Neuroendocrine tumors can secrete hormonal peptides that lead to a condition known as carcinoid syndrome. Patients with carcinoid syndrome bear additional symptom burdens that can be alleviated but not eliminated with the use of somatostatin analogs. This article examines the cost patterns of elderly patients with neuroendocrine tumors during the first year of diagnosis, taking into account the presence of carcinoid syndrome.
Keywords: Costs; Neuroendocrine; Earcinoid; Gastrointestinal; Surveillance, Epidemiology, and End Results; Medicare
Abstract
Background.
Neuroendocrine tumors (NETs) can secrete hormonal peptides that lead to additional symptom burdens. However, it is largely unknown whether and to what extent the additional symptom burdens translate into higher costs of care. This study aimed to examine the cost pattern of elderly NET patients during the first year of diagnosis, taking into account of the carcinoid syndrome status.
Methods.
We used Surveillance, Epidemiology, and End Results Medicare data to identify elderly NET patients diagnosed between January 2003 and December 2011. Patients who had at least two claims indicative of carcinoid syndrome during the 3 months before and after the NET diagnosis were considered to have carcinoid syndrome. We adopted a payer's perspective and quantified economic outcomes using the following three measures: (a) total Medicare reimbursement amount, (b) inpatient amount, and (c) outpatient amount. We used a generalized linear model (GLM) to examine the association between syndrome and costs.
Results.
Our study cohort included 6,749 elderly NET well‐differentiated and moderately differentiated patients. Of these patients, 5,633 (83%) were alive 1 year after diagnosis with continuous enrollment, and 1,116 (17%) died within 1 year. The multivariable GLM showed significant association between the syndrome and higher total, inpatient, and outpatient costs among the group who survived the whole year; the association was insignificant among the group who died within the first year of diagnosis.
Conclusion.
This population‐based study showed that NET patients with carcinoid syndrome incurred higher costs of care especially among those who survived the first year of diagnosis.
Implications for Practice.
This is the first population‐based study that examines the health care costs associated with carcinoid syndrome among neuroendocrine tumor patients. Among patients alive throughout the first year, the unadjusted analyses showed that total median monthly costs were above $1,000 higher ($3,801 vs. $2,481) for patients with carcinoid syndrome compared with patients without. A significant association was found between carcinoid syndrome and higher total inpatient and outpatient costs among the group that survived the whole year even after controlling for clinical factors, treatment received, and demographics and neighborhood socioeconomic status; the association was insignificant among the group that died within the first year of diagnosis.
摘要
背景.神经内分泌肿瘤(NET)可分泌引发额外症状负荷的激素肽。然而, 尚不清楚额外的症状负荷是否会导致更高的医疗费用以及程度如何。本研究旨在检查NET老年患者在诊断后一年内的费用模式(将类癌综合征状态考虑在内)。
方法.研究使用了监测、流行病学和最终结果医疗保险数据来识别2003年1月至2011年12月期间被诊断患有NET的老年患者。在NET诊断前后3个月内有至少两次提示类癌综合征的索赔的患者被视为罹患类癌综合征。我们采纳了一名支付人的观点并使用以下三个指标对经济结果进行了量化:(a)医疗保险总报销金额, (b)住院费用以及(c)门诊费用。研究使用广义线性模型(GLM)检查了综合征与费用之间的相关性。
结果.研究队列包括6 749名NET高分化型和中度分化型老年患者。其中, 5 633名(83%)在诊断后一年存活内并持续入组, 1 116名(17%)在诊断后一年内死亡。多变量GLM显示在诊断后一年内存活的患者组中, 综合征与总费用、住院费用以及门诊费用较高显著相关;诊断后一年内死亡的患者组中此相关性不显著。
结论.本项基于人群的研究显示, 罹患类癌综合征的NET患者的医疗费用较高, 尤其是诊断后一年内存活的患者。
对临床实践的提示:这是首项基于人群的检查神经内分泌肿瘤患者中与类癌综合征相关的医疗费用的研究。未经校正的分析显示, 在诊断后一年内存活的患者中, 罹患类癌综合征患者的总中位月费用比无类癌综合征的患者高1 000美元(3 801美元 vs. 2 481美元)。研究发现, 即使对临床因素、接受的治疗、人口统计学以及邻近的社会经济状态进行了控制, 诊断后一年内存活的患者组中类癌综合征与总住院费用和门诊费用较高仍显著相关;但诊断后一年内死亡的患者组中相关性不明显。
Introduction
Neuroendocrine tumors (NETs) start from the hormone‐producing cells of the human body's neuroendocrine system. They can be found throughout the body and are usually categorized based on either their anatomic location or their embryonic origin. The incidence of NETs has been steadily increasing. The annual age‐adjusted incidence of NETs increased more than fivefold from 1973 (1.09/100,000) to 2004 (5.25/100,000) [1]. A more recent study [2], [3] showed that the incidence is continuing to increase, possibly due to advances in diagnostic techniques such as computerized tomography and endoscopy and higher awareness among clinicians. Meanwhile, the prevalence is increasing even more substantially because the survival of NET patients has improved significantly over time. Such better survival rates of NET patients may have resulted from more patients being diagnosed at an early stage and lower grade, and also from evolution of systemic therapies for distant stage patients.
