Abstract
Culturing cells on thermoresponsive polymers enables cells to be harvested as an intact cell sheet without disrupting the extracellular matrix or compromising cell-cell junctions. Previously, cell sheet fabrication methods using methylcellulose (MC) gel and PNIPAAm were independently demonstrated. In this study, MC and PNIPAAm fabrication methods are detailed and the resulting cell sheets characterized in parallel studies for direct comparison of human adipose derived stromal/stem cell (hASCs) sheet formation, cell morphology, viability, proliferation, and osteogenic potential over 21 days. A cell viability study revealed that hASCs in MC and PNIPAAm cell sheets remained viable for 21 days and proliferated until confluency. Osteogenic cell sheets exhibited upregulation of alkaline phosphatase (ALP) at day 7, as well as calcium deposition at 21 days. Additionally, expression of osteocalcin (OCN), a late-stage marker of osteogenesis, was quantified at days 14 and 21 using RT-PCR. OCN was upregulated in MC cell sheets at day 14 and PNIPAAm cell sheets at days 14 and 21. These results indicate that hASCs formed into cell sheets commit to an osteogenic lineage when cultured in osteogenic conditions. Cell sheets composed of hASCs may be used for further studies of hASC differentiation or surgical delivery of undifferentiated cells to defect sites.
Keywords: cell sheets, PNIPAAm, adipose stem cells, hydrogel, methylcellulose
INTRODUCTION
Each year, over 1 million surgical procedures are undertaken for the repair of fractures and critical-size bone defects in the United States.1 Autografts and allografts are the current standard of treatment in bone regeneration; however, such treatments have associated risks including donor-site morbidity, risk of disease transmission, and immune rejection.2 Traditional tissue engineering methods utilize either isolated cell suspension injection or biodegradable scaffolds to support tissue formation.3 However, most conventional scaffolds used for bone repair do not adequately support cell migration or establish sufficient cell-extracellular matrix (ECM) and cell-cell interactions throughout the scaffolds.4 Additionally, current cell seeding methods can lead to insufficient nutrient transport to cells located in the interior of the scaffold, resulting in poor graft integration.4,5 Cell sheet engineering is an emerging technology developed as an alternative to engineered scaffolds to address these shortcomings. Once cells have grown to confluency on the cell sheet, they can be detached from the surface of the culture dish as a single sheet with ECM without the use of proteolytic enzymes or mechanical disruption. One advantage of this technique is the preservation of essential cell-cell interactions as well as organized ECM. Additionally, cell sheet engineering eliminates the need for biodegradable scaffolds, allowing for direct incorporation of tissue with reduced risk of a local or systemic response to degradation products.6 Cell sheets have been successfully fabricated using thermoresponsive hydrogels. Methylcellulose (MC) is a viscosity-enhancing polymer procured from cellulose which undergoes a sol to gel transition on heating. 7 Alternatively, PNIPAAm is a thermoresponsive polymer which can be grafted on tissue culture polystyrene (TCPS) dishes using a high voltage electron beam (30 kv) source, a system used by Okano et al. to develop a novel technique of cell sheet engineering for tissue reconstruction.8–12 In both systems, the polymers are permissive of cell attachment at elevated temperature (−37°C) and promote cell detachment at lower temperatures (−25°C).
In this study, two cell sheet fabrication methods using MC and poly(N isopropylacrylamide) have been evaluated for the formation of human adipose derived stromal/stem cell (hASC) sheets. Surface characterization of the hydrogels was assessed via atomic force microscopy and CryoSEM. To characterize the cell sheets and compare the osteogenic potential of hASCs cultured on each hydrogel, a parallel comparison study of cell sheet formation, cell morphology, viability, proliferation, and differentiation potential over 21 days was conducted. Cell viability and proliferation were analyzed using LIVE/DEAD® staining and PicoGreen DNA quantification assay. Osteogenic differentiation was assessed colorimetrically for alkaline phosphatase (ALP) expression and mineralization while gene expression for osteocalcin (OCN) was assessed by RT-PCR.
MATERIALS AND METHODS
Cell culture
Extracts of subcutaneous adipose tissue were acquired from three donors through liposuction. All tissues were obtained from LaCell LLC (New Orleans, La) with informed consent under a clinical protocol approved by either the Pennington Biomedical Research Center Institutional Review Board (Baton Rouge LA) or Western Institutional Review Board (Puyallup, WA). hASCs purchased from LaCell LLC have demonstrated tri-lineage differentiation potential and express class I histocompatibility antigen (HLA-ABC), CD9, CD10, CD13, CD29, CD34, CD44, CD49e, CD54, CD55, CD59, CD105, and CD166 surface markers as confirmed via flow cytometry. Unlike in human bone marrow stromal cells, the STRO-1 antigen was not detected.13
The hASC isolation procedure has been previously described.14 The primary cell culture’s initial passage is referred to as “Passage 0” and denoted as p0. The cells were removed from the dish using 5 mL trypsin followed by incubation at 37°C for 7–10 min, plated at a density of 5000 cells/cm2 (Passage 1) in T125 flasks for expansion, and allowed to grow to 80% confluency. Passage 2 cells were used for cell viability testing and osteogenesis.
