Surgical resection is the primary treatment option for retroperitoneal liposarcomas, but complete resection is difficult and postoperative recurrence and metastasis are common. This prospective study analyzed the feasibility and efficacy of microwave ablation combined with iodine‐125 seed implantation in the local control of recurrent retroperitoneal liposarcomas.
Keywords: Recurrent retroperitoneal liposarcomas, Microwave ablation, Iodine‐125 radioactive seed, Brachytherapy, Local control
Abstract
Introduction.
The objective of the present study was to evaluate the feasibility, safety, and short‐term efficacy of microwave ablation (MWA) combined with iodine‐125 (125I) seed implantation in recurrent retroperitoneal liposarcomas (rRPLs).
Materials and Methods.
From September 2012 to March 2015, 11 patients were enrolled in this prospective study. Eleven tumors (median, 9 cm; range, 5.5–12.5 cm) were treated with computerized tomography‐guided MWA for 11 sessions and 125I seed implantation for 18 sessions. 125I seed implantation was performed 4 weeks after MWA.
Results.
There were no procedure‐related deaths. Post‐MWA pain (grade ≥2) was the most common complication (6 of 11 patients, 54.5%), and fever (grade ≥2) was observed in two patients. Reversible nerve injury, defined as transient limb paresthesia or leg weakness, was observed in one patient. There were fewer complications associated with the 125I seed implantation procedure compared with the MWA procedure. All 11 patients who underwent the MWA procedure achieved a partial response (PR), according to the modified Response Evaluation Criteria in Solid Tumors, 1 month post‐ablation; after 125I seed implantation was performed, a complete response was observed in three, five, and six target tumors in 3, 6, and 12 months, respectively.
Conclusion.
In selected patients with rRPLs, MWA combined with 125I seed implantation is feasible and safe with favorable local control efficacy.
Implications for Practice.
This study evaluated the feasibility, safety, and short‐term efficacy of microwave ablation (MWA) combined with iodine‐125 (125I) seed implantation in recurrent retroperitoneal liposarcomas (rRPLs). Results suggest that a single session of MWA may be not sufficient in large‐volume rRPLs and that as a supplement treatment, 125I seed implantation is safe and easy accessible. MWA combined with 125I seed has excellent local control effectiveness, and long‐term efficacy and survival benefit still need to be more comprehensively evaluated.
摘要
引言.当前研究的目的是评估微波消融(MWA)联合碘‐125(125I)粒子植入用于复发性腹膜后脂肪肉瘤(rRPL)的可行性、安全性和短期疗效
材料和方法.2012年9月到2015年3月期间, 11名患者入组这项前瞻性研究。用计算机断层扫描引导的MWA对11个肿瘤(中位直径为9cm;范围, 5.5‐12.5cm)进行了11次治疗和18次125I粒子植入。在MWA后4周进行了125I粒子植入
结果.未出现与手术有关的死亡事件。MWA术后疼痛(≥2级)是最常见的并发症(11名患者中有6名出现, 54.5%), 2名患者出现发热症状(≥2级)。在1名患者中观察到可逆性神经损伤, 该症状定义为一过性肢体感觉异常或腿无力。与MWA术相比, 与125I粒子植入术相关的并发症较少。根据改良版实体瘤缓解评估标准, 消融后1个月, 11名接受MWA术的患者均实现了部分缓解(PR)。植入125I粒子后第3、6和12个月内分别观察到3个、5个和6个目标肿瘤完全缓解
结论.在选定rRPL患者中, MWA联合125I粒子植入是可行和安全的, 而且具有良好的局部控制效果。
对临床实践的提示:本研究评估了MWA联合125I粒子植入用于rRPL的可行性、安全性和短期疗效。结果表明, 单次MWA对于大体积rRPL可能不充分, 而125I粒子植入作为补充治疗安全且易于进行。MWA联合125I粒子具有良好的局部控制效果, 但其长期疗效和生存获益仍需进行进一步全面评估
Introduction
Surgical resection is the primary treatment option for retroperitoneal liposarcomas (RPLs) [1]. Complete resection is difficult, and postoperative recurrence and metastasis are common [2]. Moreover, recurrent RPLs have been reported to exhibit a higher degree of malignancy and a stronger invasion ability; therefore, repeated complete surgical resection becomes more difficult [1]. Despite these characteristics, effective local therapy can reduce the symptoms of these tumors by up to 75% [3]. The purpose of local therapy in this situation is not to completely excise the tumor, but to alleviate symptoms, relieve organ obstruction caused by tumor compression, and maintain organ function. Local ablative therapies, such as radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation, are established modalities in the palliative treatment of solid tumors [4], [5], [6]. In actual application, boundary residue is often inevitable in the retroperitoneum area due to the tumor size and close proximity to vital organs [7]. There is no universal agreement or reported study that partial resection can prolong survival and provide palliation in selected patients with retroperitoneal liposarcomas; this is also true for other local therapies, such as energy ablation [2]. Achieving a complete response (CR) is important when local therapy is performed in selected patients. Iodine‐125 (125I) radioactive seed implantation has been shown to be efficient in treating many types of malignant tumors [8], [9], especially in complex situations [10], [11]. This study focused on whether 125I seed implantation after MWA could improve local control in selected patients with rRPLs.
