Abstract
This commentary describes the progress of the SEAL [Surrogate Endpoints for Aggressive Lymphoma] research group and invites collaboration in sharing data to continue building a large database of individual patient data from multiple clinical trials in DLBCL.
Keywords: Biomarkers, Clinical trials, Diffuse large B‐cell lymphoma, Individual patient data, Meta‐database, Surrogate endpoints
Traditional study endpoints, such as overall survival (OS), are widely accepted in current clinical studies as valid outcomes for new drug approval. However, these established primary endpoints for oncology clinical studies often require many years to attain sufficiently mature data to provide necessary power for demonstration of a clinical benefit, especially when studying novel agents. Thus, identification of potential surrogates to accompany or replace these endpoints is important for accelerating cancer treatment development. The most common and accepted methods for surrogate endpoint evaluation require individual patient data (IPD) from multiple clinical trials. Beside surrogacy evaluation studies, other evidence‐based research also recognizes the limitations of data from a single clinical trial. For example, one‐trial data commonly cannot provide sufficient sample size to address a research question targeting rare populations or events. Combining information from several individual studies may answer questions that a single trial cannot. This type of data sharing at the patient level from individual clinical trials, and subsequent integration into a comprehensive meta‐analytic database, can provide the necessary information to support goals of identifying and evaluating potential surrogate endpoints, enhancing recognition of optimal therapies, and evaluating prognostic features in rare but important populations. Such collaborations create significant challenges because they may require cooperation across international borders and data‐sharing agreements between companies, academic institutions, or both. However, multiple meta‐database collaborations (as described below, Adjuvant Colon Cancer End Points [ACCENT], Analysis and Research in Cancers of the Digestive System [ARCAD], and Follicular Lymphoma Analysis of Surrogacy Hypothesis [FLASH]) have demonstrated that these research initiatives can be successfully performed. These meta‐database groups established the statistical methods of retrospectively combining and analyzing data from large collections of previously completed studies to provide evidence for supporting evidence‐based research.
The ACCENT group validated 3‐year disease‐free survival as a surrogate endpoint of 5‐year OS for 20,898 patients from 18 studies of adjuvant treatment in colorectal cancer (CRC) [1]. The database answered significant and important questions about early stage colon cancer, including the use of adjuvant chemotherapy in elderly patients, evidence for cure by adjuvant therapy, and factors influencing survival following recurrence [2], [3]. Similarly, the ARCAD group analyzed data from 16,762 patients with metastatic CRC who received a variety of frontline therapies in the modern era. A moderate correlation between long‐term OS and early progression or death was identified at both patient and trial levels [4]. This analysis provided an updated surrogacy evaluation of progression‐free survival (PFS) for examining newer, novel treatments.
Similar analyses have occurred in patients with follicular lymphoma. The FLASH group analyzed data from patients who had received multiple types of frontline therapy and identified a robust association between complete response (CR) at 30 months and PFS in phase III trials [5]. These data provide a significantly shorter time to recognize a clinical benefit with newer therapy, whereas a much longer follow‐up time (over twice as long) is generally required to observe a median PFS in patients with follicular lymphoma. These studies have demonstrated a viable and reproducible path for evaluating surrogate endpoints that may provide an earlier indication of clinical benefit and facilitate the new drug approval process.
Establishing surrogate endpoints is especially critical in patients with more aggressive disease, such as diffuse large B‐cell lymphoma (DLBCL). As the most common aggressive form of non‐Hodgkin lymphoma (NHL), comprising ∼30% of all types of NHL [6], [7], [8], there remains a large unmet need to identify more effective therapies for patients with DLBCL. In particular, DLBCL patients who experience early relapse or primary treatment failure following standard therapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) experience poor outcomes [9], [10]. In initial studies, PFS at 24 months has been reported to be a robust endpoint for disease‐related outcome in DLBCL [11], [12]. Studies to identify surrogate endpoints for survival are needed to more rapidly identify frontline regimens that can improve outcomes for patients who are at a high risk of relapse or progression. Thus, the Surrogate Endpoints for Aggressive Lymphoma (SEAL) collaboration was established to (a) construct a meta‐database integrating IPD from randomized clinical trials in DLBCL, (b) evaluate potential surrogate endpoints for OS in DLBCL trials, and (c) support continuous translational research such as prognostic analyses, risk classifications, subgroup analyses, etc.
