This systematic review and meta‐analysis was performed to assess the efficacy of salvage treatment in pretreated adult type soft tissue sarcomas, gastrointestinal stromal tumors excluded. The objective was to compare the main outcome endpoints (progression‐free survival, overall survival, objective response rate) between trials investigating second‐line therapy in soft tissue sarcomas to define the importance and the role of subsequent treatment after first‐line failure.
Keywords: Soft tissue sarcoma, Pretreated, Chemotherapy, Second line, Meta‐analysis
Abstract
Background.
Prognosis for patients with metastatic soft tissue sarcomas (STS) is dismal, with median overall survival (OS) of 8–12 months. The role of second‐line therapy has been inconsistently investigated over the last 20 years. This systematic review and meta‐analysis was performed to assess the efficacy of salvage treatment in pretreated adult type STS, gastrointestinal stromal tumor (GIST) excluded.
Material and Methods.
PubMed, Web of Science, SCOPUS, EMBASE, CINAHL, and The Cochrane Library were searched for randomized phase II/phase III trials exploring second‐ or beyond therapy lines in pretreated metastatic STS. Two independent investigators extracted data; the quality of eligible studies was resolved by consensus. Hazard ratio (HR) of death and progression (OS and progression‐free survival [PFS]) and odds ratio (OR) for response rate (RR) were pooled in a fixed‐ or random‐effects model according to heterogeneity. Study quality was assessed with the Cochrane's risk of bias tool, and publication bias with funnel plots.
Results.
Overall, 10 randomized trials were selected. The pooled HR for death was 0.81 (95% confidence interval [CI] 0.73–0.9). Second‐line therapy reduced the risk of progression by 49% (HR = 0.51, 95% CI 0.34–0.76). This translated into an absolute benefit in OS and PFS by 3.3 and 1.6 months, respectively. Finally, RR with new agents or chemotherapy doublets translated from 4.3% to 7.6% (OR = 1.78, 95% CI 1.22–2.50).
Conclusion.
Better survival is achieved in patients treated with salvage therapies (chemotherapy, as single or multiple agents or targeted biological agents). A 3‐months gain in OS and an almost double RR is observed. Second lines also attained a reduction by 50% the risk of progression.
Implications for Practice.
There is some evidence that salvage therapies after first‐line failure are able to improve outcome in metastatic soft tissue sarcoma (STS). Trabectedin, gemcitabine‐based therapy, and pazopanib are currently approved drugs used after conventional upfront treatment. This meta‐analysis reviews the benefit of new agents used in randomized trials in comparison with no active treatments or older agents for recurrent/progressed STS. The results show that modern drugs confer a statistically significant 3‐month benefit in terms of overall survival, and an increase in response rate. Despite a limited improvement in outcome, currently approved second‐line therapy should be offered to patients with good performance status.
Introduction
Soft tissue sarcomas (STS) are rare tumors of mesenchymal origin. They account for 1%–2% of malignant neoplasms, and more than 50 different histotypes are recognized [1]. Local control has improved over the past 30 years with the standard surgical procedure including a wide excision with R0 margins. A wide excision is followed by radiotherapy for high‐grade, deep lesions that are larger than 5 cm [2], [3].
Adjuvant chemotherapy is not accepted as standard treatment in adult STS; however, some authors recommend it for some stages of disease (e.g., in stages II and III, National Comprehensive Cancer Network guidelines provide level 2B recommendation for adjuvant chemotherapy) [4], [5], [6], [7], [8], [9].
Nonetheless, even with the optimal multidisciplinary approach, 45% of patients die from the disease at 5 years and 50% at 10 years [10]. For metastatic disease, more than 100 phase II studies and more than 20 randomized studies have been published [11], [12], [13], [14]. Among the investigated drugs, only doxorubicin, ifosfamide, and dacarbazine have given a response rate >15% [15], [16], [17], with a short progression‐free survival (PFS) of 2–4 months. The combination of these drugs results in a higher response rate ranging from 25%–40%, but median overall survival (OS) does not exceed 12 months [18], [19]. Thus, the treatment of metastatic STS usually remains palliative even if about 5% of patients with metastatic STS may be cured after multidisciplinary treatment mainly in limited, resectable lung metastasis [20].
In the last 10 years, the term “histology‐driven therapy” was coined for a new concept indicating that some agents are more active in particular families of STS. As a consequence, in some subtypes of sarcomas, some agents scarcely active or inactive in other histologies can be applied with satisfactory results [13]. Leiomyosarcoma has a limited sensitivity to ifosfamide, but trabectedin as single agent, gemcitabine plus docetaxel, and doxorubicin plus dacarbazine are valid options [13], [21], [22]. Low‐grade liposarcomas (LS) are in general chemoresistant, whereas myxoid LS is sensitive to trabectedin and undifferentiated LS to anthracyclines [23], [24]. Angiosarcoma is sensitive to paclitaxel [25], synovial sarcoma to high‐dose ifosfamide [19], and solitary fibrous tumor to temozolomide plus bevacizumab [26]. Pazopanib is the first targeted therapy with some activity in different sarcomas, such as leiomyosarcomas and synovial sarcomas, but not in adipocytic sarcomas [27].
In light of these recent results and evolving strategies, determining appropriate second‐line chemotherapy options is a current focus in STS. Second‐line therapy of a tumor describes any subsequent intervention with a new regimen after treatment failure because of disease progression or unacceptable toxicity with initial therapy. Herein, we performed a systematic review and a meta‐analysis of phase II/III randomized studies published in recent decades, including both newer and older agents. The objective of our study was to compare the main outcome endpoints: PFS, OS, and objective response rate (ORR) between trials investigating second‐line therapy in STS to define the importance and the role of subsequent treatment after first‐line failure.
Materials and Methods
In conducting the meta‐analysis, we followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis statement [28].
