be reduced. In addition, blocking LAMA4 might establish an anabolic milieu by down regulating genes involved in inflammation and cartilage degeneration.
Further studies on the role of adhesion molecules in cartilage maintenance are needed, as there might be a potential to introduce anti-degenerative mechanisms by LAMA4 blockade.
Supplementary Material
Acknowledgments
Grant sponsor: Oesterreichische Nationalbank-Jubilaeumsfond; Grant number: 14764.
This work was funded by the “Oesterreichische National-bank- Jubilaeumsfond (Project number 14764). Thanks to Dr. Andelko Hrzenjak/ZMF for technical support.
Footnotes
AUTHORS CONTRIBUTIONS
Florentine C. Moazedi-Fuerst, Scientific design, idea, manuscript preparation; Gerald Gruber, specimen collection, manuscript preparation; Martin H. Stradner, statistics, manuscript preparation; Diego Guidolin, Image analyses and statistics; Jonathan C. Jones, anti-LAMA4-antibody supply; Koppany Bodo, immunofluoresence interpretation; Karin Wagner, array analyses; Daniela Peischler, tissue culture cell observer; Jennifer Weber, array analyses; Patrick Sadoghi, specimen collection; Mathias Glehr, specimen collection; Andreas Leithner, specimen collection; Winfried B. Graninger, manuscript preparation, supervision.
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Schüettler KF, Struewer J, Rominger MB, et al. Repair of a chondral defect using a cell free scaffold in a young patient—a case report of successful scaffold transformation and colonisation. BMC Surg. 2013;13:11. doi: 10.1186/1471-2482-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marmotti A, Bonasia DE, Bruzzone M, et al. Human cartilage fragments in a composite scaffold for single-stage cartilage repair: an in vitro study of the chondrocyte migration and the influence of TGF-β1 and G-CSF. Knee Surg. Sports Traumatol Arthrosc. 2013;21:1819–1833. doi: 10.1007/s00167-012-2244-7. [DOI] [PubMed] [Google Scholar]
- 3.Kouri JB, Jimenez SA, Quintero M, et al. Ultrastructural study of chondrocytes from fibrillated and non-fibrillated human osteoarthritic cartilage. Osteoarthr Cartil. 1996;4:111–125. doi: 10.1016/s1063-4584(05)80320-6. [DOI] [PubMed] [Google Scholar]
- 4.Morales TI. Chondrocyte moves: clever strategies? Osteoarthritis Cartilage. 2007;15:861–871. doi: 10.1016/j.joca.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeppel JP, Crist JD, Anderson HC, et al. Molecular regulation of articular chondrocyte function and its significance in osteoarthritis. Histol Histopathol. 2011;26:377–394. doi: 10.14670/HH-26.377. [DOI] [PubMed] [Google Scholar]
- 6.Kouri JB, Lavalle C. Do chondrocytes undergo “activation” and “transdifferentiation” during the pathogenesis of osteoarthritis? A review of the ultrastructural and immuno-histochemical evidence. Histopathol Histol. 2006;21:793–802. doi: 10.14670/HH-21.793. [DOI] [PubMed] [Google Scholar]
- 7.Hoshiyama Y, Otsuki S, Oda S, et al. Chondrocyte clusters adjacent to sites of cartilage degeneration have characteristics of progenitor cells. J Orthop Res. 2015;33:548–555. doi: 10.1002/jor.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang C, Lauffenburger DA, Morales TI. Motile chondrocytes from newborn calf: migration properties and synthesis of collagen II. Osteoarthritis Cartilage. 2003;11:603–612. doi: 10.1016/s1063-4584(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 9.Khoshfetrat AB, Kino-Oka M, Takezawa Y, et al. Seeding density modulates migration and morphology of rabbit chondrocytes cultured in collagen gels. Biotechnol Bioeng. 2009;102:294–302. doi: 10.1002/bit.22029. [DOI] [PubMed] [Google Scholar]
- 10.Yousif LF, Di Russo J, Sorokin L. Laminin isoforms in endothelial and perivascular basement membranes. Cell Adhes Migr. 2013;7:101–110. doi: 10.4161/cam.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagato S, Nakagawa K, Harada H, et al. Down-regulation of laminin alpha4 chain expression inhibits glioma invasion in vitro and in vivo. Int J Cancer. 2005;117:41–50. doi: 10.1002/ijc.21102. [DOI] [PubMed] [Google Scholar]
