Abstract
BACKGROUND
The A(H1N1)pdm09 virus strain used in the live attenuated influenza vaccine was changed for the 2015–2016 influenza season because of its lack of effectiveness in young children in 2013–2014. The Influenza Vaccine Effectiveness Network evaluated the effect of this change as part of its estimates of influenza vaccine effectiveness in 2015–2016.
METHODS
We enrolled patients 6 months of age or older who presented with acute respiratory illness at ambulatory care clinics in geographically diverse U.S. sites. Using a test-negative design, we estimated vaccine effectiveness as (1 – OR) × 100, in which OR is the odds ratio for testing positive for influenza virus among vaccinated versus unvaccinated participants. Separate estimates were calculated for the inactivated vaccines and the live attenuated vaccine.
RESULTS
Among 6879 eligible participants, 1309 (19%) tested positive for influenza virus, predominantly for A(H1N1)pdm09 (11%) and influenza B (7%). The effectiveness of the influenza vaccine against any influenza illness was 48% (95% confidence interval [CI], 41 to 55; P<0.001). Among children 2 to 17 years of age, the inactivated influenza vaccine was 60% effective (95% CI, 47 to 70; P<0.001), and the live attenuated vaccine was not observed to be effective (vaccine effectiveness, 5%; 95% CI, −47 to 39; P = 0.80). Vaccine effectiveness against A(H1N1)pdm09 among children was 63% (95% CI, 45 to 75; P<0.001) for the inactivated vaccine, as compared with −19% (95% CI, −113 to 33; P = 0.55) for the live attenuated vaccine.
CONCLUSIONS
Influenza vaccines reduced the risk of influenza illness in 2015–2016. However, the live attenuated vaccine was found to be ineffective among children in a year with substantial inactivated vaccine effectiveness. Because the 2016–2017 A(H1N1)pdm09 strain used in the live attenuated vaccine was unchanged from 2015–2016, the Advisory Committee on Immunization Practices made an interim recommendation not to use the live attenuated influenza vaccine for the 2016–2017 influenza season. (Funded by the Centers for Disease Control and Prevention and the National Institutes of Health.)
The effectiveness of influenza vaccination and the corresponding effect of vaccination programs on the burden of influenza can vary considerably from year to year.1–4 In light of this variability, annual observational studies of influenza vaccine effectiveness are critical as ongoing evaluations of the value of influenza vaccination programs,5–7 as well as for identifying problems with licensed influenza vaccines. The Influenza Vaccine Effectiveness Network provides estimates of influenza vaccine effectiveness and of the cases of influenza averted by vaccination in the United States.3,4,8–10
A recent important finding of the Influenza Vaccine Effectiveness Network,8 later confirmed by other studies,11,12 was that the quadrivalent live attenuated influenza vaccine lacked effectiveness against A(H1N1)pdm09 viruses in young children during the 2013–2014 influenza season.8 This occurred in a season in which trivalent and quadrivalent inactivated influenza vaccines were effective at preventing influenza, indicating that the failure of the live attenuated vaccine was not due to a poor match between vaccines and circulating A(H1N1)pdm09 strains. This failure of the live attenuated vaccine was surprising, since earlier randomized trials had suggested that, among young children, the trivalent live attenuated vaccine provided protection against influenza that was superior to that provided by the trivalent inactivated vaccine.13,14 Poor thermostability of the A(H1N1)pdm09 vaccine strain was suspected as a possible cause, and the A(H1N1)pdm09 strain in the live attenuated vaccine was updated to A/Bolivia/559/2013 (an A/California/7/2009-like virus) for the 2015–2016 influenza season. Here, we report estimates of influenza vaccine effectiveness from the Influenza Vaccine Effectiveness Network for the 2015–2016 influenza season, including comparisons of vaccine effectiveness between the trivalent and quadrivalent inactivated vaccines and the quadrivalent live attenuated vaccine.
METHODS
STUDY POPULATION
Details of the Influenza Vaccine Effectiveness Network have been published previously.3,8–10 Study participants were recruited during the 2015–2016 influenza season (defined by local influenza surveillance) at study sites in Michigan, Pennsylvania, Texas, Washington, and Wisconsin. Eligible participants were patients 6 months of age or older who presented to ambulatory care clinics for acute respiratory illness with a cough of 7 or fewer days in duration at the time of the medical visit. Patients who had received antiviral agents for their current illness, who were younger than 6 months of age as of September 1, 2015, or who had enrolled in the study within the previous 14 days were ineligible. Study staff obtained informed consent from patients (or from a parent or guardian for minors) and interviewed the patients regarding demographic characteristics, influenza risk factors, health status, symptoms, and receipt of the influenza vaccine for the current season. Health conditions associated with an increased risk of severe influenza15 were defined on the basis of International Classification of Diseases codes assigned to medical encounters in the year before enrollment (see the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org).