Here we describe the progress of the SEAL clinical trials program to date and invite colleagues to collaborate in sharing data to continue building a large meta‐database of individual patient data from multiple clinical trials. The SEAL group comprises hematologists/oncologists, scientists, and statisticians, as well as partners from Celgene who provided support for the efforts of data collection and analyses. Core team members meet on a regular basis to discuss compilation database integration and analyses of proposed projects and data access to external nonmembers, and ensure appropriate ongoing management of the information. The overall goal of SEAL is to compile a large meta‐database providing adequate source information for proposing, endorsing, analyzing, reviewing, and publishing research initiatives.
The current SEAL objectives are multifold, including the following: (a) determine trial‐ (primary) and patient‐level (secondary) correlations between surrogate endpoint candidates and OS following frontline treatment for DLBCL using well‐established statistical methods based on individual patient data from a large collection of completed, multicenter, randomized controlled clinical trials, and (b) investigate potential surrogate endpoint candidates: event‐free survival, PFS, CR at 24 months or earlier, and others as they become available. Candidate endpoints that may become available in the future include more sensitive tests for residual disease such as positron emission tomography scan and circulating tumor DNA. Biologically defined DLBCL subtypes such as cell‐of‐origin may also be evaluated in the future. Detailed definitions of these candidates and evaluation strategies are preplanned before any analyses.
The initial focus of the group will be on analyzing results from clinical trials of induction therapy in patients with DLBCL, with plans to extend the analyses into maintenance therapy upon sufficient collection of data from relevant maintenance trials. These trials should include chemotherapy and an anti‐CD20 antibody, as well as biological agents that have been studied in phase III trials, to ensure that surrogate endpoints are applicable to a wide range of agents studied in phase III trials. Potential analyses beyond surrogacy include evaluating prognostic models, performing subgroup analyses, and gaining knowledge about disease processes.
The current SEAL study was initiated with a search of the published literature. Required inclusion criteria are original clinical trials published in English after January 1, 1995, or abstracts published within the last 2 years. Studies must involve randomization of ≥100 adult patients with previously untreated DLBCL or aggressive NHL by World Health Organization/Revised European American Lymphoma classification (or any synonymous abbreviation, term, or different spelling of those terms), and at least one treatment arm should include rituximab and have an active comparator. Excluded studies are those in only early‐stage (I or II) patients, pediatrics, low‐grade or human immunodeficiency virus‐related lymphoma, relapsed/refractory disease, salvage treatment, supportive care, growth factor palliative care (as the main topic), quality of life, and health economic trials.
Trials of aggressive NHL required IPD for DLBCL patients. Based on availability of IPD from each included trial, the statistical analysis plan for evaluating potential surrogate endpoints may include more than one endpoint and subpopulation analysis. Selection of potential surrogate endpoints involves teleconferences between SEAL investigators, the SEAL executive committee, and SEAL biostatisticians after review of the literature. Surrogacy evaluation within subpopulations provides insights of consistency or heterogeneity of a particular candidate endpoint.
Table 1 provides an overview of current trials that are or will be incorporated into the SEAL database. Although response rates vary based on the selected treatment regimen and patient characteristics, initial response to chemoimmunotherapy is generally very high, leading to prolonged follow‐up and OS. Given the duration of follow‐up occurring in these trials, surrogate endpoints could provide meaningful clinical value for determining the benefits of novel therapy sooner, limiting prolonged exposure to ineffective therapies, and more rapidly identifying patients in need of additional therapy.
