Key Points
Methods that use an MSKCC single 10-color tube or EuroFlow two 8-color tubes provide similar sensitivity in the detection of MRD in multiple myeloma.
Abstract
In patients with multiple myeloma, obtaining posttreatment minimal residual disease (MRD) negativity is associated with longer progression-free survival and overall survival. Here, we compared the diagnostic performance of a single 10-color tube with that of a EuroFlow 8-color 2-tube panel for MRD testing. Bone marrow samples from 41 multiple myeloma patients were tested in parallel using the 2 approaches. Compared with the sum of the cells from the EuroFlow two 8-color tubes, the Memorial Sloan Kettering Cancer Center (MSKCC) single 10-color tube had a slight reduction in total cell number with a mean ratio of 0.85 (range, 0.57-1.46; P < .05), likely attributable to permeabilization of the cells. Percent of plasma cells showed a high degree of concordance (r2 = 0.97) as did normal plasma cells (r2 = 0.96), consistent with no selective plasma cell loss. Importantly, concordant measurement of residual disease burden was seen with abnormal plasma cells (r2 = 0.97). The overall concordance between the 2 tests was 98%. In 1 case, there was a discrepancy near the limit of detection of both tests in favor of the slightly greater theoretical sensitivity of the EuroFlow 8-color 2-tube panel (analytical sensitivity limit of MSKCC single 10-color tube: 6 cells in 1 million with at least 3 million cell acquisitions; EuroFlow 8-color 2-tube panel: 2 cells in 1 million with the recommended 10 million cell acquisitions).
Visual Abstract
Introduction
Minimal residual disease (MRD) negativity is associated with longer progression-free and overall survival in multiple myeloma.1-7 Flow cytometry offers rapid, comparatively inexpensive MRD monitoring with a proven sensitivity of 2 × 10−6.8-10 The EuroFlow Consortium proposed an MRD test (including 10 specific antigens) that uses two 8-color tubes: a surface only tube and a surface/cytoplasmic tube.9 Although these methods are clearly effective, the inevitable drawback is increased costs resulting from multiple antibody duplication and labor, which may pose barriers for wide clinical adoption of the test outside dedicated centers and for applicability to patients treated outside major clinical trials. For example, in the United States, reimbursement is not provided for the increased cost and effort of implementing this resource-intensive method. Nonetheless, the updated 2016 International Myeloma Working Group (IMWG) clinical response criteria call for EuroFlow or an equivalent test to determine response to deep treatment.11
To reduce additional costs and labor burden for the laboratories, we investigated whether a streamlined approach of combining surface and cytoplasmic staining in a single 10-color tube previously proposed by the EuroFlow Consortium12 could offer similar test performance. Comparison of this approach with the EuroFlow 8-color 2-tube panel was performed in a series of 41 routinely obtained myeloma follow-up clinical samples.
Methods
Patients and samples for comparing MSKCC single 10-color tube with EuroFlow two 8-color tubes
All patients were treated at Memorial Sloan Kettering Cancer Center (MSKCC) for multiple myeloma (age, sex, and clinical status of the patients according to IMWG criteria11 before MRD measurement are summarized in Table 1). Per institutional standards, the study was approved by the local institutional review board, and the study was performed in accordance with the Declaration of Helsinki. As part of a routine clinical care follow-up, 4 mL of bone marrow aspirate (first bone marrow aspiration pull) was obtained. The samples were mixed, counted, assessed for viability and, to simulate a real-life setting, split evenly (2 mL each) between the MSKCC single 10-color tube and the EuroFlow two 8-color tubes for evaluation. Twenty million cells or the entire volume of the sample was used for each arm. Processing, staining, and sample acquisition by the 2 methods was performed in parallel within 24 hours of collection.
Table 1.
