Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a syndrome of cytokine-driven immune activation. Cardinal features include fever, hemophagocytosis, hepatosplenomegaly, lymphocytic infiltration, and hypercytokinemia that result in multisystem organ dysfunction and failure. Familial HLH is genetically driven, whereas secondary HLH (SHL) is caused by drugs, autoimmune disease, infection, or cancer. SHL is associated with worse outcomes, with a median overall survival typically of less than 1 year. This reflects difficulty in both diagnostic accuracy and in establishing reliable treatments, especially in cases of malignancy-induced SHL, which have significantly worse outcomes. Malignancy-induced HLH is seen almost exclusively with hematologic malignancies, constituting 97% of cases in the literature over the past 2 years. In these situations, the native immune response driven by CD8 T cells produces an overabundance of T helper 1 cytokines, notably interferon-γ, tumor necrosis factor-α, and interleukin-6, which establish a positive feedback loop of inflammation, enhancing replication of hematologic malignancies while leaving the host immune system in disarray. In this paper, we present 2 case studies of secondary HLH driven by HM, followed by a review of the literature discussing the cytokines driving HLH, diagnostic criteria, and current treatments used or undergoing investigation.
Case 1
A previously healthy 34-year-old male presented with progressive malaise, fevers, and abdominal discomfort. He was found to have massive splenomegaly along with pancytopenia and coagulopathy. Initial laboratory studies showed lactate dehydrogenase (LDH) 470 U/L, ferritin 4450 ng/dL, white blood cell count 1.3 × 109/L, platelets 31 × 109/L, hematocrit 22%, and fibrinogen level 130 mg/dL. A bone marrow (BM) biopsy was performed and showed lymphohistiocytic aggregation without hemophagocytosis. The patient underwent splenectomy; pathology showed splenic red pulp congestion and proliferation of sheets of normal histiocytes with marked erythrophagocytosis. No conclusive evidence of B- or T-cell lymphoma was found at that time, although features suggestive of but not diagnostic for T-cell rich diffuse large B-cell lymphoma (DLBCL) were seen in the spleen. The patient subsequently had a positron emission tomography/computed tomography scan and was found to have small hyperactive para-aortic lymph nodes that were not accessible for biopsy. The patient began therapy for HLH with dexamethasone and etoposide for 6 cycles and tolerated it well, with resolution of his laboratory abnormalities and symptoms.
A year and a half after initial diagnosis, he presented to the hospital again with back pain and fevers. A computed tomography scan showed multiple retroperitoneal and periaortic lymph nodes along with liver lesions. He was started on dexamethasone and admitted to the hospital. Multiple lymph node biopsies were performed and were inconclusive, possibly from steroid pretreatment. Further evaluation with bilateral BM biopsies reported T-cell rich DLBCL, BCL6 positive, BCL2 negative, and c-Myc negative. His International Prostate Symptom Score was 3. The patient completed 6 cycles of rituximab-cyclophosphamide, doxorubicin, vincristine, prednisone treatment for 21 days; end-of-treatment BM biopsy, brain magnetic resonance imaging, and positron emission tomography/computed tomography showed no evidence of disease. The patient continues to be in remission from DLBCL and HLH at 11 months.
Case 2
A 56-year-old male with a history of chronic lymphocytic leukemia (CLL) was initially diagnosed in 2011, when he presented with lymphocytosis with no associated adenopathy or cytopenia. An initial fluorescence in situ hybridization panel revealed multiple cytogenetic abnormalities, including 11q, 13q, and 6q deletions. The patient was observed until 2015, at which time he required therapy for his CLL because of a white blood cell count of 95 000 and associated rash and arthritis. He was started on fludarabine, cyclophosphamide, and rituximab (FCR) for 4 cycles and experienced complete remission based on his response on scans. The patient did however continue to have a mild splenomegaly measuring 16 cm. He was then noted to have severe pancytopenia and prolonged neutropenia with a white blood cell count of 0.5 × 109/L and an absolute neutrophil count of 200/mm3. This continued 2 months after his fourth cycle of FCR. A BM biopsy was performed 2 months after his last cycle of FCR and revealed hypercellular BM with no CLL, but with hemophagocytosis and a ferritin >2000 ng/dL. He was observed at this time. One month later, the patient presented with pain, fever, pancytopenia, synovitis, and rash. On admission, he had a ferritin level >4000 ng/dL. He was started on treatment of HLH with dexamethasone, etoposide, and intravenous immunoglobulin. He initially showed good response to treatment with normalization of his laboratory values and resolution of his symptoms after 2 cycles of etoposide. Genetic testing with next-generation sequencing revealed a heterozygous nonsynonymous single nucleotide polymorphism, A91V, in Perforin-1, which in silico analysis using PolyPhen-2 software1 predicted as probably damaging (0.937 [sensitivity, 0.66; specificity, 0.91]). Although the public database ClinVar yielded conflicting interpretations of pathogenicity, the variant has been previously reported as a disease-causing mutation2 and that it results in lowered levels of perforin expression.3 The patient was on treatment with intravenous immunoglobulin through the end of 2016 because etoposide treatment had to be held because of persistent thrombocytopenia. The patient is also undergoing consideration for allogeneic stem cell transplant, which would be performed if HLH recurs. He has been in remission for 7 months.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a condition of genetic or functional hypercytokinemia leading to global dysfunction. Normally, innate and adaptive immune systems allow the body to respond to a near infinite display of antigens and generate a protective response to pathogens. Unfortunately, when dysregulated, these systems produce nonspecific inflammation that prevents a targeted response. Cytokines are produced in excess, promoting edema, hyperferritinemia, hypertriglyceridemia, hypofibrinogenemia, anemia, and fever. In the short term, it appears similar to a septic response, but over time HLH produces more symptoms, leading to high morbidity and mortality.4 HLH was named for the characteristic appearance of BM macrophages on biopsy. It was originally discovered in 19525 as an idiopathic cytopenia syndrome with lymphocytic infiltrates and benign histocytosis. Clinical manifestations also include cytopenias in multiple cell lines, decreased natural killer (NK) cell activity, and increased soluble interleukin-2r (IL-2r; CD25) (Table 1).6 The vigorous immune response in HLH may be due to familial hemophagocytic lymphohistiocytosis (FHL) or secondary hemophagocytic lymphohistiocytosis (SHL). FHL is due to the inherited defects of genes involved in cell killing by NK cells and T cells.7 Cells that are targeted by the adaptive immune system for destruction are identified by CD8 (killer) T cells, which bind to exposed major histocompatibility complex class I on the cell surface. CD8 T cells then degranulate, secreting perforin and granzymes through membrane fusion. Interferon-γ (IFN-γ) and other T helper 1 (Th1) cytokines are secreted in the process to recruit other lymphocytes to the area. NK cells perform a similar function as part of the innate immune system, recognizing surface molecules called killer-cell immunoglobulin-like receptors, and through major histocompatibility complex binding.8 The NK cells also possess a regulatory function by secreting IL-10 to prevent T-cell activation9 and function to support the role of T-regulatory cells.10 Together, these checks and balances produce a targeted strike preventing the cytokine storm that develops during HLH.
Table 1.
HLH-2004 guidelines for diagnosis of HLH
| HLH-2004 requires 1 or 2 of the below to be fulfilled |
|---|
| 1. Molecular diagnosis consistent with HLH |
| 2. Diagnostic criteria with 5 of 8 of the following symptoms: |
| Fever |
| Splenomegaly |
| Cytopenias affecting >2 lineages in PB |
| Hemoglobin <9 g/dL (<10 g/dL in infants <4 wk of age) |
| Platelets <100 000/μL |
| Neutrophils <1000/μL |
| Hypertriglyceridemia: fasting triglycerides >265 mg/dL and/or hypofibrinogenemia: <1.5g/L |
| Hemophagocytosis in the BM, spleen, lymph nodes, or liver |
| Low or absent NK cell activity |
| Ferritin >500 μg/L |
| Increased soluble CD25 (IL-2r) concentration: >2400 U/mL |
The guidelines for HLH are presented in the text. Additionally, several other characteristics are associated with HLH including cerebrospinal fluid pleocytosis, elevated cerebrospinal fluid protein, liver histology consistent with chronic persistent hepatitis, cerebromeningeal symptoms, lymphadenopathy, skin rash, hypoproteinemia, hyponatremia, increased very low-density lipoprotein, or decreased HDL.