Fabrication of cell sheets using Nunc™ Dishes with UpCell™ surface
hASCs of Passage 2 from each donor (n=3) were pooled and counted. hASCs were then plated on a thermoresponsive 3.5 cm culture dish (Nunc™ Dishes with UpCell™ Surface, Thermo Scientific). Pre-warmed growth medium of 1 m (GM) (37°C) was added to the culture dish at a cell density of 1 × 106 cells per mL. The culture dish was placed on a hot plate (37°C) for 30 min for cell attachment. After a 30-min incubation, an additional 1 mL of pre-warmed GM was added. The cells were incubated at 37°C in 5% CO2 for 24 h. The culture dish was removed from the incubator after 24 h. The cell sheet detached spontaneously after 15–20 min at room temperature (25°C).
Fabrication of cell sheets using MC hydrogel coated dishes
Fabrication of cell sheets using MC hydrogel coated dishes was performed based on previous literature.15 Fourteen percent of Aqueous MC (viscosity 15cp) solutions were prepared by dissolving the MC powder in heated water at −90°C with the addition of Dulbecco’s Phosphate-Buffered Saline (DPBS) at room temperature (25°C). After being thoroughly mixed, the solutions were refrigerated overnight until homogeneous MC solutions were obtained. Five hundred microliter of 14% MC solution was poured into the center of each TCPS (3.5 cm) dish placed on ice. A thin transparent layer of the solution was evenly distributed on the TCPS dish. Subsequently, the TCPS dish was incubated at 37°C for 45 min to form an opaque gelled layer. To improve cell attachment, 1 mL of aqueous collagen type I (3 mg/mL, Rat tail collagen Type I, Sigma-Aldrich), was evenly spread over the gelled MC at 37°C. hASCs of 1.0 × 106 were then seeded evenly on the MC coated TCPS culture system on a hot plate (37°C); and a cell sheet was formed after culturing for 24 h at 37°C, 5% CO2. When moved to room temperature (25°C), the cell sheet detached gradually from the MC surface (Fig. 1).
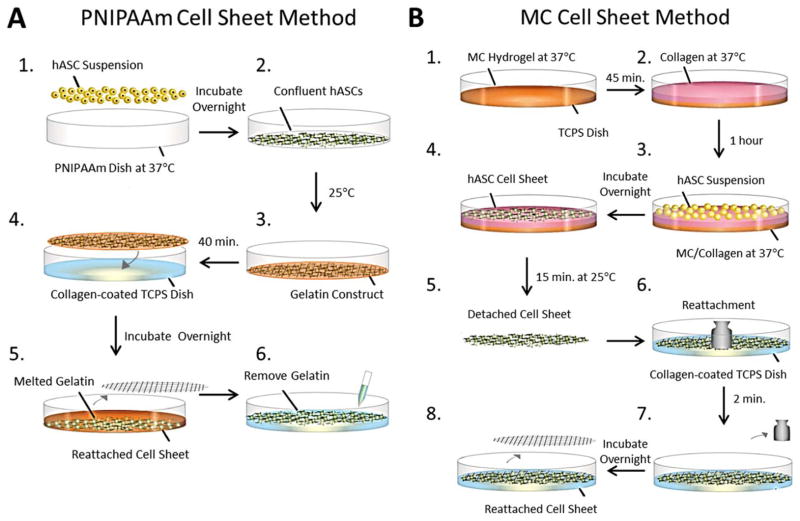
FIGURE 1.
Schematic illustrations of cell sheet fabrication, detachment, transfer, and reattachment methods using PNIPAAm and MC. A: hASCS cell sheet formed after overnight incubation using PNIPAAm. A gelatin-mesh construct was used to transfer and reattach the intact cell sheet to a TCPS dish coated with collagen. B: MC hydrogel was fabricated in a TCPS dish followed by coating with a collagen solution. Intact cell sheet was formed after overnight incubation. Cell sheet was transferred to a collagen coated TCPS dish. A metal mesh disc and weight were placed on top to facilitate reattachment.
Controlled detachment and re-attachment of cell sheets
Detachment and transfer methods were modified from Okano et al.16 A round polydimethylsiloxane (PDMS) mold with a drilled circular (3.5 cm diameter) hole was placed onto another similar intact mold. Gelatin powder of 0.75 g from bovine skin (Type B, Powder, Sigma) was added to Hank’s Balanced Salt Solution at 60°C and the final volume adjusted to 10 mL. The gelatin solution was neutralized with 1 N NaOH and sterilized using a 0.45-μm membrane filter. The gelatin was pipetted over a 30 mm stainless steel mesh disc (2.8 mm mesh size) placed in the PDMS mold. The gelatin construct was incubated for 10 min on ice, resulting in a disc-shaped gelatin gel embedded with a stainless mesh disc.
To transfer a hASC sheet fabricated using PNIPAAm, a disc-shaped gelatin construct was placed onto confluent cells cultured on Nunc™ Dishes after removal of the culture medium. Fresh GM of 1 mL was added, followed by incubation at room temperature (25°C) for 40 min to promote cell attachment to the gelatin. The gelatin construct with attached cell sheet was then transferred to a TCPS dish coated with a thin layer of collagen I (3 mg/mL, rat tail collagen, Life Technologies Co) and incubated overnight at 37°C. Following removal of the stainless steel mesh and melted gelatin, fresh culture medium was added to the TCPS dishes with reattached cell sheets [Fig. 1(A)]. The cell sheet fabricated using MC was transferred to a TCPS dish coated with a thin layer of collagen. A mesh disc was placed on top and weight (200 mg) was added for 2 min to facilitate cell attachment, as can be seen in Figure 1(B). The mesh disc was removed after overnight incubation at 37°C.
