Abstract
Approved therapies that target the B-cell receptor (BCR) signaling pathway, such as ibrutinib and idelalisib, are known to show activity in chronic lymphocytic leukemia (CLL) via their direct effects on crucial survival pathways in malignant B cells. However, these therapies also have effects on T cells in CLL by mediating toxicity and possibly controlling disease. By focusing on the effects of BCR signaling inhibitors on the T-cell compartment, we may gain new insights into the comprehensive biological outcomes of systemic treatment to further understand mechanisms of drug efficacy, predict the toxicity or adverse events, and identify novel combinatorial therapies. Here, we review T-cell abnormalities in preclinical models and patient samples, finding that CLL T cells orchestrate immune dysfunction and immune-related complications. We then continue to address the effects of clinically available small molecule BCR signaling inhibitors on the immune cells, especially T cells, in the context of concomitant immune-mediated adverse events and implications for future treatment strategies. Our review suggests potentially novel mechanisms of action related to BCR inhibitors, providing a rationale to extend their use to other cancers and autoimmune disorders.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by clonal CD5+ B-cell accumulation.1 These malignant B cells depend on constitutive B-cell receptor (BCR) signaling for survival signals. On BCR ligation, adaptor proteins SYK and LYN become phosphorylated and initiate formation of a central signalosome that includes Bruton’s tyrosine kinase (BTK) and phospholipase C (PLC)-γ2 molecules. BTK is a Tec-family kinase crucial for signal transduction through phosphorylation of PLC-γ2.2 The phosphatidylinositol 3 kinase (PI3K) δ subunit also contributes to malignant cell survival by recruiting signaling proteins to the cell membrane. Among those recruited are BTK and AKT, facilitating downstream activation of nuclear factor-κB and inhibition of proapoptotic pathways.3
Components of the BCR signaling pathway are attractive therapeutic targets in CLL and other B-cell malignancies.4 Selective inhibitors of BTK and PI3Kδ (such as ibrutinib and idelalisib, respectively) have gained attention for significant clinical activity in patients with CLL with relapsed or refractory (R/R) disease.5 Because of pathway homology, BCR inhibitors also inhibit T-cell signaling and activation.6 The effects of BCR inhibitors on T cells need to be considered to fully understand mechanisms of efficacy and occurrence of adverse events. In this review, we highlight the importance of T-cell biology in relation to CLL development and discuss its possible role in treatment efficacy and the occurrence of adverse events after current treatments.
T-cell abnormalities in patients with CLL
Investigators have widely reported immune defects, including T-cell dysfunction, occurring alongside CLL development in patients. Abnormal T cells act in collaboration with the CLL microenvironment to support the growth of malignant B cells. In addition, T-cell abnormalities are evidence of mechanisms of tumor immune-surveillance escape. Effects of T-cell changes on CLL have emerged, including imbalance in T-cell subsets, exhausted phenotypes, dysregulation of co-inhibitory molecules, increase in suppressive numbers and phenotypes, abnormal cytokine secretion, and immune synapse and cytotoxicity defects.7 Here, we review studies that support the possibility of targeting the tumor microenvironment (TME) by exploiting CLL T-cell defects.8
Imbalance of T-cell subsets
Overall changes in T-cell ratios have consistently been described in human CLL.9 One such description is inverted CD4-to-CD8 ratio being attributed to the expansion of CD8+ T cells in circulation, accompanied by Th2 preponderance and preferential expression of Th2-type chemokine receptors on T cells.10,11 The findings of all the experiments mentioned here are referenced in comparison with normal counterparts. CD4+ T cells accumulate in lymphoid tissue and associate with CLL B cells to provide survival signals12 and to drive malignant progression. Interestingly, T-cell ratios may differ between niches.13 Evidence suggests CD8+ expansion in CLL may be related to a CLL-specific adaptive immune response. Next-generation sequencing of CLL T cells has documented clonal architecture and provided evidence that antigen drive could underlie expansion in a CLL-specific context.14 Another theory postulated that chronic viral infection is a likely culprit for inducing T-cell changes in patients with CLL.15 However, T-cell defects have not been shown to correlate with cytomegalovirus-positive or cytomegalovirus-negative status in patients with CLL. Subsequent studies did not find functional impairment in cytomegalovirus-specific CD8+ T cells.16,17