INFLUENZA VACCINATION HISTORY
The virus strains recommended for the 2015–2016 Northern Hemisphere influenza vaccines were A/California/7/2009 (H1N1pdm09)–like, A/Switzerland/9715293/2013 (H3N2)–like, B/Phuket/3073/2013-like (Yamagata lineage) viruses, and (for quadrivalent vaccines) B/Brisbane/60/2008-like virus (Victoria lineage). Study staff reviewed the electronic immunization records for all participants to determine receipt of influenza vaccinations. Electronic immunization records varied among sites and included electronic health records, employee health records, and state immunization registries. During enrollment interviews, participants (or their parents or guardians) also provided information regarding whether they received an influenza vaccination for the current season, including information on vaccine type (nasal spray vs. injection) (see the Methods section in the Supplementary Appendix). Influenza vaccination history for the current season was defined with the use of electronic immunization records and data reported by the participants, as described previously.8 Vaccination status for the previous season was defined with the use of electronic immunization records.
LABORATORY METHODS
At enrollment, study staff obtained combined nasal and oropharyngeal swab specimens (only nasal swab specimens were obtained for children younger than 2 years of age). Specimens were tested for influenza viruses with the use of real-time reverse-transcriptase polymerase chain reaction (RT-PCR).9 Specimens were first tested for any influenza A or B virus; subsequent assays identified influenza A virus subtype and influenza B virus lineage. Participants who tested positive for influenza were designated as case patients, and participants who tested negative were designated as non–case patients.
A subset of influenza virus–positive specimens was sent to the Centers for Disease Control and Prevention for genetic characterization, antigenic characterization, or both. Full-length hemagglutinin sequences were obtained by means of whole-genome sequencing from original specimen samples.16 Viruses were classified into hemagglutinin genetic groups on the basis of phylogenetic analyses. In addition, A(H3N2) viruses were classified into hemagglutinin genetic groups by means of a pyrosequencing assay.17,18 Selected viruses from each clade or genetic group were grown to high titer and antigenically characterized by means of hemagglutination-inhibition or focus-reduction (for A[H3N2]) assays against the 2015–2016 vaccine reference strains.19
STATISTICAL ANALYSIS
We excluded the following participants from the primary analyses: participants with inconclusive RT-PCR results, participants who had been vaccinated less than 14 days before illness onset (as reported by the participant), children younger than 9 years of age who were partially vaccinated according to the 2015–2016 Advisory Committee on Immunization Practices (ACIP) recommendations,15 and non–case patients who were enrolled outside the periods of local influenza circulation. For the remaining participants, we calculated descriptive statistics separately for influenza case patients and non–case patients, including medians for continuous variables and distributions for categorical variables.
Influenza vaccine effectiveness was estimated with the use of a test-negative design, which compares the odds of testing positive for influenza virus among vaccinated versus unvaccinated participants.20,21 Following Jackson and Nelson,20 we considered vaccine effectiveness to be the relative difference in influenza risk between vaccinated and unvaccinated participants, expressed as a percentage and calculated as (1 – OR) × 100, where OR is the odds ratio for influenza among vaccinated persons as compared with unvaccinated persons, determined from logistic-regression models. The 95% confidence intervals for vaccine effectiveness were calculated as 1 – CIOR, is the confidence interval of the odds where CIOR ratio estimates. A priori, estimates were adjusted for network site, age (with the use of linear tail-restricted cubic splines), presence of high-risk medical conditions, and calendar time (in 2-week intervals). No additional covariates modified the estimated odds ratio by 5% or more, our predetermined inclusion threshold, so the final model included only the a priori covariates.
We estimated vaccine effectiveness for any influenza-associated illness and separately for illness due to influenza according to subtype or lineage. We also estimated vaccine effectiveness according to age group, vaccine type (overall and among children 2 to 17 years of age), and receipt of a vaccine for the 2014–2015 season (among participants ≥9 years of age). For the subtype-specific and lineage-specific estimates, case patients infected with other influenza virus subtypes or lineages were excluded. For vaccine type–specific estimates, participants who received other vaccine types were excluded. For all estimates, P values of less than 0.05 were considered to indicate statistical significance.
To assess the potential for bias from excluding partially vaccinated children younger than 9 years of age, we repeated the primary analyses (vaccine effectiveness overall and stratified according to age) with these children included. We estimated vaccine effectiveness while restricting the analysis to participants who enrolled less than 5 days after illness onset in order to identify bias resulting from potential false negative influenza virus test results among participants who presented 5 to 7 days after illness onset. We also estimated vaccine effectiveness using different definitions of vaccination: participant-reported vaccination only (excluding the Wisconsin site, where participant-reported history data were not collected) and electronic immunization records–confirmed vaccination only.
