Three case reports of patients with metastatic renal cell cancer, who had a significant response to anti‐PD‐L1‐antibody when used in combination with targeted therapy (bevacizumab), radiotherapy, and anti‐CTLA‐4 therapy (ipilimumab), are presented. A review of the literature and future scope for combination therapy are discussed.
Keywords: Renal cell carcinoma, Immunotherapy, Targeted therapy, Complete response rate, Combination therapy
Abstract
Background.
Immunotherapy has historically been of interest in the management of metastatic renal cell cancer (mRCC) because of its relative chemoresistance and the reproducible but low incidence of spontaneous remission in metastatic disease. Recently, targeted immunotherapies in the form of checkpoint inhibitors have shown durable responses in approximately 20%–30% of patients with solid tumors, with a much more acceptable side‐effect profile. Anti‐programmed death receptor 1 (PD‐1)/programmed death receptor ligand 1 antibodies rely on the presence of host T cells in the tumor microenvironment to be stimulated in order to activate an antitumor response. The presence of tumor antigens augments this stimulation. This has led to further research into combination therapy with anti‐PD‐1 inhibitors and radiotherapy, chemotherapy, or targeted therapy with the aim of increasing the response rate to these agents.
Materials and Methods.
We describe three cases of patients with mRCC treated with anti‐PD‐1 antibody therapy in combination with targeted therapy (bevacizumab), anti‐cytotoxic T lymphocyte antigen 4 therapy (ipilimumab), or radiotherapy. We perform a comprehensive literature review on combination immunotherapy and the scope for the future.
Results.
Two patients had a complete clinical response within 3 months of commencing treatment. The third patient had a further significant response to radiotherapy outside the field of treatment after initial response to anti‐PD‐1 therapy, which lasted for over 12 months.
Conclusion.
We are now in the era of immunotherapy with promising results in select patients. However, the number of complete remissions with single agents are low. This report demonstrates the potential for combination therapy in mRCC to produce complete responses and improved survival rates. Whether these results equate to cure in a subset of patients requires longer follow‐up. Further evaluation of dosing regimens, sequencing methods, and biomarkers to select patient population is required to advance this treatment strategy.
Implications for Practice.
Multiple phase I–III studies exploring the benefit of combination immunotherapy are currently under way. Further research into predictive biomarkers to identify the cohort of patients who gain this benefit is pertinent. This case series demonstrates that the combination of immunotherapy with other treatments can lead to complete responses, even in patients with initially bulky disease. Combination therapy with immunotherapy seems to cause more durable responses in patients with metastatic renal cell cancer compared with monotherapy. Significantly longer follow‐up is necessary to determine whether durable complete response confers a cure in a select group of patients.
Introduction
Renal cell cancer (RCC) is the eighth most prevalent cancer in Australia, accounting for 2.5% of all cancers [1]. Current treatment options are aimed at targeting the activity of the vascular endothelial growth factor (VEGF) and the mammalian target of rapamycin pathways. Immunotherapy has historically been of interest in the management of renal cell carcinoma because of its relative chemotherapy and radiotherapy resistance and the reproducible but low incidence of spontaneous remission in metastatic disease [2], [3]. High‐dose interleukin‐2 has been curative in 5%–7% of patients; however, its use was overshadowed by multiorgan toxicities, and response to treatment is unpredictable [4], [5]. Recently, targeted immunotherapies in the form of checkpoint inhibitors targeting cytotoxic T lymphocyte antigen 4 (CTLA‐4), programmed death receptor 1 (PD‐1), or programmed death receptor ligand 1 (PD‐L1) have shown promising and durable responses in approximately 20%–30% of patients with non‐small cell lung cancer and metastatic melanoma, with a much more acceptable side‐effect profile, particularly with the anti‐PD‐1/PD‐L1 antibodies alone [6], [7], [8]. In metastatic RCC (mRCC), response rates to anti‐PD‐1 antibodies have been in the range of 20%–30%. In early phase studies, patients who received nivolumab had a 3‐year survival rate of 44% [9].
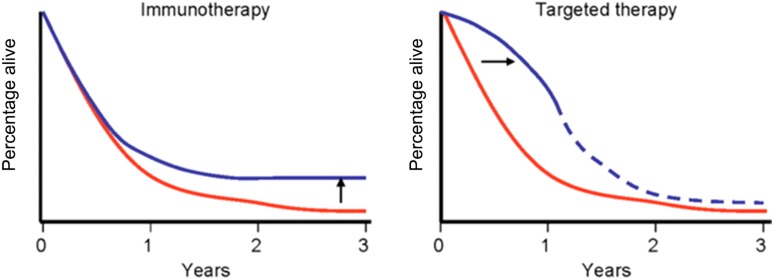
Despite demonstrating initial antitumor activity, resistance to molecular‐targeted agents often develops within the first year of treatment, signifying the need for therapies with longer, more durable responses [10], [11]. In comparison, immunotherapy has changed the pattern of response with the ability to induce long‐term remissions, albeit in a small proportion of patients, moving the survival curve up, as shown in Figure 1 [12]. One of the next ventures in mRCC management will be to try to increase this proportion of patients making up the tail end of the curve. One way in which this can be achieved is with combination treatment. Anti‐PD‐1 antibodies rely on the presence of host T cells in the tumor microenvironment to be stimulated in order to activate an antitumor response. The presence of tumor antigens augments this stimulation [13], [14].