Another description of T-cell imbalance is the observation that expansion of CD4+FoxP3+ regulatory T cells (Tregs) is characteristic of changes in T-cell ratios in CLL. These changes have been correlated with progression and prognostic markers such as IgVH mutation status and CD38 expression. Increases in CD8+CD25HIFOXP3+ cells have also been correlated with progression.18 After effective treatment, Treg decreases indicate a mutualistic relation between Tregs and malignant B cells necessary for immune homeostasis in CLL.19,20 Interestingly, Tregs are not the only suppressive population involved, as myeloid-derived suppressor cells (MDSCs) are also implicated in CLL progression.21 Evidence indicated that low T-helper 17 (Th17) numbers, interleukin-17+ (IL-17+) cytotoxic T-cell numbers, and decreased IL-17 expression levels are correlated with poor prognoses.22 Increased Th17 with lenalidomide may have a protective role against CLL progression.23 In contrast, induction of Th17 cells and associated cytokines may increase the possibility of complications such as autoimmune cytopenias. Accordingly, increased Th17 cells have been detected in patients experiencing autoimmune cytopenias, with a decreased Treg-to-Th17 ratio.24 Although still unconfirmed, it is suspected that IL-10 secretion by malignant B cells may modulate Treg/Th17 differentiation.22,23,25
Terminally differentiated and exhausted T cells
Patients with CLL have been shown to have decreased naive T cells and a shift toward the CD8+ effector memory phenotype.26-28 The accumulation of terminal memory in CLL may be a result of chronic antigen stimulation. Peptide-specific effector memory T cells recognizing the cytoskeletal proteins vimentin and cofilin-1 have been detected in patient samples, establishing possible autoantigens recognized by CLL B cells.29 In patients with CLL, exhausted T cells classically exhibit defective effector function, express inhibitory receptors, and proliferate poorly in response to chronic infection.30 CLL T cells extensively upregulate exhaustion markers PD-1, CD244, CD160, and intracellular CTLA-4, translating to defective proliferation and cytotoxicity.31,32 Furthermore, an increase of T-effector cells in patients with CLL correlated with lowered PD-1 expression and better prognoses.33
T-cell function
Differentially expressed genes in CD4+ CLL T cells occur in cell growth, differentiation, proliferation, survival, cytoskeleton formation, and vesicle trafficking pathways.34 These changes predict Th2 differentiation consistent with the data mentioned here. In CD8+ CLL T cells, differentially expressed genes are involved in cytoskeleton formation, intracellular transport, vesicle trafficking, and cytotoxicity. CLL B cells have been found to induce similar changes in normal CD4+ and CD8+ T cells after ex vivo coculture in a contact-dependent manner, suggesting a reciprocal interaction.34
Subsequent functional analyses confirmed predicted defects.35 When CLL T cells form impaired immune synapses with antigen-presenting cells (APCs), activation function is reduced. It has been proposed that degranulation of cytotoxic T lymphocytes (CTLs) is a regulatory mechanism of evasion used by malignant B cells to interfere with immune synapse formation.36 Further, abnormal cytoskeleton formation and vesicle packaging contributed toward defective cytotoxic response. Immunomodulation with lenalidomide improved immune synapse formation between CLL T cells and B cells, validating the concept of targeting T-cell dysfunction in the CLL TME.35 Cytokine profiles have also illustrated CLL-induced T-cell dysfunction. Increased IL-10 and IL-6 secretion by CLL B cells affords protection from CTLs,37 and increased IL-4 production by circulating CD4+ T cells reiterates Th2 differentiation.13 IFN-γ and TNF-α secretion by CLL T cells also provides extrinsic survival signals as part of the TME.