Analysis of cell sheets using histology
Following detachment, hASCs cell sheets were processed and stained as described previously.17,18 Briefly, the cell sheets were rinsed with DPBS, fixed in 10% formalin for 24 h and embedded in paraffin wax for sectioning. To evaluate the composition of the cell sheets, 10 μm sections were cut and stained with H&E and Masson’s Trichrome (American MasterTech, Lodi, CA, Item No. KTTRBPT) according to the manufacturer’s protocols and imaged under brightfield illumination with an Olympus BX46 microscope at 10X magnification.
Immunofluorescence staining
ActinGreen™ 488 ReadyProbes was used for F-actin staining on hASCs cell sheets. Cell sheets were fixed with 4% paraformaldehyde for 20 min, rinsed with PBS, and permeabilized with 0.1% Triton X-100 for 10 min at room temperature (25°C). Subsequently, cell sheets were incubated with two drops of ActinGreen 488 reagent per mL of medium for 30 min in dark. Cell nuclei were stained with DRAQ5™ before imaging. The Leica TCS SP2 spectral confocal & multiphoton system, a Leica DM IRE2 inverted microscope with a galvo-Z stage, was used to image the samples. Excitation lasers at 488 and 647 nm were used in imaging experiments concurrently with tuned emission wavelength windows.
Cell viability and proliferation
hASCs cell sheets were transferred into 24-well plates and maintained in GM for 21 days. LIVE/DEAD® staining (Cell viability®, Invitrogen – using a Lumar System) was performed at 1, 7, 14, and 21 days to assess hASC viability in cell sheets.
Total DNA content was used to determine the cell count of each cell sheet as previously described.19 Proteinase K of 0.5 mL (Sigma-Aldrich) at a concentration of 0.5 mg/mL was added to each well, and plates were incubated at 56°C overnight for enzymatic lysis of cells and DNA release. Aliquots (50 μL) were mixed with equal volumes of 0.1 g/mL PicoGreen dye solution (Invitrogen) in 96-well plates. Samples were then excited at 480 nm with an emission wavelength of 520 nm using a plate reader (Wallac 1420 Multilabel HTS Counter). Total DNA concentration was compared to a standard curve generated from serial dilutions of hASCs to calculate the number of the cells in each well. Samples prepared without cells served as blank controls.
Osteogenic study
Re-attached hASCs cell sheets were cultured in GM and osteogenic medium (OM) for 21 days in a 24-well plate. ALP enzyme activity was measured at 7 days as an early stage osteogenic marker. ALP histochemistry was performed on hASCs exposed to different treatments at day 7. ALP upregulation was qualitatively measured by Millipore kits (SCR 004) according to the manufacturer’s instructions. Briefly, hASCs were fixed with 4% formaldehyde for 1–2 min at room temperature and then rinsed with DPBS. The cell sheets were then stained with Fast Red Violet solution and Napthol AS-BI phosphate solution before imaging with a color camera mounted on an Olympus cellTIRF illuminator light microscope. hASCs cell sheet calcium deposition (cell sheets cultured with GM and OM) was assessed after 21 days of culture by histological staining. Cell sheets were rinsed with DPBS and fixed in 10% neutral buffered formalin for 24 h at room temperature (25°C). To evaluate mineralization, the cell sheets were stained with 2% alizarin red solution for 30 min at room temperature (25°C), and the samples were imaged under the Olympus BX46 microscope at 10x magnification. Adobe Photoshop (64 bit) was used for semi-quantitative analysis of alizarin red images. Five non-overlapping images were randomly chosen to analyze for each group. The raw image was thresholded, made into a binary black-and white image and the pixels were measured at a luminosity of 0. The number of pixels at the luminosity level 0 was divided by the total area of pixels to give a percentage of color coverage calculated as follows:
Total RNA was extracted from cell sheets as previously described.20 Total RNA to cDNA EcoDry Premix (ClonTech) was used for cDNA synthesis. qRT-PCR was performed using 2× iTaq™ SYBR® green supermix with ROX (Biorad) and primers for OCN to quantify osteogenic target gene expression of hASCs in cell sheets cultured in either GM or OM for 14 and 21 days. Reactions were performed with an MJ Mini™ Thermal Cycler (BioRad). The sequences of PCR primers (forward and backward, 50–30) were as follows: OCN, 5′-GCCCAGCGGTGCAGAGT-3′ and 5′-TAGCGCCTGGGTCTCTTCAC-3′. Samples were normalized (ΔCt) against the house keeping gene 18S rRNA and the −ΔΔCt value of OCN in cell sheets cultured in osteogenic compared to control medium was calculated using the ΔΔCt method as described in Supporting Information Data 2.21,22
Atomic force microscopy
AFM images were collected on a Bruker Dimension Icon (Bruker Nano Surfaces, Santa Barbara, CA). The probes used were MLCT-C (0.01 N/m spring constant, Bruker Nano Surfaces, Camarillo, CA) and MLCT-D (0.03 N/m spring constant, Bruker Nano Surfaces, Camarillo, CA) for the collagen hydrogel, Scanasyst Air (0.4 N/m spring constant) for the PNIPAAm topography, and OTESPA-R3 (29.13 N/m calibrated spring constant, Bruker Nano Surfaces, Camarillo, CA) for Peak Force quantitative nanomechanical mapping (PF-QNM) of PNIPAAm. PF-QNM was done using a relative calibration method and a polystyrene (2.8 GPa) reference sample. AFM analysis was done in Bruker Nanoscope Analysis 1.8 (Bruker Nano Surfaces, Santa Barbara, CA). To study protein deposition and organization on the substrates, collagen and PNIPAAm samples were incubated in Fetal Bovine Serum (FBS) solution for 4 h at 37°C.
