Key Points
The therapeutic efficacy of the AZT and IFN combination in ATL presumably reflects the inhibition of RT-related functions.
HTLV-1–RT activity from short-term cultured PBMCs may represent a predictive correlate of clinical response to AZT/IFN in ATL patients.
Introduction
The human T-lymphotropic virus type 1 (HTLV-1) retrovirus1 is the etiological agent of adult T-cell leukemia/lymphoma (ATL), HTLV-1–associated myelopathy/tropical spastic paraparesis, and a number of inflammatory diseases.2 ATL is an aggressive malignancy occurring mostly in adults 30 to 50 years after HTLV-1 infection. ATL cells are characterized by low virus expression in vivo. The prognosis is very poor, ranging from 6 to 24 months median survival. Chemotherapy has little long-term efficacy in ATL. Allogeneic stem cell transplantation can result in long-term disease control but few patients can be successfully transplanted.3
Antiretrovirals with activity against HIV-1, such as zidovudine (3′-azido-2′,3′-dideoxythymidine [AZT]) and tenofovir, and new phosphonated compounds, efficiently block HTLV-1 transmission in vitro,4-7 whereas HTLV-1 was resistant to lamivudine.8
Two preliminary phase 2 studies using the combination of AZT and interferon-α (IFN-α) reported an unexpectedly high response rate, particularly in previously untreated acute ATL patients.9-11 The efficacy of this combination was confirmed, with minor differences, in various clinical trials and in a worldwide meta-analysis.12-16 These results changed the clinical management of ATL.16-19 However, there is yet no direct proof of an antiviral mechanism of action of the drug combination, due to the absence of virological markers that strictly correlate with disease status.
In this study, we investigated HTLV-1 reverse transcriptase (RT) activity and other virological parameters, in samples from short-term cultures of peripheral blood mononuclear cells (PBMCs) collected from ATL patients before and after therapy.
Methods
Patients and samples
This retrospective cohort study was carried out using PBMCs collected and immediately frozen from 10 HTLV-1+ ATL patients. For 7 patients, PBMCs were collected both before therapy and 1 to 2 years after initiation of antiviral therapy with AZT (900 mg per day) and IFN (5 million IU/m2 per day).13 For 3 patients, only diagnostic samples were available (characteristics of the patients and further details on sample processing are available as supplemental Methods).
Cell cultures and quantification of virological parameters
PBMCs were cryopreserved until use. After 24 hours, and after 7 and 10 days in culture, supernatants were harvested and frozen, and the cell concentration was adjusted to the initial value with fresh medium. We were unable to set up cell cultures from 1 of the 7 treated patients, who was therefore excluded from further analysis.
HTLV-1 expression was evaluated in RNA isolated from cells collected after 7 days in culture, using the RNAqueous-Micro kit (Ambion), by quantitative real-time polymerase chain reaction (PCR) for tax/rex expression performed in triplicate as previously described.20
HTLV-1 p19 was detected in supernatants from ATL cultures, using an HTLV-1 p19 Gag antigen capture enzyme-linked immunosorbent assay (ZeptoMetrix Corp) according to the manufacturer’s protocols. p19 concentrations in the supernatants harvested after 24 hours of culture did not differ from those in supernatants from the same culture harvested later on. Consequently, supernatants collected at different times from the same culture were pooled.
HTLV-1 RT activity was quantified in crude whole lysates of the same pools of supernatants used for p19 detection, using our recently described cell-free HTLV-1 RT assay.7 First, we tested the reliability of our HTLV-1 RT assay using serial dilution of MT2 supernatants mimicking conditions of those from ATL PBMC cultures. DNA extracted from cells harvested after 10 days in culture was used for HTLV-1 clonality analysis of samples collected before and after therapy for 1 representative responder patient and 1 nonresponder patient (details of the assays and of the preliminary tests are given in supplemental Methods).
Results and discussion
AZT and IFN therapy decreased HTLV-1 tax/rex transcripts
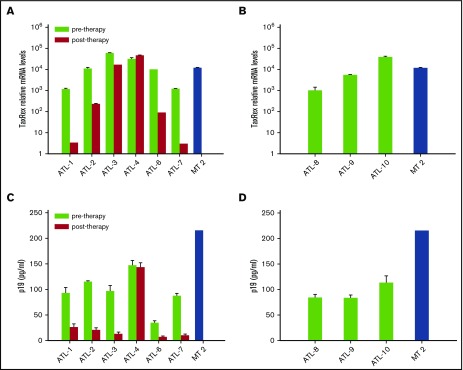
We quantified tax/rex transcripts by quantitative real-time PCR. At the time of collection of the posttherapy sample, 4 patients (ATL 1, 2, 3, 6) were in complete response and 1 (ATL 7) was in good partial response to AZT/IFN, whereas 1 patient with acute ATL (ATL 4) was resistant to this therapy (supplemental Results). The expression of tax/rex in PBMC cultures from ATL 1, 2, 6, and 7 was variably inhibited in comparison with pretreatment samples, whereas minimal change was seen in ATL 3 and no change was observed in ATL 4 (Figure 1A). Expression of tax/rex in diagnostic samples from ATL 8, 9, and 10 was comparable to pretreatment samples of treated ATL patients (Figure 1B).