PB, peripheral blood.
HLH is a very rare condition, occurring at a rate of 0.12 per 100 000, based on a Swedish survey. FHL is the most common type and comprises 5 subtypes based on the identified genetic mutation. The most common mutation is in the gene for perforin, which functions similarly to terminal complement proteins by causing membrane perforation and allowing the entrance of the pro-apoptotic granzymes into target cells.11 Defects in perforin are the cause of FHL type 2, the most commonly identified genetic defect in FHL.12 Munc13-4,13 syntaxin-11,14 and MUNC18-215 are all involved in membrane trafficking and fusion. They present with similar, though not identical, features of FHL type 2.16 FHL type 1 has a similar clinical manifestation, but it has no known genetic association. Similar findings have also been documented in 4 monogenetic disorders that result from mutations in membrane trafficking proteins including Chediak-Higashi syndrome,17 Griscelli syndrome type 2,18 Hermansky-Pudlak syndrome type 2,19 and X-linked lymphoproliferative disease.20 Still other manifestations have been characterized involving defects in the IFN-γ receptor21 and IL-2–inducible T-cell kinase.22 Because of ineffective cell lysis or absent feedback, CD8 T cells increase secretion of pro-inflammatory cytokines, which leads to further recruitment of lymphocytes and histiocytes, creating positive feedback and sustaining high levels of inflammation.15,23 SHL relies on the same pathogenesis, but requires a different trigger to initiate the inflammatory cascade.
In SHL, activation results from a trigger such as autoimmune disease, certain drugs (infliximab), infections, or malignancy.24 This may contribute to the increased mortality seen in secondary HLH,7 which ranges between 22% and 88% of treated patients.25 This risk increases further when considering HLH resulting from malignant disease, which, according to 1 study, decreased the median survival to 9 months compared with 72 months in nonmalignant SHL.26
Cytokines and malignancy in HLH
Cytokines are a diverse class of proteins that induce edema, hyperplasia, and cell death, depending on context. The importance of cytokines in malignancy-induced HLH cannot be understated. They are produced by and in response to HM, leading to activation dendritic cells (DCs), macrophages, NK cells, and CD8 T cells. Furthermore, it is likely that the balance of pro- and anti-inflammatory cytokines is just as important for determining disease morbidity and mortality. In addition to the IFN-γ produced by CD8 T cells, multiple cytokines and chemokines are produced by reactive macrophages,27 DCs, and some tumor cells28 that perpetuate the pro-inflammatory environment. Discerning the intricacies of SHL to determine more effective treatment options may rely on a better understanding of the subtle divergences between FHL and SHL despite phenotypic similarities.
The initial presentation of SHL in malignant disease occurs in 2 settings: during the chemotherapeutic treatment29 or as a result of the malignancy itself.30 The etiology of chemotherapeutic triggers can be attributed to cell lysis and necrosis, which are known to cause the release of IL-5, IL-13, IL-10, IL-6,31 tumor necrosis factor-α (TNF-α), and IL-β.32,33 In addition, tumor-infiltrating lymphocytes (TILs) have been shown to switch to a Th1 cytokine profile immediately following chemotherapy, leading to increases in IL-1, TNF-α, and IFN-γ.34,35 The combination of cell lysis and cytokine secretion by TILs and macrophages may be sufficient to trigger global dysregulation in susceptible individuals.28 In addition to activation from a cellular lysis, some cases of SHL may be the direct result of chemotherapy potentiating an underlying single nucleotide polymorphism or subclinical mutation leading to development of SHL, which likely occurred in case 2. Specific chemotherapeutic triggers are difficult to elucidate, but fludarabine29,36 (used in case 2) has been implicated as a cause of SLH, specifically in CLL. Case reports also suggest that drugs used in the treatment of childhood neuroblastoma may be implicated in development of HLH.37 Unfortunately, very little literature is available on this phenomenon, especially regarding specific drugs or a clear mechanism of action.
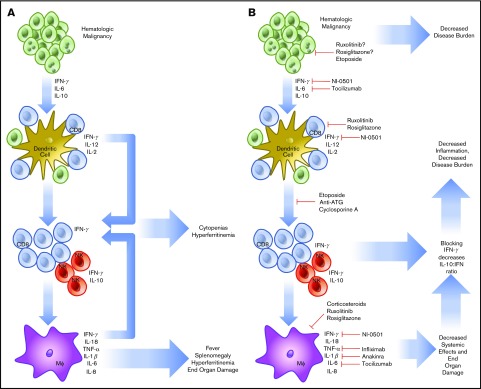
HLH that is not related to the onset of chemotherapy may be of a more complicated etiology. In HLH, activated CD8 T cells and IFN-γ are known to be indispensable for the pathogenesis of FHL in perforin-1 knockout mice or infection with lymphocyte choriomeningitis virus, the most common model systems used.38 Similar findings have been noted in degranulation39 and IFN-γ neutralization experiments.40 Rescue experiments have demonstrated that increasing perforin activity (and decreasing inflammatory activation) in human explants prevents the inflammatory cascade associated with HLH.41 The cytokines involved in malignancy-induced HLH are like those found in FHL, with high levels of IL-6, IL-10, and IFN-γ.42 To compound this, hematologic malignancies (HMs) are known to possess the ability to diffusely activate DC.43 Further, they prevent normal dendritic pruning, which leads to a larger population of activated DCs to activate host T cells and interact with HMs. Additionally, in HM, there is precedent for aberrant secretion of cytokines by tumor cells, sometimes in the high levels of IFN-γ expected to lead to cases of HLH.44,45 Although the tumor-mediated secretion of cytokines may play a small role, HLH in malignancy seems to stem primarily from an ineffective host immune response against a background of genetic susceptibility. HLH caused by monoallelic deletions may present later in life in any of the known HLH genes.46 In this case, the increased inflammatory burden of cancer leads to the same IFN-γ/Th1-dependent feedback loop causing a preferential increase in CD8 T-cell populations and leading to sustained inflammation (Figure 1).
Figure 1.
Pathophysiology and treatment strategies in malignancy-induced HLH. HMs are known to secrete cytokines (IL-6, IL-10, IFN-γ), which are able to further drive cell division and prevent the organized recruitment of CD8 T cells to the tumor microenvironment (A). These T cells and accompanying NK cells then secrete IL-2, further increasing the burden of cells, which are unable to effectively remove HM. In addition, cell turnover and cytokine production drives downstream activation of macrophages. Together, these components combine to create HLH, resulting in cytokine-driven cytopenias, hyperferritinemia, splenomegaly, and end-organ damage. To treat HLH, specific treatments to prevent T-cell recruitment, reproduction, and cytokine secretion are being used (B). The treatment regimen using etoposide, antithymocyte immunoglobulin, and cyclosporine includes the most broad approach and has demonstrated efficacy. More specific treatments are now in trial, including the anti-IFN-γ antibody, NI-0501. Approaches to alleviate activation of JAK1/2 using ruxolitinib and IL-6 secretion (tocilizumab) are also in trial. Several other agents are routinely used, including infliximab and anakinra, but are not consistently shown to demonstrate efficacy. NF-κB using rosiglitazone is also a potential target, but potentially complicated because of the wide-reaching effects of a blockade.