Table 1. Clinical trials in patients with diffuse large B‐cell lymphoma incorporated or planned for inclusion in the Surrogate Endpoints for Aggressive Lymphoma database.
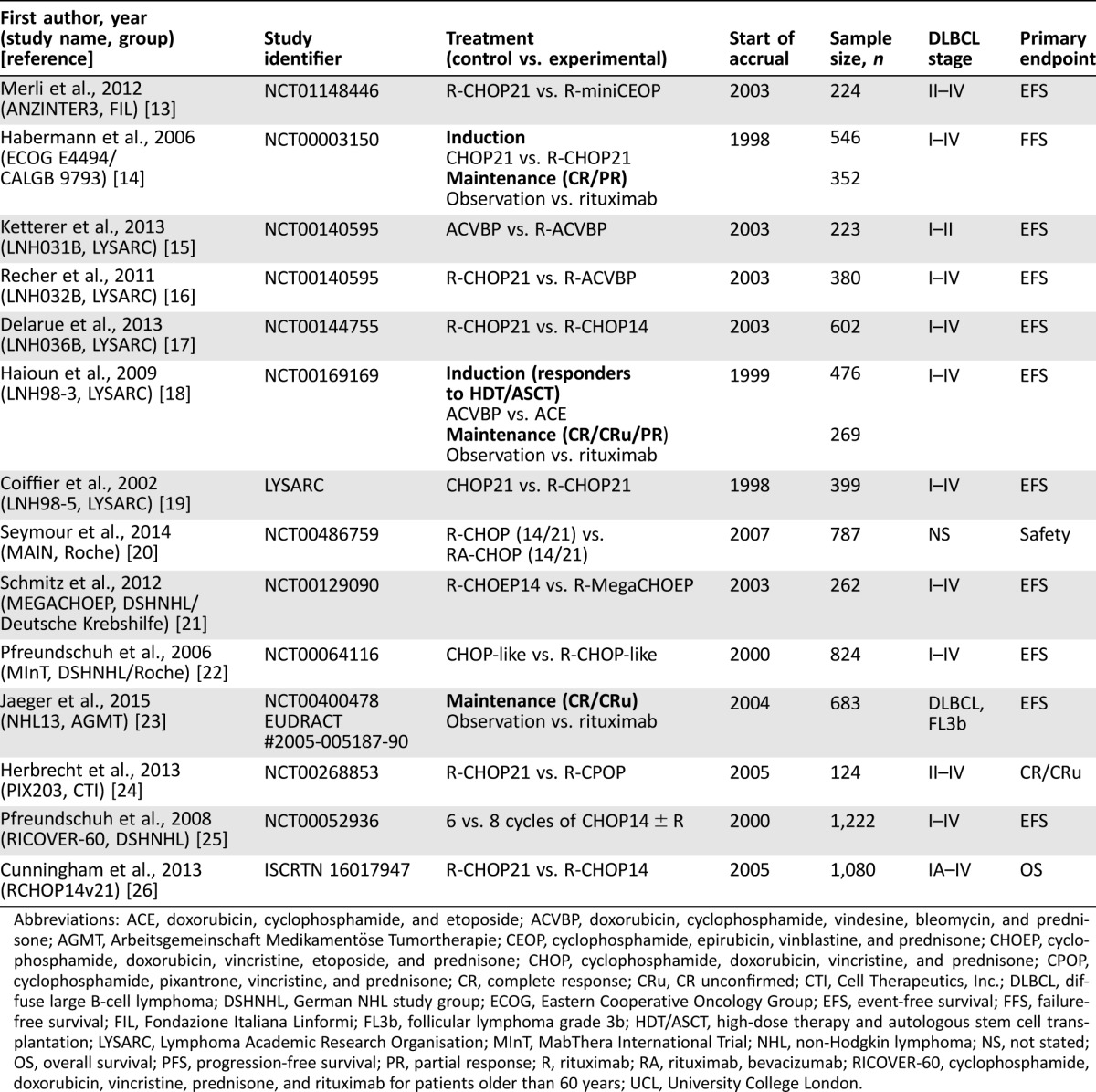
Abbreviations: ACE, doxorubicin, cyclophosphamide, and etoposide; ACVBP, doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone; AGMT, Arbeitsgemeinschaft Medikamentöse Tumortherapie; CEOP, cyclophosphamide, epirubicin, vinblastine, and prednisone; CHOEP, cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CPOP, cyclophosphamide, pixantrone, vincristine, and prednisone; CR, complete response; CRu, CR unconfirmed; CTI, Cell Therapeutics, Inc.; DLBCL, diffuse large B‐cell lymphoma; DSHNHL, German NHL study group; ECOG, Eastern Cooperative Oncology Group; EFS, event‐free survival; FFS, failure‐free survival; FIL, Fondazione Italiana Linformi; FL3b, follicular lymphoma grade 3b; HDT/ASCT, high‐dose therapy and autologous stem cell transplantation; LYSARC, Lymphoma Academic Research Organisation; MInT, MabThera International Trial; NHL, non‐Hodgkin lymphoma; NS, not stated; OS, overall survival; PFS, progression‐free survival; PR, partial response; R, rituximab; RA, rituximab, bevacizumab; RICOVER‐60, cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab for patients older than 60 years; UCL, University College London.
We encourage additional collaborators (both academic and industrial) to participate in this initiative to expand the SEAL database and contribute to the knowledge that may be derived from ongoing and future studies. At the present time, access to raw data from each trial is restricted to the SEAL coordinating statistics and data center at the Mayo Clinic because individual trials have not agreed to full public data access. Requests for specific analyses based on the SEAL database will be considered by the SEAL Steering Committee and, if approved, will be conducted by the SEAL statistics and data center in conjunction with the initiating investigator. Individual trial owners will have the ability to allow their data to be included or excluded in each specific SEAL analysis.