CryoSEM experiment
CryoSEM was performed in an FEI Helios 660 with a Quorum PolarPrep 2200T Cryo System on both PNIPAAm and collagen surfaces to study the surface characterization after soaking in FBS solution. The specimens were (1) fastened to aluminum posts with ~50/50 Tissue tek/carbon paint, (2) installed in a cryo shuttle which is plunge frozen in prepumped liquid nitrogen LN2, (3) evacuated in a cryo transfer device and (4) transferred to the cryo stage.
Statistical analysis
Statistical analysis of results was performed using GraphPad Prism. Data was analyzed for statistically significant differences with two-way ANOVA, p<0.05. Fisher’s LSD test was used for post comparisons.
RESULTS
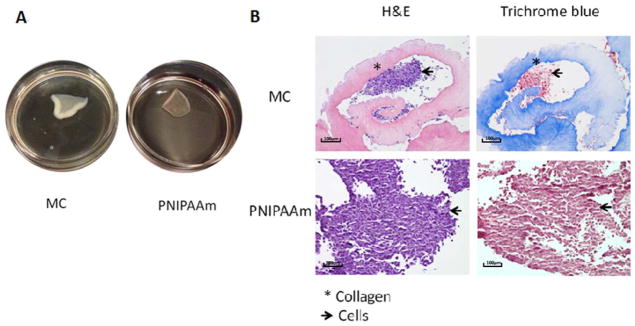
Cell sheets fabricated using MC and PNIPAAm methods spontaneously detached after incubation at room temperature. Figure 2 shows the appearance of cell sheets immediately following detachment. hASC cell sheets contract and curl once detached, as can be seen in Figure 2(A). Therefore, reattachment of cell sheets is necessary for long-term in vitro analysis. Furthermore, the fabrication methods of cell sheets determine their composition. To analyze composition, cell sheets were prepared for histology and stained with H&E and Trichrome Blue [Fig. 2(B)]. Collagen appears pink with H&E and blue with Masson’s Trichrome stains. Both H&E and Masson Trichrome blue stain confirmed the existence of a considerable amount of collagen in addition to hASCs on MC cell sheets while PNIPAAm sheets are comprised primarily of hASCs.
FIGURE 2.
Appearance (A) and histological analysis of cell sheets fabricated with MC and PNIPAAm using H&E and Tri-chrome blue staining (B).
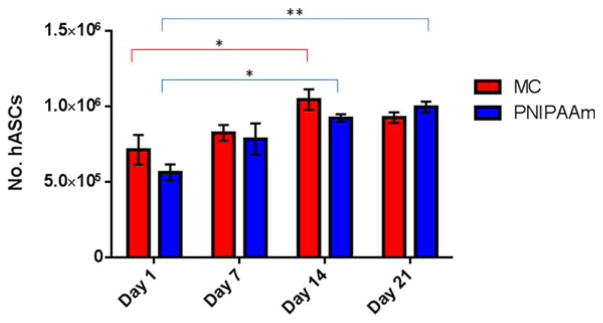
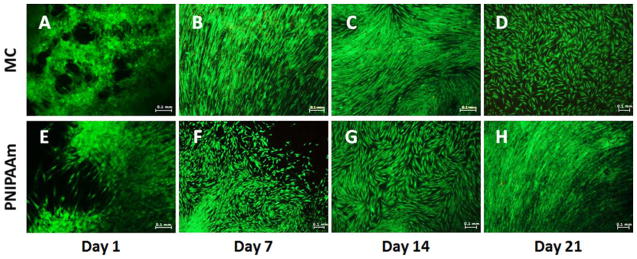
Cell viability in reattached cell sheets was analyzed over 21 days of culture in GM. PicoGreen® total DNA quantification was used to determine the number of hASCs in cell sheets at day 1, 7, 14, and 21 (Fig. 3). DNA content analysis indicates that hASCs in MC cell sheets proliferated until reaching confluence by day 14 and proliferation decreased after day 14, while hASCs in PNIPAAm cell sheets continued to proliferate until day 21 (Fig. 3). Statistical analysis indicates that hASCs in MC sheets may have begun to die between days 14 and 21 after becoming 90% confluent. The cell number at day 14 was significantly (*p<0.05) higher than day 1, but no significant difference between day 1 and day 21 was observed. Conversely, statistical analysis of PNIPAAm cell sheets revealed significant higher number of the cells at days 14 and 21 compared to day 1, with * p<0.05 and ** p<0.01, respectively (Fig. 3). hASC viability was also visualized with LIVE/DEAD® staining. Representative images of cell sheets at day 1, 7, 14, and 21 can be seen with live cells in green and dead cells in red. The LIVE/DEAD® staining indicates that hASCs in both MC and PNIPAAm cell sheets were viable and proliferated until reaching 80–90% confluency. Cells retained a healthy spindle-shaped morphology in both cell sheets (Fig. 4). Images from LIVE/DEAD® staining support the data generated from DNA content analysis that indicate the hASCs in MC cell sheets proliferated until reaching 80–90% confluency by day 14 and the proliferation stopped at day 14 [Fig. 4(A–D)]. hASCs in PNIPAAm cell sheets appear to continue to proliferate until day 21, [Fig. 4(E–H)] in agreement with DNA content analysis (Fig. 3). After re-attaching onto the regular cell culture plate, it was observed that hASCs in MC cell sheets were migrating out from the cell sheets and spreading out on the culture dish during the 21-day culture (Supporting Information Data 1).