Search Strategy and Study Selection
For the purpose of this analysis, a systematic search of the PubMed, Web of Science, SCOPUS, EMBASE, and CINAHL databases and The Cochrane Library was performed from inception to February 7, 2016. Search terms were (“sarcoma” [MeSH Terms] OR “sarcoma” [All Fields]) AND ((second [All Fields] AND “line” [All Fields])) OR pretreated [All Fields] OR (previously [All Fields] AND treated [All Fields]) OR progressing [All Fields] OR progressed [All Fields] OR failure [All Fields]) AND ((“random allocation” [MeSH Terms] OR (“random” [All Fields] AND “allocation” [All Fields]) OR “random allocation” [All Fields] OR “randomized” [All Fields]) OR randomized [All Fields]).
We included in the systematic review all randomized, controlled phase II/III trials that compared standard (single agent or doublet chemotherapy) therapy with experimental agents or placebo/best supportive care in adult patients with advanced/metastatic, progressing STS after first‐line therapy. Trials were only included if they were published in full and in English. We excluded trials that included primarily bone sarcoma, Ewing/rhabdomyosarcoma, Kaposi sarcoma, gastrointestinal stromal tumor (GIST), and childhood/adolescent sarcomas. Two authors (FP and AB) independently evaluated each reference title identified by the search and then applied the inclusion criteria. In cases of disagreement between the two reviewers, the full article was obtained and inspected independently by a third senior reviewer (AC).
Data Extraction
To be included in the analysis, a trial had to compare a modern agent (experimental drug or a modern chemotherapy), either alone or in combination with another (doublet), with a single agent (either a standard cytotoxic drug or other agents), placebo, best supportive care (BSC), or observation alone. Modern agents were those developed starting from trabectidin studies era. For each trial, the data extracted were as follows: (a) year of publication; (b) type of study; (c) number of patients enrolled in experimental and control arms; (d) treatment arms and schedules; (e) first‐line therapy; (f) ORR according to World Health Organization or Response Evaluation Criteria In Solid Tumors criteria; (g) median PFS or time to progression (TTP) with the corresponding hazard ratio (HR) and 95% confidence interval (CI) for each treatment comparison; and (h) median OS with the corresponding HR and 95% CI for each treatment comparison. Data extraction was conducted independently by two investigators (FP and AB), and any discrepancies were resolved by consensus in frequent meetings in the presence of the senior investigator (AC).
Statistical Analysis
We considered OS as the primary efficacy endpoint of this meta‐analysis, and ORR, PFS, or TTP as secondary endpoints. For the main analysis, in which all experimental arms were compared with all control arms as second‐line therapies for STS, we performed a meta‐analysis of HRs for PFS/TTP and OS and a meta‐analysis of odds ratios (ORs) for ORR using a random‐ or fixed‐effects model. A random‐effects model based on an estimate and standard error was used to pool the estimate across studies using the Der Simonian‐Laird method [29]. Heterogeneity between studies was quantified by the between‐study variance tau2 and the Cochran's Q test and/or I2 statistics, which describes the percentage of variation across studies due to heterogeneity rather than chance [30]. Studies were classified as showing low (I2 ≤ 50%), moderate (I2 > 50% and ≤75%), or high (I2 ≥ 75%) heterogeneity. A p value ≤.05 was considered statistically significant. All statistical tests were two‐sided. The Cochrane risk of bias tool was assessed for included studies.
Subgroup analysis for OS only was performed for doublet versus single‐agent studies, for single‐agent versus no active therapy studies, and experimental versus control arms with active treatment as control arms.
A quantitative analysis for publication bias for OS meta‐analysis included the Begg's and Egger's rank correlation test [31], and the Egger intercept test [32]. A 2‐sided alpha error of 0.05 was used to determine statistical significance. Publication bias analyses were performed using the RevMan v5.3 and Comprehensive Meta‐analysis version 2 (Biostat Inc., Englewood, NJ, https://www.meta-analysis.com).
Results
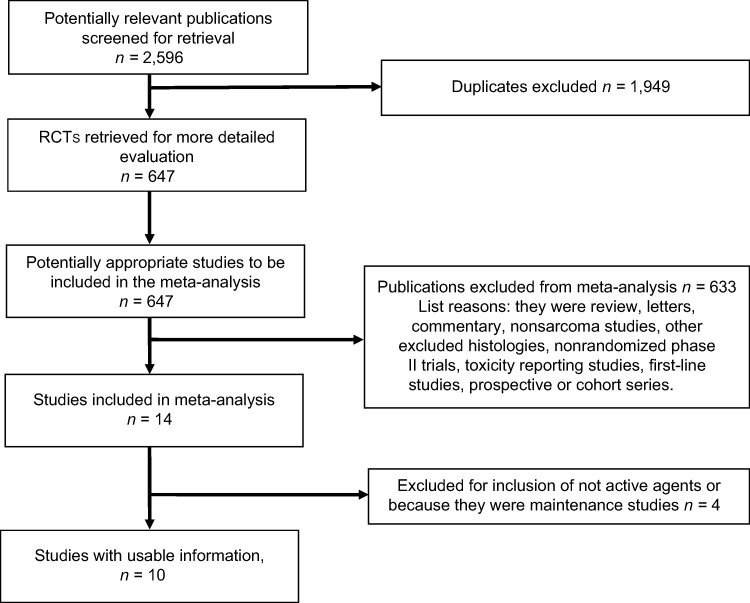
A total of 2,596 publications were identified through the electronic search. After applying the previously described criteria, we included 10 randomized trials (n = 4 phase III and n = 6 phase II trials) [21], [22], [27], [33], [34], [35], [36], [37], [38], [39]. A flow diagram describing the inclusion of trials is presented in Figure 1. These trials enrolled a total of 2,267 patients. Among these, n = 3 compared doublets versus single agents, n = 4 single agents versus single agents, and n = 3 single agents versus placebo or BSC. Experimental and control arms included 1,287 and 966 subjects, respectively. Among experimental arms, n = 2 were targeted therapies and n = 8 cytotoxic drugs (either alone or in combination). Control arms were targeted therapies, cytotoxic, and placebo (or BSC) in 0, n = 7, and n = 3 cases, respectively.
Figure 1.
Randomized controlled trials search and selection procedure.
Abbreviation: RCT, randomized controlled trial.