NETs can secrete hormonal peptides that lead to a condition known as carcinoid syndrome [4]. Patients with carcinoid syndrome bear additional symptom burdens such as flushing, diarrhea, wheezing, and fibrotic valvular heart disease [5]. The current recommended treatment for hormone secretion and associated symptoms involves the use of somatostatin analogues, which can reduce, but not eliminate, the symptom burden among the majority of patients [6], [7], [8]. Given the increasing survival and prevalence of NETs, a large number of patients suffer the symptom burden of carcinoid syndrome. However, it is largely unknown whether and to what extent the additional symptom burdens translate into higher costs of care. This study aims to examine the cost pattern of elderly NET patients during the first year of diagnosis, taking into account the presence of carcinoid syndrome.
Materials and Methods
Data Source
We used the Surveillance, Epidemiology, and End Results (SEER) registry data linked with Medicare claims for this study. The SEER program of the National Cancer Institute collects incidence and survival data on all cancers in 18 selected geographic areas and covered approximately 30% of the U.S. population [9]. The SEER registry data includes information on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow‐up for vital status. Offering linkage to Medicare claims data, the SEER‐Medicare data provides additional information on enrollment to Medicare plans, the utilization of health care resources, comorbidities, and other patient information such as neighborhood socioeconomic status (SES). The SEER‐Medicare database is a widely used data source for the study of health care resource utilizations in oncology and is considered to be representative of the U.S. elderly population [10].
Study Cohort
We first selected patients diagnosed with NETs between January 1, 2003, and December 31, 2011, based on the International Classification of Diseases for Oncology, 3rd Edition (ICD‐O‐3) codes: 8240, 8241, 8242, 8243, 8244, 8245, 8246, and 8249 (gastrointestinal and pulmonary carcinoids and neuroendocrine carcinomas). Then we excluded patients with pancreas as their primary tumor site because while pancreatic NETs may secrete hormones that cause secretory syndromes, they may not produce the typical carcinoid syndrome. We also excluded patients with stage in situ and patients less than 65 years old at the time of diagnosis. Further, we required that the patients have continuous Medicare Parts A and B enrollment and no Health Management Organization (HMO) enrollment during the 12 months before and the 12 months after the NET diagnosis to ensure the completeness of medical claims used to capture patient comorbidities and utilization of health care services, respectively, because when patients are enrolled in HMOs, we do not have detailed claims information to capture their utilization of health care services. Patients with poorly differentiated neuroendocrine carcinomas and neuroendocrine neoplasms of unspecified grade were excluded to provide a homogeneous population of patients. The final study cohort included 6,749 patients with well‐differentiated and moderately differentiated tumors. Supplemental online Fig. 1 is a detailed flowchart for the inclusion and exclusion criteria of our study cohort.
Costs of Care
We adopted a payer's perspective and examined costs of care based on Medicare payment amount. We examined three types of costs: (a) total costs, (b) inpatients costs, and (c) outpatient costs. We studied the average monthly Medicare payment amount normalized to the first half of 2016 dollars based on the medical care services consumer price index [11].
Key Explanatory Variable: Presence of Carcinoid Syndrome
We identified patients with carcinoid syndrome based on the following International Classification of Disease 9th Revision (ICD‐9) codes: flushing (782.62), diarrhea (564.5, 787.91), and carcinoid syndrome (259.2). As in prior analyses [12], [13], patients who had at least two claims with any of the above mentioned ICD‐9 codes during a 6 month time window between 3 months before and 3 months after the NET diagnosis were considered to have carcinoid syndrome.
Other Explanatory Variables
We included clinical factors, treatment received, patient demographics, comorbidities, and neighborhood SES in our analyses. The clinical factors included stage of the tumor (local/regional, distant, and unknown), histological grade (well‐differentiated, moderately differentiated), tumor site (larynx, bronchus, lung, trachea and other respiratory organs; cecum and appendix; colon; small intestine; and all others). We included the following four indicators for treatment received: (a) chemotherapy (yes/no), (b) radiotherapy (yes/no), (c) resection of primary tumors (yes/no), and (d) resection of liver metastasis (yes/no). Patient demographics included age (65–69, 70–74, 75–79, ≥80), gender (male, female), race/ethnicity (non‐Hispanic white, non‐Hispanic black, Hispanics or all others), region (Northeast, West, Midwest, South), and urban/rural status (metropolitan vs. nonmetropolitan). We used the Deyo‐Romano modified Charlson comorbidity score [14], [15], [16] categorized into four groups: (a) zero, (b) one, (c) two, and (d) at least three. We also considered neighborhood SES based on three measures in terms of the following quartiles: (a) percent with at least high school education, (b) median household income, and (c) percent living in poverty.
Statistical Analysis
We provided the descriptive statistics for the study sample by whether the patients survived throughout the first year of diagnosis. We conducted separate analyses for patients who were alive throughout the year after diagnosis and patients who died within a year because these patients have very different cost patterns due to the high cost of terminal care. We compared the group difference by the presence of carcinoid syndrome using chi‐square statistics. For comparison of costs, we used the Wilcoxon‐Mann‐Whitney test; for the occurrence of emergency room (ER) admissions and hospitalizations, we used the chi‐square test.