RESULTS
PARTICIPANT CHARACTERISTICS
From November 2, 2015, through April 15, 2016, we enrolled 7563 patients who presented to ambulatory care clinics for acute respiratory illness. We excluded 684 participants (9%) from the primary analyses, including 10 participants with inconclusive RT-PCR results, 62 participants who had been vaccinated less than 14 days before illness onset, 160 partially vaccinated children younger than 9 years of age, and 452 influenza virus–negative participants who enrolled outside the periods of influenza circulation. Of the remaining 6879 eligible participants, 1309 (19%) tested positive for influenza virus (case patients), with the number of cases peaking in calendar week 10 (Fig. S1 in the Supplementary Appendix). The influenza case patients included 765 (58%) who were infected with A(H1N1)pdm09, 72 (6%) infected with A(H3N2), 250 (19%) infected with B/Yamagata, 200 (15%) infected with B/Victoria, and 3 (<1%) coinfected with A(H1N1)pdm09 and B/Yamagata (Table S1 in the Supplementary Appendix); 16 were infected with influenza A virus but no subtype was determined, and 3 were infected with influenza B virus of undetermined lineage. A total of 3396 participants (49%) were vaccinated 14 or more days before illness onset.
As compared with non–case patients, case patients were more likely to be 9 to 64 years of age, male, and black and non-Hispanic, to report “excellent” general health, and to have a shorter interval from illness onset to enrollment (Table 1, and Table S1 in the Supplementary Appendix). Case patients were less likely than non–case patients to be obese (body-mass index [the weight in kilograms divided by the square of the height in meters], ≥30), to have a high-risk medical condition, or to have received influenza vaccine. Among non–case patients, those who had been vaccinated tended to be older, female, and white and non-Hispanic and to have one or more high-risk medical conditions and an educational level of bachelor’s degree or higher (Table 1, and Table S1 in the Supplementary Appendix).
Table 1.
Characteristics of the Study Participants.
| Characteristic | All Participants | Influenza Virus–Negative Participants | ||
|---|---|---|---|---|
| Influenza Virus– Negative (N = 5570) | Influenza Virus– Positive (N = 1309) | Unvaccinated (N = 2668) | Vaccinated (N = 2902) | |
| number of patients (percent) | ||||
| Age | ||||
|
| ||||
| 6 mo to 8 yr | 1272 (23) | 254 (19) | 642 (24) | 630 (22) |
|
| ||||
| 9 to 17 yr | 694 (12) | 164 (13) | 417 (16) | 277 (10) |
|
| ||||
| 18 to 49 yr | 1957 (35) | 499 (38) | 1116 (42) | 841 (29) |
|
| ||||
| 50 to 64 yr | 918 (16) | 283 (22) | 356 (13) | 562 (19) |
|
| ||||
| ≥65 yr | 729 (13) | 109 (8) | 137 (5) | 592 (20) |
|
| ||||
| 2 to 17 yr | 1655 (30) | 392 (30) | 942 (35) | 713 (25) |
|
| ||||
| Study site | ||||
|
| ||||
| Michigan | 751 (13) | 243 (19) | 330 (12) | 421 (15) |
|
| ||||
| Pennsylvania | 1254 (23) | 356 (27) | 652 (24) | 602 (21) |
|
| ||||
| Texas | 1147 (21) | 192 (15) | 644 (24) | 503 (17) |
|
| ||||
| Washington | 1399 (25) | 321 (25) | 579 (22) | 820 (28) |
|
| ||||
| Wisconsin | 1019 (18) | 197 (15) | 463 (17) | 556 (19) |
|
| ||||
| Race and ethnic group* | ||||
|
| ||||
| White, non-Hispanic | 4233 (76) | 919 (70) | 1928 (72) | 2305 (79) |
|
| ||||
| Black, non-Hispanic | 402 (7) | 157 (12) | 246 (9) | 156 (5) |
|
| ||||
| Other, non-Hispanic | 456 (8) | 132 (10) | 224 (8) | 232 (8) |
|
| ||||
| Hispanic | 455 (8) | 98 (7) | 258 (10) | 197 (7) |
|
| ||||
| Unknown | 24 (<1) | 3 (<1) | 12 (<1) | 12 (<1) |
|
| ||||
| Vaccinated | 2902 (52) | 494 (38) | — | — |
Race and ethnic group were reported by the patients. Percentages may not total 100 because of rounding.
ANTIGENIC AND GENETIC CHARACTERIZATION OF VIRUSES
All antigenically characterized viruses — 67 A(H1N1)pdm09 viruses, 16 A(H3N2) viruses, 43 B/Yamagata viruses, and 47 B/Victoria viruses — were antigenically similar to the respective vaccine reference strains. Among the 73 genetically sequenced A(H1N1)pdm09 viruses, 72 (99%) were of hemagglutinin genetic group 6B.1, and 1 (1%) was of group 6B. Among the 73 A(H3N2) viruses that were tested by sequencing or pyrosequencing, 34 (47%) were of hemagglutinin group 3C.2a, and 39 (53%) were of group 3C.3a. Among the sequenced influenza B viruses, 33 of 34 B/Yamagata viruses (97%) belonged to clade 3, and all 35 B/Victoria viruses belonged to clade 1A.