Because of the immunologic origin of HM, cell-signaling pathways that initiate and sustain inflammation are known to be dysregulated. Beginning from the outside of the cell, Toll-like receptors (TLRs) form the basis of innate immunity at the subcellular level. By recognizing pathogen-associated molecular patterns and damage-associated molecular patterns, TLRs alert surrounding cells to the presence of pathogens or cellular damage.47 TLRs are activated through a conformational change upon binding a target ligand. This causes the activation of MyD88, which activates a signaling cascade leading to IRAK1 phosphorylation, IκB inactivation, and NF-κB nuclear translocation. To this end, a possible route of pathogenesis for SHL may be facilitated through TLRs and not cytokines. When repeatedly triggered in mouse models, agonists of MyD8848 or TLR949 lead to SHL development. TLRs are also becoming recognized as vital players in HM and other cancers48 because changes within this pathway may increase proliferation in HM.50 Myelodysplastic syndromes with a 5q deletion51 produce excess IL-6 through constitutive NF-κB activation. Further, certain mutant TLRs that are constitutively active or more prone to activation have been described and predispose to the development of lymphoma.52 Subtypes of acute myeloid leukemia that increase TLR2/4 transcripts are associated with significantly lower overall survival. In these cases, TLR2 acts as an independently poor risk factor.53 In DLBCL, mutations in MyD88 promote pro-inflammatory cytokine production.54 The final component of the signaling cascade is NF-κB, which facilitates the development of HLH by increasing CD8 T-cell activation55 and promoting cytokine production. Although more potentially problematic, NF-κB may also represent a useful drug target in HLH through inhibitors such as rosiglitazone (Figure 1).56
HMs also use the JAK-STAT system, which is used in healthy cells to transduce cytokine signaling. JAK1 is activated by signaling through the type I IFN (IFN-α/β), type II IFN (IFN-γ), IL-2, IL-6, and IL-10 receptors.57 JAK2 acts via the Epo, growth hormone, Prl, IL-3, and IFN-γ receptors and is associated with polycythemia vera, essential thrombocythemia, myelodysplastic syndrome, and myelofibrosis.58 JAK3 associates with the IL-2, IL-4, IL-7, IL-9, and IL-21 receptors.59 The deficiency of JAK3 leads to the development of severe combined immunodeficiency and is associated with T-cell/NK-cell malignancy. JAKs form the transduction machinery of HLH and JAK inhibitors such as ruxolitinib are attractive targets for both (Figure 1).60 In summation, it seems likely that HMs can contribute to and thrive in the pro-inflammatory environment of HLH; however, this remains speculative because direct evidence of growth enhancement of HMs by HLH has not yet been shown.
The cytokine profile associated with HLH is almost exclusively of the Th1 type, demonstrating high levels of IL-1β, IL-6, IFN-γ, TNF-α, and macrophage activation that drives the process (Figure 1).61,62 Each cytokine contributes to the constellation of findings within HLH. IL-1β and TNF-α contribute to macrophage priming, fever, and nonspecific inflammation.63 IL-6 production may lead to renal failure and fever.64 IFN-γ produced in high levels leads in part to BM cytopenias, hyperferritinemia, hepatosplenomegaly, and hemophagocytosis.65 IL-10, an anti-inflammatory cytokine, is produced by NK cells as a pro-inflammatory cytokine level increase. IL-18, a neutrophil-attracting cytokine, is known to be secreted by macrophages and DCs with IL-1β, which enhances the activity of IL-12 at promoting cell-mediated immunity.66 The activity of IL-18 is antagonized by IL-18 binding protein, which is secreted in response to IFN-γ. However, in HLH, IL-18 binding protein is not produced in response to IFN-γ, and IL-18 levels are increased, further enhancing the cycle of inflammation.67 In a trial of 728 patients, Xu et al found that the primary cytokines involved in HLH were IFN-γ and IL-10, and determined the combined effectiveness in a cohort with positive systemic inflammatory response syndrome criteria. Using IFN-γ >75 pg/mL and IL-10 >60 pg/mL achieved and sensitivity of 93% and specificity of 99%.42 Further, IL-10 has been shown as a predominant protective factor in mouse models. More serum IL-10 and a larger NK cell population maintain improved outcomes and decreased morbidity.68 Other cytokines have known associations without clear parts in disease evolution. IL-16 has been shown to increase gradually after significantly elevating in the acute phase of HLH.69 Macrophage-secreted chemokines such as IL-8, macrophage inflammatory protein-1β, and monocyte chemotactic protein-1 are also significantly elevated, which further promotes monocyte recruitment and inflammatory overflow.70 Although many more play a role in establishment or exacerbation, they have not yet been elucidated.
Diagnosis and prognosis
Currently, a clinical diagnosis of HLH currently requires 5 of the 8 criteria originally based on the Treatment Protocol for Hemophagocytic Lymphohistiocytosis 2004 (HLH-2004) trial (Table 1) or a molecular diagnosis using the genetic tests of FHL types 2 through 5. The primary difficulty in all cases of HLH is maintaining an early suspicion of disease before end-organ damage is too great. In the case of HLH in malignancy, the situation is complicated further because many of the signs and symptoms of HLH may result from HM, HLH, or both. Herald symptoms such as fever, multiple cytopenias, and hepatosplenomegaly may be missed on initial presentation, especially given copresentation with HM or chemotherapeutic treatment. These symptoms, when considered together, are useful in identifying HLH, but some symptoms present later in disease course, whereas others may not develop at all in cases of malignancy-induced HLH.71
Attempts to improve on the HLH-2004 criteria by using a modified H-score,72 which comprises fever (<38.4), 33 (38.4-39.4), or 49 (>39.4), organomegaly (0 [no], 23 [hepatomegaly or splenomegaly], or 38 [hepatomegaly and splenomegaly]), cytopenias (0 [1 lineage], 24 [2 lineages], or 34 [3 lineages]), ferritin (0 [2000), 35 [2000-6000], or 50 [>6000]), triglycerides (0 [<1.5], 44 [1.5-4], or 64 [>4]), fibrinogen (0 [(>2.5] or 30 [≤2.5]), hemophagocytosis (0 [no] or 35 [yes]), aspartate aminotransferase (0 [<30] or 19 [≥30]), known underlying immunosuppression (0 [no]or 18 [yes]), or a BM score73 have achieved superiority in small trials, but remain more useful for childhood HLH. High soluble IL-2r and NK cell activity, both included in HLH-2004 are specific but not widely available measures of HLH, which has limited further validation. One study found that the ratio of soluble IL-2R to ferritin was useful in diagnosing lymphoma-induced HLH,74 whereas others using adenosine deaminase,75 NK cell degranulation flow cytometry,76 and cytokine analysis42 are promising but require larger trials for widespread validation.
In a study by Lim et al that analyzed 170 cases of malignancy-induced HLH vs nonmalignancy-induced HLH, fever was found to be the most common presenting symptom (68% of cases).26 The most common physical finding was splenomegaly (55%); the most common laboratory findings were hyperferritinemia (≥500 μg/L, 89%) and elevated LDH levels (84%). LDH levels and elevated transaminases are features more specific to adult HLH and highlight the need to recognize different presenting symptoms and a timeline of presentation if possible.25 Given the nonspecific nature of these symptoms and laboratory abnormalities, having a low threshold to perform a BM biopsy may be useful in rapidly diagnosing malignancy-induced HLH (case 2).
Establishing a diagnosis of HLH may be difficult and requires a high degree of suspicion on presentation. This difficulty is compounded in malignancy-induced HLH. As in case 1, HLH may be the presenting symptom of an underlying malignancy or may occur as the result of an existing condition as in case 2. Although the presentation varies, HM is by far the most common associated condition. Based on work by Ramos-Casals et al,24 cancer was responsible for 48% of SHL. Of those 94% were HMs, primarily NK or T-cell lymphomas with an almost equal distribution of B-cell lymphomas. Hodgkin lymphoma was responsible for 6% of cases and solid tumors for only 3%. For continuity, we began our review on the end date of Ramos-Casals et al’s search (30 September 2011 through 31 December 2016) using the term “hemophagocytic lymphohistiocytosis cancer”; we identified 334 articles. These were screened and excluded if they referred to FHL, secondary HLH of infectious, rheumatic, or other etiology not directly related to malignancy; this left 119 articles that contained 822 cases. Although concurrent infectious diseases were excluded, HHV8 leading to Castleman disease and Kaposi sarcoma are established but rare causes of HLH.77-79 Epstein-Barr virus (EBV) is also a well-known infectious cause of HLH. Although the contribution of EBV to the development of HLH is beyond the scope of this article, it is notable that EBV-driven lymphomas contributed significantly to the results. Although this may not be an effect of EBV itself, it is difficult to know the degree to which EBV contributes to the inflammatory environment in HMs.