Pooled ORR, Median PFS, and OS in Control and Experimental arms
Overall, n = 10 control arms provided a pooled ORR of 4.3% (95% CI 1.8%–6.8% according to the random‐effects model), and conversely, the corresponding experimental arms were associated with a pooled ORR of 7.6% (95% CI 6.4%–8.8% according to the random‐effects model).
In all studies with data necessary for analysis (median PFS or TTP), the pooled median PFS was 2.35 months for control arms and 3.95 months for experimental arms. In n = 10 and n = 9 control and experimental arms with data for OS available (median OS), the pooled median OS was 10.1 and 13.4 months, respectively.
Meta‐Analysis of ORR, PFS, and OS
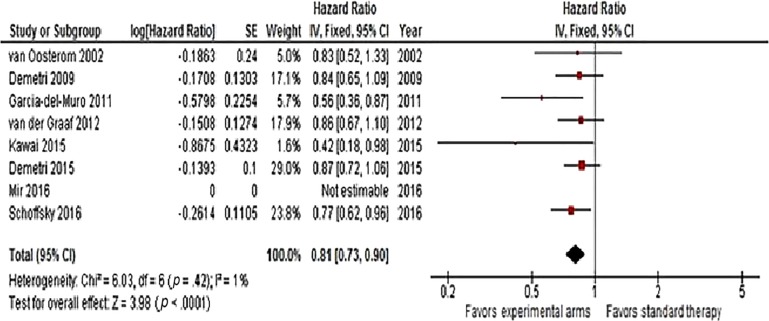
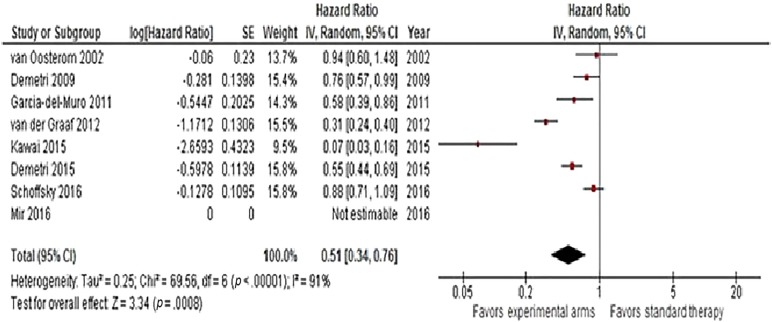
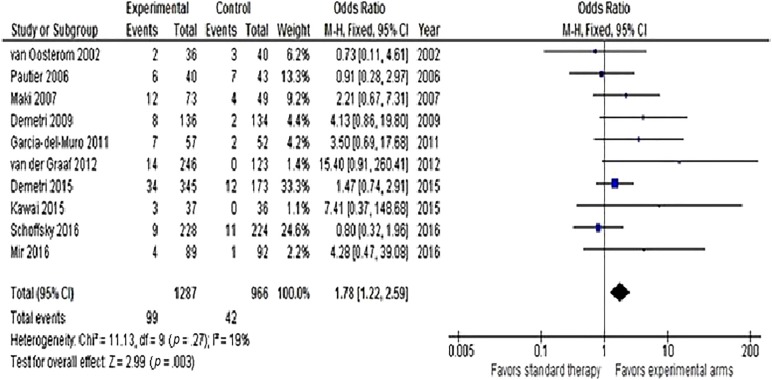
Hazard ratios for OS were available for n = 8 trials. A significant reduction in the risk of death (HR = 0.81, 95% CI 0.73–0.9, p < .0001) was observed with second‐line therapy, as shown in Figure 2. No heterogeneity was detected. Progression‐free survival was available in n = 8 studies (including Van Oosterom et al. trial that reported TTP). Overall, second‐line therapy reduced the risk of progression or death by 49% (HR = 0.51, 95% CI 0.34–0.76, p = .0008). Heterogeneity was high, so a random‐effects model was used (Fig. 3). In the trial of Van Osterom et al., only one death due to progressive disease was not accounted in each arm for TTP analysis. Finally, ORR was determined in all articles. The chance of having a response with recent second‐line doublets or single agents was almost doubled (OR = 1.78, 95% CI 1.22–2.59, p = .003; Fig. 4). Low heterogeneity was observed.
Figure 2.
Forest plot of hazard ratios comparing overall survival for all patients who received modern versus conventional (or best supportive care/placebo) second‐line chemotherapy (n = 10 studies).
Abbreviations: CI, confidence interval; IV, intravenous; SE, standard error.
Figure 3.
Forest plot of hazard ratios comparing progression‐free survival for patients who received modern versus conventional (or best supportive care/placebo) second‐line chemotherapy (n = 11 studies).
Abbreviations: CI, confidence interval; IV, intravenous; SE, standard error.
Figure 4.
Forest plot of odds ratios comparing overall response rate for patients who received modern versus conventional (or best supportive care/placebo) second‐line chemotherapy (n = 14 studies).
Abbreviations: CI, confidence interval; M‐H, Mantel‐Haenszel.
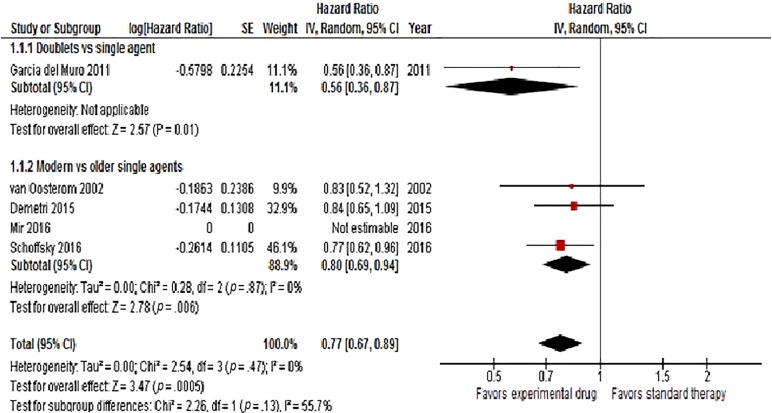
When the analysis was restricted to studies comparing experimental drugs with any active therapies as control (single agents) arms, the result remained significant (HR = 0.77, 95% CI 0.67–0.89, p = .0005; Fig. 5). When splitting studies comparing doublets with single agents and newer single agents with older single agents, the results were slightly different (doublets vs. single agents: HR = 0.56; 95% CI 0.36–0.87, p = .01; newer single agents vs. older single agents: HR = 0.80; 95% CI 0.69–0.94, p = .006; Fig. 5).