With improved understanding of the TME, novel targets continue to emerge. CD4+ CLL T cells were shown to internalize vesicles containing miR-363 secreted by CLL B cells, and silencing of miR-363 prevented T-cell alteration.38 Genome-wide analyses comparing CD8+ CLL T cells with normal CD8+ T cells identified differentially methylated immune-regulatory genes, including PD-1 promoter, KLRG1, and CCR6, confirming epigenetic reprogramming in CLL.11 We previously demonstrated that treatment of CTLs from patients with CLL with hypomethylating agent 5-aza-2-deoxycytidine caused a potentially beneficial Th1 polarization through demethylation of Th1-specific promoters.39 In a later study, the epigenetic modifiers 5-aza-2-deoxycytidine and LAQ824 effectively restored immunogenicity in CLL cell lines and primary CLL patient cells.40 The combination treatment simultaneously improved T-cell activation and APC function of CLL B cells.
Lessons from preclinical CLL models
Mouse models are advantageous for investigating CLL immunobiology. Examining the CLL T-cell compartment has resulted in novel mechanisms for CLL progression and interest in immunotherapeutic strategies.41-43 To study the dependence of CLL B cells on immune subsets, carboxyfluorescein succinimidyl ester-labeled human CLL cells were injected into NSG mice alongside various immune components, such as CD34+ progenitor cells, mesenchymal stromal cells, or mature APCs.44 Data demonstrated that T cells activated by allogeneic APCs were required for leukemic cell survival and proliferation. Administration of anti-CD3 or anti-CD4 antibodies consistently reduced leukemic growth. Another study proved that blockade of B-cell maturation is imposed by TME signals in CLL.45 These findings relate to the efficacy of CLL therapies that function to eliminate autologous T-cell support for leukemic cells or improve T-cell immune surveillance to augment response to CLL antigens.
Immunological studies in euTCL1 transgenic mice46 confirmed that leukemic B cells impaired T-cell function, which could be reversed by lenalidomide treatment. In an accelerated euTCL1 model, T-cell alterations induced by disease progression were found to be antigen-driven and clonally skewed.42 McClanahan et al investigated CLL T-cell function in aging and accelerated euTCL1 models.43 CD8+ T-cell proliferation was increased in the spleen, in contrast to previous data reported from peripheral blood of late-stage patients with CLL. EuTCL1 B and T cells upregulated expression of PD-L1/PD-L2 and PD-1, respectively, to produce T-cell dysfunction. Other inhibitory receptors, KLRG-1, 2B4, and LAG-3, also showed CLL-induced upregulation. Interestingly, PD-1+ euTCL1 T cells exhibited heterogeneous functionality. Investigations of T-cell function in the spleen indicated that immune synapse formation was increased at 6 months but impaired at 12 months, indicating overcompensation during early-stage disease. Considering these data, the authors speculated that T-cell exhaustion is not irreversible in CLL.43
Gassner et al also reported that euTCL1-derived T cells exhibited exhaustion.47 CD4+ T cells upregulated PD-1 and LAG-3 expression in periphery and lymphoid tissues, and CD8+ T cells upregulated LAG-3 expression. PD-L1 expression was modestly increased in CLL B cells from periphery tissue, but was significantly increased in CLL cells from lymph nodes, implying a TME effect. To investigate whether blocking the PD-1/PD-L1 axis in CLL has therapeutic potential, euTCL1 mice were engrafted with a mixture of fluorescently labeled syngeneic leukemic cells, some of which had been treated ex vivo with PD-L1 blocking antibody. These experiments implied that PD-1/PD-L1 blockade could reactivate the CTL response within the euTCL1 transgenic recipients to target malignant cells; however, overall survival was not reported.47
Together, these exciting reports reiterate previously established trends in human CLL and provide evidence that CLL T cells can be targeted with the intention of improving prognoses.48
Effect of BCR signaling inhibitors on T cells and other immune subsets, and future therapeutic implications