Figure 1.
Changing pattern of survival with immunotherapy.
This has led to further research into combination therapy with anti‐PD‐1 antibodies and radiotherapy, chemotherapy, or targeted therapy with the aim of increasing the long‐term benefit of these agents.
Here we describe three cases of patients with mRCC who had a significant response to anti‐PD‐L1 antibody when used in combination with targeted therapy (bevacizumab), radiotherapy, and anti‐CTLA‐4 therapy (ipilimumab) and review the literature and future scope of combination therapy.
Case Report 1
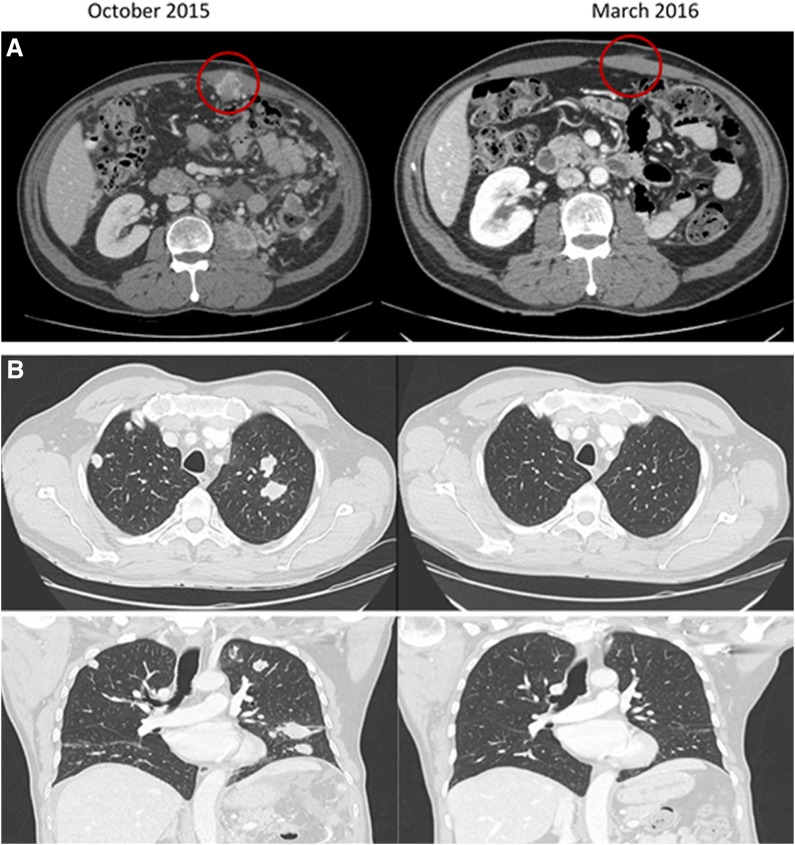
In August 2015, Mr. G.S. presented with painless hematuria, and a computed tomography scan demonstrated a large left renal mass with multiple lung and peritoneal masses. He underwent a cytoreductive laparoscopic left nephrectomy for a 135 × 80 mm clear cell RCC, nuclear grade 4, with areas of necrosis. He entered a randomized controlled study and was allocated to receive atezolizumab and bevacizumab for mRCC. He commenced treatment on November 4, 2015, with no infusion reaction. On November 19, Mr. G.S. presented with a 4‐day history of coryzal symptoms with fevers, chills, and abdominal pain. Laboratory examination revealed hyponatremia and mildly elevated C reactive protein. A palpable mass in the left lower quadrant enlarged clinically consistent with a “flare” reaction. Septic workup was negative, and Mr. G.S. recovered with supportive care and 48 hours of intravenous antibiotics. He remained on study treatment, and subsequently there was a reduction in the size of the upper abdominal mass, and staging scans showed almost complete response in all lesions (Fig. 2). He continued on treatment with no further adverse events and remained in complete remission 18 months after starting therapy.
Figure 2.
Resolution of metastases after atezolizumab and bevacizumab. (A): Anterior abdominal wall mass (red circle). (B): Pulmonary metastases.
Bevacizumab and Anti‐PD‐L1 Antibody
The tumor's ability to immunosuppress its microenvironment includes adapting the tumor vasculature [15]. VEGF is a potent angiogenic factor that regulates angiogenesis and increases proliferation, migration, and metastasis. VEGF may also have an effect in immune regulation because it has been shown to inhibit dendritic cell maturation and T‐cell responses, thereby suppressing antitumor immune responses [16], [17]. In a study by Yuan et al., patients with metastatic melanoma treated with ipilimumab, pretreatment serum levels of vascular endothelial growth factor‐A (VEGF‐A) (but not change in VEGF‐A level) correlated with overall survival [18], leading to a phase I study of ipilimumab and bevacizumab in patients with metastatic melanoma. The study demonstrated an objective response rate of 19.6% and median survival of 25.1 months, longer than that seen with ipilimumab alone (10.1 months) [19]. The combination treatment was relatively well tolerated. Biopsy samples taken after the treatment further revealed lymphocyte‐mediated destruction of blood vessels feeding the tumor deposits.