We used a generalized linear model (GLM) with log link function and gamma distribution for the multivariable regression analyses of costs. The estimated impact in term of dollars, the 95% confidence interval, and p values are presented. As explained above, we separately analyzed the cost pattern of patients who survived the whole first year after diagnosis and patients who died within a year.
To examine the time trend of incurred cost during the year, we provided figures showing the total, inpatient, and outpatient costs incurred in each month after diagnosis for the following five groups of NET patients: (a) patients who were alive throughout the whole year, (b) patients who died within 3 months, (c) patients who died within 6 months, (d) patients who died within 9 months, and (e) patients who died within 12 months.
All statistical analyses were conducted in SAS Enterprise Guide 6.1 (analytical software; SAS Institute, Cary, NC, http://www.sas.com). The Institutional Review Board at The University of Texas MD Anderson Cancer Center exempted this study for approval because all patients in the database had been deidentified.
Results
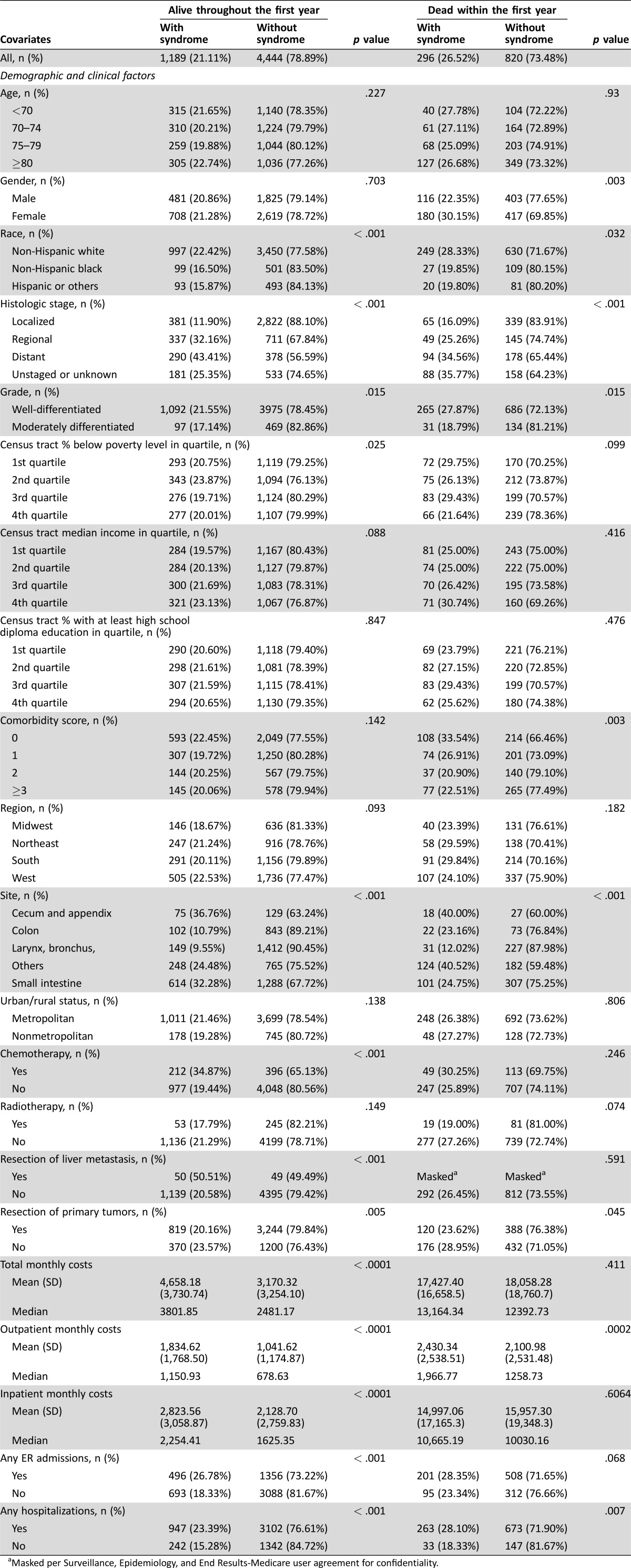
Table 1 provides the descriptive statistics of the study sample by vital status and whether carcinoid syndrome was present. There were 5,633 patients who were alive throughout the first year and 1,116 patients who died within the first year of diagnosis. Among the patients that survived the whole first year after diagnosis, 1,189 (21%) had carcinoid syndrome; there were group differences in terms of the presence of carcinoid syndrome by gender; race; histologic stage; grade; primary tumor site; and treatment received, including chemotherapy, radiotherapy, and resection of liver metastasis. Among the group who died within the first year, 296 (26.5%) had carcinoid syndrome. In this group, we found differences in the proportion with carcinoid syndrome by gender, comorbidities, histologic stage, grade, primary tumor site, radiotherapy, and resection of primary tumors.
Table 1. Description of the study sample by vital status and whether carcinoid syndrome was observed.
Masked per Surveillance, Epidemiology, and End Results‐Medicare user agreement for confidentiality.