The aim of this prospective study was to analyze the feasibility and efficacy of MWA combined with 125I seed implantation in the local control of rRPLs. To the best of our knowledge, the present study is the first one to evaluate the feasibility, safety, and efficacy of MWA combined with 125I seed implantation in patients with rRPLs.
Materials and Methods
Ethics
This prospective study was performed in accordance with the Declaration of Helsinki of the World Medical Association. It was approved by the Sun Yat‐sen University Cancer Center Institutional Review Board, and eligible patients provided written, informed consent.
Patient Characteristics
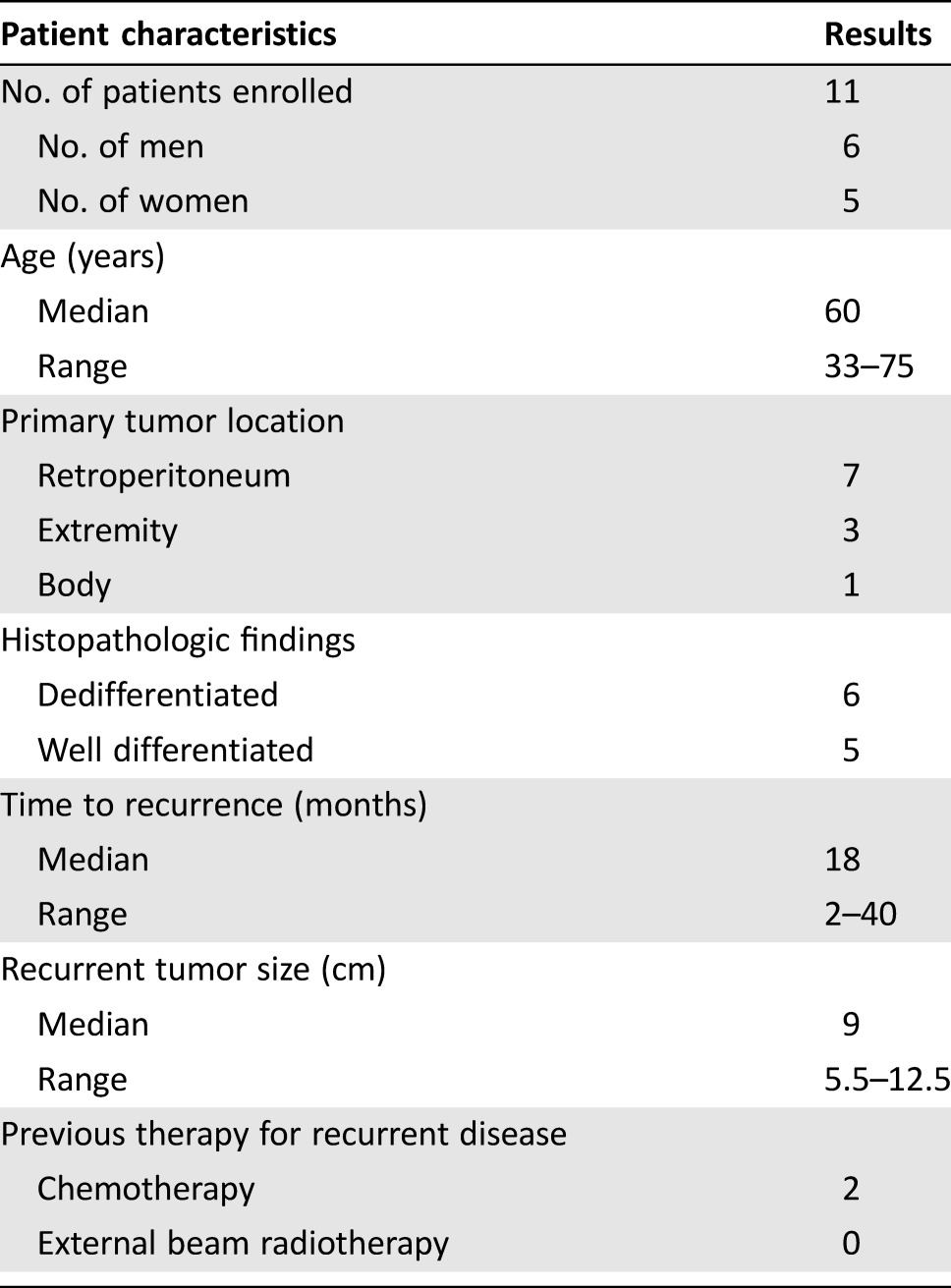
From September 2012 to March 2015, 11 patients (median age 60 years, range 33–75 years), including six male and five female patients, with solitary rRPLs were enrolled in this study and treated with computerized tomography (CT)‐guided MWA combined with 125I seed implantation at the Cancer Center of Sun Yat‐sen University. The median size of these tumors was 9 cm (range, 5.5–12.5 cm) at the largest diameter. The time from surgery to recurrence ranged from 2 to 40 months (median, 18 months). Dedifferentiated (DD) type and well‐differentiated (WD) types were identified in six and five samples, respectively, according to histological findings (Table 1). The patients enrolled in this study met the following criteria: (a) Eastern Cooperative Oncology Group performance status of 1 or lower, (b) histologically confirmed liposarcoma, (c) treated with surgical resection at least one time, (d) only one recurrent tumor located in the retroperitoneum, and (e) not suitable for repeated resection or refused repeated surgical resection. Patients with unstable cardiorespiratory function, severe hypocoagulability, and active infection were excluded from this study.