Figure 1.
tax/rex expression and p19 detection in samples from short-term PBMC cultures of ATL patients pre– and post–in vivo therapy with AZT/IFN. (A) tax/rex messenger RNA (mRNA) expression was detected by quantitative real-time PCR from cells collected after 7 days of short-term PBMC cultures of ATL patients. The results represent the mean values of triplicate samples expressed as relative tax/rex mRNA expression ± standard deviation (SD) in log scale, with respect to the untreated samples with least tax/rex expression. (B) tax/rex mRNA expression, detected and expressed as in panel A, referring to diagnostic samples from ATL patients (ATL 8, 9, 10). (C) p19 level (mean ± SD of quadruplicate samples, in picograms per milliliter) measured by antigen capture assay in pools of PBMC culture supernatants from each ATL patient or control HTLV-1–transformed cell line MT-2. (D) p19 level, detected and expressed as in panel C, referring to diagnostic samples from ATL patients (ATL 8, 9, 10).
AZT and IFN therapy reduced p19 release
Supernatants from PBMC cultures of treated patients ATL 1, 2 and 3, 6, and 7 exhibited a decrease of p19 production by 75% to 88% in comparison with those of the same patients collected before treatment (Figure 1C). Conversely, no difference occurred in ATL 4 before vs after treatment. Levels of p19 in samples from newly diagnosed ATL patients (ATL 8, 9, 10) were similar to pretreatment samples of treated patients (Figure 1D).
AZT and IFN therapy completely inhibited RT activity and modified the clonality pattern in samples from responding ATL patients
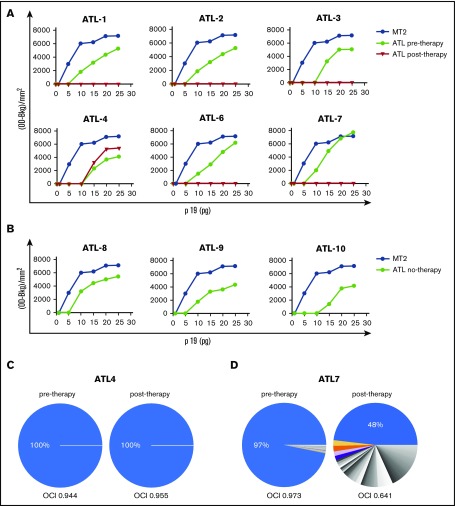
RT preparations consisting of samples containing 5 to 25 pg of p19 in 10 μL, following readjustment from the crude whole lysates of supernatants, were used as a source of RT in the in vitro quantitative real-time PCR assay. The amplification products were assayed by densitometry (Figure 2A). In all samples, the plateau of amplification was reached in the presence of 20 to 25 pg of the p19 equivalent amount of RT preparation. However, the RT activity detected in 20-pg p19 equivalent RT preparations from ATL samples before treatment was around 30% less than that detected in the same p19 equivalent amount of MT-2 supernatants. The RT preparations from ATL patients (1, 2, 3, 6, 7) before treatment exhibited an activity similar to newly diagnosed ATL patients (8, 9, 10) (Figure 2B). The RT activity was completely inhibited in samples collected after therapy in ATL 1, 2, 3, 6, and 7 patients at all p19 equivalent amounts tested (Figure 2A). Conversely, the RT activity detected in the sample from the ATL 4 patient after treatment was similar to that of the same patient before treatment.
Figure 2.