A phase I study evaluating MPDL3280A (anti‐PD‐L1 antibody) in combination with bevacizumab in ten patients with mRCC demonstrated an overall response rate of 40% and was well tolerated, with no synergistic toxicity seen [20].
Similarly, high activity was seen in a phase I study of nivolumab in combination with sunitinib (S) or pazopanib (P) with response rates of 52% (17/33 patients on S) and 45% (9/20 patients on P). However, the safety profile was significantly high, with grade 3–4‐related adverse events (AEs) observed in 27/33 patients (82%) in arm S and 14/20 patients (70%) in arm P. Most common related grade 3–4 AEs were elevated alanine aminotransferase (ALT) and hypertension (18% each); hyponatremia and lymphocyte count decreased (15% each) in arm S, and elevated ALT and aspartate aminotransferase as well as diarrhea (20% each) and fatigue (15%), occurred in arm P. Grade 3–4‐related AEs led to discontinuation in 10/33 patients (30%; 2 N2 mg/kg, 8 N5 mg/kg) in arm S and 4/20 patients (20%) in arm P [21]. Phase I–III studies are currently under way, further examining the combination of anti‐PD‐L1 antibodies and targeted therapies (Table 1).
Table 1. Combination targeted therapies and immunotherapy in renal cell cancer.
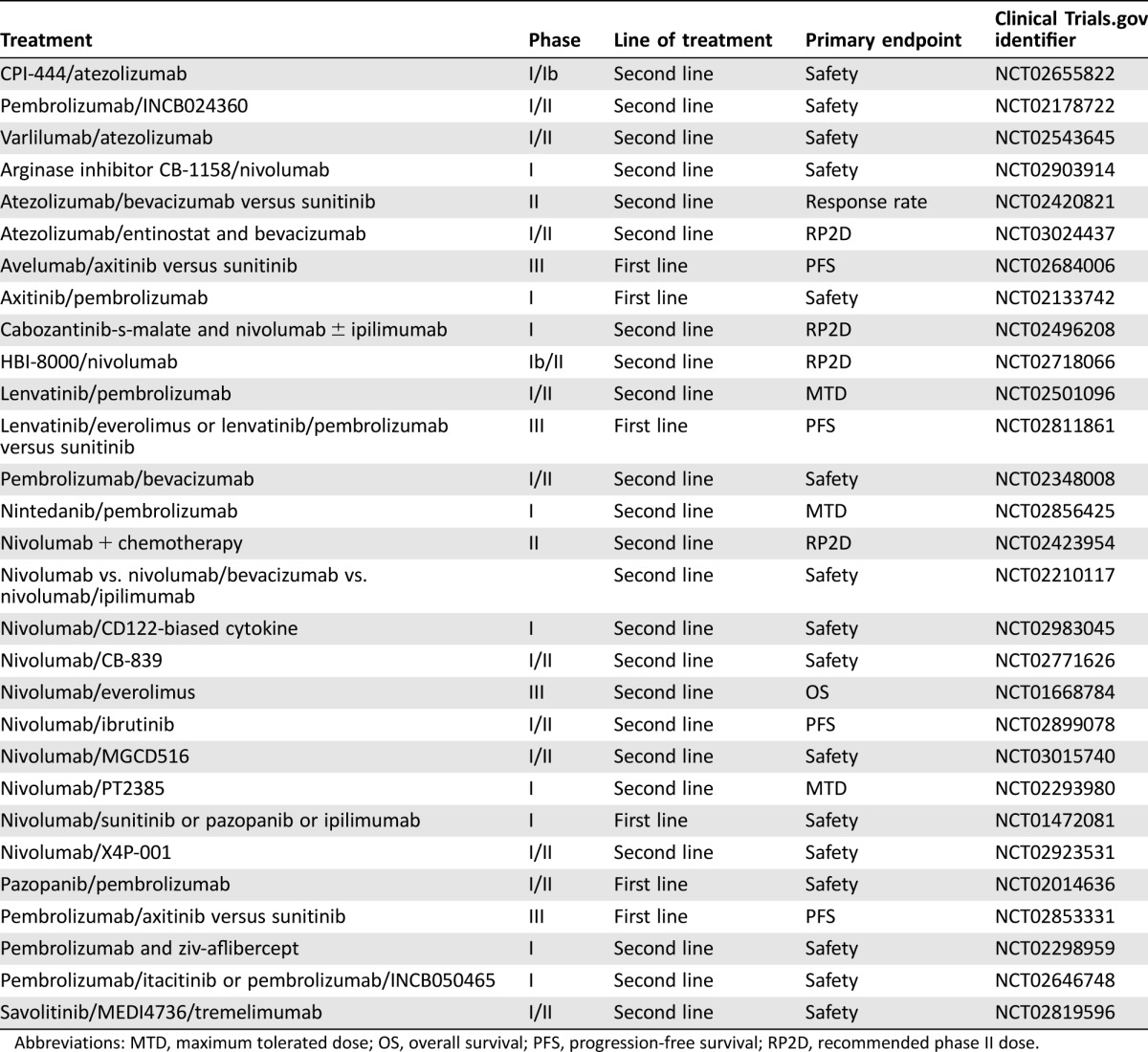
Abbreviations: MTD, maximum tolerated dose; OS, overall survival; PFS, progression‐free survival; RP2D, recommended phase II dose.