Table 1. Characteristics of patients with recurrent retroperitoneal liposarcomas treated with combination therapy.
Technical Procedures
Microwave Ablation Procedure.
MWA procedures were performed by experienced interventional radiologists, and intravenous conscious sedation (remifentanil 0.5–1 µg/kg) was used during the procedure. All procedures were guided by CT scan images (Brilliance; Philips Healthcare, The Netherlands, http://www.usa.philips.com/healthcare; Fig. 1) obtained with the following parameters: voltage, 120 kV; 200 mA per section; section thickness, 5 mm; rotation time, 0.75 seconds; planned CT dose index, 10.0 mGy. Eleven tumors in total were treated. The equipment used in the MWA procedure consisted of a commercially available system (FORSEA; Qinghai Microwave Electronic Institute, Nanjing, China, http://www.visonmedicalusa.com/), coupled to a 16‐gauge cooled‐shaft coagulation antenna (Qinghai Microwave Electronic Institute, Nanjing, China, http://www.visonmedicalusa.com/). The power was generally set at 60 or 70 W. The median ablation time was 10 minutes. All of the 11 tumors were larger than 5 cm; therefore, ablation was performed with multiple antennas. CT scanning was performed again to evaluate the immediate necrotic conditions, defined as tumor density reduction after ablation, and to determine the presence of any complications, such as bleeding.
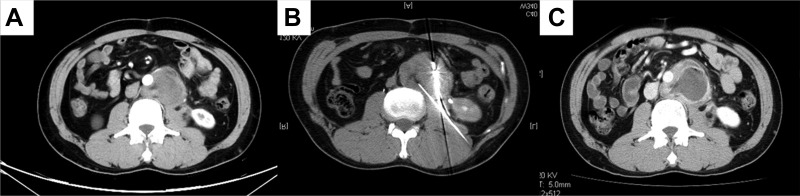
Figure 1.
Microwave ablation (MWA) procedure and outcome. A 54‐year‐old male with recurrent retroperitoneal liposarcoma (dedifferentiated type) treated with MWA combined with iodine‐125 seed implantation. (A): Recurrent liposarcoma located in the retroperitoneum; computerized tomography scan with enhancement. (B): Procedure of MWA, high‐density images indicate the ablation antennas. (C): One month after MWA, necrosis appeared in the central portion of the tumor, and enhancement was observed in the surrounding area.
125I Seed Implantation and Dose Verification.
The radioactivity of the 125I seeds (CIAE‐6711; Chinese Atomic Science Institution, Beijing, China, http://www.ciae.ac.cn/eng/CIAE/index.htm) used in this study was 2.59 × 107 Bq. Before implantation, a treatment plan (TP) was generated for each patient using a computerized treatment planning system (TPS; RT‐RSI, Beijing Atom and High Technique Industries, Inc., Beijing, China, http://www.atom-hitech.com/) to determine the number of seeds to be implanted and the best implantation location (Fig. 2A and 2B). One radiation physicist and two radiologists generated the TP. Patients underwent a detailed tumor volume study using enhanced CT scans performed 4 weeks post‐MWA. Careful delineations of the residual tumor volume, the planning target volume, and surrounding vital organs (e.g., the bowel) were performed for every CT section. The planning target volume was defined as 0.5 cm of expansion external to the gross tumor volume. The prescribed dose was 120 Gy, which was chosen based on our previous studies [12], [13]. All 125I seed implantations were guided by CT scan (Brilliance; Philips Healthcare, The Netherlands) with the same CT scanning parameters described in the MWA procedure. Local infiltration anesthesia (1% lidocaine; Yimin, Yichang, China) was induced before puncture, and then 18‐gauge needles (Atom High Tech) were inserted into the tumor and positioned against its deepest margin (Fig. 2C). An implantation instrument (Atom High Tech) was then attached to the needles for implantation. Every 125I seed was placed 0.5–1.0 cm apart, in line with the TPS as far as possible. Postimplantation dose verification was performed to verify the therapeutic dose according to the TPS. For tumors that had been insufficiently dosed, repeated implantation was performed during the following 4 weeks. A D90 (Dose contains 90% target volume) value of >120 Gy at last implantation was regarded as a success.