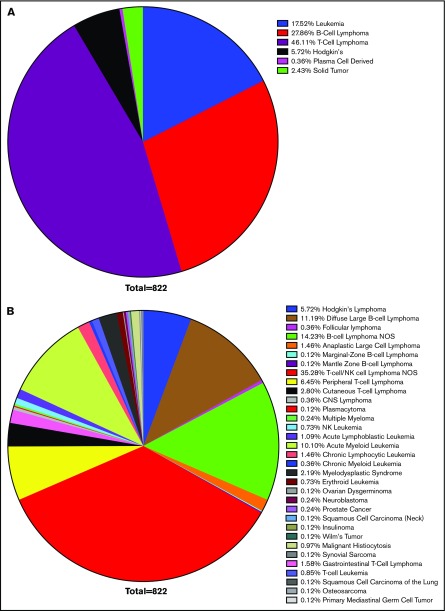
Confirming earlier results, leukemias and lymphomas were the most common malignancy associated with HLH, accounting for 97% of cases. T-cell lymphoma accounted for 46%, B-cell lymphoma accounted for 28%, leukemia 18%, Hodgkin lymphoma 6%, and solid tumors 2%. Within these larger categories, most cases of B- and T-cell lymphomas were unspecified. DLBCL made up the largest defined subtype, but CLL, acute myeloid leukemia, cutaneous T-cell lymphoma, and peripheral T-cell lymphoma also composed significant totals (Figure 2). This corresponds with previous data concluding that lymphomas and leukemia are most likely to be associated with malignancy-induced HLH.
Figure 2.
Survey of HLH cancer publications. We surveyed the literature with regard to the etiology of neoplasms, which led to HLH and resulted in selection of 119 publications. Within this cohort, 822 cases were identified as instances of malignancy-induced HLH. Cases of infectious or genetic causes were excluded, as were concurrent infections in which a clear etiology could not be identified. The most common neoplastic cause was lymphoma (80% of cases), of which T-cell lymphoma accounted for 46%, B-cell lymphoma 28%, leukemia 18%, Hodgkin lymphoma 6%, and solid tumors 2% of HLH (A). Although nonspecific TIL lymphoma was the most commonly cited cause (35%), DLBCL was the most commonly cited cause of a specific etiology (11%), followed by acute myeloid leukemia (10%), peripheral T-cell lymphoma (6%), and cutaneous T-cell lymphoma (3%) (B). CNS, central nervous system; NOS, not otherwise specified.
Why lymphomas are more likely to lead to HLH seems it should be intuitive, but no definitive evidence has shown a mechanism. One hypothesis supports that HMs can lead to diffuse DC and macrophage activation.43 Another suggests that rearrangement and genetic modifications between different lymphomas predispose to HLH. For example, in a small study of the Mitelman database, HLH in DLBCL was found to occur in older patients more often than other lymphomas. This involved the rearrangement of more immune-related genes, especially 9p24, which contains PD-1 and PD-2. DLBCL cases also featured more rearrangements is in the IFN-λ loci, perhaps leading to enhanced T-cell surveillance and activation.80
Prognosis
Prognosis in malignancy-induced HLH is poor overall compared with other forms of HLH, but varies based several distinct factors. Prognostic findings for HLH are summarized in Table 2. Age >60 years was found to have a poorer overall prognosis.81 One study found that male sex lead to poorer outcomes, although this has not been corroborated by other studies.82 Laboratory values are perhaps the most useful tool in determining the long-term outcomes of HLH. Bilirubin 2 times above the upper limit of normal or 2 mg/dL correlate to a poor outcome.78 Levels of ferritin above are known to correlate to increased morbidity and mortality, and in malignancy-induced HLH, a ferritin level >50 000 μg/L correlated to 30-day mortality.83 On physical examination, edema and splenomegaly were also associated with a poor outcome.
Table 2.
Prognostic findings of HLH
| Prognostic indicator | Favorable factor | Adverse factor | Reference |
|---|---|---|---|
| General | |||
| Age | >2 y, <60 y | <2 y, >60 y | 81,82,99-102 |
| Sex | Female | Male | 82 |
| Laboratory values | |||
| Ferritin | <2 000 μg/L | >50 000 μg/L | 83,101,103 |
| Bilirubin level | <2 mg/mL | >2 mg/mL | 78,99,104 |
| Lactate level | <2 000 U/L | >2 000 U/L | 99 |
| Albumin | >20 g/L | <20 g/L | 104 |
| β-2 microglobulin | <4.03 mg/L | >4.03 mg/L | 105 |
| Neutrophils | >500/μL | <500/μL | 104 |
| CD3+ cells | Increased | Decreased | 106 |
| CD8+ cells | >45% of CD3+ cells | <45% of CD3+ cells | 106 |
| CD4:CD8 ratio | Decreased | Maintained or Increased | 106,107 |
| NK cell level | <3% of lymphocytes | >3% of lymphocytes | 107,108 |
| Platelet count | >80 000/μL | <80 000/μL | 100,101 |
| Platelet count normalization | <2 wk | >2 wk | 104 |
| aPTT | <60 s | >60 s | 102 |
| Cholinesterase | >2 000 U/L | <2 000 U/L | 108 |
| IL-10 | >2 000 pg/mL | <2 000 pg/mL | 109 |
| C16 ceramide:sphingosine | Low | High | 110 |
| AST or ALT | <800 IU/L | >800 IU/L | 102 |
| DIC score | <5 | >5 | 101 |
| Physical examination | |||
| Edema | Absent | Present | 78 |
| Splenomegaly | Absent | Present | 82 |
| Spleen size | <4 cm | >4 cm | 111 |
| Disease | |||
| Malignancy | Absent | Present | 83 |
| Lymphoma | Absent | Present | 81 |
| Cancer type | B-cell NHL | T/NK-cell lineage | 78,84 |
| CNS involvement | No involvement | Involvement | 100 |
| Clinical parameters | PET parameters of marrow | PET negative | 112 |
| Treatment | 112 | ||
| Initial therapy | HLH94/04 | Not HLH94/04 | 112 |
| Clinical response at 2 wk | Yes | No | 101 |
| Treatment | Etoposide | No etoposide | 81,100 |
| HSCT stem cell source | BM, PB | Cord | 113 |
| HSCT donor type | MRD, MUD | MMUD | 113 |
Prognostic outcomes specific to malignancy-induced HLH are italicized. Many prognostic implications have been drawn from studies of HLH, but relatively few are isolated to adult HLH, with even fewer being related to malignancy-induced HLH. General features of HLH that are known to yield an adverse prognostic impact are age, ferritin level, bilirubin level, lactate, albumin, β2-microglobulin, neutropenias, thrombocytopenia, and an elevated aPTT. Less conventional laboratory values including elevated IL-10, ratio CD4:CD8, NK-cell levels, cholinesterase, and C16 ceramide:sphingosine ratio are also able to predict poor outcome.
aPTT, activated partial thromboplastin time; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DIC, disseminated intravascular coagulation; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
In studies that compared malignancy-induced HLH with SHL resulting from another cause, malignancy was an adverse prognostic indicator.83 Lymphoma is an adverse risk factor, but within lymphoma B-cell lymphomas were found to have positive outcomes over T-/NK cell–derived lymphomas.84 This may be due to a superior prognosis of B lymphomas in general, but analysis of cases within the Mitelman database demonstrated that there was no significant difference in survival.80 Treatment regimen used also had prognostic implications. Treatment that did not involve etoposide was an independent prognostic risk because etoposide is useful for the treatment of T-cell lymphoma and HLH because of its ability to halt T-cell division. In both aspects, etoposide is an effective treatment option.
Treatment
Untreated HLH is nearly uniformly fatal, therefore early diagnosis and prompt treatment are critical.81, The treatment of HLH should be aimed at suppressing the immune system and treating the underlying disorder. The first international treatment protocol for HLH included an 8-week induction therapy with etoposide, high-dose dexamethasone, and intrathecal methotrexate for patients with central nervous system involvement followed by maintenance with cyclosporine; however, this regimen is used mainly in FHL. Etoposide selectively depletes activated T cells, leading to suppression of inflammatory cytokines and improved survival, suggesting that T-cell deletion, rather than suppression of activation, was most effective.85 The Histiocyte Society’s collaborative therapeutic (HLH-94) protocol reported survival of 55%, with a median follow-up of 3.1 years.86 The main goal of induction therapy is to suppress the life-threatening inflammatory process that underlies HLH. This regimen resulted in improved outcomes; however, there were a significant number of early relapses.87 This resulted in the HLH-2004 protocol, which included cyclosporine as part of the induction regimen and addition of hydrocortisone to intrathecal methotrexate. For patients that are not enrolled in clinical trial, the current practice is still to treat with HLH-94 because the results of 2004 are pending (Table 3).