Case Report 2
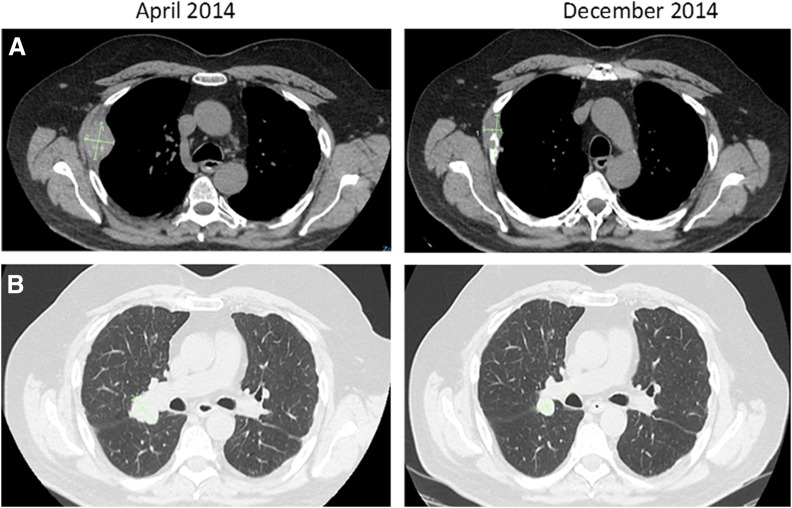
In 2010, J.M. was diagnosed with RCC after investigations for vague abdominal pain revealed a large right renal mass and multiple lung and bone metastases. Cytoreductive nephrectomy revealed a nuclear grade 4 clear cell carcinoma with focal extension in the perirenal fat. Lung metastases progressed, and in November 2015, he commenced sunitinib. Despite an initial response, J.M. had progressive disease manifest as pleural effusion and bronchial obstruction, resulting in massive hemoptysis. He commenced on anti‐PD‐1 antibody, nivolumab, in April 2013 with an immediate partial response. In January 2014, he underwent a single fraction of palliative radiotherapy for a lytic lesion to the right fourth rib causing severe pain, while continuing with two weekly treatments of nivolumab. After a transient increase in the size of the lytic lesion, J.M. again received a dramatic response clinically and radiologically in all lesions, including those outside the radiotherapy field (abscopal effect) (Fig. 3). As part of the known spectrum of immune toxicities, J.M. developed autoimmune arthritis and was treated successfully with a weaning dose of oral prednisone. The clinical response after radiotherapy lasted for over 12 months; however, in August 2015, J.M. had further disease progression in the spine, managed through surgical decompression and adjuvant radiotherapy. Nivolumab was discontinued, and he commenced on axitinib approximately 5 years after his initial diagnosis for metastatic renal cell carcinoma.
Figure 3.
Response to radiotherapy and immunotherapy including out of field response (abscopal effect). (A): Right rib lesion receiving radiotherapy shows healing of bone and reduction in size of mass. (B): Lung lesion not in the field of radiotherapy shows further response to treatment.
Synergistic Effects of Radiotherapy and Immune Therapy
Abscopal or off‐target effects have been reported in patients with metastatic melanoma in which the combination of radiotherapy and ipilimumab led to widespread tumor response outside the field of radiation [22].
Radiotherapy has been shown to increase the expression of the immunosuppressive protein PD‐L1 on tumor cells and has the potential to overcome many of the mechanisms of tumor immune escape through the release of immunogenic private antigens, enhanced tumor expression of Major Histocompatibility Class I (MHC‐I), release of the toll like receptor 4 (TLR4) agonist HMGB‐1, and the generation of tumor‐specific cytotoxic T cells [23]. Xenograft models of breast cancer, malignant melanoma, glioblastoma, and colon cancer have demonstrated that the addition of radiotherapy to the anti‐PD‐1 antibodies synergistically improves antitumor immunity by increasing antigen presentation and promoting an immune‐rich tumor microenvironment [24], [25], [26].
It is unclear what dosing regimens, radiotherapy dose, or optimal site of radiotherapy or sequencing of treatment will give the best response, and the combination needs to be carefully evaluated with regards to toxicity. Clinical trials examining the utility of the combination of radiotherapy and immunotherapy are currently under way (Table 2).
Table 2. Combination radiotherapy and immunotherapy studies in renal cell cancer.