OVERALL VACCINE EFFECTIVENESS
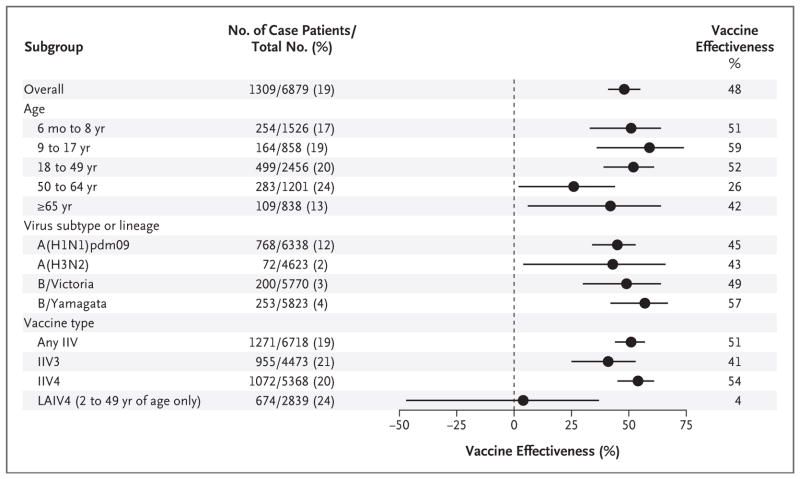
After adjustment for potential confounders, the estimated vaccine effectiveness against any influenza virus was 48% (95% confidence interval [CI], 41 to 55; P<0.001) (Fig. 1, and Table S2 in the Supplementary Appendix). Significant vaccine effectiveness was observed in all age groups (with point estimates ranging from 26 to 59%; P≤0.04 for all estimates) and for each virus subtype or lineage (with point estimates ranging from 43 to 57%; P≤0.03 for all estimates) (Fig. 1, and Table S2 in the Supplementary Appendix). Among participants of all ages, significant vaccine effectiveness was observed for the inactivated influenza vaccine (P<0.001) but not for the quadrivalent live attenuated influenza vaccine (P=0.86) (Fig. 1).
Figure 1. Adjusted Estimates of Influenza Vaccine Effectiveness, Overall and Stratified According to Age, Virus Subtype or Lineage, and Vaccine Type.
In each subgroup, the number of case patients is the number of participants in that subgroup who tested positive for influenza virus; the total number in each subgroup is the number of participants in the subgroup who tested positive or negative. For the analysis according to virus subtype or lineage, the total number included all patients who tested negative plus all those who tested positive for the subtype or lineage of interest, with the exception that participants from the Wisconsin site were excluded from the analysis of A(H3N2). Vaccine effectiveness was calculated as (1 – OR) × 100, in which OR is the odds ratio for testing positive for influenza virus among vaccinated versus unvaccinated participants in each subgroup. Horizontal bars indicate 95% confidence intervals. IIV denotes inactivated influenza vaccine, IIV3 trivalent IIV, IIV4 quadrivalent IIV, and LAIV4 quadrivalent live attenuated influenza vaccine.
VACCINE TYPE COMPARISONS
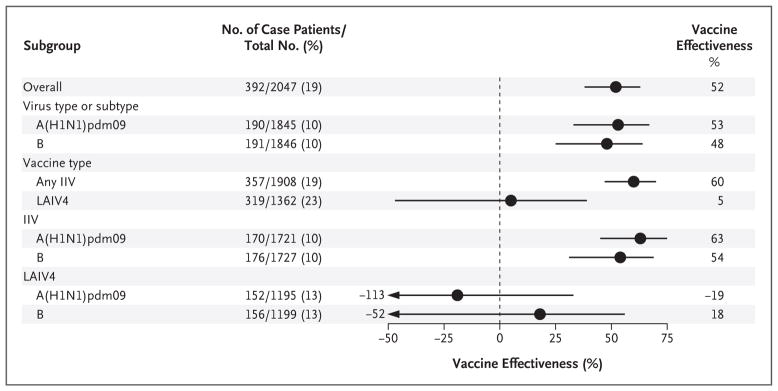
Among children 2 to 17 years of age, the risk of influenza was not significantly lower among those who received the quadrivalent live attenuated influenza vaccine than among those who received no vaccine; the effectiveness of the vaccine in this group was not significant for the prevention of influenza overall (vaccine effectiveness, 5%; 95% CI, −47 to 39; P = 0.80), of A(H1N1)pdm09 influenza (vaccine effectiveness, −19%; 95% CI, −113 to 33; P = 0.55), or of influenza B (vaccine effectiveness, 18%; 95% CI, −52 to 56; P = 0.53) (Fig. 2, and Table S2 in the Supplementary Appendix). In contrast, the risk of influenza was significantly lower among children who received any inactivated influenza vaccine than among those who received no vaccine; the effectiveness of the inactivated vaccine in this group was significant against influenza caused by any virus (vaccine effectiveness, 60%; 95% CI, 47 to 70; P<0.001), by A(H1N1)pdm09 (vaccine effectiveness, 63%; 95% CI, 45 to 75; P<0.001), and by influenza B virus (vaccine effectiveness, 54%; 95% CI, 31 to 69; P = 0.001). In a comparison between the 136 children (2 to 17 years of age) who received quadrivalent live attenuated vaccine and the 682 children who received the trivalent or quadrivalent inactivated vaccine, the live attenuated vaccine was associated with significantly higher odds of influenza (odds ratio, 2.7; 95% CI, 1.6 to 4.6; P<0.001) (Fig. 3).