Patients’ characteristics
| Sex | Age (y) | Clinical status |
|---|---|---|
| F | 62 | VGPR |
| M | 77 | sCR |
| F | 57 | sCR |
| M | 56 | sCR |
| F | 45 | PD |
| F | 55 | CLR |
| M | 41 | PR |
| M | 41 | sCR |
| F | 50 | PD |
| M | 74 | sCR |
| F | 55 | VGPR |
| F | 59 | SD |
| F | 47 | sCR |
| M | 59 | sCR |
| M | 70 | SD |
| M | 46 | sCR |
| F | 62 | sCR |
| M | 43 | sCR |
| M | 36 | CR |
| M | 41 | sCR |
| M | 63 | SD |
| M | 68 | CR |
| F | 75 | CLR |
| F | 64 | CR |
| M | 54 | sCR |
| F | 58 | CR |
| M | 69 | sCR |
| M | 70 | VGPR |
| F | 30 | PR |
| F | 70 | sCR |
| F | 51 | sCR |
| F | 80 | PR |
| F | 64 | PD |
| F | 43 | Diagnostic |
| M | 66 | PR |
| F | 54 | Unknown |
| F | 60 | VGPR |
| M | 64 | sCR |
| F | 54 | CR |
| M | 83 | PR |
| M | 65 | PD |
CLR, clinical relapse; CR, complete response; F, female; M, male; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
Evaluation procedure for comparison testing
Technical details and validation of both methods have been reported elsewhere.10,13 Briefly, the MSKCC 10-color single-tube MRD method uses EuroFlow bulk lysis followed by surface antibody staining, which consists of a surface antibody cocktail of CD117PC5.5 (104D2D1), CD19PC7 (J3-119), CD138APC (B-A38), and CD81PacificBlue (JS64) all from Beckman Coulter; CD56APC-R700 (NCAM16.2), CD38BV510 (HIT2), and CD27BV605 (L128), all from BD Horizon; and CD45APC-H7 (2D1) from BD Biosciences, followed by fixation and/or permeabilization and staining with anti-κ fluorescein isothiocyanate and anti-λ phycoerythrin antibodies. The EuroFlow 8-color 2-tube method uses surface-only staining in 1 tube that consists of individually added antibodies and a separate tube that uses a 2-step procedure of surface staining followed by cytoplasmic light chain staining.10 All samples were acquired by using 1 of 3 standardized FACSCanto 10-color flow cytometers (BD Biosciences) at MSKCC. To maintain identical fluorescence and scatter readout over time, daily controls were used in accordance with guidelines from the US Food and Drug Administration (FDA) and the National Cancer Institute (NCI).14 MSKCC-derived settings were used for 10-color analysis13 whereas a EuroFlow photomultiplier tube and forward scatter settings were used for 8-color analysis.15 Analysis of electronic flow cytometry standard files for both sets of samples was performed at MSKCC and at Universidad de Salamanca. Expert-based nonautomated data analysis with Infinicyt software (Cytognos, Salamanca, Spain) was used for both the merged 8-color and the 10-color flow cytometry standard data files at Universidad de Salamanca; custom Woodlist software (gift of Dr Brent Wood, University of Washington, Seattle, WA) was used at MSKCC.