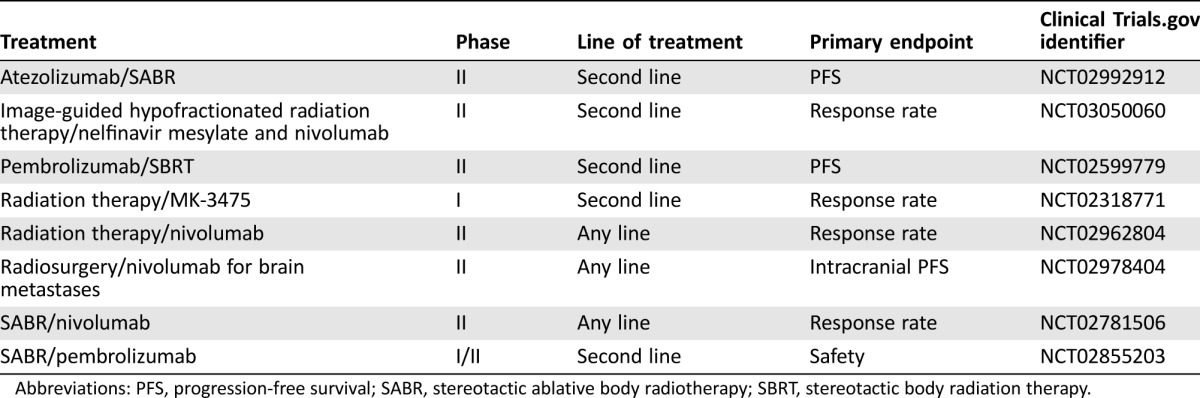
Abbreviations: PFS, progression‐free survival; SABR, stereotactic ablative body radiotherapy; SBRT, stereotactic body radiation therapy.
Case 3
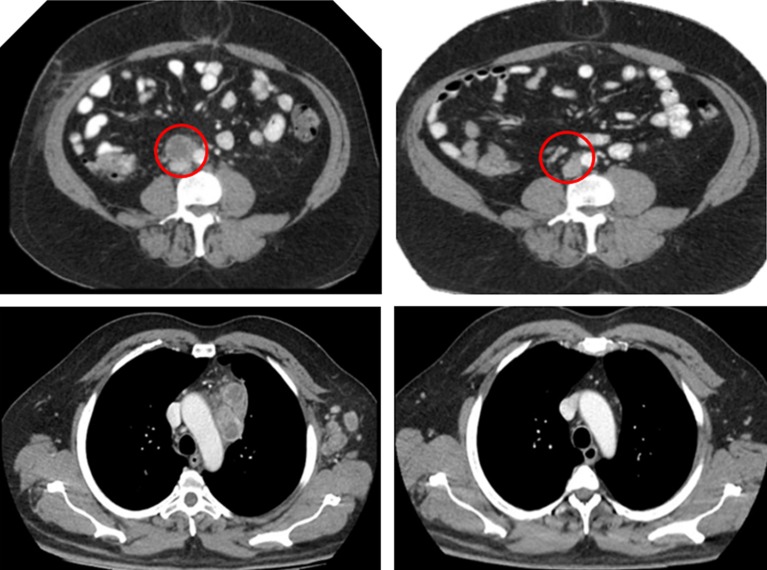
Mr. A.G. presented with a 6‐month history of fatigue and weight loss in January 2015, and investigations led to the diagnosis of mRCC with extensive para‐aortic, supraclavicular, and mediastinal lymphadenopathy. He underwent a cytoreductive nephrectomy, which revealed a nuclear grade 4 clear cell carcinoma. He was recruited to the phase III Bristol‐Myers Squibb CA209‐214 study of ipilimumab plus nivolumab versus sunitinib, in which he was randomized to receive the combination immunotherapy protocol. A.G. sustained an immediate and complete response on routine staging scans after four cycles of combination treatment (Fig. 4). Treatment was well tolerated except for the development of autoimmune thyroiditis, resulting in hypothyroidism, which was managed uneventfully with thyroxine. A.G. continues with twice weekly single‐agent nivolumab and remains in complete response.
Figure 4.
Resolution of para‐aortic lymphadenopathy (red circle).
Ipilimumab and Anti‐PD‐1 Antibody
Combination CTLA‐4 and PD‐1/PD‐L1 blockade has a strong scientific rationale with some evidence of synergy. In a B16 melanoma model, synergistic activity between CTLA‐4, anti‐PD‐1, and anti‐PD‐L1 antibodies was demonstrated, with inhibition of CTLA‐4 increasing the proportion of express PD‐1‐expressing, tumor‐infiltrating lymphocytes (TILs) [27]. Furthermore, PD‐1 blockade leads to upregulation of CTLA‐4 on TILs. The efficacy of PD‐1 blockade may be compromised by the suboptimal activation of tumor‐specific effector T cells mediated by CTLA‐4, which also may be aggravated by upregulation of CTLA‐4 induced by the PD‐1 inhibition itself. A phase I study evaluating the safety and tolerability of ipilimumab and nivolumab demonstrated a response rate of 29%–39% in mRCC, with an acceptable safety profile, and a phase III trial of this combination has finished recruitment (clinicaltrials.gov identifier NCT02231749). Clinical trials examining the utility of the combination of radiotherapy and immunotherapy are currently underway (Table 3).
Table 3. Combination immunotherapy studies in renal cell cancer.