Figure 5.
Forest plot of hazard ratios in subgroup analysis comparing overall survival for patients who received modern versus conventional second‐line chemotherapy in doublets versus single agents trials (with control arms including only active therapy).
Abbreviations: CI, confidence interval; IV, intravenous; SE, standard error.
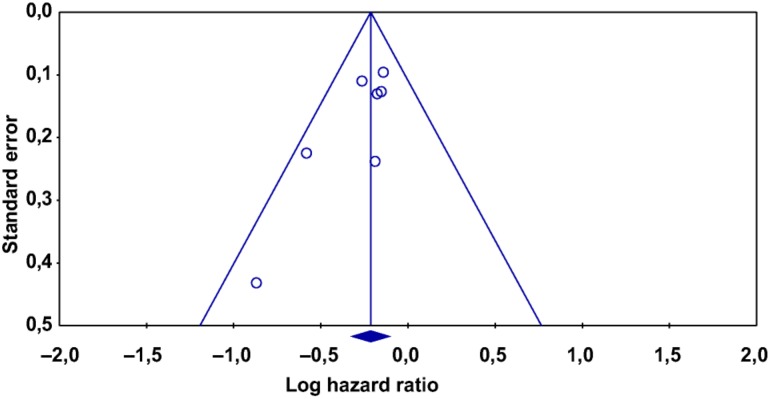
Risk of bias for included trials is reported in Table 2. All randomized patients were included in the analyses, confirming that the issue of incomplete outcome data was addressed. With regard to selective data reporting, outcomes of interest were reported in all studies. Low risk of bias was reported for n = 2 studies only. Finally, no obvious publication bias was observed through inspection of the funnel plot (Fig. 6). The Egger test found significant evidence of bias for OS analysis; the Begg and Mazumdar rank correlation test found evidence of publication bias (p = .066); and the Duval and Tweedie and trim and fill tests extrapolated the probable absence of n = 1 and n = 29 studies for OS analysis, respectively. This meant that in the first case, there were only n = 1 asymmetric (negative) studies that when put to the right of the mean value of HR would change the final result, and that second, we would need to locate and include n = 29 “negative or neutral” studies for the combined two‐tailed p value to exceed .050.
Table 2. Assessment of risk of bias.
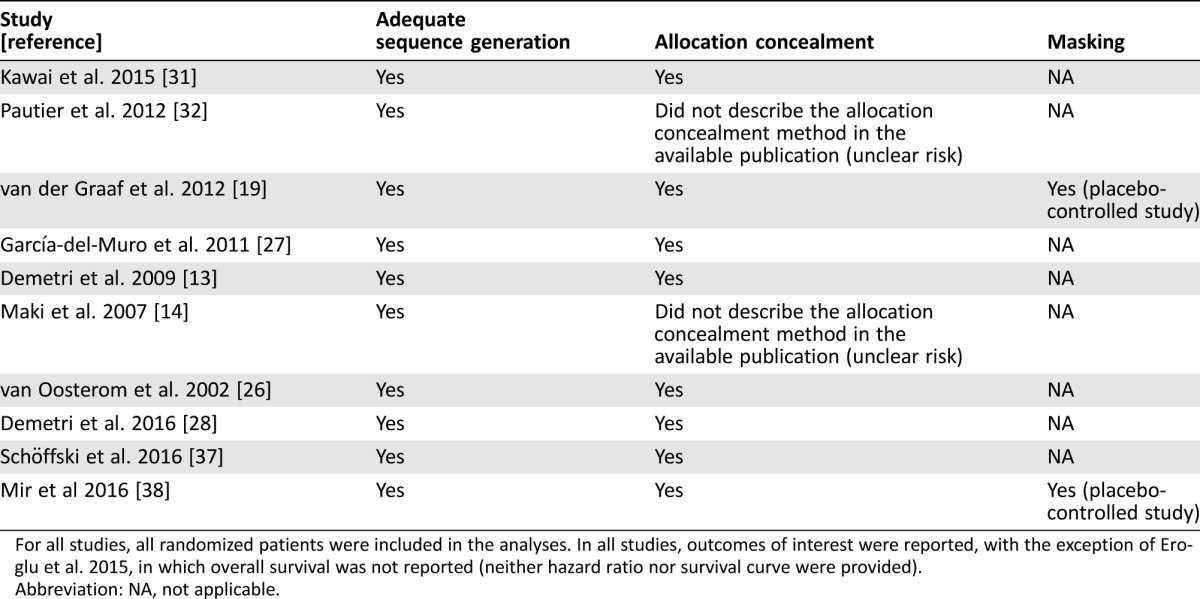
For all studies, all randomized patients were included in the analyses. In all studies, outcomes of interest were reported, with the exception of Eroglu et al. 2015, in which overall survival was not reported (neither hazard ratio nor survival curve were provided).
Abbreviation: NA, not applicable.
Figure 6.
Funnel plot of standard error by hazard ratio (HR) for overall survival (OS). The funnel plot of n = 8 studies included in the meta‐analysis of OS illustrates the standard error of log HR in Y axis and HRs for OS in X axis.