Figure 2. Adjusted Estimates of Influenza Vaccine Effectiveness among Children 2 to 17 Years of Age, Overall and Stratified According to Virus Subtype or Lineage and Vaccine Type.
In each subgroup, the number of case patients is the number of children 2 to 17 years of age who tested positive for influenza virus (or for the type or subtype of interest, in the subgroups that are based on these factors); the total number includes unvaccinated children who tested negative for influenza virus, children vaccinated with the relevant vaccine type who tested negative for influenza virus, and children who were vaccinated with the relevant vaccine and tested positive for influenza virus (or for the type or subtype of interest, in the subgroups that are based on these factors). Vaccine effectiveness was calculated as (1 – OR) × 100, in which OR is the odds ratio for testing positive for influenza virus among vaccinated versus unvaccinated participants in each subgroup. Horizontal bars indicate 95% confidence intervals.
Figure 3. Odds Ratios for Medically Attended Influenza, Overall and According to Age Group.
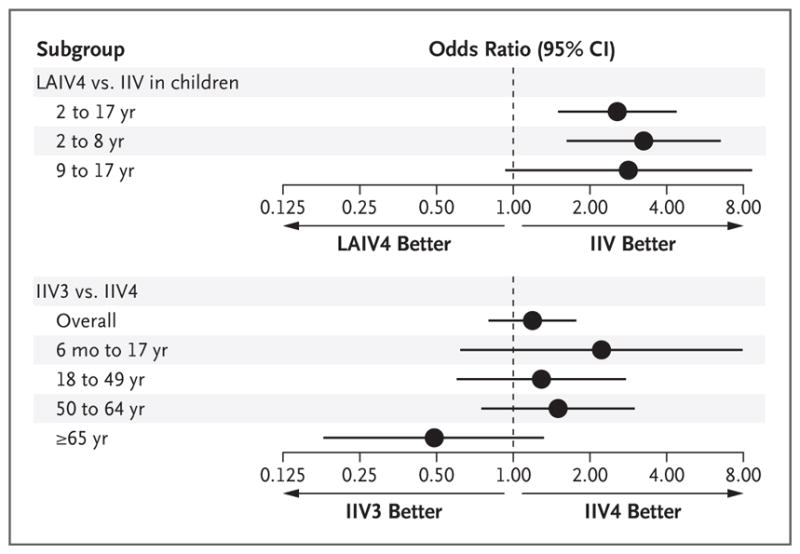
Horizontal bars indicate 95% confidence intervals.
Vaccine-effectiveness estimates tended to be higher for the quadrivalent inactivated vaccine than for the trivalent inactivated vaccine, both overall and when participants were stratified according to age group (Table S2 in the Supplementary Appendix), but the differences were not significant. In a comparison between the 781 participants who received the standard-dose trivalent inactivated vaccine and the 1885 who received the quadrivalent inactivated vaccine, the trivalent vaccine was not associated with significantly higher odds of influenza, either overall (odds ratio, 1.2; 95% CI, 0.8 to 1.7) or according to age group (Fig. 3). Against B/Victoria viruses, which were not included in the trivalent inactivated vaccine, we observed significantly higher vaccine effectiveness for the quadrivalent inactivated vaccine than for the trivalent vaccine (57% [95% CI, 36 to 71] vs. −99% [95% CI, −311 to 4]; P = 0.01 for the comparison).
EFFECTS OF PREVIOUS INFLUENZA VACCINATION
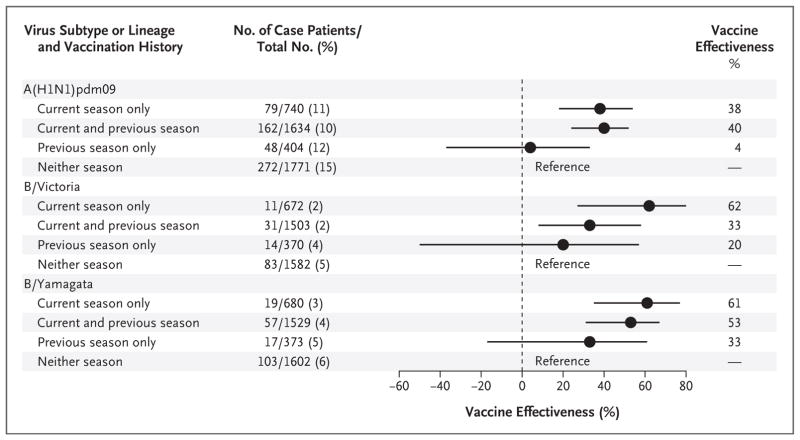
Against the A(H1N1)pdm09 and B/Yamagata strains, 2015–2016 season vaccine effectiveness among participants 9 years of age or older was similar between patients who had also been vaccinated in the previous season (2014–2015) and those who had not (Fig. 4). Against B/Victoria, the estimate of 2015–2016 vaccine effectiveness was lower among participants who had been vaccinated in 2014–2015 than among participants who had not, although the difference was not significant (33% [95% CI, −8 to 58] vs. 62% [95% CI, 27 to 80]; P = 0.10).