More importantly, Table 1 shows that there are significant differences in terms of costs for patients with and without carcinoid syndrome. Among the cohort who survived the whole first year after diagnosis, the patients with carcinoid syndrome had a median monthly total cost of $3,802, which is significantly higher (p < .0001) than $2,481 for patients without syndrome. Both outpatient and inpatient median monthly costs were also significantly higher (p < .0001) for patients with carcinoid syndrome compared with those without syndrome ($1,151 vs. $679 and $2,254 vs. $1,625, respectively). Similarly, among patients who died within the first year, the presence of carcinoid syndrome was associated with higher costs. Patients who died within the year had much higher monthly costs than patients who survived the first year as expected. We also found that among patients who survived the first year, those with the syndrome were significantly (p < .001) more likely to have ER admissions and hospitalization; among patients who died within the first year, the association remained significant (p = .007) for hospitalizations but was insignificant (p = .068) for ER admissions.
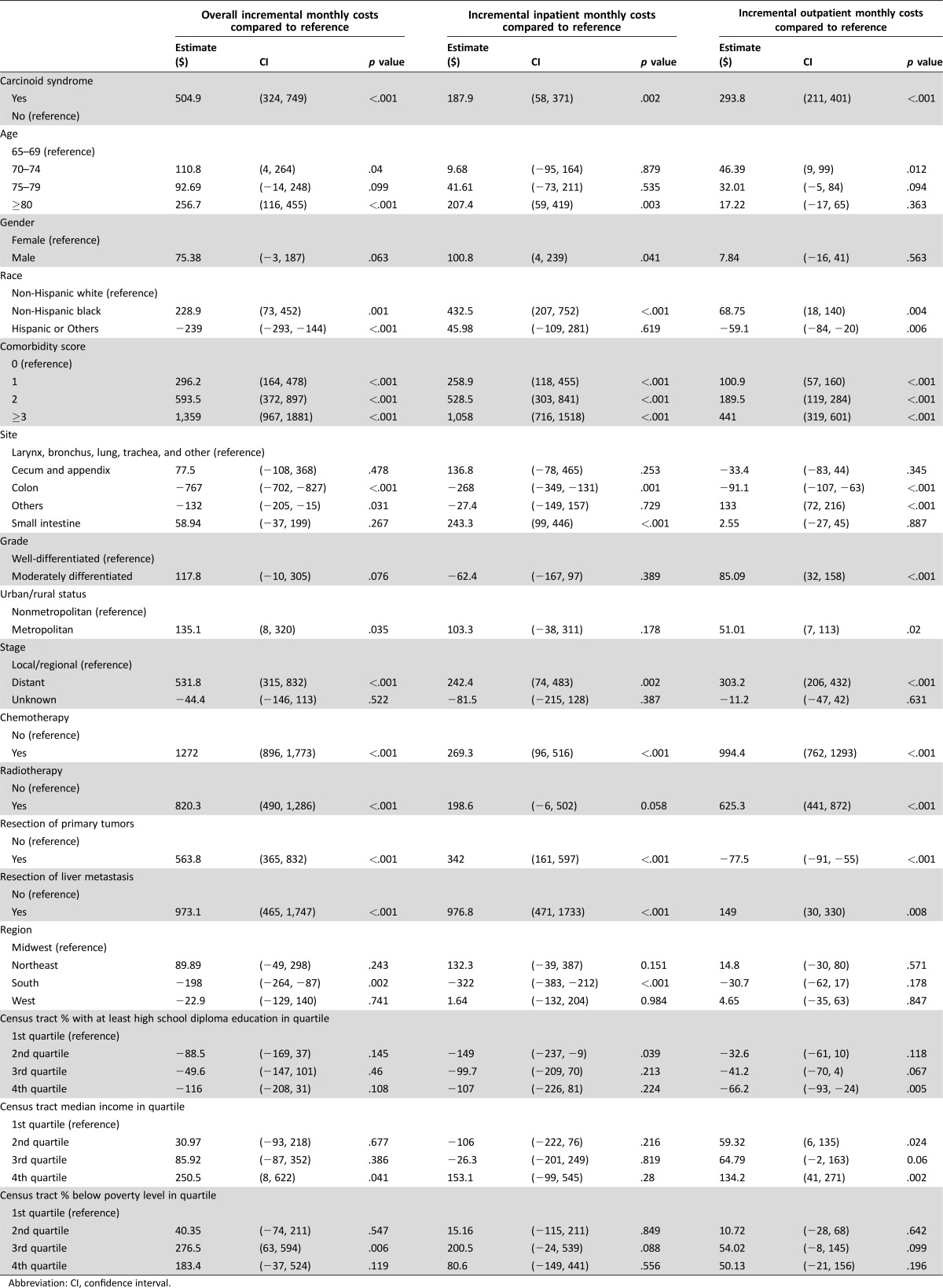
The results from the multivariable GLM analyses are presented in Table 2 and Table 3 for the cohort alive throughout the first year and the cohort who died within the first year, respectively. Among patients alive throughout the first year of diagnosis, carcinoid syndrome was associated with significantly higher total costs ($505), higher inpatient costs ($188), and higher outpatient costs ($294), with p values less than .005 even after controlling for clinical factors, treatment received, patient demographics, comorbidities, and neighborhood SES. The analyses also showed that the comorbidities had a large impact on the health care costs. Patients who had a comorbidity score above two were estimated to incur $594 more total monthly costs compared with patients who had zero comorbidity. Patients with distant stage NET had higher overall, inpatient, and outpatient costs than patients who had local or regional stage disease ($532, p < .001; $242, p = .002; and $303, p < .001, respectively).