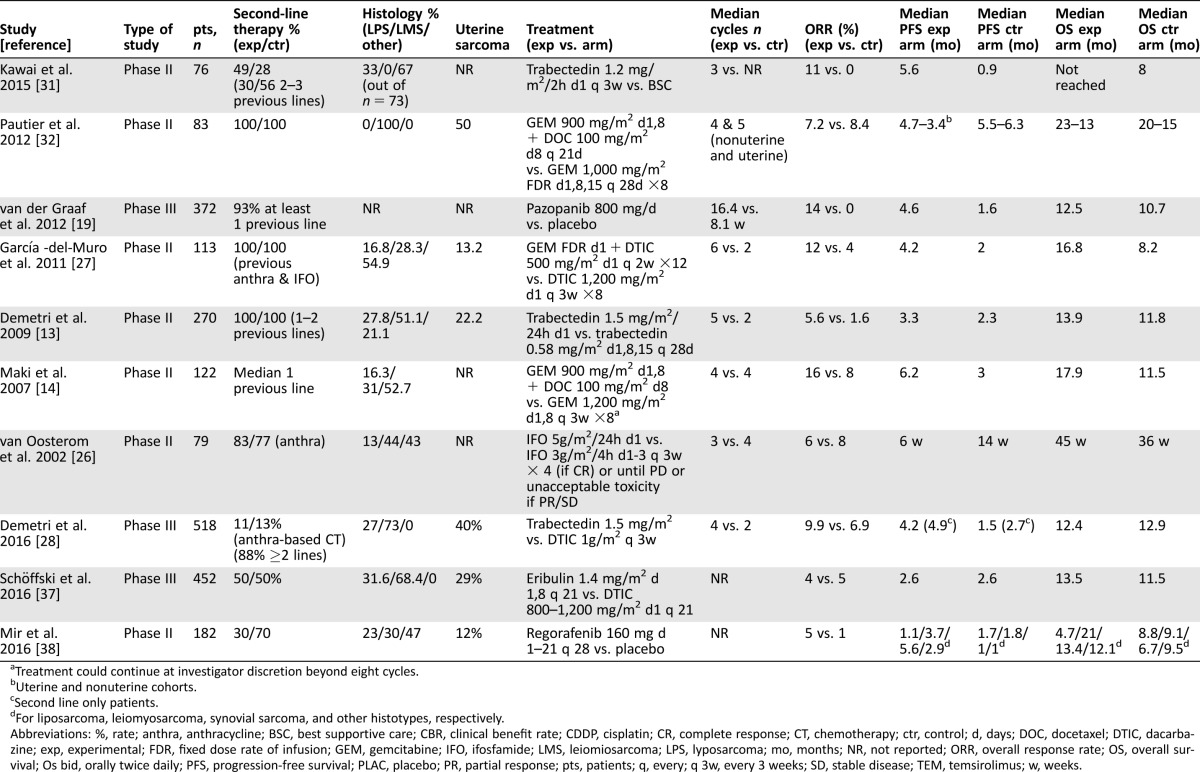
Table 1. Characteristics of the included studies.
Treatment could continue at investigator discretion beyond eight cycles.
Uterine and nonuterine cohorts.
Second line only patients.
For liposarcoma, leiomyosarcoma, synovial sarcoma, and other histotypes, respectively.
Abbreviations: %, rate; anthra, anthracycline; BSC, best supportive care; CBR, clinical benefit rate; CDDP, cisplatin; CR, complete response; CT, chemotherapy; ctr, control; d, days; DOC, docetaxel; DTIC, dacarbazine; exp, experimental; FDR, fixed dose rate of infusion; GEM, gemcitabine; IFO, ifosfamide; LMS, leiomiosarcoma; LPS, lyposarcoma; mo, months; NR, not reported; ORR, overall response rate; OS, overall survival; Os bid, orally twice daily; PFS, progression‐free survival; PLAC, placebo; PR, partial response; pts, patients; q, every; q 3w, every 3 weeks; SD, stable disease; TEM, temsirolimus; w, weeks.
Discussion
This systematic review aimed to assess the role of salvage therapy in metastatic pretreated STS. The meta‐analysis was based on data extracted from literature reviews of all published trials that had randomized patients in phase II–III studies receiving second‐line chemotherapy or biological agent versus conventional therapy. Second and further line of chemotherapy in metastatic STS was prescribed in good performance status patients even before the pubblication of conclusive studies. The oldest studies, performed from 1980 to 1990, compared historical agents in second‐line therapy without a histological subanalysis of the STS treated. Notwithstanding, Bramwell et al. [39] demonstrated a higher activity of ifosfamide (24% ORR) versus cyclophosphamide (5%), and Verveij demonstrated that doxorubicin was better than docetaxel [15]. The conclusions were that docetaxel was inactive in STS, and that its use was not recommended. As a consequence, in daily practice after first line containing antracyclines, salvage monotherapy with high dose ifosfamide, or dacarbazine, or vinorelbine were usually proposed [17], [40], [41].
A phase III trial by Maki et al. comparing gemcitabine + docetaxel versus gemcitabine alone in different subtypes of STS reported a higher activity of the combination on ORR, PFS, and OS in undifferentiated pleomorphic sarcomas and in uterine and nonuterine leiomyosarcomas [22] . Following that study, gemcitabine + docetaxel became the standard mainly in the reported subtypes. A few years later, Pautier and colleagues did not confirm the superiority of the combination over gemcitabine alone, and nowadays this drug as single agent is a common second‐line therapy mainly in leiomyosarcomas, undifferentiated pleomorphic sarcomas, and angiosarcomas [37], [42]. Garcia del Muro et al., in a cooperative Spanish study, demonstrated better activity of the combination gemcitabine + dacarbazine than dacarbazine alone in different STS and specifically in leiomyosarcoma [34]. At present, gemcitabine monochemotherapy or in combination is an accepted treatment after first‐line progression.
In 2009, Verveij confirmed in an article in the Journal of Clinical Oncology [43] and Scurr [13] demonstrated in 2011 that a specific drug can be more active in particular subtypes of STS. The histology‐driven theory was very well investigated over the last 10 years in studies on second‐line therapy in STS. The revisited old drug ifosfamide showed a specific activity with doxorubicin in synovial sarcoma as ORR in the Edmonson's study [19]. Demetri showed a high level of activity of the newer drug trabectedin in patients with advanced myxoid or round cell liposarcoma and leiomyosarcoma after failure of the first‐line chemotherapy including anthracyclines, ifosfamide, and other traditional agents [21], [35].
Trabectedin is a marine‐derived antineoplastic compound isolated from the Caribbean tunicate Ecteinascidia turbinate that is approved for the treatment of patients with advanced STS. The drug binds the minor groove of DNA and interferes with DNA transcription to RNA by inhibiting the binding of transcription factors [44]. This drug demonstrated specific activity against L‐type sarcomas and showed a higher ORR and PFS as compared with the active control with dacarbazine. A recent French study with trabectedin as second‐line therapy suggests that the treatment should not be discontinued because its maintenance use significantly reduces the risk of progression in patients with stable disease or in response [45].