Although frontline therapy with chemoimmunotherapy is still appropriate for younger patients with CLL with mutated IgVH, it is often not well-tolerated in the older population.49,50 Alternative drugs ibrutinib and idelalisib have been developed to meet the needs of these patients. To date, ibrutinib is approved as both a frontline and R/R CLL patient therapy, whereas idelalisib is approved for R/R patients in combination with rituximab.51
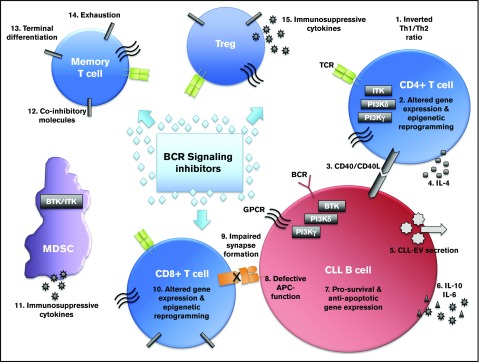
Although consequences of the BCR signaling blockade are well-characterized in the targeted malignant B cell, BCR inhibitors, including ibrutinib, idelalisib, and others, can interact with multiple immune cell types, especially T cells. How these drugs interact with immune deficiency occurring in CLL is currently unknown; however, the complex interaction of immune cells in this environment, as illustrated in Figure 1, makes the effects of BCR inhibitors in immune subsets especially interesting.
Figure 1.
The complex interplay of immune subsets in chronic lymphocytic leukemia that may be affected by B-cell receptor signaling inhibitors. BCR signaling inhibitors, such as ibrutinib, idelalisib, and others, act directly on the malignant B cell. However, recent research demonstrates that BCR inhibitors act on various immune cell types within the CLL microenvironment that control immune dysfunction and immune-related serious adverse events occurring in patients. Novel mechanisms of action for B-cell receptor inhibitors characterized in these immune cells may lead to repurposing of the drug for use in other cancers or autoimmune conditions.
PI3K inhibitors and toxicity
PI3Kδ shows higher intrinsic activity in leukemic B cells compared with normal B cells and can be therapeutically targeted in CLL. Idelalisib (targeting PI3Kδ) and duvelisib (also known as IPI-145, targeting PI3Kγ/δ) have both demonstrated clinical responses attributed to their direct effects on malignant B cells’ dependence on intrinsic and extrinsic survival signals.52,53 Nevertheless, their reported toxicities are a cause for concern. Although data from early idelalisib CLL trials in R/R patients reported only 3% occurrence of serious adverse event (SAE) diarrhea, a subsequent trial in treatment-naive patients reported a 42.2% occurrence of diarrhea or colitis SAEs, followed by high discontinuation rates. Other SAEs reported included hepatotoxicity and pneumonitis.54-56 Grade 3/4 diarrhea and hepatotoxicity were reported in clinical trials with duvelisib57 and the pan-PI3K inhibitor pilaralisib.58 These data indicate that this toxicity pattern may be a class effect of PI3K inhibitors.
T cells represent a secondary target cell population for PI3K inhibitors because of their dependence on PI3Kδ and PI3Kγ isoforms for different signaling pathways. Early in vitro studies reported no cytotoxic properties of idelalisib against normal CD3+ T cells, but described a reduction of inflammatory and anti-apoptotic cytokines.59 Recently, preliminary studies have reinvestigated PI3K inhibitors in specific T-cell subsets to determine culprits with a role in autoimmune toxicity. Matos et al reported that patients who received idelalisib showed decreased Treg number and function. Tregs in patients experiencing autoimmune toxicities showed decreased expression of the functional markers GITR, T-bet, CXCR3, granzyme-B, and TIM-3. Tregs also downregulated prosurvival BCL-2 and increased proapoptotic CD95.60 Our group subsequently described the differential effect of TGR-120261,62 in normal human T cells and murine CLL T cells compared with idelalisib and duvelisib.63,64 Treg numbers and function were depleted by all 3 inhibitors, but were maintained closer to normal levels after TGR-1202 treatment. These data are of relevance to determining why this inhibitor causes fewer incidences of SAEs in patients. Further, Deng et al recently reported a previously unknown off-target effect of TGR-1202. TGR-1202 silenced c-myc translation in leukemia and lymphoma cells through inhibition of casein kinase 1 ε (CK1ε).65 The effect of this inhibition on T cells is currently unknown.