HTLV-1 RT activity and clonality analysis in samples from short-term PBMC cultures from ATL patients pre– and post–in vivo therapy with AZT/IFN. (A) Serial amounts of 5 to 25 pg p19-equivalent RT preparations obtained from supernatants of short-term cultured PBMCs from ATL patients, pre– and post–in vivo therapy with AZT/IFN, were added in the quantitative real-time PCR and HTLV-1 RT activity was assessed. The yield of PCR-amplified products, quantified by densitometry, is shown on the vertical axis. HTLV-1 RT activity in RT preparations obtained from supernatants of MT-2 cells was assayed as a control. (B) HTLV-1 activity, detected and expressed as in panel A, referring to diagnostic samples from ATL patients (8, 9, 10). (C-D) HTLV-1 clonality analysis. The relative abundance of HTLV-1–infected T-cell clones was quantified by linker-mediated PCR and high-throughput sequencing, as previously described.21 Each sector of the pie chart denotes a single clone of HTLV-1–infected cells, a clone being defined by the genomic integration site of the (single-copy) provirus. Clones with a relative abundance >1% are colored; lower-abundance clones are shown in gray. The width of each sector indicates the relative abundance of that clone. An OCI > 0.7 is characteristic of ATL.22 The HTLV-1 proviral loads were as follows: ATL4 (pretherapy, 57.12%; posttherapy, 53.47%); ATL7 (pretherapy, 11.65%; posttherapy, 13.93%). OD-Bkg, optical density–background.
Whereas no difference was observed in the HTLV-1 proviral loads, the clonality analysis showed a dramatic shift from a monoclonal pretherapy pattern to a more polyclonal posttherapy pattern in samples from the responder patient 7, with oligoclonality index (OCI) decreasing from 0.97 to 0.64; no change was observed in nonresponder patient 4 with OCI at 0.94 and 0.95 before and after therapy, respectively (Figure 2C).
The mechanisms underlying the therapeutic efficacy of the combination of AZT and IFN in ATL patients are still elusive. Several reports suggest that either AZT or IFN alone can control HTLV-1 infection in vitro and in animal models. Nevertheless, no clear evidence was provided concerning the effect of the AZT/IFN combination on virus infectivity in ATL patients. In the present study, we report for the first time that long-term in vivo therapy with AZT and IFN caused complete inhibition of RT activity, reductions in 2 other virological parameters, and a dramatic change in the clonality pattern, in short-term cultures of PBMCs from ATL patients who responded to therapy. A previous study on PBMCs freshly collected from ATL patients showed no evidence of RT-mediated viral replication.19 Hence, we assume that short-term culture allowed a more substantial recovery of virus release in the supernatants.
The findings we report here suggest that HTLV-1 replication, presumably in HTLV-1–infected nonleukemic cells, might contribute to the evolution of ATL. Furthermore, the HTLV-1 RT assay may be considered as predictive of response to therapy or early relapse; this conclusion needs to be validated by a large prospective study.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by the Italian Ministry of University and Research, Projects of National Interest (PRIN; A.B. and B.M.); Istituto Superiore di Sanità, AIDS Project; the University of Rome Tor Vergata (B.M.); and the University of Messina and the Institute of Translational Pharmacology (A. Mastino).
Authorship
Contribution: B.M., A. Mastino, and A.B. searched for literature, designed the study, interpreted the data, and wrote the paper; A.B., O.H., and A. Marçais enrolled the patients and followed the clinical study; E.B. performed the primary cell cultures and viral load of fresh ATL mononuclear cells; C.F. and F.M.-M. carried on the HTLV-1 RT assay and were in charge of virus preparation and virus capture assay; S.G. and E.V. carried out the real-time PCR assay; E.B., C.F., and B.M. performed elaboration and graphical representation of the data; and J.T. and C.R.B. performed the clonality analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonio Mastino, Department of Chemical, Biological, Pharmaceutical, and Environmental Sciences, University of Messina, Via F. Stagno d’Alcontres 31, 98166 Messina, Italy; e-mail: amastino@unime.it; and Ali Bazarbachi, Department of Internal Medicine, American University of Beirut, P.O. Box 113-6044, Beirut, Lebanon; e-mail: bazarbac@aub.edu.lb.