Activity has also been reported with pazopanib, a synthetic imidazole pyrimidine, which is a multitarget tyrosine kinase inhibitor with activity against vascular endothelial growth factor receptor (VEGFR) 1, 2, and 3, and platelet derived growth factor receptor (PDGFR). In the PAzopanib ExpLorEd in SofT‐Tissue Sarcoma ‐ A phasE III study (PALETTE) study, pazopanib was compared with placebo in many different pretreated STS. The drug showed a better PFS but not OS in leiomyosarcoma and synovial sarcoma [27]. A previous phase II study [46] had shown an inferior PFS in adipocytic STS.
A recent French study with trabectedin as second‐line therapy suggests that the treatment should not be discontinued because its maintenance use significantly reduces the risk of progression in patients with stable disease or in response.
A second targeted agent, regorafenib, has recently demonstrated some activities in second or later lines of therapy in STS [47]. Regorafenib is an oral agent, multikinase inhibitor active against VEGFR 1, VEGFR 2, VEGFR 3, Kit, PDGFR, and Ras. Compared with placebo, it showed an interesting activity on PFS in all groups of sarcomas except adipocitic subtypes.
In 2016, inhibition of PDGFR‐α by the monoclonal antibody Olaratumab showed promising clinical activity if associated to doxorubicin both in OS and in PFS, as first‐line therapy only [48]. This is going to become another active targeted agent in STS treatment, but no data are currently available in second‐line treatment.
Eribulin mesylate, a compound isolated from the marine sponge Halichondria that acts as an inhibitor of microtubule dynamics, was shown to have a particular activity against liposarcomas and general activity against fibroblastic sarcoma, epithelioid sarcoma, and malignant solitary fibrous tumor, even in a small number of patients included in the study [49]. The benefit was demonstrated in ORR and PFS at 12 weeks. More recently, eribulin demonstrated such activity but only in OS and not in PFS [38].
Besides these four drugs discussed above, no other new agent has shown significant activity either in STS overall or in a specific subtype. Neither selumetinib [50] nor thrombospondin [51] and ombrabulin [52] showed a sufficient activity in metastatic STS to support their use in clinical practice or justify further studies. Limited experiences are reported on the activity of palbociclib in well‐differentiated/dedifferentiated liposarcoma [53] and sunitinib in solitary fibrous tumor [54]. Both agents are not approved in many countries for this specific use.
There was a different situation concerning ridaforolimus, an mammalian target of rapamycin (mTOR) inhibitor used as second‐line/maintenance treatment in metastatic STS, which achieved an objective response or stable disease compared with first‐line therapy. However, the drug failed to demonstrate a clinical impact to maintain the response, although a small statistical benefit was recorded compared with placebo [55]. Some studies included in our meta‐analysis investigated the role of the dose or schedule of a specific drug in second‐line therapy. Van Oosterom showed better activity in a nonhistology‐driven study of a fractionated schedule of ifosfamide versus an only 1‐day schedule [33]. Similarly, Demetri et al. showed a better activity of trabectedin administered every 3 weeks instead of weekly administration [21].
The limitations of these studies should be noted. The meta‐analysis was based on data extracted from the literature and could suffer from some possible bias. Only eight trials investigated the OS benefit of second‐line therapy; PFS was available only in eight, whereas ORR evaluation was present in all studies. The main weakness is the lack of a formal analysis according to histology; in fact, most studies reported efficacy in mixed populations. Some of the studies compared the investigational agent or combination with placebo, failing to give an active drug in these patients. Most of the studies did not include a formal quality of life measurement in spite of the fact that the drugs were not curative and had limited benefit in terms of response or survival. Otherwise, this is the first meta‐analysis exploring the efficacy of salvage treatments with novel agents compared with no therapy or use of older ineffective drugs in STS. Finally, the benefit of palliative surgery and of symptomatic radiotherapy cannot be accounted, but it is likely that multidisciplinary treatment would be only exceptionally permitted in this population and investigated in large randomized trials. The benefit was significant for both OS and PFS, and for ORR despite the fact that <10% was almost double compared with historical agents. The heterogeneity for the primary analysis was virtually absent, and no obvious bias was found in funnel plots.
The reduction by about 40% of the risk of progression highlights the strong activity of new agents even in these previously treated and refractory patients. The importance of the results was confirmed by a significant OS benefit, despite that postprogression therapies or crossover to active agents could have diluted the absolute gain.
The reduction by about 40% of the risk of progression highlights the strong activity of new agents even in these previously treated and refractory patients. The importance of the results was confirmed by a significant OS benefit, despite that postprogression therapies or crossover to active agents could have diluted the absolute gain. In experimental arms, postprogression survival accounted for more than 70% of the entire OS. Because the number of new drugs against metastatic STS is increasing, it will be important to define which is the best endpoint to be considered (e.g., OS, PFS, percentage of PFS at 3 or 6 months, ORR, or TTP) [56].
Conclusion
Although the results of this meta‐analysis on ORR, OS, and PFS of salvage therapy in metastatic STS were positive, the prognosis of these patients remains unsatisfactory, with a medium survival of 8–12 months. More research is needed to find a particular biomarker or targets to treat patients not only on the basis of histology‐driven activity but also by targeting potentially “druggable” molecular defects.
Acknowledgments
We thank Ray Hill, an independent medical writer, who provided English‐language editing and journal styling prior to submission on behalf of Health Publishing & Services Srl.
Footnotes
For Further Reading: Silvia Stacchiotti, Olivier Mir, Axel Le Cesne et al. Activity of Pazopanib and Trabectedin in Advanced Alveolar Soft Part Sarcoma. The Oncologist first published on July 28, 2017.