PI3K inhibition in immune subsets
Class I PI3-kinases are composed of a regulatory (p85 or p55) and a catalytic (class IA p110 α, β, δ, or class IB γ) subunit. On recruitment of subunits to the membrane at phosphorylated YXXM motifs, PI3K signaling is initiated, which acts downstream to control varied cellular functions, such as growth, proliferation, and apoptosis. Although α and β subunits are ubiquitously expressed, δ and γ are mainly expressed by leukocytes.66 At this time, relative expression of the 4 class I PI3K catalytic subunit isoforms in immune cell types has not been comprehensively characterized.
Data previously reported from genetically silenced mouse models might be useful in predicting the modulation of immune subsets by PI3K inhibitors. The PI3Kδ-inactive mouse model showed normal thymic development and T-subset ratios, but T cells exhibited reduced CD44 expression, indicating a role for the δ subunit in the differentiation of effector and/or memory T cells. PI3Kδ-inactive T cells exhibited diminished proliferation and IL-2 production poststimulation. PI3Kδ-inactive mice developed mild inflammatory bowel disease characterized by intestinal leukocyte infiltration, which was thought to result from dysfunctional Tregs.67 Impairment of the T-cell-mediated immune response has been partly explained by the recent discovery that PI3Kδ promotes CD4+ T-cell interactions with APCs through LFA-1 binding to ICAM-1.65 In addition, PI3Kδ-inactive Tregs produced less IL-10 and expressed lower levels of CD38+, correlating to defective suppressive function.68 PI3Kδ-inactive Tregs were incapable of protecting against a model of induced colitis. RAG-knockout (KO)/PI3Kδ inactive mice receiving CD4+ T cells developed severe colitis, showing increased percentages of IFN-γ- and IL-17A-producing lamina propia CD4+ T cells compared with RAG-KO mice.69 Despite overall reduced inflammatory T-cell response, PI3Kδ-inactive mice exhibited improved resistance to bacterial infections, possibly as a result of reduced Treg expansion and tissue homing.70,71
Investigations with PI3Kγ-inactive or PI3Kγ-KO mice have also highlighted roles for this catalytic isoform in T-cell development, trafficking, activation, and Th1 and Th17 responses. Unlike PI3Kδ, PI3Kγ has been linked to chemokine-receptor signaling through G-protein-coupled receptors, but not T-cell receptors (TCRs). The tumor-reactive CD8+ effector T-cell population is of interest because of its involvement in antitumor immunity.72 Murine PI3Kγ-KO CD8+ effector T cells displayed impaired migration after viral challenge,73 suggesting γ could regulate tumor-reactive CD8+ effector T cells. PI3Kγ is also involved in the regulation of dendritic cell (DC), neutrophil, and monocyte migration.74 Further, studies of PI3Kγ/δ double KO mice showed dramatic reductions in T cells in peripheral blood, lymph nodes, and spleen alongside symptoms of lymphopenia.75 Functionally, Tregs of γ-KO/δ-inactive mice were deficient in suppressive assays and expressed low levels of GITR and FoxP3. It was noted that lymphopenia is associated with autoimmunity; however, a secondary factor is necessary to induce autoimmune disease, such as dysfunctional Tregs or local tissue inflammation.76
Although there is now evidence that peripheral Treg function is compromised after PI3Kδ or γ/δ genetic inactivation, experiments using PI3K inhibitors to interrogate Treg function in vitro have reported variable results, likely because of the context of dosing and stimulation. Tregs derived from normal human peripheral blood mononuclear cells were selectively spared by PI3Kα, PI3Kδ, or MEK inhibitors when compared with CD4+ CD25− (Tcon) and CD8+ (Teff) subsets.77 Tregs retained closer to normal levels of proliferation, activation, and suppressive capacity after anti-CD3/CD28 stimulation in the presence of these inhibitors. Most interestingly, PI3Kδ and PI3Kα protein expression levels were lower in Tregs than in Tcons. This is consistent with observations that PI3K signaling inhibition can differentially affect Tregs vs Tcon or Teff and suggests protein expression of the PI3K catalytic isoforms should be quantified and compared among immune subsets. Thus far, the clinical application of PI3K inhibitors resembles the PI3Kδ-inactive mouse model, as peripheral Treg numbers and functions are compromised in patients after PI3K inhibitor treatments.