References
- 1.Iwanaga M, Watanabe T, Yamaguchi K. Adult T-cell leukemia: a review of epidemiological evidence. Front Microbiol. 2012;3:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okajima R, Casseb J, Sanches JA. Co-presentation of human T-cell lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis and adult-onset infective dermatitis associated with HTLV-1 infection. Int J Dermatol. 2013;52(1):63-68. [DOI] [PubMed] [Google Scholar]
- 3.Kato K, Akashi K. Recent advances in therapeutic approaches for adult t-cell leukemia/lymphoma. Viruses. 2015;7(12):6604-6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macchi B, Faraoni I, Zhang J, et al. AZT inhibits the transmission of human T cell leukaemia/lymphoma virus type I to adult peripheral blood mononuclear cells in vitro. J Gen Virol. 1997;78(Pt 5):1007-1016. [DOI] [PubMed] [Google Scholar]
- 5.Hill SA, Lloyd PA, McDonald S, Wykoff J, Derse D. Susceptibility of human T cell leukemia virus type I to nucleoside reverse transcriptase inhibitors. J Infect Dis. 2003;188(3):424-427. [DOI] [PubMed] [Google Scholar]
- 6.Chiacchio U, Rescifina A, Iannazzo D, et al. Phosphonated carbocyclic 2′-oxa-3′-azanucleosides as new antiretroviral agents. J Med Chem. 2007;50(15):3747-3750. [DOI] [PubMed] [Google Scholar]
- 7.Balestrieri E, Matteucci C, Ascolani A, et al. Effect of phosphonated carbocyclic 2′-oxa-3′-aza-nucleoside on human T-cell leukemia virus type 1 infection in vitro. Antimicrob Agents Chemother. 2008;52(1):54-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balestrieri E, Forte G, Matteucci C, Mastino A, Macchi B. Effect of lamivudine on transmission of human T-cell lymphotropic virus type 1 to adult peripheral blood mononuclear cells in vitro. Antimicrob Agents Chemother. 2002;46(9):3080-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill PS, Harrington W Jr, Kaplan MH, et al. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med. 1995;332(26):1744-1748. [DOI] [PubMed] [Google Scholar]
- 10.Hermine O, Bouscary D, Gessain A, et al. Brief report: treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N Engl J Med. 1995;332(26):1749-1751. [DOI] [PubMed] [Google Scholar]
- 11.Bazarbachi A, Hermine O. Treatment with a combination of zidovudine and alpha-interferon in naive and pretreated adult T-cell leukemia/lymphoma patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(suppl 1):S186-S190. [DOI] [PubMed] [Google Scholar]
- 12.White JD, Wharfe G, Stewart DM, et al. The combination of zidovudine and interferon alpha-2B in the treatment of adult T-cell leukemia/lymphoma. Leuk Lymphoma. 2001;40(3-4):287-294. [DOI] [PubMed] [Google Scholar]
- 13.Hermine O, Allard I, Lévy V, Arnulf B, Gessain A, Bazarbachi A; French ATL therapy group. A prospective phase II clinical trial with the use of zidovudine and interferon-alpha in the acute and lymphoma forms of adult T-cell leukemia/lymphoma. Hematol J. 2002;3(6):276-282. [DOI] [PubMed] [Google Scholar]
- 14.Matutes E, Taylor GP, Cavenagh J, et al. Interferon alpha and zidovudine therapy in adult T-cell leukaemia lymphoma: response and outcome in 15 patients. Br J Haematol. 2001;113(3):779-784. [DOI] [PubMed] [Google Scholar]
- 15.Ratner L, Harrington W, Feng X, et al. ; AIDS Malignancy Consortium. Human T cell leukemia virus reactivation with progression of adult T-cell leukemia-lymphoma. PLoS One. 2009;4(2):e4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazarbachi A, Plumelle Y, Carlos Ramos J, et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28(27):4177-4183. [DOI] [PubMed] [Google Scholar]
- 17.Kinpara S, Kijiyama M, Takamori A, et al. Interferon-α (IFN-α) suppresses HTLV-1 gene expression and cell cycling, while IFN-α combined with zidovudine induces p53 signaling and apoptosis in HTLV-1-infected cells. Retrovirology. 2013;10(10):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bazarbachi A, Suarez F, Fields P, Hermine O. How I treat adult T-cell leukemia/lymphoma. Blood. 2011;118(7):1736-1745. [DOI] [PubMed] [Google Scholar]
- 19.Nasr R, El Hajj H, Kfoury Y, de Thé H, Hermine O, Bazarbachi A. Controversies in targeted therapy of adult T cell leukemia/lymphoma: ON target or OFF target effects? Viruses. 2011;3(6):750-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balestrieri E, Pizzimenti F, Ferlazzo A, et al. Antiviral activity of seed extract from Citrus bergamia towards human retroviruses. Bioorg Med Chem. 2011;19(6):2084-2089. [DOI] [PubMed] [Google Scholar]
- 21.Gillet NA, Malani N, Melamed A, et al. The host genomic environment of the provirus determines the abundance of HTLV-1-infected T-cell clones. Blood. 2011;117(11):3113-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowan AG, Witkover A, Melamed A, et al. T cell receptor Vβ staining identifies the malignant clone in adult T cell leukemia and reveals killing of leukemia cells by autologous CD8+ T cells. PLoS Pathog. 2016;12(11):e1006030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.