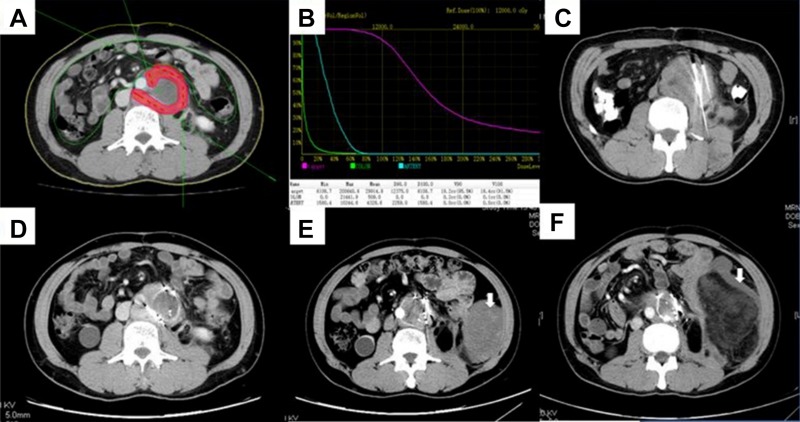
Figure 2.
Iodine‐125 (125I) seed implantation and follow‐up. A 54‐year‐old male with recurrent retroperitoneal liposarcoma (dedifferentiated type) treated with microwave ablation (MWA) combined with 125I seed implantation. (A, B): The treatment planning system results pre‐implantation. (C): 125I seed implantation procedure; the high‐density images indicate the implantation needles. (D): Four weeks after implantation, enhancement was still observed in the surrounding area and repeated implantation was performed. (E): Six months after initial therapy, tumor shrinkage was observed, and enhancement in the surrounding area was not obvious. A new lesion was observed (white arrow). (F): Eighteen months after initial therapy, the target lesion was still under control, but the new lesion had increased in size (white arrow).
Follow‐Up and Local Control Assessment
After each treatment, all patients received continuous electrocardiogram monitoring for 12 hours in the recovery ward. Medication was administered when patients complained of pain, fever, vomiting, etc. All patients were hospitalized for at least 3 days after each procedure. Follow‐up imaging (mainly enhanced CT scans) was performed monthly post‐procedure in the first 3 months and every 3 months after the initial 3 months to evaluate the therapeutic effectiveness of the combined therapy. Post‐procedure chemotherapy, if recommended by the medical oncologist and the patient was willing, was allowed in this study during the follow‐up time. Target tumor response was evaluated according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [14]. Images were evaluated by three radiologists independently, with findings including CR, PR, stable disease, and progressive disease. The response rate (RR) was determined by the sum of the CR and PR rates. The final follow‐up appointment for this study was in April 2016.
Results
Tumor Characteristics and Treatment Procedures
Tumor diameter ranged from 5.5–12.5 cm, with a median size of 9 cm. Eleven sessions of MWA were performed in 11 target tumors. Double antennas were used in six tumors, and triple antennas were used in five tumors. The MWA power setting was 60 or 70 W, and the ablation time ranged from 6 to 12 minutes, with a median time of 10 minutes. In total, 18 sessions of 125I seed implantation were performed in 11 target tumors. Three sessions of implantation were performed in two tumors, two sessions of implantation were performed in three tumors, and one session of implantation was performed in the remaining tumors. A total of 432 125I seeds were implanted. D90 ranged from 124 to 141 Gy before seed implantation and from 124 to 148 Gy after seed implantation (Table 2). The success rate was 100% in 11 sessions of MWA and 18 sessions of 125I seed implantation.
Table 2. Tumor characteristics and treatment procedures of 11 target lesions.
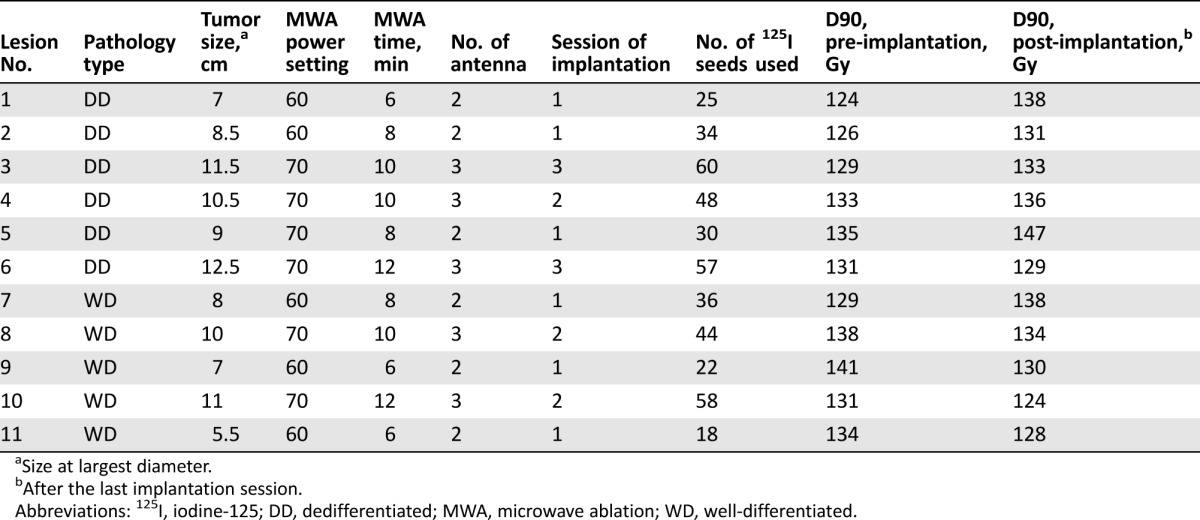
Size at largest diameter.