Table 2. Multivariate Regression Analysis for the cohort alive throughout the first year.
Abbreviation: CI, confidence interval.
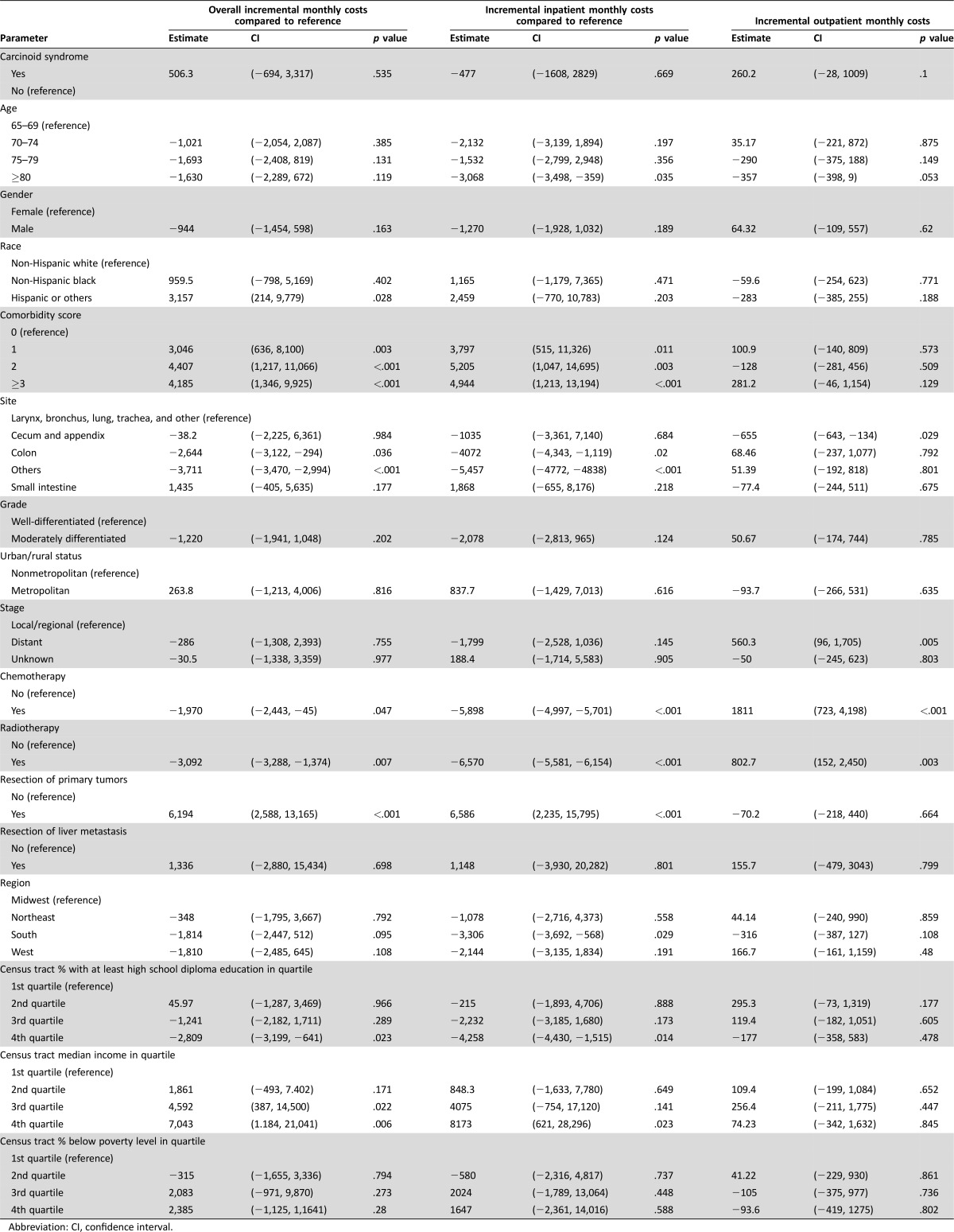
Table 3. Multivariate Regression Analysis for the cohort that died within the first year.
Abbreviation: CI, confidence interval.
Among the group who died within the first year of diagnosis, the association between carcinoid syndrome and costs were much less prominent. The associations for total, inpatient, and outpatient monthly costs were no longer significant at p values of 0.535, 0.669, and 0.1, respectively.