Implications for Practice: This retrospective study, conducted among the world reference centers for treatment of sarcoma, confirms the value of pazopanib in patients with advanced alveolar soft part sarcoma (ASPS), with dimensional and durable responses, whereas trabectedin shows a limited activity. Alveolar soft part sarcoma is resistant to conventional cytotoxic chemotherapy. Pazopanib and trabectedin are licensed for treatment of sarcoma from second line; in the lack of prospective clinical trials, these results are relevant to defining ASPS best management and strongly support initiatives aimed at obtaining the approval of pazopanib in the front line of the disease.
Author Contributions
Conception/design: Alessandro Comandone, Fausto Petrelli, Antonella Boglione, Sandro Barni
Provision of study material or patients: Fausto Petrelli, Antonella Boglione
Collection and/or assembly of data: Fausto Petrelli, Antonella Boglione, Sandro Barni
Data analysis and interpretation: Alessandro Comandone, Antonella Boglione, Sandro Barni
Manuscript writing: Alessandro Comandone, Fausto Petrelli, Antonella Boglione, Sandro Barni
Final approval of manuscript: Alessandro Comandone, Fausto Petrelli, Antonella Boglione, Sandro Barni
Disclosures
The authors indicated no financial relationships.
References
- 1.Fletcher CDM, Bridge JA, Hogendoorn PCW. et al. (eds). WHO Classification of Tumours of Soft Tissue and Bone. Lyon, France: IARC, 2013. [Google Scholar]
- 2. Cahlon O, Brennan MF, Jia X et al. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb‐sparing surgery without adjuvant radiation. Ann Surg 2012;255:343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beane JD, Yang JC, White D et al. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20‐year follow‐up of a randomized prospective trial. Ann Surg Oncol 2014;21:2484–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pervaiz N, Colterjohn N, Farrokhyar F et al. A systematic meta‐analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft‐tissue sarcoma. Cancer 2008;113:573–581. [DOI] [PubMed] [Google Scholar]
- 5. Frustaci S, Gherlinzoni F, De Paoli A et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: Results of the Italian randomized cooperative trial. J Clin Oncol 2001;19:1238–1247. [DOI] [PubMed] [Google Scholar]
- 6. Woll PJ, Reichardt P, Le Cesne A et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft‐tissue sarcoma (EORTC 62931): A multicentre randomised controlled trial. Lancet Oncol 2012;13:1045–1054. [DOI] [PubMed] [Google Scholar]
- 7. Gronchi A, Frustaci S, Mercuri M et al. Short, full‐dose adjuvant chemotherapy in high‐risk adult soft tissue sarcomas: A randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012;30:850–856. [DOI] [PubMed] [Google Scholar]
- 8. Le Cesne A, Ouali M, Leahy MG et al. Doxorubicin‐based adjuvant chemotherapy in soft tissue sarcoma: Pooled analysis of two STBSG‐EORTC phase III clinical trials. Ann Oncol 2014;25:2425–2432. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network . Soft-tissue sarcoma, version 2.2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf. Accessed June 14, 2017. [DOI] [PubMed]
- 10. De Angelis R, Sant M, Coleman MP et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE–5‐A population‐based study. Lancet Oncol 2014;15:23–34. [DOI] [PubMed] [Google Scholar]
- 11. Santoro A, Tursz T, Mouridsen H et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first‐line treatment of advanced soft tissue sarcomas: A randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–1545. [DOI] [PubMed] [Google Scholar]
- 12. Antman K, Crowley J, Balcerzak SP et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol 1993;11:1276–1285. [DOI] [PubMed] [Google Scholar]
- 13. Scurr M. Histology‐driven chemotherapy in soft tissue sarcomas. Curr Treat Options Oncol 2011;12:32–45. [DOI] [PubMed] [Google Scholar]
- 14. Nielsen OS, Judson I, van Hoesel Q et al. Effect of high‐dose ifosfamide in advanced soft tissue sarcomas. A multicentre phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2000;36:61–67. [DOI] [PubMed] [Google Scholar]
- 15. Verweij J, Lee SM, Ruka W et al. Randomized phase II study of docetaxel versus doxorubicin in first‐ and second‐line chemotherapy for locally advanced or metastatic soft tissue sarcomas in adults: A study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 2000;18:2081–2086. [DOI] [PubMed] [Google Scholar]
- 16. Palumbo R, Neumaier C, Cosso M et al. Dose‐intensive first‐line chemotherapy with epirubicin and continuous infusion ifosfamide in adult patients with advanced soft tissue sarcomas: A phase II study. Eur J Cancer 1999;35:66–72. [DOI] [PubMed] [Google Scholar]
- 17. Zucali PA, Bertuzzi A, Parra HJ et al. The “old drug” dacarbazine as a second/third line chemotherapy in advanced soft tissue sarcomas. Invest New Drugs 2008;26:175–181. [DOI] [PubMed] [Google Scholar]
- 18. Frustaci S, Foladore S, Buonadonna A et al. Epirubicin and ifosfamide in advanced soft tissue sarcomas. Ann Oncol 1993;4:669–672. [DOI] [PubMed] [Google Scholar]
- 19. Edmonson JH, Ryan LM, Falkson CI et al. Phase II study of ifosfamide+doxorubicin in patients with advanced synovial sarcomas (E1793): A trial of the Eastern Cooperative Oncology Group. Sarcoma 2003;7:9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blackmon SH, Shan N, Roth JA et al. Resection of pulmonary sarcomatous metastases is associated with long‐term survival. Ann Thorac Surg 2009;88:877–884. [DOI] [PubMed] [Google Scholar]
- 21. Demetri GD, Chawla SP, von Mehren M et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: Results of a randomized phase II study of two different schedules. J Clin Oncol 2009;27:4188–4196. [DOI] [PubMed] [Google Scholar]
- 22. Maki RG, Wathen JK, Patel SR et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: Results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007;25:2755–2763. [DOI] [PubMed] [Google Scholar]
- 23. Grosso F, Jones RL, Demetri GD et al. Efficacy of trabectedin (ecteinascidin‐743) in advanced pretreated myxoid liposarcomas: A retrospective study. Lancet Oncol 2007;8:595–602. [DOI] [PubMed] [Google Scholar]