FIGURE 3.
Cell proliferation in reattached MC and PNIPAAm cell sheets during 21 day culture period. Total number of hASCs was determined using PicoGreen DNA quantification assay. Error bars represent the standard error of the mean, for experimental and technical triplicates. * p<0.05, ** p<0.01.
FIGURE 4.
LIVE/DEAD® staining assessment over 21 day culture period. LIVE/DEAD® assay stains live cells green and dead cells red. Representative overlay images of MC (A-D) and PNIPAAm (E-H) cell sheets at day 1, 7, 14, and 21.
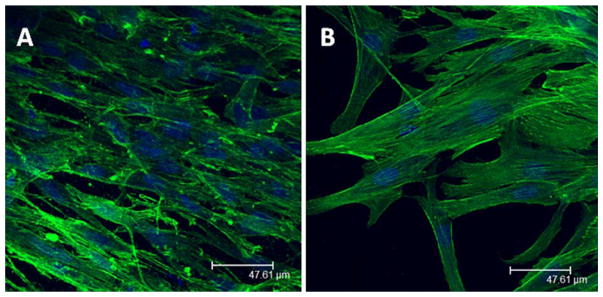
MC and PNIPAAm cell sheets were stained to show F-actin distribution in hASCs one day after formation and reattachment of cell sheets. F-actin in MC cell sheets, imaged as a z-stack, appeared to be less organized as cells migrated into the 3-dimensional structure of the collagen gel [Fig. 5(A)] compared to hASCs in PNIPAAm cell sheets which had a more elongated F-actin structure [Fig. 5(B)]. AFM and CryoSEM demonstrated distinct surface characteristics of PNIPAAm and MC that explain the difference in F-actin organization (Supporting Information Data 3–7).
FIGURE 5.
Confocal images of hASCs in cell sheets one day after reattachment. Immunofluorescent staining shows cell nuclei (blue) and F-actin (green) in (A) Representative z-stack of MC cell sheet, (B) Representative image of PNIPAAm cell sheet.
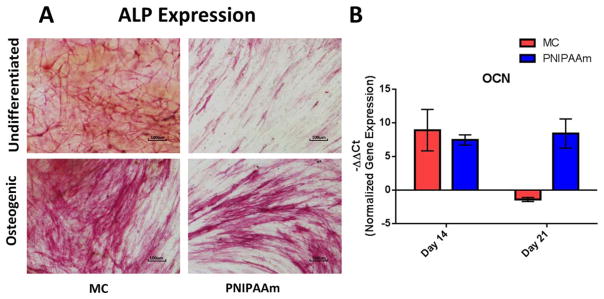
To demonstrate the osteogenic potential of hASCs cultured in sheets using MC and PNIPAAm methods, cell sheets were cultured in OM for 21 days and compared to hASC cell sheet controls cultured in GM. Osteogenesis results are summarized in Figures 6 and 7. Upregulation of ALP expression was observed in osteogenic cell sheets at day 7 [Fig. 6(A)]. For further validation of osteogenesis, OCN expression was quantified via RT-PCR at days 14 and 21. There was an increase in OCN expression of MC cell sheets cultured in osteogenic conditions compared to undifferentiated conditions at day 14, with a -ΔΔCt value of 8.895± 3.132. OCN expression of PNIPAAm cell sheets was upregulated at days 14 and 21 with an increased −ΔΔCt value from 7.435± 0.621 at day 14 to 8.395± 2.223 at day 21[Fig. 6(B)].
FIGURE 6.
Markers of osteogenesis of hASCs in MC and PNIPAAm cell sheets. A: ALP expression of hASCs on day 7 cultured in GM compared to osteogenic conditions. B: Upregulation of OCN in cell sheets at days 14 and 21 cultured in osteogenic conditions compared to undifferentiated conditions. Gene expression was normalized by comparing target gene to 18S housekeeping gene. Error bars represent the standard error of the mean for experimental and technical duplicates.
FIGURE 7.
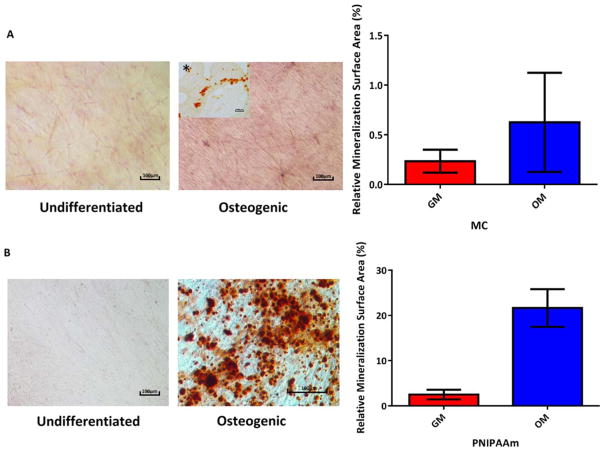
Calcium deposition and semi quantitative analysis of Alizarin Red staining in osteogenic (A) MC cell sheets (Insert on the left upper corner shows histology slice of the MC cell sheet, also stained with Alizarin Red). B: PNIPAAm cell sheets cultured in complete growth medium and osteogenic medium after 21 days.