Results
Comparison of methods using MSKCC single 10-color tube vs EuroFlow two 8-color tubes
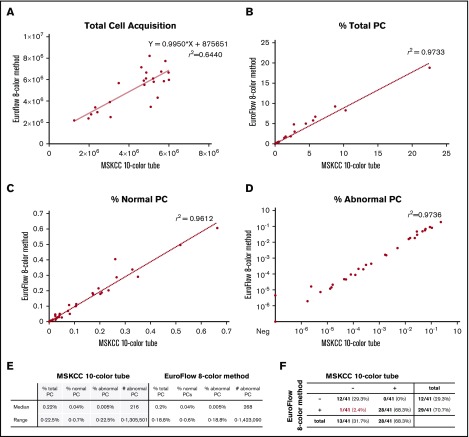
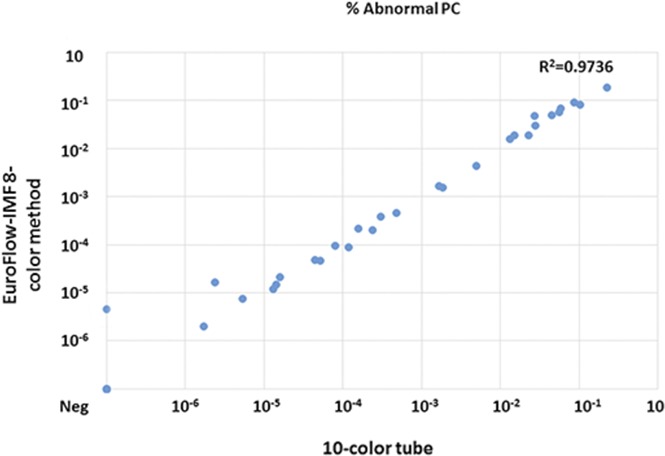
The evaluation was performed on FACSCanto 10-color flow cytometers to support both 8- and 10-color analyses. The EuroFlow 8-color 2-tube method was initially reported on a FACSCanto II cytometer; EuroFlow settings on the FACSCanto 10-color cytometer have not been validated by the EuroFlow group, but downstream data analysis was accomplished per the normal EuroFlow protocol. To mimic standard laboratory conditions, bone marrow samples were evenly split for the EuroFlow 8-color 2-tube method and the MSKCC 10-color single-tube method, and recoveries were calculated. We first assessed total cell and plasma cell recovery from the 2 panels. Twenty-four samples did not achieve maximal target events (6 million cells per tube) and had all events in the tube collected. Compared with the sum of the cells from the EuroFlow two 8-color tubes, the MSKCC single 10-color tube had a slight reduction in total cell numbers with a mean ratio of 0.85 (range, 0.57-1.46; P < .05), which was likely attributable to permeabilization of the cells (Figure 1A; supplemental Data). Thirty-five (85%) of 41 samples assessed by the MSKCC 10-color single-tube panel vs 36 (88%) of 41 samples assessed by the EuroFlow 8-color 2-tube method achieved acquisition of 3 million cells per tube as recommended by the NCI myeloma working group panel; data regarding achieved numbers by the MSKCC single 10-color tube and the EuroFlow two 8-color tubes are provided in the supplemental Data. The proportion of total plasma cells within the white blood cell gate showed a high degree of concordance (r2 = 0.97), as did the percentage of normal plasma cells (r2 = 0.96) and abnormal plasma cells (r2 = 0.97) (Figure 1B-D). These data suggest only a minor but statistically significant loss of cells; no selective loss of plasma cells occurred with permeabilization in bone marrow samples.
Figure 1.
Comparison of MSKCC single 10-color tube and EuroFlow two 8-color tubes. (A) Total cell acquisition, percentage of (B) total plasma cells (PCs), (C) normal plasma cells, and (D) abnormal plasma cells, along with (E-F) tables containing a summary of the data.