Figure 4. Adjusted Estimates of Influenza Vaccine Effectiveness, Stratified According to Receipt of Vaccines for the Current Season (2015–2016) and Previous Season (2014–2015).
In each subgroup, the number of case patients is the number of participants with the specified vaccination history who tested positive for the relevant influenza virus subtype or lineage; the total number in each subgroup is the number of participants with the specified vaccination history who tested negative for influenza or tested positive for the specified subtype or lineage. Vaccine effectiveness was calculated as (1 – OR) × 100, in which OR is the odds ratio for testing positive for influenza virus among participants who were vaccinated in the season or seasons of interest versus participants who received no vaccination in either season (the reference group). Horizontal bars indicate 95% confidence intervals.
SENSITIVITY ANALYSES
Our findings were robust to the method of determining influenza vaccination (participant report only, electronic immunization records plus plausible participant report, or electronic immunization records only) (Table S3 in the Supplementary Appendix). When the analysis was restricted to participants who enrolled less than 5 days after illness onset, the estimate of vaccine effectiveness was higher among adults 65 years of age or older than in the primary analysis population, although not significantly so (69% [95% CI, 42 to 83] vs. 42% [95% CI, 6 to 64]). In other age groups, restricting the analysis to participants who enrolled less than 5 days after illness onset did not meaningfully change the estimates of vaccine effectiveness. In the analysis of vaccine effectiveness among children younger than 18 years of age, the inclusion of partially vaccinated children did not change the estimates of effectiveness, either overall or according to vaccine type, by more than 4 percentage points.
DISCUSSION
For the 2015–2016 influenza season, influenza vaccines were effective against influenza viruses in general and against A(H1N1)pdm09 in particular. The overall vaccine effectiveness was 48%, a finding consistent with other seasons in which vaccine virus strains were antigenically similar to circulating virus strains,3,8,9 and inactivated vaccines were effective against both A(H1N1)pdm09 viruses and influenza B viruses. However, despite the change to the A(H1N1)pdm09 strain in the quadrivalent live attenuated vaccine,8 we again observed that this vaccine was not effective among children, particularly against A(H1N1)pdm09.
In June 2016, preliminary 2015–2016 vaccine-effectiveness estimates from the Influenza Vaccine Effectiveness Network and from several other observational studies were presented to the ACIP. In each of these studies, estimated vaccine effectiveness against A(H1N1)pdm09 was lower for the live attenuated vaccine than for the inactivated vaccine. Preliminary estimates from the Department of Defense for children 2 to 17 years of age indicated a nonsignificant vaccine effectiveness of 15% for the quadrivalent live attenuated vaccine against A(H1N1)pdm09, as compared with a significant 68% effectiveness for the trivalent or quadrivalent inactivated vaccine.22 The Influenza Clinical Investigation for Children (ICICLE) study (ClinicalTrials.gov number, NCT01997450) estimated vaccine effectiveness among children 2 to 17 years of age at study sites in eight U.S. states. For A(H1N1)pdm09, the interim results of that study showed a non-significant vaccine effectiveness of 50% for the quadrivalent live attenuated vaccine and a significant vaccine effectiveness of 71% for the trivalent or quadrivalent inactivated vaccine.23 A Finnish cohort study involving 2-year-old children showed a significant vaccine effectiveness against influenza A — predominantly against A(H1N1)pdm09 — of 48% for the quadrivalent live attenuated vaccine and 80% for the trivalent inactivated vaccine.24 Finally, a U.K. study involving children 2 to 17 years of age showed a non-significant vaccine effectiveness of 42% against A(H1N1)pdm09 for the quadrivalent live attenuated vaccine, as compared with an effectiveness of 100% for the trivalent inactivated vaccine.25 In light of these data, the ACIP made an interim recommendation not to use the quadrivalent live attenuated vaccine in the United States for the 2016–2017 influenza season.26
Although these observational studies consistently showed lower effectiveness for the live attenuated vaccine than for the inactivated vaccine, point estimates for the effectiveness of the live attenuated vaccine varied widely among studies. The reasons for this are unclear. The quadrivalent live attenuated vaccines in the United States, Finland, and the United Kingdom were all produced by the same manufacturer at the same plant,24 so it is unlikely that differences in estimated vaccine effectiveness are due to variations in the vaccine product. The observed variations could be due to chance, since the vaccine-effectiveness point estimates for all five studies have relatively wide confidence intervals. Vaccination history among children probably differs between populations in the U.S. and European studies. In the United States, children following the ACIP recommendations would have received at least one dose of inactivated influenza vaccine before 2 years of age and the quadrivalent live attenuated vaccine thereafter.27 In contrast, the Joint Committee on Vaccination and Immunisation in the United Kingdom recommends influenza vaccination beginning at 2 years of age for healthy children, using the quadrivalent live attenuated vaccine, and the Finnish study predominantly included children who had never received an influenza vaccine. Thus, children in the U.S. studies differed from their European counterparts in the number of previous influenza vaccine doses received and in the formulation of their first vaccinations. None of these studies was well-powered to examine differences in vaccine effectiveness according to vaccination history, so it is difficult to explore this in more detail. This is not a complete explanation, since discrepant results were also seen among the U.S. studies, which may have similar populations with regard to previous vaccination.