At day 21, cell sheets were stained with alizarin red to show calcium deposition. Mineralization was observed in MC cell sheets cultured in OM [Fig. 7(A)] compared to sheets cultured in GM. Due to the presence of the collagen layer, mineralization in MC cell sheets is easier to visualize by microtome sectioning via histology compared to staining the whole sheet [Fig. 7(A*)]. Semi-quantitative analysis of Alizarin Red in MC cell sheets shows increase in calcium deposition in osteogenic samples relative to those cultured in GM. However, no significant difference was observed for MC sheets.
Substantial mineral deposition was observed in PNIPAAm cell sheets after 21 days in OM compared to sheets cultured in GM [Fig. 7(B)]. Semi-quantitative analysis of Alizarin Red also revealed the difference in OM and GM groups was statistically significant in PNIPAAm cell sheets. No direct comparison was made between Alizarin Red staining of MC and PNIPAAm cell sheets due to the variation caused by the presence of a collagen layer in MC cell sheets.
DISCUSSION
MC hydrogels are prepared by pouring aqueous MC mixed with defined salt content on TCPS dishes at 25°C followed by gelling via 37°C incubation. Once confluence is reached, a continuous monolayer cell sheet is formed on the surface of the collagen-coated hydrogel. When removed from the incubator, the cell sheet detaches gradually at room temperature. 7 MC is a thermoresponsive hydrogel which is hydrophilic at low temperatures (~25°C) and hydrophobic at high temperatures (~37°C). At lower temperatures, water molecules surround hydrophobic groups causing cellulose derivatives to become soluble in water.23 At higher temperatures, increased phase separation between the hydrophobic and hydrophilic groups results in the formation of insoluble hydrophobic aggregates in water and, subsequently, the formation of a gel network structure. Addition of salts lowers the gel formation temperature by complexing water molecules around the salts and increasing hydrophobic aggregations.24,25
Similarly, PNIPAAm is a thermoresponsive polymer based on hydrophilic–hydrophobic changes. PNIPAAm is dehydrated at temperatures above 32°C, at which cell attachment occurs, and is hydrated at temperatures below 32°C which causes detachment of cells as an intact sheet. Cells adhere directly to the hydrophobic surface of a PNIPAAm hydrogel with 15–20 nm thickness at 37°C. In contrast, MC does not support cell adhesion.6 An ECM adhesion layer (~100 μm thickness) must be coated on MC hydrogel to improve cell adhesion. These two different fabrication methods for making cell sheets are expected to affect the biological behavior of cell sheets.
Viability assays (LIVE/DEAD® staining and PicoGreen®) results indicate viable and proliferating cells in the MC and PNIPAAm cell sheets. Interestingly, hASCs migrated out of the MC cell sheets onto the culture dish based on LIVE/ DEAD® staining images, suggesting reorganization after reattachment of cell sheets. The high rate of cell proliferation based on PicoGreen® assay in MC cell sheets is also in agreement with LIVE/DEAD® staining images. The collagen layer embedded with the MC cell sheet may contribute to the faster cell proliferation as the collagen served as a 3D ECM structure, thus providing not only a physical support, but also outside-in signals that regulate cell adhesion, migration, proliferation, and differentiation.26,27 As is well known, it is difficult to maintain the proliferation potential of hASCs for an extended period.28 Cell sheets were fabricated with hASCs at passage 2 and after 21 days exhibited a decrease in the proliferation of cells, possibly as a result of hASCs committing to differentiation pathways.29
The presence of the collagen layer in MC cell sheets not only affects cell proliferation and migration but also has an influence on morphology of the cells. F-actin filaments were more elongated and uniform along the long axis of the cell in PNIPAAm hASC sheets than in MC hASC sheets. Random and unaligned striations of the F-actin filaments were observed in the MC cell sheet. The difference in sarcomeric structure and alignment of F-actin with different fabrication methods suggests that the substrate stiffness affected cellular structure of hASCs in cell sheets. The difference in stiffness and protein deposition also affects the differentiation behavior of MSCs.30,31 Engler et al. observed that substrate stiffness affects cytoskeleton organization and differentiation pathways in MSCs.32 Protein adsorption on the surface affects adhesion of mammalian cells within a biological environment. 33,34 Substrate stiffness (<0.5 KPa for collagen and 3.04 Gpa for PNIPAAm) and protein deposition were studied using AFM and CryoSEM (Supporting Information Data 3–7). This difference between MC and PNIPAAm cell sheets is expected as collagen type I coating was used for the MC method to increase hASC attachment on MC substrate. As a result, hASCs first bound and then detached along with the collagen gel, preserving all the native and foreign ECM. Similar findings have also been reported by others using MC methods.7,35
ALP staining results indicate ALP saturation of osteoblast plasma membranes. High levels of ALP observed in cell sheets may indicate that osteoblast maturation occurred in culture.36 ALP, as one of the early membrane-bound protein enzymes expressed during osteogenesis, decreases as other markers, such as OCN, are upregulated later in the developmental program. No significant difference was observed between PNIPAAm and MC. At day 21, extracellular mineral deposits on MC and PNIPAAm were stained by Alizarin Red, indicating that osteogenesis was initiated by 21 days after culturing reattached cell sheets in OM.