- 12.Aumailley M, Smyth N. The role of laminins in basement membrane function. J Anat. 1998;193:1–21. doi: 10.1046/j.1469-7580.1998.19310001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenne E, Soehnlein O, Genové G, et al. Immune cell recruitment to inflammatory loci is impaired in mice deficient in basement membrane protein laminin alpha4. J Leukoc Biol. 2010;88:523–528. doi: 10.1189/jlb.0110043. [DOI] [PubMed] [Google Scholar]
- 14.Fuerst FC, Gruber G, Stradner MH, et al. Regulation of MMP3 by laminin alpha 4 in human osteoarthritic cartilage. Scand J Rheumatol. 2011;40:494–496. doi: 10.3109/03009742.2011.605392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moazedi-Fuerst FC, Hofner M, Gruber G, et al. Epigenetic differences in human cartilage between mild and severe OA. J Orthop Res. 2014;32:1636–1645. doi: 10.1002/jor.22722. [DOI] [PubMed] [Google Scholar]
- 16.Camplejohn KL, Allard SA. Limitations of safranin “O” staining in proteoglycan-depleted cartilage demonstrated with monoclonal antibodies. Histochemistry. 1988;89:185–188. doi: 10.1007/BF00489922. [DOI] [PubMed] [Google Scholar]
- 17.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 18.DeHahn KC, Gonzales M, Gonzalez AM, et al. The alpha4 laminin subunit regulates endothelial cell survival. Exp Cell Res. 2004;294:281–289. doi: 10.1016/j.yexcr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aigner T, McKenna L. Molecular pathology and patho-biology of osteoarthritic cartilage. Cell Mol Life Sci. 2002;59:5–18. doi: 10.1007/s00018-002-8400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura M, Mie M, Nakamura M, et al. Construction of multi-functional extracellular matrix proteins that inhibits migration and tube formation of endothelial cells. Biotechnol Lett. 2012;34:1571–1577. doi: 10.1007/s10529-011-0788-0. [DOI] [PubMed] [Google Scholar]
- 22.Koelling S, Kruegel J, Irmer M, et al. Migratory Chondrogenic Progenitor Cells from Repair Tissue during the Later Stages of Human Osteoarthritis. Cell Stem Cell. 2009;4:324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 23.McGlashan SR, Haycraft CJ, Jensen CG, et al. Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol. 2007;26:234–246. doi: 10.1016/j.matbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 24.McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- 25.Jensen CG, Poole CA, McGlashan SR, et al. Ultra-structural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int. 2004;28:101–110. doi: 10.1016/j.cellbi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Fan L, Mellor H. The small Rho GTPase Rif and actin cytoskeletal remodelling. Biochem Soc Trans. 2012;40:268–272. doi: 10.1042/BST20110625. [DOI] [PubMed] [Google Scholar]
- 27.Faix J, Breitsprecher D, Stradal TEB, et al. Filopodia: Complex models for simple rods. Int J Biochem Cell Biol. 2009;41:1656–1664. doi: 10.1016/j.biocel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Sun YM, Cooper M, Finch S, et al. Rest-mediated regulation of extracellular matrix is crucial for neural development. PLoS One. 2008:3. doi: 10.1371/journal.pone.0003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimune H, Valdez G, Jarad G, et al. Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. J Cell Biol. 2008;182:1201–1215. doi: 10.1083/jcb.200805095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayan MD, Gago-Fuentes R, Carpintero-Fernandez P, et al. Articular chondrocyte network mediated by gap junctions: role in metabolic cartilage homeostasis. Ann Rheum Dis. 2015;74:275–284. doi: 10.1136/annrheumdis-2013-204244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaji S, Suzuki A, Sugiyama Y, et al. A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell-substrate interaction. J Cell Biol. 2001;153:1251–1264. doi: 10.1083/jcb.153.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaji S, Suzuki A, Kanamori H, et al. Affixin interacts with alpha-actinin and mediates integrin signaling for reorganization of F-actin induced by initial cell-substrate interaction. J Cell Biol. 2004;165:539–551. doi: 10.1083/jcb.200308141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo J, Sato F, Kusumi T, et al. Claudin-1 expression is induced by tumor necrosis factor-alpha in human pancreatic cancer cells. Int J Mol Med. 2008;22:645–649. [PubMed] [Google Scholar]