Other findings from this study merit comment. With the exception of the quadrivalent live attenuated vaccine in children, the 2015–2016 influenza vaccines were effective in persons of all ages and against all circulating virus subtypes and lineages. Some studies have suggested that previous-season vaccination inhibits the effectiveness of the vaccine for the current season. As in previous seasons investigated by the Influenza Vaccine Effectiveness Network, we found that vaccine effectiveness among participants who had been vaccinated in the previous season was lower than that among those who had not been vaccinated in the previous season, although the difference was not significant.3,8,9 We also found that the quadrivalent inactivated vaccine was significantly more effective than the trivalent inactivated vaccine against B/Victoria viruses, which were not included in the 2015–2016 trivalent vaccine. In some previous seasons, the trivalent inactivated vaccine has been seen to protect against both B lineages.3 Our finding of superior vaccine effectiveness of the quadrivalent relative to the trivalent inactivated vaccine against B/Victoria suggests that switching to the quadrivalent inactivated vaccine may indeed prevent more cases of influenza B than the trivalent vaccine during some seasons; this idea needs to be tested.
Several limitations of our study should be considered. As with any observational study, we cannot rule out unmeasured confounding as an explanation for our findings. In addition, the test-negative design is still comparatively new. This design may be subject to biases that are not fully understood,28–31 although it is unlikely that these biases would be differential between the live attenuated vaccine and the inactivated vaccine. Our overall study population was large, but the study lacked power to estimate vaccine effectiveness precisely in certain subgroups of interest, particularly when participants were stratified according to both age and influenza virus subtype or lineage. Strengths of this study include the prospective screening and enrollment of participants meeting predefined clinical criteria, the geographically diverse study sites, and the use of a highly specific test for influenza virus.32 In addition, our findings were robust to a wide range of assumptions regarding study eligibility, choice of variables to include in adjusted vaccine-effectiveness models, and definition of vaccination status.
During the 2015–2016 influenza season, the quadrivalent live attenuated influenza vaccine was found to be ineffective against A(H1N1)pdm09 in children, whereas there was substantial effectiveness of the inactivated influenza vaccine. Potential explanations for the live attenuated vaccine having lower effectiveness than the inactivated vaccine in this group remain unclear but may include poor replicative fitness of its A(H1N1)pdm09 strains or vaccine-virus interference.33 Although the quadrivalent live attenuated vaccine remains licensed in the United States, the ACIP did not recommend this vaccine for the 2016–2017 influenza season.