Abu-Eid et al showed that PI3K-Akt pathway inhibition decreased Treg infiltration of the TC-1 tumor and enhanced the antitumor effects of tumor-specific vaccinations.78 In another study, Ali et al found that both genetic and pharmacological inactivation of PI3Kδ in Tregs conferred resistance to solid tumor growth (4T1, EL4, and LLC) in mice. Although PI3Kδ blockade weakened the cytotoxic T-cell response, Treg-mediated immune suppression was overridden and an antitumor effect was achieved. PI3Kδ may be more essential for Treg response than effector T-cell response,79 and PI3Kδ blockade may reinvigorate adaptive antitumor responses, implying additional mechanisms for PI3K inhibitor efficacy against hematological and solid malignancies.
Alongside Tregs, other immune subsets have been implicated in PI3K-mediated antitumor immunity. It was found that PI3K inhibition could relieve immunosuppression to augment the use of Toll-like receptor (TLR) agonist for improving antitumor immunity in combination with DC vaccines.80 In a comprehensive analysis using inhibitors of all class I PI3K catalytic isoforms, class I PI3K inhibition in DCs suppressed IL-10 and TGF-β secretion. However, PI3K inhibition did not hinder pro-inflammatory induction of IL-12 and IL-1B after TLR5 ligand (flagellin) treatment. The combination of pathogen-derived flagellin with pan-PI3K inhibition suppressed tumor growth in subcutaneous B16, CT26, and LLC solid tumor models, with and without DC vaccines. Here, it was demonstrated that both DC and tumor-infiltrating lymphocyte populations were functionally modulated by PI3Ki treatment, and that both were involved in the antitumor response.
Ibrutinib in T cells and other immune cells
Although ibrutinib is known for its clinical success as an irreversible BTK inhibitor, it is also the first clinically available inhibitor of inducible T-cell kinase (ITK). ITK is expressed in T cells and belongs to the Tec kinase family. It is a major player in TCR signaling, activating PLC-γ and downstream NFAT, nuclear factor-κB, MAPK pathway, calcium mobilization, cytoskeleton reorganization, and synapse formation and adhesion.81 Gomez-Rodriguez et al demonstrated the importance of ITK in regulating Th17/Treg lineage differentiation.82 Considering the immunomodulatory effects of ITK inhibition, it is possible for ibrutinib to be repurposed for use in other contexts.
Dubovsky et al explored the effects of ibrutinib in T cells using healthy human T cells, T cells of patients with CLL, Jurkat T cells, and mouse models.83 This study established that ibrutinib treatment irreversibly inhibited ITK to decrease downstream activation in Th2 cells, but not Th1 or CD8+ T cells, as a result of compensation by redundant resting lymphocyte kinase. Ibrutinib-treated CLL T cells exhibited Th1 skewing via T-bet upregulation and JunB downregulation. Systemic treatment confirmed these effects in euTCL1 mice. These data imply that ibrutinib may function through a 2-pronged approach to target malignant B cells directly, while reinvigorating a beneficial inflammatory T-cell response in CLL. Using mouse models of leishmaniasis, leukemia, and listeriosis, this study highlighted the therapeutic potential of ibrutinib in other diseases involving disproportionate polarization of Th2 immunity.