- 34.Shiozaki A, Bai X, Shen-Tu G, et al. Claudin 1 Mediates TNFα-Induced Gene Expression and Cell Migration in Human Lung Carcinoma Cells. PLoS One. 2012;7:e38049. doi: 10.1371/journal.pone.0038049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaturvedi P, Pratta M, Steplewski K, et al. Functional characterization of an orphan nuclear receptor, Rev-ErbAalpha, in chondrocytes and its potential role in osteoarthritis. Arthritis Rheum. 2006;54:3513–3522. doi: 10.1002/art.22170. [DOI] [PubMed] [Google Scholar]
- 36.Ranhotra HS. The NR4A orphan nuclear receptors: mediators in metabolism and diseases. J Recept Signal Transduct Res. 2015;35:184–188. doi: 10.3109/10799893.2014.948555. [DOI] [PubMed] [Google Scholar]
- 37.Marzaioli V, McMorrow JP, Angerer H, et al. Histamine contributes to increased RANKL to osteoprotegerin ratio through altered nuclear receptor 4A activity in human chondrocytes. Arthritis Rheum. 2012;64:3290–3301. doi: 10.1002/art.34554. [DOI] [PubMed] [Google Scholar]
- 38.Minowada G, Jarvis LA, Chi CL, et al. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 39.Roach HI, Aigner T, Soder S, et al. Pathobiology of osteoarthritis: pathomechanisms and potential therapeutic targets. Curr Drug Targets. 2007;8:271–282. doi: 10.2174/138945007779940160. [DOI] [PubMed] [Google Scholar]
- 40.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43:1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 41.Blaney Davidson EN, van der Kraan PM, van den Berg WB. TGF-beta and osteoarthritis. Osteoarthritis Cartilage. 2007;15:597–604. doi: 10.1016/j.joca.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Blom AB, van Lent PL, van der Kraan PM, et al. To seek shelter from the WNT in osteoarthritis? WNT-signaling as a target for osteoarthritis therapy. Curr Drug Targets. 2010;11:620–629. doi: 10.2174/138945010791011901. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita H, Goto C, Tajima R, et al. Cryptic fragment alpha4 LG4-5 derived from laminin alpha4 chain inhibits de novo adipogenesis by modulating the effect of fibroblast growth factor-2. Dev Growth Differ. 2008;50:97–107. doi: 10.1111/j.1440-169X.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- 44.Eguchi T, Kubota S, Kawata K, et al. Novel transcription-factor-like function of human matrix metal-loproteinase 3 regulating the CTGF/CCN2 gene. Mol Cell Biol. 2008;28:2391–2413. doi: 10.1128/MCB.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laurent M, Martinerie C, Thibout H, et al. NOVH increases MMP3 expression and cell migration in glioblastoma cells via a PDGFR-alpha-dependent mechanism. FASEB J. 2003;17:1919–1921. doi: 10.1096/fj.02-1023fje. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Wang Y, Yu L, et al. MiR-146b-5p inhibits glioma migration and invasion by targeting MMP16. Cancer Lett. 2013;339:260–269. doi: 10.1016/j.canlet.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Yamanaka H, Makino K, Takizawa M, et al. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in rheumatoid synovium. Lab Invest. 2000;80:677–687. doi: 10.1038/labinvest.3780071. [DOI] [PubMed] [Google Scholar]
- 48.Lin L, Shen Q, Zhang C, et al. Assessment of the profiling MicroRNA expression of differentiated and dedifferentiated human adult articular chondrocytes. J Orthop Res. 2011;29:1578–1584. doi: 10.1002/jor.21423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.