Increased levels of OCN correlate with the mineralization of the extracellular matrix during osteogenic differentiation of hASCs.23 Positive ALP staining and a significant increase in the level of OCN expression further indicates hASCs in both MC and PNIPAAm cells sheets are committed to osteogenic differentiation. Although the default pathway of hASCs is adipogenesis, osteogenic induction of cell sheets by bone morphogenetic protein 2 (BMP2) or micro-RNA148b may be used in vivo to modulate the hASC differentiation pathway in future studies.17,37,38 Increased mineralization of the hASC cell sheets fabricated by PNIPAAm compared to MC cells sheets could potentially be explained by the substrate stiffness. Stiffness of grafted PNIPAAm is about 3.04 GPa while stiffness of collagen type I substrate is<0.5 kPa. Extracellular-matrix tethering, organization, and substrate stiffness have been correlated with the differentiation fate of mesenchymal stem cells, as the cells exert tractional forces and gauge the feedback during the initiation of the differentiation process.39,40 Another potential explanation is that the osteogenic differentiation protocols optimized for planar culture in TCPS may not be adequate to drive robust mineralization in the quasi 3-D collagen matrix associated with the MC fabrication method.
CONCLUSION
In this study, a facile method for cell sheet transfer was established. The technique allows users to transfer and manipulate the cell sheets in vitro while maintaining sheet morphology, ECM structure and cell-cell interactions largely intact. Using this user-friendly “gelatin-mesh hybrid” transferring method, re-attachment and long term in vitro study of cell sheets could be achieved. Cell sheets fabricated using MC or PNIPAAm methods maintained cell viability for up to 21 days. It is clear that hASCs cell sheets made by either MC or PNIPAAm have potential as a research tool to examine complex cell-cell interactions and for potential clinical use in tissue engineering applications. hASCs in cell sheets fabricated with both MC and PNIPAAm methods committed to an osteogenic lineage when cultured in OM. Cell sheets made by PNIPAAm method demonstrated greater ossification compared with the MC method, indicating the PNIPAAm fabrication approach may be better suited for application in bone tissue engineering research. Furthermore, cell sheet stacking enables fabrication of quasi-three-dimensional tissues with limited or no integration of synthetic materials while allowing cell-cell junctions to remain intact. Cell sheets may provide an informative model system for studying cell interactions and spatial organization in a controlled microenvironment. Future study will include explorations of the potential of cells sheets fabricated by both methods for different applications in tissue engineering.
Supplementary Material
Acknowledgments
Contract grant sponsors: Louisiana State University Agricultural Center and the National Institute Dental and Craniofacial Research of National Institutes of Health under award R01 DE024790
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The author would like to thank Caroline Copeland (LSU BAE Undergraduate) for her assistance in graphic design and the image semi-quantitative analysis. The author also would like to thank Trevor Clark for his assistance in CryoSEM experiment.
Footnotes
No benefit of any kind will be received either directly or indirectly by the authors
Additional Supporting Information may be found in the online version of this article.
References
- 1.Jahangir AA, Mehta S, Sharan A the Washington Health Policy Fellows. Bone-graft substitutes in orthopaedic surgery. American Academy of Orthopaedic Surgeons; 2008. [Google Scholar]
- 2.Rutledge K, Cheng Q, Pryzhkova M, Harris GM, Jabbarzadeh E. Enhanced differentiation of human embryonic stem cells on extracellular matrix-containing osteomimetic scaffolds for bone tissue engineering. Tissue Eng Part C Methods. 2014;20:865–874. doi: 10.1089/ten.tec.2013.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Yamato M, Kohno C, Nishimoto A, Sekine H, Fukai F, Okano T. Cell sheet engineering: Recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26:6415–6422. doi: 10.1016/j.biomaterials.2005.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Kumar PRA, Sreenivasan K, Kumary TV. Alternate method for grafting thermoresponsive polymer for transferring in vitro cell sheet structures. J Appl Polym Sci. 2007;105:2245–2251. [Google Scholar]
- 5.Anil Kumar PR, Varma HK, Kumary TV. Cell patch seeding and functional analysis of cellularized scaffolds for tissue engineering. Biomed Mater (Bristol, England) 2007;2:48–54. doi: 10.1088/1748-6041/2/1/008. [DOI] [PubMed] [Google Scholar]
- 6.Thirumala S, Gimble JM, Devireddy RV. Methylcellulose based thermally reversible hydrogel system for tissue engineering applications. Cells (2073-4409) 2013;2:460–475. doi: 10.3390/cells2030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C-H, Chang Y, Wang C-C, Huang C-H, Huang C-C, Yeh Y-C, Hwang S-M, Sung H-W. Construction and characterization of fragmented mesenchymal-stem-cell sheets for intramuscular injection. Biomaterials. 2007;28:4643–4651. doi: 10.1016/j.biomaterials.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Liang H-F, Hong M-H, Ho R-M, Chung C-K, Lin Y-H, Chen C-H, Sung H-W. Novel method using a temperature-sensitive polymer (methylcellulose) to thermally gel aqueous alginate as a pH-sensitive hydrogel. Biomacromolecules. 2004;5:1917–1925. doi: 10.1021/bm049813w. [DOI] [PubMed] [Google Scholar]