Other studies have explored the effects of ibrutinib on the CLL immune microenvironment. Niemann et al reported decreased T-cell activation, proliferation, and PD-1 expression in patients with CLL after ibrutinib therapy.84 Consistent with prior findings in ITK knockout mice, the authors demonstrated reduced proliferation of circulating Th17 cells in these patients and inhibition of Th17 differentiation in ex vivo assays. In addition, ibrutinib treatment disrupted CLL–macrophage interactions in bone marrow specimens. Yin et al described an increase in TCR diversity in patients with CLL after 1 year of ibrutinib therapy.85 Using next-generation sequencing, this paper demonstrated that pretherapy TCRβ clones decreased, whereas the number of productive, unique clones increased during treatment. TCR diversity was positively correlated with clinical efficacy and lower infection rates. In CLL mouse models, Chen et al determined that ibrutinib treatment deregulated B-cell surface membrane CXCR4 expression and signaling, disrupted the homing of B-CLL cells to lymphoid tissue, and ultimately contributed to improved survival via this novel mechanism.86 The effect of ibrutinib on CLL T cell migration and homing, however, is yet to be studied.
In a pioneering work, Sagiv-Barfi et al harnessed the immunomodulatory capacity of ibrutinib in combination with an immune checkpoint blockade (anti-PD-L1 antibody) to treat hematological malignancies without intrinsic sensitivity to ibrutinib and solid tumors without BTK expression.87 In ibrutinib-insensitive PD-L1+ A20 lymphoma established in mice, combining ibrutinib with anti-PD-L1 synergistically delayed tumor growth and prolonged survival. Tumor-specific T cells were detected in mice treated with the combination. This result was recapitulated in ibrutinib-insensitive J558 myeloma and 4T1 (breast cancer) with appreciably fewer metastatic lesions. Similar results were seen with CT26 (colon cancer) tumor growth, with detection of T cells specific to the CT26 tumor antigen. The cured mice displayed long-term memory to CT26 antigens. Ibrutinib may therefore enhance response to T-cell therapies.
In a simultaneous study, Sagiv-Barfi et al explored the concept of repurposing ibrutinib by combining intratumoral vaccine with ibrutinib treatment in a mouse model of subcutaneous lymphoma.88 Intratumoral administration of CpG activated local natural killer cells, macrophages, and DCs through TLR9 agonist activity, resulting in local tumor regression but not systemic antitumor response. Systemic administration of ibrutinib alone caused mild tumor growth delay at all sites. In contrast, combining CpG and ibrutinib resulted in complete and permanent tumor regression at all sites. This effect was found to be CD4+ and CD8+ T-cell dependent. Infused T cells that had been pretreated with the combination prevented the outgrowth of tumors in new recipient mice.
The full extent of the immunomodulatory properties of ibrutinib remains unclear. Although natural killer T cells and mast cells also express ITK, their responses to ibrutinib treatment are not yet known. Natarajan et al identified that ibrutinib treatment decreased expression of MHCII and CD86 but increased expression of CD80 on DCs. Ibrutinib-treated DCs also promoted T-cell proliferation and enhanced IL-17 cytokine production in coculture via TLR4-modulated activation.89 In addition, in a report from Stiff et al on the effects of ibrutinib treatment on MDSCs, which express BTK, ibrutinib inhibited BTK phosphorylation.90 Together with reduced cell migration and inhibition of in vitro MDSC generation, ibrutinib treatment also reduced MDSCs in spleen and EMT6 murine mammary tumors. Further, ibrutinib treatment reduced MDSCs in a melanoma model in a BTK-dependent fashion. These data potentiate alternative modes of action for ibrutinib in previously unexplored immune cell types.
Acalabrutinib is a more selective BTK inhibitor than ibrutinib, demonstrating on par antitumor efficacy in CLL, both in preclinical models and patient trials.91,92 Preliminary comparison of patients treated with ibrutinib or acalabrutinib suggests the immunomodulatory capacity of acalabrutinib is differentiated from that of ibrutinib, likely because of fewer off-target effects on Tec-family kinases, including ITK. Ibrutinib, but not acalabrutinib, increased absolute numbers of CD4+ and CD8+ T cells enriched for effector memory subsets over naive and central memory subsets. Acalabrutinib did not change Treg-to-CD4 ratio or activation-induced cell death. Both treatment groups did, however, exhibit reduced PD-1 and CTLA-4 expression in CD4+ and CD8+ T cells.