Table 3.
Ongoing clinical trials in HLH
| Research group | Clinical trial identifier | Drug or treatment | Region | Years | Overall survival, % | Reference | Target | Status |
|---|---|---|---|---|---|---|---|---|
| Beijing Friendship Hospital | NCT02631109 | Pegaspargase, doxorubicin, etoposide, methylprednisolone | Beijing, China | 2015-present | 46 | 114 | EBV-HLH | Recruiting |
| Children's Hospital of Philadelphia | NCT02007239 | Tocilizumab | Philadelphia, PA | 2013-present | NA | NA | IL-6 receptor | Recruiting |
| Children's Hospital Medical Center Cincinnati | NCT01104025 | ATG, etoposide, dexamethasone/hydrocortisone | Cincinnati, OH | 2010-2016 | NA | NA | T cells, proliferation | Complete, data pending |
| Karolinska University Hospital | NCT00426101 | Dexamethasone, etoposide, cyclosporine A | Multiple | 2007-present | 55 | 6 | Proliferation | Ongoing |
| Beijing Friendship Hospital | NCT02862054 | Splenectomy | Beijing, China | 2016-present | 25 | 115 | Splenectomy | Ongoing |
| Assistance Publique - Hôpitaux de Paris | NCT02472054 | Alemtuzumab, prednisone/methylprednisolone, cyclosporine A | Paris, France | 2015-present | NA | NA | CD-52, proliferation | Ongoing |
| University of Michigan Cancer Center | NCT02400463 | Ruxolitinib | Ann Arbor, MI | 2015-present | NA | NA | Jak1/2 | Ongoing |
| NovImmune SA | NCT01818492 | NI-0501 (anti-IFN-γ) | Multiple | 2013-present | NA | NA | IFN-γ | Ongoing |
| Peking University People's Hospital | NCT02569463 | IL-2 | Beijing, China | 2014-present | NA | NA | BM suppression | Ongoing |
| Baylor College of Medicine | NCT01494103 | T cells with iCaspase-9 | Houston, TX | 2011-present | NA | 116 | BM suppression | Ongoing |
| Masonic Cancer Center | NCT00176865 | HSCT | Minneapolis, MN | 2002-present | NA | NA | BM suppression | Ongoing |
| Masonic Cancer Center | NCT00176826 | HSCT | Minneapolis, MN | 2000-present | NA | NA | BM suppression | Ongoing |
| Washington University School of Medicine | NCT01821781 | HSCT | St. Louis, MO | 2013-present | NA | NA | BM suppression | Ongoing |
| Masonic Cancer Center | NCT01652092 | HSCT | Minneapolis, MN | 2012-present | NA | NA | BM suppression | Ongoing |
| Pusan National University Medical School | — | CHOP | Busan, South Korea | 1999-2004 | 44 | 117 | Proliferation | Complete |
Current trials for treatment of HLH are limited in regard to established data. The HLH protocol remains the most effective therapy option to date (55%), but new innovations, especially in immunotherapy and kinase inhibitors, present new opportunities for increased efficacy. Some trials have looked at augmenting the HLH-2004 protocol with ATG or pegaspargase, but results have been limited by comparison with the HLH-2004 trial.
NA, not available.
Patients with a genetic predisposition to HLH and recurrent disease should eventually be taken to allogenic hematopoietic stem cell transplant (HSCT). In 1986, Fischer et al described the first cure for FHL through allogenic HSCT.88 HSCT should be avoided in patients with active disease because of cytokine storm and increased risk of graft-versus-host disease. Alemtuzumab, a monoclonal antibody to CD52, can be used in patients with active disease who need a bridge to transplant as well in patients that are transplant ineligible.89
The majority of HLH seen in adults is secondary to malignancy, infections and rheumatologic disorders. EBV is one of the most common infections associated with HLH, and it carries a poor prognosis. Etoposide has shown to be effective for EBV-HLH and for some cases of rheumatologic-associated HLH.90 In EBV-related HLH, the patients who received no etoposide within 4 weeks of HLH diagnosis have been reported to have 14 times more increased mortality rate compared with those who have received etoposide. Rituximab has also proven to be beneficial in EBV-HLH; it works by eliminating EBV-infected B cells.91 In patients with HLH accompanying sepsis, the standard treatment approach is antibiotics and supportive treatment; however, a course of corticosteroids and/or intravenous immunoglobulin may be used to control the hyperinflammation and hypercytokinemia in patients who do not improve with antibiotics and supportive measures. HLH associated with autoimmune diseases (macrophage activation syndrome) should be treated initially with steroids alone and usually do not require etoposide. If they do not respond to steroids, additional immunosuppressive agents such as cyclosporine or agents that address the underlying rheumatologic disorder are recommended.
Lymphoma is the most common cause of malignancy-related HLH and accounts for 27% of secondary HLH. The overall mortality associated with malignancy-associated HLH is >90%, and chemotherapy may not salvage these patients because of end-organ dysfunction at presentation.92,93 Treatment of lymphoma-associated HLH (LA-HLH) involves HLH-94/HLH-2004 protocol and treating the underlying B- or T-cell lymphoma with chemotherapy. The majority of B-cell lymphoma–related HLH was DLBCL and treatment consisted of rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone. Mortality associated with LA-HLH is significantly high, therefore early recognition and treatment with intensive chemotherapy followed by HSCT is critical in improving overall survival.86 Because etoposide-based regimens have shown success in FHL, they need to be investigated further in LA-HLH. Patients with LA-HLH are clinically unstable and may not be able to tolerate toxicity associated with high-dose chemotherapy; therefore, more targeted therapy needs to be investigated. A small retrospective study suggested that DLBCL-HLH showed more frequent rearrangements of immune-related loci in contrast to DLBCL not associated with HLH.80
Another regimen investigated for HLH included prednisone, antithymocyte globulin (ATG), and cyclosporine, which is followed by HSCT. Overall survival was identical to HLH-94 at 55%; however, ATG protocol had a higher relapse rate.94 There are also reports of successful treatment using certain monoclonal antibodies, such as daclizumab,95 alemtuzumab,96 and infliximab.97 In the case of infliximab, however, there are isolated reports of causing HLH, so caution should be taken until further research is conducted.98 Novel strategies evaluating immunomodulatory agents, to more specifically target these genes, need to be investigated.
Conclusions
Malignancy-induced HLH represents an ongoing challenge in diagnosis and management. Through a deeper understanding of the underlying pathology, especially regarding immune surveillance mechanisms, we can begin to understand where this disease falls on the spectrum of sepsis and the factors that predispose to it. Despite advances in understanding, TLRs, cytokines, and diffuse immune activation remain somewhat ambiguous in their contributions to pathophysiology in malignancy-induced HLH. To pursue new agents and reduce deaths, more of the underlying signaling needs to be explored. Furthermore, although new agents and protocols will no doubt improve morbidity and mortality, early diagnosis remains the key in prevention of catastrophic effects.
Acknowledgments
The authors thank Matthew Stein, the West Cancer Center, and the University of Tennessee Health Science Center for helping to facilitate this collaboration.
Authorship
Contribution: E.J.V., K.P., P.P. and M.G.M. were all involved in the conceptual design and analysis planning of the project and in acquisition of the data; E.J.V., K.P., and P.P. drafted the manuscript; all authors were all involved in critical revision of the manuscript; and M.G.M. supervised all aspects of the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mike G. Martin, Department of Hematology and Oncology, West Cancer Center, 1588 Union Ave, Memphis TN 38104; e-mail: mmartin@westclinic.com.