The SEAL's success to date provides another example of how patient‐level data can be integrated together to answer specific key questions in the field. These types of efforts complement those of larger clinical data‐sharing efforts. A major emphasis of the Cancer Moonshot, a U.S. presidential initiative to accelerate cancer research, is to assimilate data from decades of trials and thousands of individual patients across different malignancies and treatments. Publicly accessible databases, such as Project Data Sphere, where researchers can share, analyze, and integrate all available cancer research data, are becoming a critical part of cancer research communities. The goal of these larger all‐encompassing repositories is to bring all the information together upfront, which will allow researchers to ask specific questions as they arise. Databases such as SEAL are an important first step in this initiative to bring cancer researchers and their data together to answer disease‐level questions. Following the data‐sharing integration analysis model developed through the already established international meta‐database initiatives (ACCENT, ARCAD, and FLASH), SEAL collaboration can provide new insights to enhance these types of international collaborations.
We encourage you to reach out to collaborate with us on this important initiative. Contact information for inquiries about the SEAL group can be directed to the corresponding author.
Acknowledgments
We would like to thank Celgene Corporation for sponsoring the study. Editorial support for this manuscript was provided by Bio Connections LLC, which was funded by Celgene Corporation. The SEAL group (in alphabetical order) consists of the following members: Bertrand Coiffier, David Cunningham, Jocelyne Flament, Christopher R. Flowers, Tommy Fu, Herve Ghesquieres, Thomas M. Habermann, Corinne Haioun, Raoul Herbrecht, Ulrich Jaeger, Matthew J. Maurer, Francesco Merli, Tina Nielsen, Fang‐Shu Ou, Michael Pfreundschuh, Daniel J. Sargent, Norbert Schmitz, John F. Seymour, Qian Shi, Hervé Tilly, Lixia Wang, and Marita Ziepert.