- 9.Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J Biomed Mater Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 10.Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials. 1995;16:297–303. doi: 10.1016/0142-9612(95)93257-e. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24:2309–2316. doi: 10.1016/s0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 12.Yamato M, Okano T. Cell sheet engineering. Mater Today. 2004;7:42. [Google Scholar]
- 13.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi AT, Chen C, Shah F, Thomas-Porch C, Gimble JM, Hayes DJ. Human adipose-derived stromal/stem cell isolation, culture, and osteogenic differentiation. Methods Enzymol. 2014;538:67–88. doi: 10.1016/B978-0-12-800280-3.00005-0. [DOI] [PubMed] [Google Scholar]
- 15.Chen C-H, Tsai C-C, Chen W, Mi F-L, Liang H-F, Chen S-C, Sung H-W. Novel living cell sheet harvest system composed of thermoreversible methylcellulose hydrogels. Biomacromolecules. 2006;7:736–743. doi: 10.1021/bm0506400. [DOI] [PubMed] [Google Scholar]
- 16.Haraguchi Y, Shimizu T, Sasagawa T, Sekine H, Sakaguchi K, Kikuchi T, Sekine W, Sekiya S, Yamato M, Umezu M, Okano T. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat Protoc. 2012;7:850–858. doi: 10.1038/nprot.2012.027. [DOI] [PubMed] [Google Scholar]
- 17.Levi B, James AW, Nelson ER, Vistnes D, Wu B, Min L, Gupta A, Longaker MT. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010;5:1–11. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan DC, Pomerantz JH, Brunet LJ, Kim J-B, Chou Y-F, Wu BM, Harland R, Blau HM, Longaker MT. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem. 2007;282:26450–26459. doi: 10.1074/jbc.M703282200. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Cen L, Yin S, Chen L, Liu G, Chang J, Cui L. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and β-TCP ceramics. Biomaterials. 2008;29:4792–4799. doi: 10.1016/j.biomaterials.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 20.Zanetti AS, McCandless GT, Chan JY, Gimble JM, Hayes DJ. Characterization of novel akermanite:poly-ε-caprolactone scaffolds for human adipose-derived stem cells bone tissue engineering. J Tissue Eng Regen Med. 2015;9:389–404. doi: 10.1002/term.1646. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Regular article: Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Watkins-Curry P, Smoak M, Hogan K, Deese S, McCandless GT, Chan JY, Hayes DJ. Targeting calcium magnesium silicates for polycaprolactone/ceramic composite scaffolds. ACS Biomater Sci Eng. 2015;1:94. [Google Scholar]
- 23.Hubbell JA, Massia SP, Desai NP, Drumheller PD. Endothelial cell-selective materials for tissue engineering in the vascular graft via a new receptor. Nat Biotechnol (Nature Publishing Company) 1991;9:568–572. doi: 10.1038/nbt0691-568. [DOI] [PubMed] [Google Scholar]
- 24.Nasatto PL, Pignon F, Silveira JLM, Duarte MER, Noseda MD, Rinaudo M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers (20734360) 2015;7:777–803. [Google Scholar]
- 25.Li L, Thangamathesvaran PM, Yue CY, Tam KC, Hu X, Lam YC. Gel network structure of methylcellulose in water. Langmuir. 2001;17:8062. [Google Scholar]
- 26.Eslaminejad MB, Mirzadeh H, Nickmahzar A, Mohamadi Y, Mivehchi H. Type I collagen gel in seeding medium improves murine mesencymal stem cell loading onto the scaffold, increases their subsequent proliferation, and enhances culture mineralization. J Biomed Mater Res B. 2009;90:659–667. doi: 10.1002/jbm.b.31332. [DOI] [PubMed] [Google Scholar]
- 27.Chen HT, Lee MJ, Chen CH, Chuang SC, Chang LF, Ho ML, Hung SH, Fu YC, Wang YH, Wang HI, Wang GJ, Kang L, Chang JK. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. 2012;16:582–593. doi: 10.1111/j.1582-4934.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safwani WKZW, Makpol S, Sathapan S, Chua K. Impact of adipogenic differentiation on stemness and osteogenic gene expression in extensive culture of human adipose-derived stem cells. Arch Med Sci. 2014;10:597–606. 510. doi: 10.5114/aoms.2014.43753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P-Y, Yu J, Lin J-H, Tsai W-B. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater. 2011;7:3285–3293. doi: 10.1016/j.actbio.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 31.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95:3479–3487. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 33.Ostuni E, Chapman RG, Liang MN, Meluleni G, Pier G, Ingber DE, Whitesides GM. Self-assembled monolayers that resist the adsorption of proteins and the adhesion of bacterial and mammalian cells. Langmuir. 2001;17:6336–6343. [Google Scholar]
- 34.Zheng J, Song W, Huang H, Chen H. Protein adsorption and cell adhesion on polyurethane/Pluronic® surface with lotus leaf-like topography. Colloids Surf B Biointerfaces. 2010;77:234–239. doi: 10.1016/j.colsurfb.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 35.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 36.Tsai M-T, Li W-J, Tuan RS, Chang WH. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J Orthop Res. 2009;27:1169–1174. doi: 10.1002/jor.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tapp H, Hanley EN, Jr, Patt JC, Gruber HE. Adipose-derived stem cells: Characterization and current application in orthopaedic tissue repair. Exp Biol Med (Maywood, NJ) 2009;234:1–9. doi: 10.3181/0805/MR-170. [DOI] [PubMed] [Google Scholar]
- 38.Qureshi AT, Monroe WT, Dasa V, Gimble JM, Hayes DJ. miR-148b–Nanoparticle conjugates for light mediated osteogenesis of human adipose stromal/stem cells. Biomaterials. 2013;34:7799–7810. doi: 10.1016/j.biomaterials.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WT. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 40.Slater JH, Culver JC, Long BL, Hu CW, Hu J, Birk TF, Qutub AA, Dickinson ME, West JL. Recapitulation and modulation of the cellular architecture of a user-chosen cell of interest using cell-derived, biomimetic patterning. ACS Nano. 2015;9:6128–6138. doi: 10.1021/acsnano.5b01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.