References
- 1.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clementi R, zur Stadt U, Savoldi G, et al. Six novel mutations in the PRF1 gene in children with haemophagocytic lymphohistiocytosis. J Med Genet. 2001;38(9):643-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voskoboinik I, Thia M-C, Trapani JA. A functional analysis of the putative polymorphisms A91V and N252S and 22 missense perforin mutations associated with familial hemophagocytic lymphohistiocytosis. Blood. 2005;105(12):4700-4706. [DOI] [PubMed] [Google Scholar]
- 4.Rosado FGN, Kim AS. Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139(6):713-727. [DOI] [PubMed] [Google Scholar]
- 5.Farquhar JW, Claireaux AE. Familial haemophagocytic reticulosis. Arch Dis Child. 1952;27(136):519-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henter J-I, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. [DOI] [PubMed] [Google Scholar]
- 7.Usmani GN, Woda BA, Newburger PE. Advances in understanding the pathogenesis of HLH. Br J Haematol. 2013;161(5):609-622. [DOI] [PubMed] [Google Scholar]
- 8.Podack ER, Munson GP. Killing of microbes and cancer by the immune system with three mammalian pore-forming killer proteins. Front Immunol. 2016;7:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S-H, Kim K-S, Fodil-Cornu N, Vidal SM, Biron CA. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med. 2009;206(10):2235-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Cava A, Van Kaer L, Fu-Dong-Shi. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27(7):322-327. [DOI] [PubMed] [Google Scholar]
- 11.Ishii E. Hemophagocytic lymphohistiocytosis in children: pathogenesis and treatment. Front Pediatr. 2016;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286(5446):1957-1959. [DOI] [PubMed] [Google Scholar]
- 13.Feldmann J, Callebaut I, Raposo G, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell. 2003;115(4):461-473. [DOI] [PubMed] [Google Scholar]
- 14.zur Stadt U, Schmidt S, Kasper B, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14(6):827-834. [DOI] [PubMed] [Google Scholar]
- 15.Côte M, Ménager MM, Burgess A, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119(12):3765-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brisse E, Wouters CH, Matthys P. Hemophagocytic lymphohistiocytosis (HLH): a heterogeneous spectrum of cytokine-driven immune disorders. Cytokine Growth Factor Rev. 2015;26(3):263-280. [DOI] [PubMed] [Google Scholar]
- 17.Nagai K, Ochi F, Terui K, et al. Clinical characteristics and outcomes of Chédiak-Higashi syndrome: a nationwide survey of Japan. Pediatr Blood Cancer. 2013;60(10):1582-1586. [DOI] [PubMed] [Google Scholar]
- 18.Meeths M, Bryceson YT, Rudd E, et al. Clinical presentation of Griscelli syndrome type 2 and spectrum of RAB27A mutations. Pediatr Blood Cancer. 2010;54(4):563-572. [DOI] [PubMed] [Google Scholar]
- 19.Jessen B, Bode SFN, Ammann S, et al. The risk of hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type 2. Blood. 2013;121(15):2943-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pachlopnik Schmid J, Canioni D, Moshous D, et al. Clinical similarities and differences of patients with X-linked lymphoproliferative syndrome type 1 (XLP-1/SAP deficiency) versus type 2 (XLP-2/XIAP deficiency). Blood. 2011;117(5):1522-1529. [DOI] [PubMed] [Google Scholar]
- 21.Tesi B, Sieni E, Neves C, et al. Hemophagocytic lymphohistiocytosis in 2 patients with underlying IFN-γ receptor deficiency. J Allergy Clin Immunol. 2015;135(6):1638-1641. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh S, Bienemann K, Boztug K, Borkhardt A. Interleukin-2-inducible T-cell kinase (ITK) deficiency - clinical and molecular aspects. J Clin Immunol. 2014;34(8):892-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eife R, Janka GE, Belohradsky BH, Holtmann H. Natural killer cell function and interferon production in familial hemophagocytic lymphohistiocytosis. Pediatr Hematol Oncol. 1989;6(3):265-272. [DOI] [PubMed] [Google Scholar]
- 24.Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503-1516. [DOI] [PubMed] [Google Scholar]
- 25.Hayden A, Park S, Giustini D, Lee AYY, Chen LYC. Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: A systematic scoping review. Blood Rev. 2016;30(6):411-420. [DOI] [PubMed] [Google Scholar]
- 26.Lim SH, Park S, Jang JH, et al. Clinical significance of bone marrow hemophagocytosis in adult patients with malignancy and non-malignancy-induced hemophagocytic lymphohistiocytosis. Ann Hematol. 2016;95(2):325-335. [DOI] [PubMed] [Google Scholar]
- 27.Bracaglia C, de Graaf K, Pires Marafon D, et al. Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2017;76(1):166-172. [DOI] [PubMed] [Google Scholar]
- 28.Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43(3):374-379. [DOI] [PubMed] [Google Scholar]
- 29.Tiong IS, Lau MBY, Toumoua S, Chiruka S. A case of hemophagocytic lymphohistiocytosis in a patient with chronic lymphocytic leukemia after treatment with fludarabine, cyclophosphamide, and rituximab chemotherapy, with autopsy findings. Case Rep Hematol. 2012;2012:326053. [DOI] [PMC free article] [PubMed]
- 30.Janka GE, Lehmberg K. Hemophagocytic syndromes--an update. Blood Rev. 2014;28(4):135-142. [DOI] [PubMed] [Google Scholar]
- 31.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome [published correction appears in Blood. 2015;126(8):1048]. Blood. 2014;124(2):188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lachmann HJ, Quartier P, So A, Hawkins PN. The emerging role of interleukin-1β in autoinflammatory diseases. Arthritis Rheum. 2011;63(2):314-324. [DOI] [PubMed] [Google Scholar]
- 33.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539-545. [DOI] [PubMed] [Google Scholar]
- 34.Sepehrizadeh Z, Mohammadi M, Emami A, et al. Assessment of cytokine expression profile in acute myeloid leukemia patients before and after chemotherapy. Turk J Haematol. 2014;31(2):149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto T, Kimura T, Ueta E, Tatemoto Y, Osaki T. Characteristic cytokine generation patterns in cancer cells and infiltrating lymphocytes in oral squamous cell carcinomas and the influence of chemoradiation combined with immunotherapy on these patterns. Oncology. 2003;64(4):407-415. [DOI] [PubMed] [Google Scholar]
- 36.Jayakody Arachchillage D, Hurst E, Carey P, et al. Haemophagocytic lymphohistiocytosis following fludarabine/cyclophosphamide chemotherapy for chronic lymphocytic leukaemia. Br J Haematol. 2014;166(1):142-145. [DOI] [PubMed] [Google Scholar]
- 37.Nascimento FA, Nery J, Trennepohl J, Pianovski MAD. Hemophagocytic lymphohistiocytosis after initiation of chemotherapy for bilateral adrenal neuroblastoma. J Pediatr Hematol Oncol. 2016;38(1):e13-e15. [DOI] [PubMed] [Google Scholar]
- 38.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104(3):735-743. [DOI] [PubMed] [Google Scholar]
- 39.Binder D, van den Broek MF, Kägi D, et al. Aplastic anemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus. J Exp Med. 1998;187(11):1903-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pachlopnik Schmid J, Ho C-H, Chrétien F, et al. Neutralization of IFNgamma defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol Med. 2009;1(2):112-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiwari S, Hontz A, Terrell CE, et al. High level of perforin expression is required for effective correction of hemophagocytic lymphohistiocytosis [published online ahead of print 29 July 2016] Hum Gene Ther. doi:10.1089/hum.2016.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu X-J, Tang Y-M, Song H, et al. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J Pediatr. 2012;160(6):984-90.e1. [DOI] [PubMed] [Google Scholar]