The overall relative yield and quantitation of abnormal plasma cells were highly similar between the 2 tests (r2 = 0.97) (Figure 1D-E). Highly concordant analytical results were obtained with an overall qualitative concordance of 98% (Figure 1F). A single discrepancy occurred near the limit of detection for both panels (the EuroFlow two 8-color tubes were MRD positive and the MSKCC single 10-color tube was MRD negative), favoring a slightly higher theoretical sensitivity for the EuroFlow two 8-color tubes, as previously suggested.10
Discussion
We demonstrate that the performance of a single 10-color tube is comparable to that of the EuroFlow 2-tube approach. The analytical sensitivity limit of the MSKCC single 10-color tube was recently evaluated and found to be 6 × 10−6 (ie, 6 cells in 1 million) with at least 3 million cell acquisitions13 compared with the theoretical limit of detection for the 2-tube 8-color approach of 2 × 10−6 (ie, 2 cells in 1 million) for the EuroFlow recommended targeted acquisition of 10 million cells.10 Both tests have been validated to detect at least 20 abnormal cells in a sample. The real-world sensitivity of both tests strongly depends on the quality of the samples. Thus, the documented sensitivity limit and the published recommendations regarding MRD test development for both the MSKCC single 10-color tube and the EuroFlow 2-tube approaches are in accord with FDA-NCI guidelines,14 international consensus recommendations for myeloma flow cytometry-based MRD quality control,16,17 and the IMWG clinical response criteria for MRD negativity.11
Further efforts to wide adoption of the specific 10-color single-tube test still remain. For example, although the EuroFlow 8-color 2-tube method uses automated analysis, which provides a hint to the potential for hemodilution, is supplemented by expert review, and relies on a database of normal samples,9 there is no such database for the 10-color single-tube method.10,13 In our study, this potential pitfall did not result in degradation of test interpretation, but the problem may arise when the test is transferred to different institutions with various degrees of expertise. However, in principle there should be no barrier to creating a database of normal samples for a single institution that uses proprietary Infinicyt software or for forming a collaborative group that uses standardized sample processing and acquisition procedures after full validation of the procedure, as has already been done for the EuroFlow 8-color 2-tube approach.9 Conversely, the analytic approach used in this study for the MSKCC single 10-color tube does not require specific proprietary software. We chose to use the Woodlist software as a visualization tool for the flow cytometry data in this study, but most flow cytometry visualization software tools could be used with both the 8-color and the 10-color panels. Overall, the MSKCC 10-color single-tube MRD approach, which is based on a combination of markers previously proposed by EuroFlow,12 allows laboratories to implement the method within their existing analytical workflow.
Wide adoption of myeloma MRD testing will require a continuous quality assurance program that includes standardization of reporting and a proficiency program to ensure uniform and accurate reporting between laboratories.16 An external quality assurance (proficiency) program is currently available for the EuroFlow 8-color 2-tube panel, but it has not yet been developed for the MSKCC 10-color single-tube approach. Given very high concordance between the 2 approaches, it is likely that the same proficiency program would be applicable for both. As with any other medical test, one may conjecture that 2 separate measurements of the same sample (ie, EuroFlow 8-color 2-tube method) could provide a quality check for consistency in flow cytometry MRD measurements in clinical settings, especially for very low MRD levels. In this study, the overall results generated by the 2 methods were highly similar (r2 = 0.97; Figure 1D-E). Studies that use a larger number of samples with low-level infiltration by MRD are needed to confirm and expand on our results.
As is true for all flow cytometry tests that rely on surface-antigen staining, introducing surface-antigen targeting therapies may result in degradation of test performance unless alternative clones and reagents are used such as the multiepitope CD38 reagent proposed and validated by the EuroFlow Consortium for patients treated with anti-CD38 drugs (eg, daratumumab).10 We are in the process of developing the MSKCC assay for multiple myeloma patients treated with various monoclonal antibodies. Having the flexibility to make adjustments in response to the development of new drugs, competing logistic pressures, various regulatory approvals, drug availability across multiple countries, and new more informative markers will require standardization based on rigorous analytical criteria.16 In parallel with the ongoing development of flow cytometry assays to match the fast pace of FDA approvals of new drugs that promote rapid and deep responses to treatment (ie, more patients will achieve low-level MRD),18 there is a need for flow cytometry–based MRD isolation assays to facilitate molecular characterization of residual cells by next-generation sequencing.
This study was designed to compare MSKCC’s 10-color single-tube approach with the established 8-color 2-tube panel developed and extensively validated by the EuroFlow group in a multicenter setting.10 The EuroFlow group12 recognizes that there might be alternative approaches that, once they are appropriately designed, tested, and validated, will contribute to the overall advancement of science and improved patient care.
The 10-color single-tube approach is streamlined, which may lead to its wider adoption and acceptance in laboratories where logistic and/or financial barriers play a major role. At MSKCC, the 10-color single-tube method reduces the costs of performing the test (associated with reagents, instrument time, and labor) without losing analytical performance. In summary, we report high concordance in a single-center setting between the EuroFlow 8-color 2-tube panel and the MSKCC 10-color single-tube panel.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by the International Myeloma Foundation and Memorial Sloan Kettering Core Grant P30 CA008748 from the National Institutes of Health, National Cancer Institute.