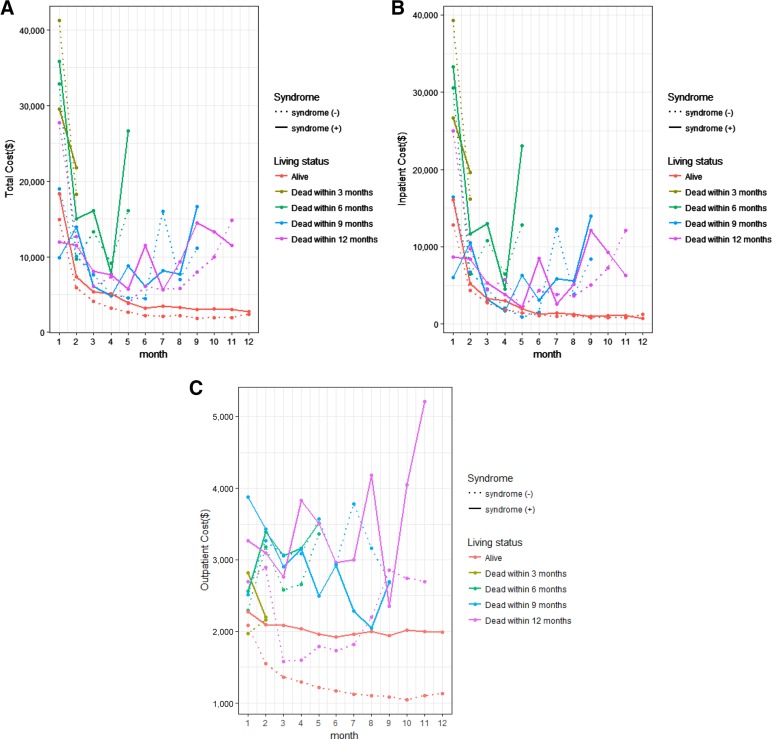
Figure 1A–1C depict the time trend pattern of monthly costs during the first year of diagnosis for total, inpatient, and outpatient costs. Figure 1A shows that all patients incurred high costs in the initial months; patients who survived the whole year had much lower costs after the initial time period, while patients who died within the first year incurred high costs towards the end of life and showed a classic U‐shape with high costs during initial and terminal phases. Figure 1A also shows that patients with carcinoid syndrome had higher costs than patients without. Figure 1B showed a similar time trend for inpatient costs. Patient who survived the whole year had a downward trend in inpatient costs, while patients who died had a U‐shaped curve. The difference between patients with and without syndrome was not as obvious as in Figure 1A. Figure 1C shows the pattern for outpatient costs. It is interesting that among patients alive throughout the first year, those without the syndrome had a substantial drop in outpatient costs, while patients with the syndrome maintained a stable outpatient cost during the year. Figure 1C also showed that the outpatient costs for patients who died within the year did not show the U‐shape observed in total and inpatient costs.
Figure 1.
(A): Total costs by month after diagnosis. (B): Inpatient costs by month after diagnosis. (C): Outpatient costs by month after diagnosis.
Abbreviations: syndrome, carcinoid syndrome.
Discussion
This study is the first one in the literature that examines the health care costs associated with carcinoid syndrome among NET patients. We compared the overall, inpatient, and outpatient costs between patients with and without the syndrome from the payer's perspective. We separately analyzed patients who survived the first year of diagnosis and patients who died within the first year. Among patients alive throughout the first year, the unadjusted analyses showed that total median monthly costs were above $1,000 higher ($3,801 vs. $2,481) for patients with the syndrome compared with patients without, with approximately half of the difference each coming from inpatient and outpatient costs. We found that carcinoid syndrome was significantly associated with higher health care costs including overall, inpatient and outpatient costs even after controlling for clinical factors, treatment received, demographics, and neighborhood SES among patients alive throughout the first year. We found that patients with a comorbidity score above two incurred much higher costs compared to patients who had no comorbidities. This finding is consistent with the literature on the impact of comorbidities on health care costs among cancer patients. For example, one study on newly diagnosed cancer patients found that patients with four or more comorbid conditions were much more likely (OR = 2.5, 95% CI: 2.26 to 2.76) to be in the top 10% in terms of health spending [17]. Another study estimated that the increase in cancer treatment cost associated with the chronic conditions during the first 6 months after cancer diagnosis ranged from $4,385 for cardiac disease to $11,009 for mental health disorders [18]. Our estimates of increase in health care costs per month ranging from $296 to $1,359 depending on the number of comorbidities is in line with the above estimates and confirms the large impact of comorbidities on health care costs in the NET population.
As patients with the syndrome were much more likely to receive somatostatin analog, which could be a major source of higher cost, we further examined the overall, inpatient, and outpatient costs, excluding somatostatin analog related claims. We identified claims related to somatostatin analog usage based on Healthcare Common Procedure Coding System codes (J‐2353, J‐2352, J‐1930) and National Drug Code codes (00078034061, 00078034084, 00078034161, 00078034184, 00078034261, 00078034284, 15054006001, 15054009001, 15054012001, 15054012002). The detailed results are presented in Table 4. We found that the median overall monthly cost of patients with the syndrome was reduced by about $600 a month after excluding somatostatin analog‐related costs with most of the difference originating from outpatient costs, while the median costs for patients without the syndrome did not change much after the exclusion. After excluding somatostatin analog‐related costs, the difference in outpatient costs was much smaller at around $200. However, patients with the syndrome still had higher inpatient costs than patients without the syndrome. It suggests that patients with the syndrome have a higher disease burden even after controlling for the use of symptom control drugs, or that initial therapy involves more frequent use of surgery and/or selective internal radiotherapy in the inpatient setting to reduce symptom burden in patients with carcinoid syndrome. This is in line with our finding in Table 1 that patients with the syndrome were much more likely to have ER admission and hospitalizations. This could be related to delayed initiation of somatostatin analogue therapy after diagnosis. Secondly, even in patients initiated on somatostatin analogue therapy, they could be on subtherapeutic doses. In fact, we have previously shown that the majority of elderly patients with carcinoid syndrome do not have somatostatin analogue therapy initiated within the first 6 months after diagnosis [19], and even in those who received somatostatin analogue therapy, 36% received dosages lower than the recommended 20 mg per 28 days [20]. Further, data from clinical studies of octreotide showed that patients who received appropriate therapy may have improved but still residual diarrhea [8]. Finally, these inpatient admissions and ER visits could be related to other complications of carcinoid syndrome, such as abdominal cramping or carcinoid heart disease.