- 43.Terrell CE, Jordan MB. Perforin deficiency impairs a critical immunoregulatory loop involving murine CD8(+) T cells and dendritic cells. Blood. 2013;121(26):5184-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.López-Alvarez MR, Martínez-Sánchez MV, Salgado-Cecilia MG, et al. Association of monoclonal expansion of Epstein-Barr virus-negative CD158a+ NK cells secreting large amounts of gamma interferon with hemophagocytic lymphohistiocytosis. Clin Vaccine Immunol. 2009;16(1):142-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brady MT, Lee J, Ferrone S, Wang ES, Wetzler M. Interferon-γ secretion by t(9;22) acute lymphoblastic leukemia-derived dendritic cells. Leuk Res. 2011;35(2):275-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tesi B, Lagerstedt-Robinson K, Chiang SCC, et al. Targeted high-throughput sequencing for genetic diagnostics of hemophagocytic lymphohistiocytosis. Genome Med. 2015;7:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leifer CA, Medvedev AE. Molecular mechanisms of regulation of Toll-like receptor signaling. J Leukoc Biol. 2016;100(5):927-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krebs P, Crozat K, Popkin D, Oldstone MB, Beutler B. Disruption of MyD88 signaling suppresses hemophagocytic lymphohistiocytosis in mice. Blood. 2011;117(24):6582-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Behrens EM, Canna SW, Slade K, et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest. 2011;121(6):2264-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monlish DA, Bhatt ST, Schuettpelz LG. The role of Toll-like receptors in hematopoietic malignancies. Front Immunol. 2016;7(390):390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Starczynowski DT, Kuchenbauer F, Argiropoulos B, et al. Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype. Nat Med. 2010;16(1):49-58. [DOI] [PubMed] [Google Scholar]
- 52.Mollaki V, Georgiadis T, Tassidou A, et al. Polymorphisms and haplotypes in TLR9 and MYD88 are associated with the development of Hodgkin’s lymphoma: a candidate-gene association study. J Hum Genet. 2009;54(11):655-659. [DOI] [PubMed] [Google Scholar]
- 53.Rybka J, Butrym A, Wróbel T, et al. The expression of Toll-like receptors in patients with acute myeloid leukemia treated with induction chemotherapy. Leuk Res. 2015;39(3):318-322. [DOI] [PubMed] [Google Scholar]
- 54.Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bronk CC, Yoder S, Hopewell EL, et al. NF-κB is crucial in proximal T-cell signaling for calcium influx and NFAT activation. Eur J Immunol. 2014;44(12):3741-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chuang H-C, Lay J-D, Hsieh W-C, Su I-J. Pathogenesis and mechanism of disease progression from hemophagocytic lymphohistiocytosis to Epstein-Barr virus-associated T-cell lymphoma: nuclear factor-kappa B pathway as a potential therapeutic target. Cancer Sci. 2007;98(9):1281-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368(2):161-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282(28):20059-20063. [DOI] [PubMed] [Google Scholar]
- 59.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121-S131. [DOI] [PubMed] [Google Scholar]
- 60.Das R, Guan P, Sprague L, et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood. 2016;127(13):1666-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Billiau AD, Roskams T, Van Damme-Lombaerts R, Matthys P, Wouters C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-γ-producing lymphocytes and IL-6- and TNF-α-producing macrophages. Blood. 2005;105(4):1648-1651. [DOI] [PubMed] [Google Scholar]
- 62.Osugi Y, Hara J, Tagawa S, et al. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997;89(11):4100-4103. [PubMed] [Google Scholar]
- 63.Yang C, Zhang D-M, Song Z-B, et al. Protumoral TSP50 regulates macrophage activities and polarization via production of TNF-α and IL-1β, and activation of the NF-κB signaling pathway. PLoS One. 2015;10(12):e0145095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weber J, Yang JC, Topalian SL, et al. Phase I trial of subcutaneous interleukin-6 in patients with advanced malignancies. J Clin Oncol. 1993;11(3):499-506. [DOI] [PubMed] [Google Scholar]
- 65.Créput C, Galicier L, Buyse S, Azoulay E. Understanding organ dysfunction in hemophagocytic lymphohistiocytosis. Intensive Care Med. 2008;34(7):1177-1187. [DOI] [PubMed] [Google Scholar]
- 66.Biet F, Locht C, Kremer L. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J Mol Med (Berl). 2002;80(3):147-162. [DOI] [PubMed] [Google Scholar]
- 67.Nold-Petry CA, Lehrnbecher T, Jarisch A, et al. Failure of interferon γ to induce the anti-inflammatory interleukin 18 binding protein in familial hemophagocytosis. PLoS One. 2010;5(1):e8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sepulveda FE, Maschalidi S, Vosshenrich CAJ, et al. A novel immunoregulatory role for NK-cell cytotoxicity in protection from HLH-like immunopathology in mice. Blood. 2015;125(9):1427-1434. [DOI] [PubMed] [Google Scholar]
- 69.Takada H, Ohga S, Mizuno Y, Nomura A, Hara T. Increased IL-16 levels in hemophagocytic lymphohistiocytosis. J Pediatr Hematol Oncol. 2004;26(9):567-573. [DOI] [PubMed] [Google Scholar]
- 70.Tamura K, Kanazawa T, Tsukada S, Kobayashi T, Kawamura M, Morikawa A. Increased serum monocyte chemoattractant protein-1, macrophage inflammatory protein-1beta, and interleukin-8 concentrations in hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;51(5):662-668. [DOI] [PubMed] [Google Scholar]
- 71.Hanoun M, Dührsen U. The maze of diagnosing hemophagocytic lymphohistiocytosis: single-center experience of a series of 6 clinical cases. Oncology. 2017;92(3):173-178. [DOI] [PubMed] [Google Scholar]
- 72.Debaugnies F, Mahadeb B, Ferster A, et al. Performances of the H-score for diagnosis of hemophagocytic lymphohistiocytosis in adult and pediatric patients. Am J Clin Pathol. 2016;145(6):862-870. [DOI] [PubMed] [Google Scholar]
- 73.Wang H-Y, Yang C-F, Chiou T-J, et al. Risk of hemophagocytic lymphohistiocytosis in adults with fevers of unknown origin: the clinical utility of a new scoring system on early detection [published online ahead of print 28 July 2016] Hematol Oncol. doi:10.1002/hon.2333. [DOI] [PubMed] [Google Scholar]
- 74.Tsuji T, Hirano T, Yamasaki H, Tsuji M, Tsuda H. A high sIL-2R/ferritin ratio is a useful marker for the diagnosis of lymphoma-associated hemophagocytic syndrome. Ann Hematol. 2014;93(5):821-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen W, Zhang S, Zhang W, et al. Elevated serum adenosine deaminase levels in secondary hemophagocytic lymphohistiocytosis. Int J Lab Hematol. 2015;37(4):544-550. [DOI] [PubMed] [Google Scholar]
- 76.Chung HJ, Park CJ, Lim JH, et al. Establishment of a reference interval for natural killer cell activity through flow cytometry and its clinical application in the diagnosis of hemophagocytic lymphohistiocytosis. Int J Lab Hematol. 2010;32(2):239-247. [DOI] [PubMed] [Google Scholar]
- 77.Grossman WJ, Radhi M, Schauer D, Gerday E, Grose C, Goldman FD. Development of hemophagocytic lymphohistiocytosis in triplets infected with HHV-8. Blood. 2005;106(4):1203-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cattaneo C, Oberti M, Skert C, et al. Adult onset hemophagocytic lymphohistiocytosis prognosis is affected by underlying disease and coexisting viral infection: analysis of a single institution series of 35 patients [published online ahead of print 3 June 2016] Hematol Oncol. doi:10.1002/hon.2314. [DOI] [PubMed] [Google Scholar]
- 79.Re A, Facchetti F, Borlenghi E, et al. Fatal hemophagocytic syndrome related to active human herpesvirus-8/Kaposi sarcoma-associated herpesvirus infection in human immunodeficiency virus-negative, non-transplant patients without related malignancies. Eur J Haematol. 2007;78(4):361-364. [DOI] [PubMed] [Google Scholar]