After the last implantation session.
Abbreviations: 125I, iodine‐125; DD, dedifferentiated; MWA, microwave ablation; WD, well‐differentiated.
Complications
The severity of the reported toxicity was evaluated with the Common Terminology Criteria for Adverse Events (CTCAE version 4.0). No procedure‐related deaths occurred, and no patients needed to immediately withdraw during these procedures. All patients tolerated the procedures well. With the help of intravenous conscious sedation, no patients complained of severe pain during MWA application. Post‐ablation pain was noted in the treatment of nine (81.8%) tumors, and only six (54.5%) patients experienced grade 2 pain. The number of patients experiencing fever, emesis, and nerve injury was five (45.5%), two (18.2%), and one (9.0%), respectively. All of the complications mentioned above were mild to moderate and easy to handle. Nerve injury was defined as limb paresthesia or leg weakness and was present in one patient who recovered completely in 2 months without special therapy. After 18 sessions of 125I seed implantation, the number of patients experiencing local pain, fever, bleeding, seed migration, and radiation enteritis was four (22.2%), three (16.7%), three (16.7%), two (11.1%), and zero (0%), respectively. All procedure‐related complications were reported grade 1 and grade 2. The rate of complications in the 125I seed implantation procedure was less than that in the MWA procedure (Table 3).
Table 3. Complications of MWA and 125I seed implantation.
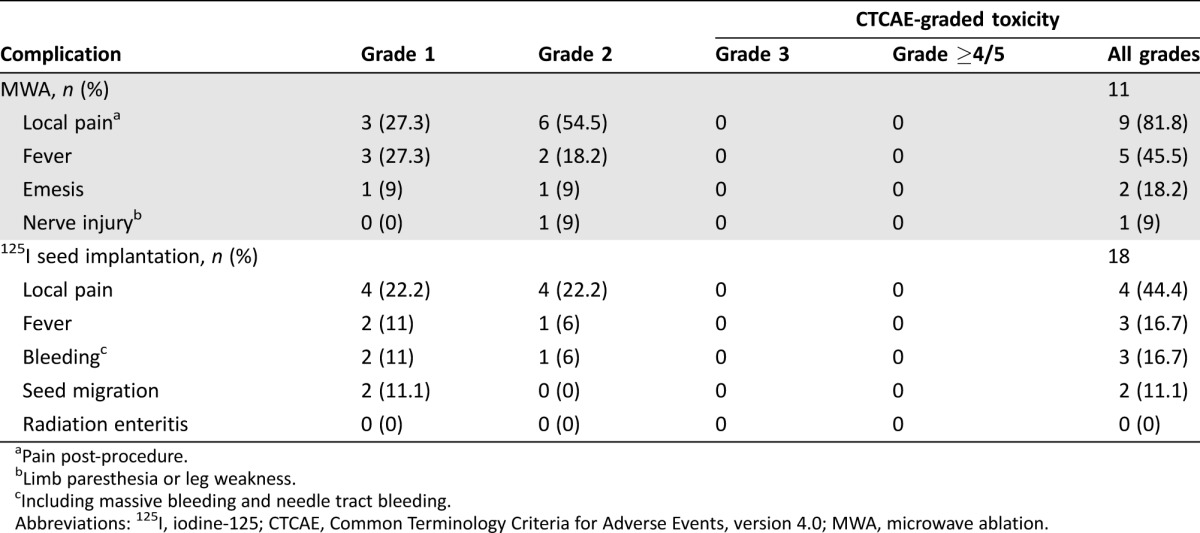
Pain post‐procedure.
Limb paresthesia or leg weakness.
Including massive bleeding and needle tract bleeding.
Abbreviations: 125I, iodine‐125; CTCAE, Common Terminology Criteria for Adverse Events, version 4.0; MWA, microwave ablation.
Follow‐Up and Local Control
The median follow‐up period was 20 months, ranging from 13 to 33 months. One patient was lost to follow‐up 33 months after procedure, and the other 10 patients survived through the last follow‐up. One patient experienced tumor progression and local recurrence (DD patient) at 6‐months follow‐up, and two patients experienced tumor progression and local recurrence (WD patient) and a lung metastatic lesion (DD patient), respectively, at 12‐months follow‐up. In total, only three patients experienced tumor progression; median progression‐free survival time interval has not yet been reached. Two patients with DD rRPLs accepted chemotherapy post‐procedure, and one patient with DD rRPLs accepted chemotherapy when tumor progressed. In this prospective study, all 11 (100%) patients who underwent the MWA procedure achieved a PR according to mRECIST 1 month post‐ablation, and no CR was observed. After 125I seed implantation was performed, CR was observed in three, five, and six target tumors in 3, 6, and 12 months, respectively, according to routine exam results. The RR at 3, 6, and 12 months after the initial MWA procedure was 100% in the 11 targeted tumors (Table 4).