Table 4. Comparison of the non‐somatostatin‐analogue‐related costs between patients with or without syndrome in the cohort alive throughout the first year.
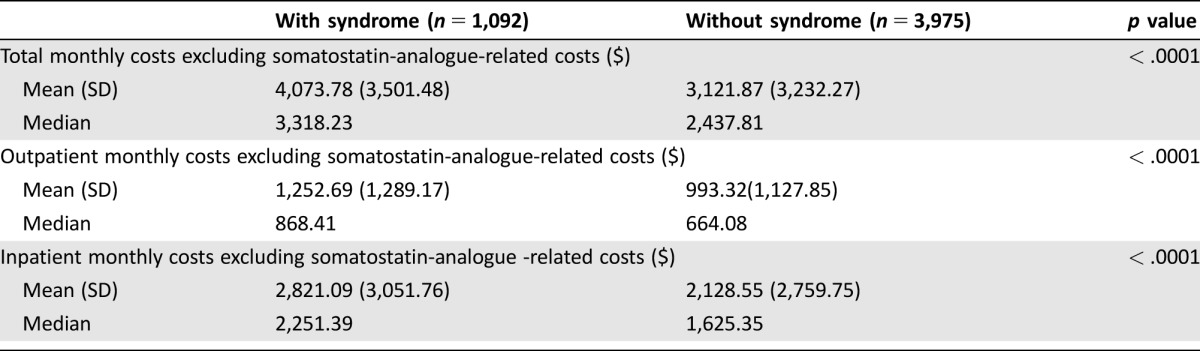
Patients who died within the first year incurred much higher costs, with total monthly costs above $15,000 regardless of carcinoid syndrome presence. Among this group, after adjusting for clinical factors, treatment received, demographics, and neighborhood SES, the significant association between carcinoid syndrome and costs vanished. This is probably due to the overwhelmingly higher cost of terminal care, which is often accompanied by more frequent hospital admissions than earlier phases of disease.
Additionally, we showed the time pattern of health care costs incurred during the first year. All NET patients incurred very high costs during the initial months, when a substantial amount of diagnostic testing and possible interventions (such as surgeries) occur. The total cost in the first month was above $10,000 for almost all patients and was above $30,000 for some groups. Patients who survived had a clear downward trend in total costs, stabilizing around $3,000 in later months, while patients who died had the typical U‐shaped curve showing high terminal care costs. The figures also showed that the high terminal care was mostly originated from high inpatient costs, while outpatient costs were much lower. One interesting observation is that among patients who survived the whole year, outpatient costs dropped significantly towards the later months for those who did not have carcinoid syndrome, while outpatient costs remained high during the whole year for those who had carcinoid syndrome. This is probably because some patients who did not have syndrome might have been on active surveillance without much need for office visits, while patients with the syndrome visited the physician's office regularly for somatostatin analogues treatment to control the symptoms. Additionally, since carcinoid syndrome is generally associated with more advanced stages of disease, it is possible that patients with carcinoid syndrome were seen more often due to complications of more advanced disease.
Our study is based on SEER‐Medicare data and, therefore, has the limitations common among observational studies. First, the presence of carcinoid syndrome was derived from Medicare claims. It is possible that there might be miscoding and inaccuracies in the claims. Second, in this study we focused on the health care costs of NET patients during the first year after diagnosis, for which we have sufficient complete data. In examining the health care cost time pattern for patients who survived the full year and patients who died within the year, we found that patients who survived the first year had relatively stable costs during the second half of the year, while patients who died showed high terminal care costs. Therefore, we expect that patients who survived for more than 1 year will continue to have reasonably stable health care costs at a level close to the end of the first year until they reach their terminal phase when the costs rise up again. Last, we focused on elderly patients because the data source used was SEER‐Medicare and the Medicare program mainly covers patients above 65 years old. This may limit the generalizability of this study to younger groups who are more likely to have private insurance, possibly of greatest relevance in thymic and appendiceal NETs, which have younger median ages of diagnosis. Nevertheless, as Medicare is the single largest payer for health care in the U.S. and Medicare reimbursement rate is widely accepted as a benchmark in the industry, studying the health care costs from the perspective of Medicare is common and widely accepted in the literature [21].
Of particular note, the temporal pattern of resource utilization is different for patients with carcinoid syndrome as compared with that of patients with nonfunctional tumors. Therefore, it is less likely that patients with carcinoid syndrome merely represent a more advanced stage of the same disease, and, rather, suggests that carcinoid syndrome is an independent predictor of health care costs. An economic argument for improved carcinoid syndrome control is therefore feasible, depending upon the cost of any intervention to further reduce symptom burden and the resultant health care costs.