Disclosures
Qian Shi: Bayer HealthCare Pharmaceuticals Inc (H); Christopher Flowers: Genentech/Roche, Gilead, AbbVie, Celgene, Seattle Genetics, Prescription Solutions, Clinical Care Options, American Society of Hematology, American Society of Clinical Oncology (C/A), AbbVie, Acerta, Celgene, Gilead Sciences, Infinity Pharmaceuticals, Janssen Pharmaceutical, Millennium/Takeda, Spectrum, Onyx Pharmaceuticals, Eastern Cooperative Oncology Group, Southwest Oncology Group, Mayo Clinic (RF); Norbert Schmitz: Celgene and Roche (RF), Janssen, Riemser, Roche, Takeda (H), Celgene, Janssen, Riemser, Roche, Takeda (Other: travel grants); Jocelyne Flament: Celgene Corporation (E, OI); Tommy Fu: Celgene Corporation (E, OI); Bertrand Coiffier: AstraZeneca, Celgene, Celltrion, Gilead, Mundipharma, Novartis, Pfizer (H, C/A, SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Sargent DJ, Wieand HS, Haller DG et al. Disease‐free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2005;23:8664–8670. [DOI] [PubMed] [Google Scholar]
- 2. O'Connell MJ, Campbell ME, Goldberg RM et al. Survival following recurrence in stage II and III colon cancer: Findings from the ACCENT data set. J Clin Oncol 2008;26:2336–2341. [DOI] [PubMed] [Google Scholar]
- 3. Sargent D, Sobrero A, Grothey A et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2009;27:872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi Q, de Gramont A, Grothey A et al. Individual patient data analysis of progression‐free survival versus overall survival as a first‐line end point for metastatic colorectal cancer in modern randomized trials: Findings from the analysis and research in cancers of the digestive system database. J Clin Oncol 2015;33:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi Q, Flowers CR, Hiddemann W et al. Thirty‐month complete response as a surrogate end point in first‐line follicular lymphoma therapy: An individual patient‐level analysis of multiple randomized trials. J Clin Oncol 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6. Teras LR, DeSantis CE, Cerhan JR et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.A clinical evaluation of the International Lymphoma Study Group classification of non‐Hodgkin's lymphoma. The Non‐Hodgkin's Lymphoma Classification Project. Blood 1997;89:3909–3918. [PubMed] [Google Scholar]
- 8. Al‐Hamadani M, Habermann TM, Cerhan JR et al. Non‐Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol 2015;90:790–795. [DOI] [PubMed] [Google Scholar]
- 9. Coiffier B, Sarkozy C. Diffuse large B‐cell lymphoma: R‐CHOP failure‐what to do? Hematology Am Soc Hematol Educ Program 2016;2016:366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costa LJ, Maddocks K, Epperla N et al. Diffuse large B‐cell lymphoma with primary treatment failure: Ultra‐high risk features and benchmarking for experimental therapies. Am J Hematol 2017;92:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi Q, Schmitz N, Flowers CR et al. Evaluation of progression‐free survival (PFS) as a surrogate endpoint for overall survival (OS) in first‐line therapy for diffuse large B‐cell lymphoma (DLBCL): Findings from the Surrogate Endpoint in Aggressive Lymphoma (SEAL) analysis of individual patient data from 7507 patients. Blood (ASH Annual Meeting Abstracts) 2016;128:4196a. [Google Scholar]
- 12. Maurer MJ, Habermann TM, Shi Q et al. Utility of progression‐free survival at 24 months (PFS24) to predict subsequent outcome for patients with diffuse large B‐cell lymphoma (DLBCL) enrolled on randomized clinical trials: Findings from a Surrogate Endpoint in Aggressive Lymphoma (SEAL) analysis of individual patient data from 5853 patients Blood (ASH Annual Meeting Abstracts) 2016;128:3027a. [Google Scholar]