Authorship
Contribution: M.R., O.L., A.D., and B.G.M.D. analyzed data, designed the research, and wrote the paper; J.A.F.-M. analyzed the data and provided critical portions for Figure 1; Q.G. performed research and analyzed data; M.K. and J.A.F.-M. performed research; J.W. performed experiments, organized data, reviewed the drafted manuscript, provided input, and accepted the final version; B.G.M.D., M.R., O.L., and A.D. designed the research; A.O. analyzed data and edited the manuscript; and O.L. designed and directed the research and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ola Landgren, Myeloma Service, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: landgrec@mskcc.org.
References
- 1.Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol. 2010;28(15):2612-2624. [DOI] [PubMed] [Google Scholar]
- 2.de Tute RM, Rawstron AC, Gregory WM, et al. . Minimal residual disease following autologous stem cell transplant in myeloma: impact on outcome is independent of induction regimen. Haematologica. 2016;101(2):e69-e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Lopez J, Lahuerta JJ, Pepin F, et al. . Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123(20):3073-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paiva B, Gutiérrez NC, Rosiñol L, et al. ; PETHEMA/GEM (Programa para el Estudio de la Terapéutica en Hemopatías Malignas/Grupo Español de Mieloma) Cooperative Study Groups. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119(3):687-691. [DOI] [PubMed] [Google Scholar]
- 5.Rawstron AC, Child JA, de Tute RM, et al. . Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31(20):2540-2547. [DOI] [PubMed] [Google Scholar]
- 6.Rawstron AC, Gregory WM, de Tute RM, et al. . Minimal residual disease in myeloma by flow cytometry: independent prediction of survival benefit per log reduction. Blood. 2015;125(12):1932-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landgren O, Devlin S, Boulad M, Mailankody S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: a meta-analysis. Bone Marrow Transplant. 2016;51(12):1565-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paiva B, van Dongen JJ, Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015;125(20):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores-Montero J, de Tute R, Paiva B, et al. . Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytometry B Clin Cytom. 2016;90(1):61-72. [DOI] [PubMed] [Google Scholar]
- 10.Flores-Montero J, Sanoja-Flores L, Paiva B, et al. . Next generation flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma [published online ahead of print on 10 March 2017]. Leukemia. doi:10.1038/leu.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S, Paiva B, Anderson KC, et al. . International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328-e346. [DOI] [PubMed] [Google Scholar]
- 12.Van Dongen J, Orfao de Matos Correia e Vale J, Flores Montero J, et al. Methods, Reagents, and Kits for Detecting Minimal Residual Disease. 2013. Patent application #14/407,268. Publication #20150160226.
- 13.Royston DJ, Gao Q, Nguyen N, Maslak P, Dogan A, Roshal M. Single-Tube 10-Fluorochrome Analysis for Efficient Flow Cytometric Evaluation of Minimal Residual Disease in Plasma Cell Myeloma. Am J Clin Pathol. 2016;146(1):41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landgren O, Gormley N, Turley D, et al. . Flow cytometry detection of minimal residual disease in multiple myeloma: Lessons learned at FDA-NCI roundtable symposium. Am J Hematol. 2014;89(12):1159-1160. [DOI] [PubMed] [Google Scholar]
- 15.Kalina T, Flores-Montero J, van der Velden VHJ, et al. ; EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 2012;26(9):1986-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldaker TA, Wallace PK, Barnett D. Flow cytometry quality requirements for monitoring of minimal disease in plasma cell myeloma. Cytometry B Clin Cytom. 2016;90(1):40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawstron AC, Paiva B, Stetler-Stevenson M. Assessment of minimal residual disease in myeloma and the need for a consensus approach. Cytometry B Clin Cytom. 2016;90(1):21-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mailankody S, Korde N, Lesokhin AM, et al. . Minimal residual disease in multiple myeloma: bringing the bench to the bedside. Nat Rev Clin Oncol. 2015;12(5):286-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.