Conclusion
This study demonstrated and quantified for the first time in the literature the additional costs associated with carcinoid syndrome in elderly NET patients during the first year of diagnosis. Such information may help stakeholders in the health care industry to efficiently allocate resources for NET patients.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This study used the linked the Surveillance, Epidemiology, and End Results (SEER) Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. We acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the SEER Program tumor registries in the creation of the SEER‐Medicare database. This work was supported in part by Ipsen. The funder sponsored the purchase of Surveillance, Epidemiology, and End Results Medicare data and provided funding for analytical support. All authors had unrestricted access to the final study data on request, were responsible for data interpretation, manuscript preparation, and the decision to submit for publication, and attest to the completeness and accuracy of the data and statistical analysis.
Author Contributions
Conception/design: Chan Shen, Daniel M. Halperin, Arvind Dasari, Shouhao Zhou, James C. Yao, Ya‐Chen Tina Shih
Collection and/or assembly of data: Chan Shen, Yiyi Chu, Shouhao Zhou
Data analysis and interpretation: Chan Shen, Yiyi Chu, Daniel M. Halperin, Arvind Dasari, Shouhao Zhou, Ying Xu, James C. Yao, Ya‐Chen Tina Shih
Manuscript writing: Chan Shen, Yiyi Chu, Daniel M. Halperin, Arvind Dasari, Shouhao Zhou, Ying Xu, James C. Yao, Ya‐Chen Tina Shih
Final approval of manuscript: Chan Shen, Yiyi Chu, Daniel M. Halperin, Arvind Dasari, Shouhao Zhou, Ying Xu, James C. Yao, Ya‐Chen Tina Shih
Disclosures
Daniel Halperin: Novartis (C/A, RF), Ipsen, Dicerna, Genentech/Roche (RF), Oxigene (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Yao JC, Hassan M, Phan A et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 2. Shen C, Dasari A, Zhao B et al. Incidence and prevalence of neuroendocrine tumors in the United States 1973–2012. Paper presented at: NANETS Symposium 2016; Jackson, Wyoming.
- 3. Dasari A, Shen C, Halperin D et al. Survival trends of neuroendocrine tumors and associated prognostic factors. Paper presented at: NANETS Symposium 2016; Jackson, Wyoming.
- 4. Beaumont JL, Cella D, Phan AT et al. Comparison of health‐related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas 2012;41:461–466. [DOI] [PubMed] [Google Scholar]
- 5. Davis Z, Moertel CG, McIlrath DC. The malignant carcinoid syndrome. Surg Gynecol Obstet 1973;137:637–644. [PubMed] [Google Scholar]
- 6. Kvols LK, Martin JK, Marsh HM et al. Rapid reversal of carcinoid crisis with a somatostatin analogue. N Engl J Med 1985;313:1229–1230. [DOI] [PubMed] [Google Scholar]
- 7. Kvols LK, Moertel CG, O'Connell MJ et al. Treatment of the malignant carcinoid syndrome. Evaluation of a long‐acting somatostatin analogue. N Engl J Med 1986;315:663–666. [DOI] [PubMed] [Google Scholar]
- 8. Rubin J, Ajani J, Schirmer W et al. Octreotide acetate long‐acting formulation versus open‐label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol 1999;17:600–666. [DOI] [PubMed] [Google Scholar]
- 9.Overview of the SEER Program. Available at http://seer.cancer.gov/about/overview.html. Accessed May 19, 2017.
- 10. Warren JL, Klabunde CN, Schrag D et al. Overview of the SEER‐Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care 2002;40(suppl 8):IV3–IV18. [DOI] [PubMed] [Google Scholar]
- 11.Databases, Tables & Calculators by Subject. Available at http://www.bls.gov/data/. Accessed May 19, 2017.
- 12. Shen C, Shih YC, Xu Y et al. Octreotide long‐acting repeatable among elderly patients with neuroendocrine tumors: A survival analysis of SEER‐Medicare data. Cancer Epidemiol Biomarkers Prev 2015;24:1656–1665. [DOI] [PubMed] [Google Scholar]
- 13. Halperin DM, Shen C, Dasari A et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: A population‐based study. Lancet Oncol 2017;18:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 15. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 16. Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: Differing perspectives. J Clin Epidemiol 1993;46:1075–1079. [DOI] [PubMed] [Google Scholar]
- 17. Wodchis W, Arthurs E, Khan A et al. Cost trajectories for cancer patients. Curr Oncol 2016;23(suppl 1):S64–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Subramanian S, Tangka FK, Sabatino SA et al. Impact of chronic conditions on the cost of cancer care for Medicaid beneficiaries. Medicare Medicaid Res Rev: 2013;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen C, Shih YC, Xu Y et al. Octreotide long‐acting repeatable use among elderly patients with carcinoid syndrome and survival outcomes: A population‐based analysis. Cancer 2014;120:2039–2049. [DOI] [PubMed] [Google Scholar]
- 20. Shen C, Xu Y, Dasari A et al. Octreotide LAR dosage and survival among elderly patients with distant‐stage neuroendocrine tumors. The Oncologist 2016;21:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riley GF. Administrative and claims records as sources of health care cost data. Med Care 2009;47:S51–S55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.