- 13. Merli F, Luminari S, Rossi G et al. Cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab versus epirubicin, cyclophosphamide, vinblastine, prednisone and rituximab for the initial treatment of elderly “fit” patients with diffuse large B‐cell lymphoma: Results from the ANZINTER3 trial of the Intergruppo Italiano Linfomi. Leuk Lymphoma 2012;53:581–588. [DOI] [PubMed] [Google Scholar]
- 14. Habermann TM, Weller EA, Morrison VA et al. Rituximab‐CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B‐cell lymphoma. J Clin Oncol 2006;24:3121–3127. [DOI] [PubMed] [Google Scholar]
- 15. Ketterer N, Coiffier B, Thieblemont C et al. Phase III study of ACVBP versus ACVBP plus rituximab for patients with localized low‐risk diffuse large B‐cell lymphoma (LNH03‐1B). Ann Oncol 2013;24:1032–1037. [DOI] [PubMed] [Google Scholar]
- 16. Recher C, Coiffier B, Haioun C et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B‐cell lymphoma (LNH03‐2B): An open‐label randomised phase 3 trial. Lancet 2011;378:1858–1867. [DOI] [PubMed] [Google Scholar]
- 17. Delarue R, Tilly H, Mounier N et al. Dose‐dense rituximab‐CHOP compared with standard rituximab‐CHOP in elderly patients with diffuse large B‐cell lymphoma (the LNH03–6B study): A randomised phase 3 trial. Lancet Oncol 2013;14:525–533. [DOI] [PubMed] [Google Scholar]
- 18. Haioun C, Mounier N, Emile JF et al. Rituximab versus observation after high‐dose consolidative first‐line chemotherapy with autologous stem‐cell transplantation in patients with poor‐risk diffuse large B‐cell lymphoma. Ann Oncol 2009;20:1985–1992. [DOI] [PubMed] [Google Scholar]
- 19. Coiffier B, Lepage E, Briere J et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large‐B‐cell lymphoma. N Engl J Med 2002;346:235–242. [DOI] [PubMed] [Google Scholar]
- 20. Seymour JF, Pfreundschuh M, Trneny M et al. R‐CHOP with or without bevacizumab in patients with previously untreated diffuse large B‐cell lymphoma: Final MAIN study outcomes. Haematologica 2014;99:1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmitz N, Nickelsen M, Ziepert M et al. Conventional chemotherapy (CHOEP‐14) with rituximab or high‐dose chemotherapy (MegaCHOEP) with rituximab for young, high‐risk patients with aggressive B‐cell lymphoma: An open‐label, randomised, phase 3 trial (DSHNHL 2002‐1). Lancet Oncol 2012;13:1250–1259. [DOI] [PubMed] [Google Scholar]
- 22. Pfreundschuh M, Trumper L, Osterborg A et al. CHOP‐like chemotherapy plus rituximab versus CHOP‐like chemotherapy alone in young patients with good‐prognosis diffuse large‐B‐cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006;7:379–391. [DOI] [PubMed] [Google Scholar]
- 23. Jaeger U, Trneny M, Melzer H et al. Rituximab maintenance for patients with aggressive B‐cell lymphoma in first remission: Results of the randomized NHL13 trial. Haematologica 2015;100:955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herbrecht R, Cernohous P, Engert A et al. Comparison of pixantrone‐based regimen (CPOP‐R) with doxorubicin‐based therapy (CHOP‐R) for treatment of diffuse large B‐cell lymphoma. Ann Oncol 2013;24:2618–2623. [DOI] [PubMed] [Google Scholar]
- 25. Pfreundschuh M, Schubert J, Ziepert M et al. Six versus eight cycles of bi‐weekly CHOP‐14 with or without rituximab in elderly patients with aggressive CD20+ B‐cell lymphomas: A randomised controlled trial (RICOVER‐60). Lancet Oncol 2008;9:105–116. [DOI] [PubMed] [Google Scholar]
- 26. Cunningham D, Hawkes EA, Jack A et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B‐cell non‐Hodgkin lymphoma: A phase 3 comparison of dose intensification with 14‐day versus 21‐day cycles. Lancet 2013;381:1817–1826. [DOI] [PubMed] [Google Scholar]