Table 4. Local control of MWA combined with 125I seed implantation in 11 target recurrent retroperitoneal liposarcomas.
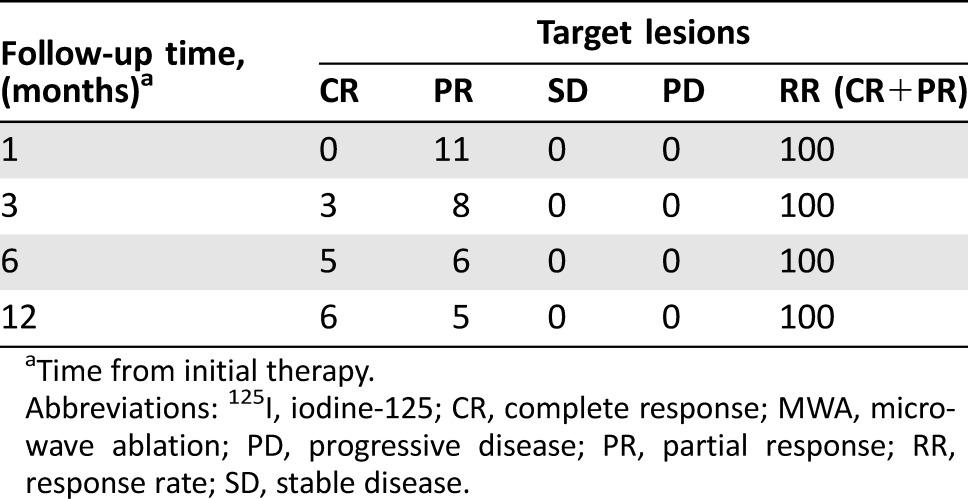
Time from initial therapy.
Abbreviations: 125I, iodine‐125; CR, complete response; MWA, microwave ablation; PD, progressive disease; PR, partial response; RR, response rate; SD, stable disease.
Discussion
RPL is relatively uncommon in adults, but it is the most common soft tissue sarcoma in the retroperitoneum area [1]. The most important predictors of sarcoma are histological subtype and the presence of gross positive margins after surgical resection [1], [15]. The presence of a mass of extreme size at the initial time of diagnosis and the location of the mass in the retroperitoneum make complete surgical resection difficult [16]. Furthermore, recurrence and metastasis are common even after successful surgical resection. Repeated surgery remains the primary treatment modality for recurrent RPLs [17], but the willingness to accept repeated resections tends to decrease in patients who have suffered from recurrence or metastasis after surgical resection. In this study, only three patients underwent repeated resection after initial surgical resection.
The efficacy of chemotherapy differs according to histological subtype and grade; previous studies described a 25% RR in patients with DD liposarcoma, and no response in patients with WD liposarcoma [18]. In the current study, the 11 patients all presented with DD and WD liposarcomas in histological findings; therefore, only two patients received chemotherapy before combined therapy. Two patients with DD rRPLs accepted chemotherapy post‐procedure, and one patient with DD rRPLs accepted chemotherapy when tumor progressed. Radiation therapy is also an important treatment for primary or metastatic retroperitoneal tumors [19], [20], but there is little high‐quality evidence for external beam radiotherapy application in the treatment of rRPLs. The application of conventional radiation therapy may be restricted due to the complex anatomy of the retroperitoneal area and the presence of many radiation‐sensitive organs in the retroperitoneal area [21]. In the current study, no patient accepted external beam radiation therapy before or after combined therapy.
As microinvasive and effective treatment modalities, energy ablative techniques such as RFA and cryoablation have been investigated in the treatment of RPLs [7], [22]; however, data on the tolerability and efficacy of MWA as a palliative treatment for rRPLs are still lacking. In general, thermal ablation has been adapted for application in lower‐volume tumors [23], [24]. Increasingly, studies have demonstrated the efficacy of MWA in larger tumors [25], [26]. In our opinion, when MWA was performed in patients with rRPLs, boundary residue was often inevitable due to tumor size and the close proximity of vital organs. Our study demonstrated a PR of 100% in all 11 tumors after a single MWA procedure, but no CR was observed. To deal with the boundary residue post‐MWA, 125I seeds were used in this study. As a low‐dose‐rate brachytherapy, the 125I seed emits continuous γ‐rays, which can inhibit tumor cell mitosis and decrease the resistance of hypoxic cells to radiation with mild normal tissue damage [27]. After 125I seed implantation was performed, complete response was observed in three, five, and six target tumors at 3, 6, and 12 months, respectively, according to routine exam results. The success of 125I brachytherapy for malignances is dependent on accurate dose distribution [28]. Our data indicated that D90 ranged from 124 to 141 Gy before seed implantation and from 124 to 148 Gy after seed implantation and that such doses ensure the efficacy of 125I seed brachytherapy. Twelve‐month 100% RR of target tumors demonstrated that MWA combined with 125I seed brachytherapy is an effective local treatment in patients with rRPLs. During the follow‐up time, one patient presented with disease progression evidenced by new tumors at 6 months, and two patients presented with disease progression evidenced by new tumors at 12 months. Of these three patients, two patients experienced local recurrence and one patient experienced lung metastatic disease. It seems that DD rRPLs are more susceptible to recurrence and metastasis than WD rRPLs, and this is consistent with other studies [2]. This result also reflects the limitations of MWA combined with 125I seed implantation as a local treatment. Because only three patients experienced tumor progression and the median progression‐free survival time interval has not yet been reached, it is difficult to compare our result with others.