- 24. Jones RL, Fisher C, Al‐Muderis O et al. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer 2005;41:2853–2860. [DOI] [PubMed] [Google Scholar]
- 25. Italiano A, Cioffi A, Penel N et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012;118:3330–3336. [DOI] [PubMed] [Google Scholar]
- 26. Park MS, Patel SR, Ludwig JA et al. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer 2011;117:4939–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Graaf WT, Blay JY, Chawla SP et al. Pazopanib for metastatic soft‐tissue sarcoma (PALETTE): A randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 2012;379:1879–1886. [DOI] [PubMed] [Google Scholar]
- 28. Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med 2009;151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 29. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 31. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101. [PubMed] [Google Scholar]
- 32. Egger M, Davey Smith G, Schneider M et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Oosterom AT, Mouridsen HT, Nielsen OS et al. Results of randomised studies of the EORTC Soft Tissue and Bone Sarcoma Group (STBSG) with two different ifosfamide regimens in first‐ and second‐line chemotherapy in advanced soft tissue sarcoma patients. Eur J Cancer 2002;38:2397–2406. [DOI] [PubMed] [Google Scholar]
- 34. García‐Del‐Muro X, López‐Pousa A, Maurel J et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: A Spanish Group for Research on Sarcomas study. J Clin Oncol 2011;29:2528–2533. [DOI] [PubMed] [Google Scholar]
- 35. Demetri GD, von Mehren M, Jones RL et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016;34:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawai A, Araki N, Sugiura H et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation‐related sarcoma: A randomised, open‐label, phase 2 study. Lancet Oncol 2015;16:406–416. [DOI] [PubMed] [Google Scholar]
- 37. Pautier P, Floquet A, Penel N et al. Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: A Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study). The Oncologist 2012;17:1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schöffski P, Chawla S, Maki RG et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open‐label, multicentre, phase 3 trial. Lancet 2016;387:1629–1637. [DOI] [PubMed] [Google Scholar]
- 39. Bramwell VH, Mouridsen HT, Santoro A et al. Cyclophosphamide versus ifosfamide: A randomized phase II trial in adult soft‐tissue sarcomas. The European Organization for Research and Treatment of Cancer [EORTC], Soft Tissue and Bone Sarcoma Group . Cancer Chemother Pharmacol 1993;31(suppl 2):S180–S184. [PubMed] [Google Scholar]
- 40. Le Cesne A, Antoine E, Spielmann M et al. High‐dose ifosfamide: Circumvention of resistance to standard‐dose ifosfamide in advanced soft tissue sarcomas. J Clin Oncol 1995;13:1600–1608. [DOI] [PubMed] [Google Scholar]
- 41. Dileo P, Morgan JA, Zahrieh D et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: Results of a phase II trial. Cancer 2007;109:1863–1869. [DOI] [PubMed] [Google Scholar]
- 42. Stacchiotti S, Palassini E, Sanfilippo R et al. Gemcitabine in advanced angiosarcoma: A retrospective case series analysis from the Italian Rare Cancer Network. Ann Oncol 2012;23:501–508. [DOI] [PubMed] [Google Scholar]
- 43. Verweij J. Soft tissue sarcoma trials: One size no longer fits all. J Clin Oncol 2009;27:3085–3087. [DOI] [PubMed] [Google Scholar]
- 44. Germano G, Frapolli R, Belgiovine C et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013;23:249–262. [DOI] [PubMed] [Google Scholar]
- 45. Le Cesne A, Blay JY, Domont J et al. Interruption versus continuation of trabectedin in patients with soft‐tissue sarcoma (T‐DIS): A randomised phase 2 trial. Lancet Oncol 2015;16:312–319. [DOI] [PubMed] [Google Scholar]
- 46. Sleijfer S, Ray‐Coquard I, Papai Z et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European organisation for research and treatment of cancer‐soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol 2009;27:3126–3132. [DOI] [PubMed] [Google Scholar]
- 47. Mir O, Brodowicz T, Italiano A et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): A randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Oncol 2016;17:1732–1742. [DOI] [PubMed] [Google Scholar]
- 48. Tap WD, Jones RL, Van Tine BA et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft‐tissue sarcoma: An open‐label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schöffski P, Ray‐Coquard IL, Cioffi A et al. Activity of eribulin mesylate in patients with soft‐tissue sarcoma: A phase 2 study in four independent histological subtypes. Lancet Oncol 2011;12:1045–1052. [DOI] [PubMed] [Google Scholar]
- 50. Eroglu Z, Tawbi HA, Hu J et al. A randomised phase II trial of selumetinib vs selumetinib plus temsirolimus for soft‐tissue sarcomas. Br J Cancer 2015;112:1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baker LH, Rowinsky EK, Mendelson D et al. Randomized, phase II study of the thrombospondin‐1‐mimetic angiogenesis inhibitor ABT‐510 in patients with advanced soft tissue sarcoma. J Clin Oncol 2008;26:5583–5588. [DOI] [PubMed] [Google Scholar]
- 52. Blay JY, Pápai Z, Tolcher AW et al. Ombrabulin plus cisplatin versus placebo plus cisplatin in patients with advanced soft‐tissue sarcomas after failure of anthracycline and ifosfamide chemotherapy: A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2015;16:531–540. [DOI] [PubMed] [Google Scholar]
- 53. Dickson MA, Schwartz GK, Keohan ML et al. Progression‐free survival among patients with well‐differentiated or dedifferentiated liposarcoma treated with CDK4 inhibitor palbociclib: A phase 2 clinical trial. JAMA Oncol 2016;2:937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stacchiotti S, Negri T, Libertini M et al. Sunitinib malate in solitary fibrous tumor (SFT). Ann Oncol 2012;23:3171–3179. [DOI] [PubMed] [Google Scholar]
- 55. Demetri GD, Chawla SP, Ray‐Coquard I et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol 2013;31:2485–2492. [DOI] [PubMed] [Google Scholar]
- 56. Van Glabbeke M, Verweij J, Judson I et al. Progression‐free rate as the principal end‐point for phase II trials in soft‐tissue sarcomas. Eur J Cancer 2002;38:543–549. [DOI] [PubMed] [Google Scholar]