- 80.Patel K, Lee SS, Valasareddy P, Vontela NR, Prouet P, Martin MG. Genetic analysis of B-cell lymphomas associated with hemophagocytic lymphohistiocytosis. Blood Adv. 2016;1:205-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arca M, Fardet L, Galicier L, et al. Prognostic factors of early death in a cohort of 162 adult haemophagocytic syndrome: impact of triggering disease and early treatment with etoposide. Br J Haematol. 2015;168(1):63-68. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Wang Q, Zheng W, et al. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93(2):100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90(3):220-224. [DOI] [PubMed] [Google Scholar]
- 84.Sano H, Kobayashi R, Tanaka J, et al. Risk factor analysis of non-Hodgkin lymphoma-associated haemophagocytic syndromes: a multicentre study. Br J Haematol. 2014;165(6):786-792. [DOI] [PubMed] [Google Scholar]
- 85.Johnson TS, Terrell CE, Millen SH, Katz JD, Hildeman DA, Jordan MB. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. J Immunol. 2014;192(1):84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henter J-I, Samuelsson-Horne A, Aricò M, et al. ; Histocyte Society. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367-2373. [DOI] [PubMed] [Google Scholar]
- 87.Trottestam H, Horne A, Aricò M, et al. ; Histiocyte Society. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(17):4577-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fischer A, Cerf-Bensussan N, Blanche S, et al. Allogeneic bone marrow transplantation for erythrophagocytic lymphohistiocytosis. J Pediatr. 1986;108(2):267-270. [DOI] [PubMed] [Google Scholar]
- 89.Strout MP, Seropian S, Berliner N. Alemtuzumab as a bridge to allogeneic SCT in atypical hemophagocytic lymphohistiocytosis. Nat Rev Clin Oncol. 2010;7(7):415-420. [DOI] [PubMed] [Google Scholar]
- 90.Weitzman S. Approach to hemophagocytic syndromes. Hematology Am Soc Hematol Educ Program 2011;2011:178-183. [DOI] [PubMed]
- 91.Imashuku S, Kuriyama K, Teramura T, et al. Requirement for etoposide in the treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. J Clin Oncol. 2001;19(10):2665-2673. [DOI] [PubMed] [Google Scholar]
- 92.Johnson B, Giri S, Nunnery SE, et al. Comorbidities drive outcomes for both malignancy-associated and non-malignancy-associated hemophagocytic syndrome. Clin Lymphoma Myeloma Leuk. 2016;16(4):230-236. [DOI] [PubMed] [Google Scholar]
- 93.Yu J-T, Wang C-Y, Yang Y, et al. Lymphoma-associated hemophagocytic lymphohistiocytosis: experience in adults from a single institution. Ann Hematol. 2013;92(11):1529-1536. [DOI] [PubMed] [Google Scholar]
- 94.Mahlaoui N, Ouachée-Chardin M, de Saint Basile G, et al. Immunotherapy of familial hemophagocytic lymphohistiocytosis with antithymocyte globulins: a single-center retrospective report of 38 patients. Pediatrics. 2007;120(3):e622-e628. [DOI] [PubMed] [Google Scholar]
- 95.Olin RL, Nichols KE, Naghashpour M, et al. Successful use of the anti-CD25 antibody daclizumab in an adult patient with hemophagocytic lymphohistiocytosis. Am J Hematol. 2008;83(9):747-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marsh RA, Allen CE, McClain KL, et al. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer. 2013;60(1):101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Henzan T, Nagafuji K, Tsukamoto H, et al. Success with infliximab in treating refractory hemophagocytic lymphohistiocytosis. Am J Hematol. 2006;81(1):59-61. [DOI] [PubMed] [Google Scholar]
- 98.Oda Y, Urushidani Y, Ooi S, et al. Hemophagocytic lymphohistiocytosis in a rheumatoid arthritis patient treated with infliximab. Intern Med. 2012;51(6):655-657. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y-R, Qiu Y-N, Bai Y, Wang X-F. A retrospective analysis of 56 children with hemophagocytic lymphohistiocytosis. J Blood Med. 2016;7:227-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu X-J, Wang H-S, Ju X-L, et al. ; Histiocytosis Study Group of the Chinese Pediatric Society. Clinical presentation and outcome of pediatric patients with hemophagocytic lymphohistiocytosis in China: a retrospective multicenter study. Pediatr Blood Cancer. 2017;64(4):e26264. [DOI] [PubMed] [Google Scholar]
- 101.Kaya Z, Bay A, Albayrak M, Kocak U, Yenicesu I, Gursel T. Prognostic factors and long-term outcome in 52 Turkish children with hemophagocytic lymphohistiocytosis. Pediatr Crit Care Med. 2015;16(6):e165-e173. [DOI] [PubMed] [Google Scholar]
- 102.Koh KN, Im HJ, Chung NG, et al. ; Korea Histiocytosis Working Party. Clinical features, genetics, and outcome of pediatric patients with hemophagocytic lymphohistiocytosis in Korea: report of a nationwide survey from Korea Histiocytosis Working Party. Eur J Haematol. 2015;94(1):51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grangé S, Buchonnet G, Besnier E, et al. The use of ferritin to identify critically ill patients with secondary hemophagocytic lymphohistiocytosis. Crit Care Med. 2016;44(11):e1045-e1053. [DOI] [PubMed] [Google Scholar]
- 104.Bin Q, Gao J-H, Luo J-M. Prognostic factors of early outcome in pediatric hemophagocytic lymphohistiocytosis: an analysis of 116 cases. Ann Hematol. 2016;95(9):1411-1418. [DOI] [PubMed] [Google Scholar]
- 105.Jiang T, Ding X, Lu W.. The prognostic significance of Beta2 microglobulin in patients with hemophagocytic lymphohistiocytosis. Dis Markers 2016;2016:1523959. [DOI] [PMC free article] [PubMed]
- 106.Dalal BI, Vakil AP, Khare NS, Wang SY, Richards MJ, Chen LY. Abnormalities of the lymphocyte subsets and their immunophenotype, and their prognostic significance in adult patients with hemophagocytic lymphohistiocytosis. Ann Hematol. 2015;94(7):1111-1117. [DOI] [PubMed] [Google Scholar]
- 107.Xiao L, Xian Y, Dai BT, et al. Clinical features and outcome analysis of 83 childhood Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis with HLH-2004 protocol [in Chinese]. Zhonghua Xue Ye Xue Za Zhi. 2011;32(10):668-672. [PubMed] [Google Scholar]
- 108.Li YG, Mao YN, Liu W, et al. Analysis of prognostic risk factors in childhood hemophagocytic syndrome [in Chinese]. Zhonghua Xue Ye Xue Za Zhi. 2011;32(12):836-839. [PubMed] [Google Scholar]
- 109.My LT, Lien B, Hsieh W-C, et al. Comprehensive analyses and characterization of haemophagocytic lymphohistiocytosis in Vietnamese children. Br J Haematol. 2010;148(2):301-310. [DOI] [PubMed] [Google Scholar]
- 110.Jenkins RW, Clarke CJ, Lucas JT Jr, et al. Evaluation of the role of secretory sphingomyelinase and bioactive sphingolipids as biomarkers in hemophagocytic lymphohistiocytosis. Am J Hematol. 2013;88(11):E265-E272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xue H, Chen C, Li W, et al. Analysis of prognostic risk factors in children with Epstein-Barr virus-associated hemophagocytic syndrome. Minerva Pediatr. 2015;67(3):251-261. [PubMed] [Google Scholar]
- 112.Zheng Y, Hu G, Liu Y, et al. The role of (18)F-FDG PET/CT in the management of patients with secondary haemophagocytic lymphohistiocytosis. Clin Radiol. 2016;71(12):1248-1254. [DOI] [PubMed] [Google Scholar]
- 113.Yoon HS, Im HJ, Moon HN, et al. The outcome of hematopoietic stem cell transplantation in Korean children with hemophagocytic lymphohistiocytosis. Pediatr Transplant. 2010;14(6):735-740. [DOI] [PubMed] [Google Scholar]
- 114.Wang Y, Huang W, Hu L, et al. Multicenter study of combination DEP regimen as a salvage therapy for adult refractory hemophagocytic lymphohistiocytosis. Blood. 2015;126(19):2186-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jing-Shi W, Yi-Ni W, Lin W, Zhao W. Splenectomy as a treatment for adults with relapsed hemophagocytic lymphohistiocytosis of unknown cause. Ann Hematol. 2015;94(5):753-760. [DOI] [PubMed] [Google Scholar]
- 116.Zhou X, Dotti G, Krance RA, et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood. 2015;125(26):4103-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shin H-J, Chung JS, Lee J-J, et al. Treatment outcomes with CHOP chemotherapy in adult patients with hemophagocytic lymphohistiocytosis. J Korean Med Sci. 2008;23(3):439-444. [DOI] [PMC free article] [PubMed] [Google Scholar]