All patients tolerated the procedures well; no procedure‐related deaths occurred, and no patients requested to immediately stop treatment during these procedures. Complications of MWA were mild to moderate and easy to handle, as previous studies reported [6], [25]. Post‐ablation pain (grade ≥2) was noted in the treatment of six (54.5%) tumors, and the number of patients experiencing fever, emesis, and nerve injury were five (45.5%), two (18.2%), and one (9.0%), respectively. Nerve injury, defined as limb paresthesia or leg weakness, was detected in one patient, who recovered completely in 2 months without special therapy. Numerous studies had proven the safety of 125I seed implantation brachytherapy [10], [12], [13], [29]. In this study, fewer complications were observed in the 125I seed implantation procedure than in the MWA procedure. After a total of 18 sessions of 125I seed implantation, the number of patients experiencing local pain, fever, bleeding, seed migration, and radiation enteritis was four (22.2%), three (16.7%), three (16.7%), two (11.1%), and zero (0%), respectively. Finally, 11 sessions of MWA and 18 sessions of 125I seed implantation were performed in 11 tumors with 100% technological success rate.
Our study also had some limitations. First, this study was not a randomized controlled trial. Second, only 11 patients were included. Finally, survival analysis was not performed in this study. More participants and longer follow‐up periods are required for future investigations.
Conclusion
MWA combined with 125I seed implantation is a safe and effective alternative for patients with recurrent RPLs. It exhibits excellent local control effectiveness with fewer complications, mild side effects, and acceptable tolerability. However, as a local therapy, disease progression was still observed after effective local control. The long‐term effects of MWA combined with 125I seed implantation treatment must be comprehensively evaluated.
Acknowledgments
The authors are grateful for the support of the National Natural Science Foundation of China (NSFC): grant number 81371654. The authors thank all patients who participated in this study, as well as the nursing team that assisted.
Contributed equally.
Contributor Information
Jiaping Li, Email: jpli3s@126.com.
Fujun Zhang, Email: doctorzhangfj@163.com.
Author Contributions
Conception/Design: Fujun Zhang, Jiaping Li
Provision of study material or patients: Fujun Zhang, Mingjian Lu
Collection and/or assembly of data: Wang Yao, Tao Zhang
Data analysis and interpretation: Fujun Zhang, Wang Yao, Wenzhe Fan, Zhihui Zhong
Manuscript writing: Fujun Zhang, Mingjian Lu
Final approval of manuscript: Fujun Zhang, Jiaping Li
Disclosures
The authors indicated no financial relationships.
References
- 1. Lewis JJ, Leung D, Woodruff JM et al. Retroperitoneal soft‐tissue sarcoma: Analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;228:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singer S, Antonescu CR, Riedel E et al. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg 2003;238:358–370; discussion 370–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shibata D, Lewis JJ, Leung DH et al. Is there a role for incomplete resection in the management of retroperitoneal liposarcomas? J Am Coll Surg 2001;193:373–379. [DOI] [PubMed] [Google Scholar]
- 4. Belfiore MP, Ronza FM, Della Volpe T et al. Long‐term local disease control in a recurrent soft‐tissue sarcoma of the thigh treated by radiofrequency ablation. J Surg Oncol 2015;111:708–710. [DOI] [PubMed] [Google Scholar]
- 5. Iannuccilli JD, Dupuy DE, Beland MD et al. Effectiveness and safety of computed tomography‐guided radiofrequency ablation of renal cancer: A 14‐year single institution experience in 203 patients. Eur Radiol 2016;26:1656–1664. [DOI] [PubMed] [Google Scholar]
- 6. Yu J, Liang P, Yu XL et al. US‐guided percutaneous microwave ablation of renal cell carcinoma: Intermediate‐term results. Radiology 2012;263:900–908. [DOI] [PubMed] [Google Scholar]
- 7. Fan W, Niu L, Wang Y et al. Percutaneous computed tomography‐guided cryoablation for recurrent retroperitoneal soft tissue sarcoma: A study of safety and efficacy. Oncotarget 2016;7:42639–42649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo JH, Teng GJ, Zhu GY et al. Self‐expandable esophageal stent loaded with 125i seeds: Initial experience in patients with advanced esophageal cancer. Radiology 2008;247:574–581. [DOI] [PubMed] [Google Scholar]