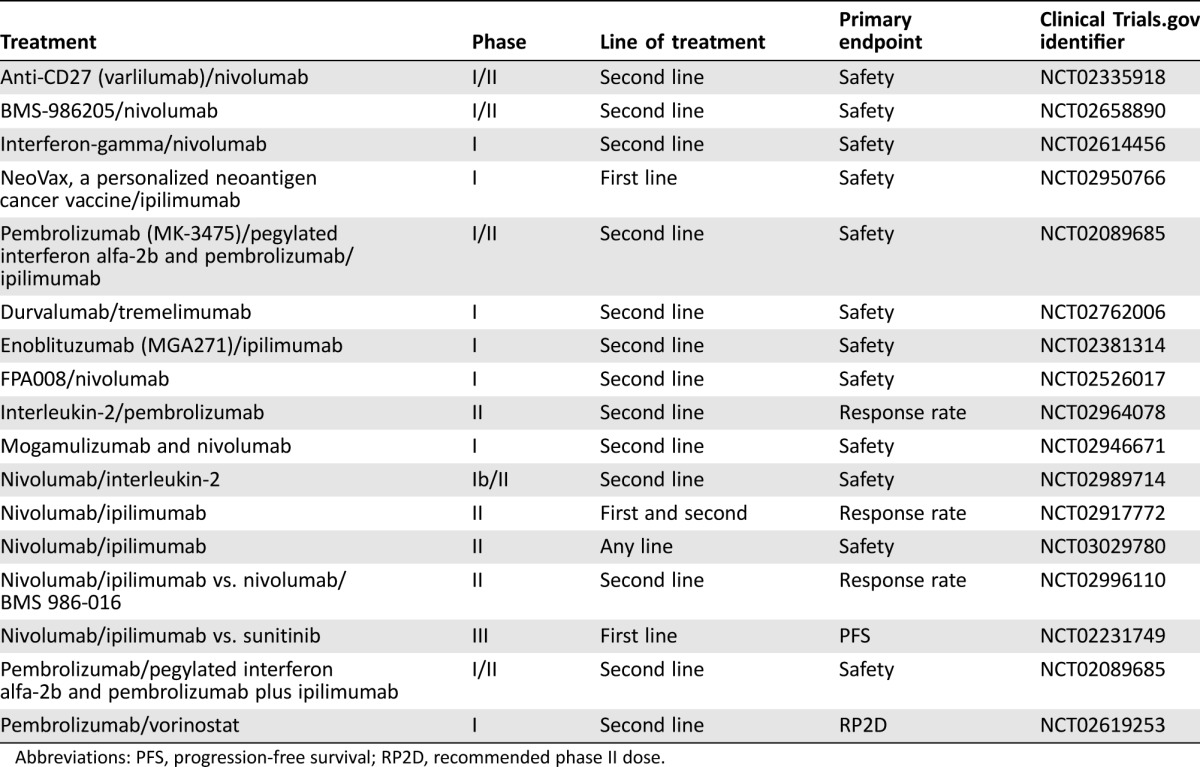
Abbreviations: PFS, progression‐free survival; RP2D, recommended phase II dose.
Discussion
In this case series, we have described three cases of patients on anti‐PD‐1/PD‐L1 antibodies in combination with another mode of treatment: radiotherapy, anti‐VEGF targeted therapy, and anti‐CTLA‐4 therapy. These significant clinical responses suggest that combination treatment with anti‐PD‐1/PD‐L1 antibodies in mRCC may be the gateway to achieve maximum potential response.
The human body is innately automated to prevent overactivation of the immune system to prevent harm to healthy tissue. Cancer takes advantage of this self‐protective mechanism and evades the immune system via multiple escape mechanisms, one of which involves the checkpoint inhibitor PD‐1, an inhibitory T‐cell receptor found primarily within the tumor microenvironment. The selectivity of the immune suppression from the cancer makes PD‐1 inhibition generally better tolerated with less off‐target toxicity, especially when compared with CTLA‐4 antibodies. Large studies have now been conducted demonstrating the utility of anti‐PD‐1 and anti‐PD‐L1 antibodies, with a response rate of approximately 20% in some tumor types [7], [8], [28]. Nivolumab has shown a survival advantage when compared with everolimus, with a median overall survival of 25 months in patients previously treated with first‐line tyrosine kinase inhibitors. However, the overall response rates are only 25%, indicating the need for more effective therapy [12], [29]. In contrast, however, phase I/II studies have demonstrated responses doubling this with the combination of nivolumab and ipilimumab (response rate [RR] 45%) [30], [31] and with nivolumab and sunitinib (RR 52%) [21]. Remarkably, complete responses to combination immunotherapy with targeted therapy are significantly more than those seen using monotherapy (complete response [CR] 9% and CR 6% vs. CR 0%–2%) [21], [30].
This phenomenon has already been well established in metastatic melanoma. The CHECKMATE 067 study of nivolumab and ipilimumab combination immunotherapy has already demonstrated superior response rates compared with either treatment alone. These results are independent of PD‐L1 or BRAF mutation status. Patients who do respond tend to have a deep nadir and continue to respond. Although a significant proportion of patients do stop treatment due to toxicity (40%), 68% of patients who discontinued treatment demonstrated an objective response, half of these after the treatment had stopped (Table 4).
Table 4. Selected trials of combination therapy in metastatic melanoma.