Supplementary Material
Acknowledgments
Supported by the Centers for Disease Control and Prevention through cooperative agreements with Kaiser Permanente Washington Health Research Institute (U01 IP000466), the University of Michigan (U01 IP000474), the Marshfield Clinic Research Foundation (U01 IP000471), Baylor Scott and White Health (U01 IP000473), and the University of Pittsburgh (U01 IP000467) and by grants from the National Institutes of Health (UL1 RR024153 and UL1TR000005, to the University of Pittsburgh).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–45. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 2.Belongia EA, Kieke BA, Donahue JG, et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis. 2009;199:159–67. doi: 10.1086/595861. [DOI] [PubMed] [Google Scholar]
- 3.McLean HQ, Thompson MG, Sundaram ME, et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis. 2015;211:1529–40. doi: 10.1093/infdis/jiu647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson ML, Jackson LA, Kieke B, et al. Incidence of medically attended influenza infection and cases averted by vaccination, 2011/2012 and 2012/2013 influenza seasons. Vaccine. 2015;33:5181–7. doi: 10.1016/j.vaccine.2015.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly H, Jacoby P, Dixon GA, et al. Vaccine effectiveness against laboratory-confirmed influenza in healthy young children: a case-control study. Pediatr Infect Dis J. 2011;30:107–11. doi: 10.1097/INF.0b013e318201811c. [DOI] [PubMed] [Google Scholar]
- 6.Pebody R, Andrews N, McMenamin J, et al. Vaccine effectiveness of 2011/12 trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: evidence of waning intra-seasonal protection. Euro Surveill. 2013;18:18. doi: 10.2807/ese.18.05.20389-en. [DOI] [PubMed] [Google Scholar]
- 7.Skowronski DM, Chambers C, Sabaiduc S, et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis. 2016;63:21–32. doi: 10.1093/cid/ciw176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaglani M, Pruszynski J, Murthy K, et al. Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013–2014 in the United States. J Infect Dis. 2016;213:1546–56. doi: 10.1093/infdis/jiv577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58:319–27. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmerman RK, Nowalk MP, Chung J, et al. 2014–2015 Influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis. 2016;63:1564–73. doi: 10.1093/cid/ciw635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caspard H, Gaglani M, Clipper L, et al. Effectiveness of live attenuated influenza vaccine and inactivated influenza vaccine in children 2–17 years of age in 2013–2014 in the United States. Vaccine. 2016;34:77–82. doi: 10.1016/j.vaccine.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Cost AA, Hiser MJ, Hu Z, et al. Brief report: mid-season influenza vaccine effectiveness estimates for the 2013–2014 influenza season. MSMR. 2014;21(6):15–7. [PubMed] [Google Scholar]
- 13.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356:685–96. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 14.Grohskopf LA, Olsen SJ, Sokolow LZ, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 influenza season. MMWR Morb Mortal Wkly Rep. 2015;64:818–25. doi: 10.15585/mmwr.mm6430a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou B, Donnelly ME, Scholes DT, et al. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and swine origin human influenza a viruses. J Virol. 2009;83:10309–13. doi: 10.1128/JVI.01109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flannery B, Zimmerman RK, Gubareva LV, et al. Enhanced genetic characterization of influenza A(H3N2) viruses and vaccine effectiveness by genetic group, 2014–2015. J Infect Dis. 2016;214:1010–9. doi: 10.1093/infdis/jiw181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishin VP, Baranovich T, Garten R, et al. A pyrosequencing-based approach to high-throughput identification of influenza A(H3N2) virus clades harboring antigenic drift variants. J Clin Microbiol. 2016;55:145–54. doi: 10.1128/JCM.01840-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther. 2011;9:669–83. doi: 10.1586/eri.11.51. [DOI] [PubMed] [Google Scholar]
- 20.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31:2165–8. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 21.Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine. 2013;31:3104–9. doi: 10.1016/j.vaccine.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Flannery B, Chung C. US Flu VE Network, 2015–16. Atlanta: Centers for Disease Control and Prevention; Jun 22, 2016. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents. ( https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf) [Google Scholar]
- 23.Caspard H, Belongia E, Bernatoniene J, et al. Multicenter study of the effectiveness of live attenuated influenza vaccine and inactivated influenza vaccine in children during the 2015–2016 season in the United States. Presented at the Options IX for the Control of Influenza Conference; Chicago. August 24–28, 2016. [Google Scholar]
- 24.Nohynek H, Baum U, Syrjänen R, Ikonen N, Sundman J, Jokinen J. Effectiveness of the live attenuated and the inactivated influenza vaccine in two-year-olds — a nationwide cohort study Finland, influenza season 2015/16. Euro Surveill. 2016;21:21. doi: 10.2807/1560-7917.ES.2016.21.38.30346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pebody R, Warburton F, Ellis J, et al. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Euro Surveill. 2016;21:21. doi: 10.2807/1560-7917.ES.2016.21.38.30348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep. 2016;65(RR-5):1–54. doi: 10.15585/mmwr.rr6505a1. [DOI] [PubMed] [Google Scholar]
- 27.Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR-7):1–60. [PubMed] [Google Scholar]
- 28.Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol. 2016;184:345–53. doi: 10.1093/aje/kww064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill. 2013;18:37. doi: 10.2807/1560-7917.es2013.18.37.20585. [DOI] [PubMed] [Google Scholar]
- 30.Ferdinands JM, Shay DK. Magnitude of potential biases in a simulated case-control study of the effectiveness of influenza vaccination. Clin Infect Dis. 2012;54:25–32. doi: 10.1093/cid/cir750. [DOI] [PubMed] [Google Scholar]
- 31.Sundaram ME, McClure DL, Van-Wormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis. 2013;57:789–93. doi: 10.1093/cid/cit379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson ML, Rothman KJ. Effects of imperfect test sensitivity and specificity on observational studies of influenza vaccine effectiveness. Vaccine. 2015;33:1313–6. doi: 10.1016/j.vaccine.2015.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambrose CS, Bright H, Mallory R. Potential causes of the decreased effectiveness of the influenza A(H1N1)pdm09 strain in live attenuated influenza vaccines. Euro Surveill. 2016;21:45. doi: 10.2807/1560-7917.ES.2016.21.45.30394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.