- 9. Taira AV, Merrick GS, Galbreath RW et al. Long‐term outcomes of prostate cancer patients with Gleason pattern 5 treated with combined brachytherapy and external beam radiotherapy. Brachytherapy 2013;12:408–414. [DOI] [PubMed] [Google Scholar]
- 10. Lu M, Pu D, Zhang W et al. Trans‐bronchoscopy with implantation of 125i radioactive seeds in patients with pulmonary atelectasis induced by lung cancer. Oncol Lett 2015;10:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhongmin W, Yu L, Fenju L et al. Clinical efficacy of CT‐guided iodine‐125 seed implantation therapy in patients with advanced pancreatic cancer. Eur Radiol 2010;20:1786–1791. [DOI] [PubMed] [Google Scholar]
- 12. Gao F, Li C, Gu Y et al. CT‐guided 125i brachytherapy for mediastinal metastatic lymph nodes recurrence from esophageal carcinoma: Effectiveness and safety in 16 patients. Eur J Radiol 2013;82:e70–e75. [DOI] [PubMed] [Google Scholar]
- 13. Zhang T, Lu M, Peng S et al. CT‐guided implantation of radioactive 125i seed in advanced non‐small‐cell lung cancer after failure of first‐line chemotherapy. J Cancer Res Clin Oncol 2014;140:1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52–60. [DOI] [PubMed] [Google Scholar]
- 15. Clark MA, Fisher C, Judson I et al. Soft‐tissue sarcomas in adults. N Engl J Med 2005;353:701–711. [DOI] [PubMed] [Google Scholar]
- 16. Sato T, Nishimura G, Nonomura A et al. Intra‐abdominal and retroperitoneal liposarcomas. Int Surg 1999;84:163–167. [PubMed] [Google Scholar]
- 17. Ikeguchi M, Urushibara S, Shimoda R et al. Surgical treatment of retroperitoneal liposarcoma. Yonago Acta Med 2014;57:129–132. [PMC free article] [PubMed] [Google Scholar]
- 18. Jones RL, Fisher C, Al‐Muderis O et al. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer 2005;41:2853–2860. [DOI] [PubMed] [Google Scholar]
- 19. Paumier A, Bonvalot S, Beaudré A et al. [Intensity modulated radiotherapy as adjuvant post‐operative treatment for retroperitoneal sarcoma: Acute toxicity]. Cancer Radiother 2011;15:413–420. [DOI] [PubMed] [Google Scholar]
- 20. Paumier A, Le Péchoux C, Beaudré A et al. IMRT or conformal radiotherapy for adjuvant treatment of retroperitoneal sarcoma? Radiother Oncol 2011;99:73–78. [DOI] [PubMed] [Google Scholar]
- 21. Pezner RD, Liu A, Chen YJ et al. Full‐dose adjuvant postoperative radiation therapy for retroperitoneal sarcomas. Am J Clin Oncol 2011;34:511–516. [DOI] [PubMed] [Google Scholar]
- 22. Keil S, Bruners P, Brehmer B et al. Percutaneous radiofrequency ablation for treatment of recurrent retroperitoneal liposarcoma. Cardiovasc Intervent Radiol 2008;31(suppl 2):S213–S216. [DOI] [PubMed] [Google Scholar]
- 23. Groeschl RT, Pilgrim CH, Hanna EM et al. Microwave ablation for hepatic malignancies: A multiinstitutional analysis. Ann Surg 2014;259:1195–1200. [DOI] [PubMed] [Google Scholar]
- 24. Kim JW, Kim JH, Sung KB et al. Transarterial chemoembolization vs. radiofrequency ablation for the treatment of single hepatocellular carcinoma 2 cm or smaller. Am J Gastroenterol 2014;109:1234–1240. [DOI] [PubMed] [Google Scholar]
- 25. Si ZM, Wang GZ, Qian S et al. Combination therapies in the management of large (≥5 cm) hepatocellular carcinoma: Microwave ablation immediately followed by transarterial chemoembolization. J Vasc Intervent Radiol 2016;27:1577–1583. [DOI] [PubMed] [Google Scholar]
- 26. Thamtorawat S, Hicks RM, Yu J et al. Preliminary outcome of microwave ablation of hepatocellular carcinoma: Breaking the 3‐cm barrier? J Vasc Intervent Radiol 2016;27:623–630. [DOI] [PubMed] [Google Scholar]
- 27. DeWeese TL, Shipman JM, Dillehay LE et al. Sensitivity of human prostatic carcinoma cell lines to low dose rate radiation exposure. J Urol 1998;159:591–598. [DOI] [PubMed] [Google Scholar]
- 28. Nath R, Anderson LL, Luxton G et al. Dosimetry of interstitial brachytherapy sources: Recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med Phys 1995;22:209–234. [DOI] [PubMed] [Google Scholar]
- 29. Li C, Zhang F, Zhang W et al. Feasibility of (125)i brachytherapy combined with sorafenib treatment in patients with multiple lung metastases after liver transplantation for hepatocellular carcinoma. J Cancer Res Clin Oncol 2010;136:1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]