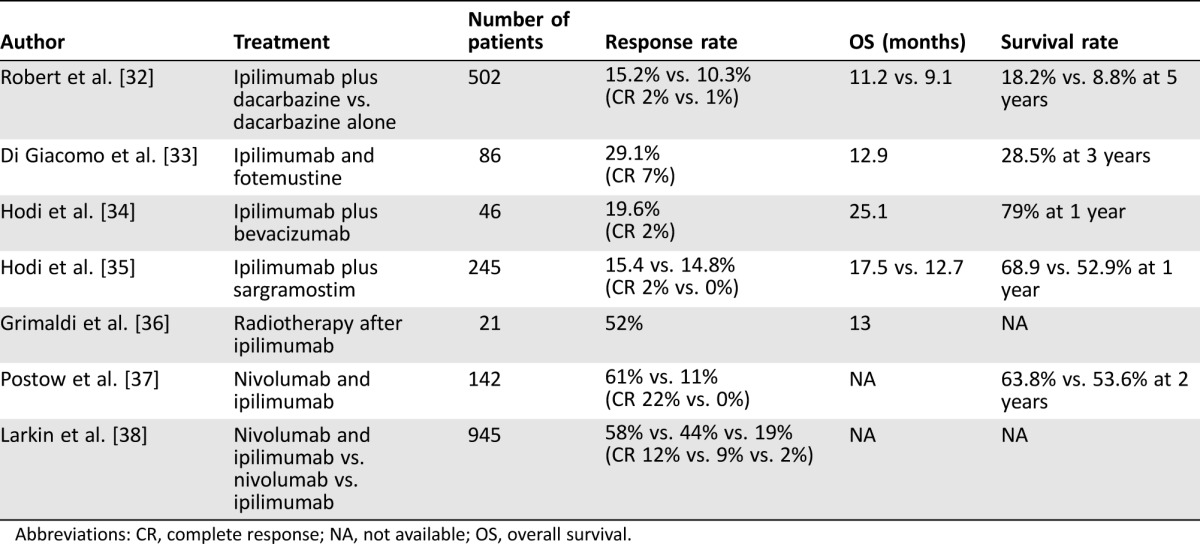
Abbreviations: CR, complete response; NA, not available; OS, overall survival.
Studies evaluating the effect of combining BRAF inhibition with immune‐directed therapy have so far encountered challenges related to the increased autoimmune toxicities. The first study of vemurafenib and ipilimumab had to be terminated due to significant hepatotoxicity [39]. Another study of sequential vemurafenib after ipilimumab showed a significant increase in the rate of high grade skin toxicity, not responsive to steroids [40]. Subsequently, MEK inhibitors used in combination with BRAF inhibitors are now being trialed with immunotherapy in the hope that the MEK inhibition can dampen the toxicity and balance the potential tumor response.
From the melanoma data, it is clear that combination immunotherapy certainly holds promise with long durable responses; however, the potential adverse effects of combining such therapies need to be carefully considered when selecting patients for this treatment.
Conclusion
Considerable advances have been made in the treatment of mRCC over the last decade. Current treatment paradigms for mRCC involve targeted therapies, which result in high response rates. Before long, however, resistance to treatment ensues, with subsequent disease progression.
Immunotherapy has shown a great deal of promise in the management of many solid tumors, including melanoma, non‐small cell lung carcinoma, and RCC, with durable benefit, albeit in a select group of patients, with the number of complete remissions with monotherapy still low. Combination treatment seems to be the next rational approach that may increase durable survival rates, with a growing body of evidence to support this. In metastatic melanoma, combination therapy has seen long‐term disease control, with a significant number of complete responses, albeit at the cost of enhanced toxicity that has been seen with some combination approaches. Whether these results equate to cure in a subset of patients requires longer follow‐up. This case series shows the same potential for combination therapy in mRCC, with dual immunotherapy agents with different targets, immunotherapy with anti‐VEGF therapy, or in combination with radiotherapy.
Larger cohort studies are currently under way exploring these exact mechanisms. Further evaluation of dosing regimens, sequencing methods, and biomarkers to select patient population will offer a substantial advance in the treatment of this disease.
Author Contributions
Conception/design: Dhanusha Sabanathan
Administrative support: Dhanusha Sabanathan; John J. Park; Manuel Marquez; Louise Francisco; Natalie Byrne; Howard Gurney
Provision of study material or patients: Dhanusha Sabanathan; John J. Park; Manuel Marquez; Louise Francisco; Natalie Byrne; Howard Gurney
Collection and/or assembly of data: Dhanusha Sabanathan; John J. Park; Manuel Marquez; Louise Francisco; Natalie Byrne; Howard Gurney
Data analysis and interpretation: Dhanusha Sabanathan; John J. Park; Manuel Marquez; Louise Francisco; Natalie Byrne; Howard Gurney
Manuscript writing: Dhanusha Sabanathan; John Park; Howard Gurney
Final approval of manuscript: Dhanusha Sabanathan; John J. Park; Manuel Marquez; Louise Francisco; Natalie Byrne; Howard Gurney
Disclosures
Howard Gurney: Bristol‐Myers Squibb, Pfizer, Merck, Roche (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Australian Institute of Health and Welfare. Cancer in Australia: An Overview , 2014. Cancer series no. 78. Canberra, Australia: Australian Institute of Health and Welfare, 2014. [Google Scholar]
- 2. Ray A, Ghosh SK. Long natural history and spontaneous regression: A rare outcome of metastatic renal cell carcinoma. Clin Cancer Investig J 2014;3:447–449. [Google Scholar]
- 3. Edwards MJ, Anderson JA, Angel JR et al. Spontaneous regression of primary and metastatic renal cell carcinoma. J Urol 1996;155:1385. [PubMed] [Google Scholar]
- 4. Fyfe G, Fisher RI, Rosenberg SA et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high‐dose recombinant interleukin‐2 therapy. J Clin Oncol 1995;13:688–696. [DOI] [PubMed] [Google Scholar]
- 5. Gore ME, Griffin CL, Hancock B et al. Interferon alfa‐2a versus combination therapy with interferon alfa‐2a, interleukin‐2, and fluorouracil in patients with untreated metastatic renal cell carcinoma (MRC RE04/EORTC GU 30012): An open‐label randomised trial. Lancet 2010;375:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non–small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 9. McDermott DF, Drake CG, Sznol M et al. Survival, durable response, and long‐term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 2015;33:2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Motzer RJ, Hutson TE, Cella D et al. Pazopanib versus sunitinib in metastatic renal‐cell carcinoma. N Engl J Med 2013;369:722–731. [DOI] [PubMed] [Google Scholar]
- 11. Brower V. Second‐line everolimus treatment in advanced renal‐cell cancer. Lancet Oncol 2012;13(suppl 6):e235. [Google Scholar]
- 12. Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer immunology research 2015;3:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quezada SA, Peggs KS. Exploiting CTLA‐4, PD‐1 and PD‐L1 to reactivate the host immune response against cancer. Br J Cancer 2013;108:1560–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang Y, Yuan J, Righi E et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA 2012;109:17561–17566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dikov MM, Ohm JE, Ray N et al. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol 2005;174:215–222. [DOI] [PubMed] [Google Scholar]
- 17. Oyama T, Ran S, Ishida T et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor‐kappa B activation in hemopoietic progenitor cells. J Immunol 1998;160:1224–1232. [PubMed] [Google Scholar]
- 18. Yuan J, Zhou J, Dong Z et al. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 2014;2:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hodi FS, Lawrence D, Lezcano C et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2014;2:632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wallin JJ, Bendell JC, Funke R et al. Atezolizumab in combination with bevacizumab enhances antigen‐specific T‐cell migration in metastatic renal cell carcinoma. Nature Commun 2016;7:12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amin A, Plimack ER, Infante JR et al. Nivolumab (anti‐PD‐1; BMS‐936558, ONO‐4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2014;32(suppl 15):5010a. [Google Scholar]
- 22. Postow MA, Callahan MK, Barker CA et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalbasi A, June CH, Haas N et al. Radiation and immunotherapy: A synergistic combination. J Clin Invest 2013;123:2756–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deng L, Liang H, Burnette B et al. Irradiation and anti‐PD‐L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharabi AB, Nirschl CJ, Kochel CM et al. Stereotactic radiation therapy augments antigen‐specific PD‐1‐mediated antitumor immune responses via cross‐presentation of tumor antigen. Cancer Immunol Res 2015;3:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeng J, See AP, Phallen J et al. Anti‐PD‐1 blockade and stereotactic radiation produce long‐term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 2013;86:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Curran MA, Montalvo W, Yagita H et al. PD‐1 and CTLA‐4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 2010;107:4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Topalian SL, Sznol M, Brahmer JR et al. Nivolumab (anti‐PD‐1; BMS‐936558; ONO‐4538) in patients with advanced solid tumors: Survival and long‐term safety in a phase I trial. J Clin Oncol 2013;31(suppl 15):3002a. [Google Scholar]
- 29. Motzer RJ, Rini BI, McDermott DF et al. Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Oncol 2015;33:1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hammers HJ, Plimack ER, Infante JR et al. Expanded cohort results from CheckMate 016: A phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). J Clin Oncol 2015;33(suppl 15):4516a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hammers HJ, Plimack ER, Sternberg C et al. CheckMate 214: A phase III, randomized, open‐label study of nivolumab combined with ipilimumab versus sunitinib monotherapy in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2015;33(suppl 15):4578a. [Google Scholar]
- 32. Robert C, Thomas L, Bondarenko I et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 33. Di Giacomo AM, Ascierto PA, Queirolo P et al. Three‐year follow‐up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian Network for Tumor Biotherapy (NIBIT)‐M1 phase II study. Ann Oncol 2015;26:798–803. [DOI] [PubMed] [Google Scholar]
- 34. Hodi FS, Lawrence D, Lezcano C et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2014;2:632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hodi FS, Lee S, McDermott DF et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: A randomized clinical trial. JAMA 2014;312:1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grimaldi AM, Simeone E, Giannarelli D et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014;3:e28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Postow MA, Chesney J, Pavlick AC et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ribas A, Hodi FS, Callahan M et al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 2013;368:1365–1366. [DOI] [PubMed] [Google Scholar]
- 40. Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med 2012;366:866–868. [DOI] [PubMed] [Google Scholar]