Conclusions
CLL T-cell dysregulation trends have been well described, showing that altered subset ratios and gene expression and function are necessary for and supportive of malignant progression. In addition to supporting malignant B cells directly, CLL T cells orchestrate immune dysfunction and immune-related SAEs. Immunomodulatory agents, monoclonal antibodies, and epigenetic modifiers targeting CLL T-cell dysfunction have yielded promising preclinical results. Clinically administered BCR inhibitors also display immunomodulatory properties, affecting a wide range of immune cell categories. Emerging data describing the immunomodulatory capacity of BCR inhibitors predict feasible combination strategies for ibrutinib with other immunomodulatory agents in CLL, such as lenalidomide or histone deacetylase inhibitors. In addition, the modulation of T cells by ibrutinib or PI3K inhibitors could augment antitumor responses from checkpoint blockade in CLL, such as with anti-PD-L1 antibody. Our review of the effect of BCR inhibitors on T cells and other immune compartments suggests potentially novel mechanisms of action, providing a rationale to extend their use to other cancers and autoimmune disorders.
Authorship
Contribution: Material in this review was written by K.M. and E.S. and was reviewed and revised by J.P.-I.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Javier Pinilla-Ibarz, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: javier.pinilla@moffitt.org.
References
- 1.Scarfò L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169-182. [DOI] [PubMed] [Google Scholar]
- 2.Zhong Y, Byrd JC, Dubovsky JA. The B-cell receptor pathway: a critical component of healthy and malignant immune biology. Semin Hematol. 2014;51(3):206-218. [DOI] [PubMed] [Google Scholar]
- 3.Brown JR. The PI3K pathway: clinical inhibition in chronic lymphocytic leukemia. Semin Oncol. 2016;43(2):260-264. [DOI] [PubMed] [Google Scholar]
- 4.Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013;34(12):592-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awan FT, Byrd JC. New strategies in chronic lymphocytic leukemia: shifting treatment paradigms. Clin Cancer Res. 2014;20(23):5869-5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120(6):1175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riches JC, Gribben JG. Immunomodulation and immune reconstitution in chronic lymphocytic leukemia. Semin Hematol. 2014;51(3):228-234. [DOI] [PubMed] [Google Scholar]
- 8.Nicholas NS, Apollonio B, Ramsay AG. Tumor microenvironment (TME)-driven immune suppression in B cell malignancy. Biochim Biophys Acta. 2016;1863(3):471-482. [DOI] [PubMed] [Google Scholar]
- 9.Kay NE, Johnson JD, Stanek R, Douglas SD. T-cell subpopulations in chronic lymphocytic leukemia: abnormalities in distribtuion and in in vitro receptor maturation. Blood. 1979;54(2):540-544. [PubMed] [Google Scholar]
- 10.Podhorecka M, Dmoszynska A, Rolinski J, Wasik E. T type 1/type 2 subsets balance in B-cell chronic lymphocytic leukemia--the three-color flow cytometry analysis. Leuk Res. 2002;26(7):657-660. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Xu X, Lee EJ, et al. . Phenotypic alteration of CD8+ T cells in chronic lymphocytic leukemia is associated with epigenetic reprogramming. Oncotarget. 2016;7(26):40558-40570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghia P, Strola G, Granziero L, et al. . Chronic lymphocytic leukemia B cells are endowed with the capacity to attract CD4+, CD40L+ T cells by producing CCL22. Eur J Immunol. 2002;32(5):1403-1413. [DOI] [PubMed] [Google Scholar]
- 13.Wallace ME, Alcantara MB, Minoda Y, Kannourakis G, Berzins SP. An emerging role for immune regulatory subsets in chronic lymphocytic leukaemia. Int Immunopharmacol. 2015;28(2):897-900. [DOI] [PubMed] [Google Scholar]
- 14.Vardi A, Vlachonikola E, Karypidou M, et al. . Restrictions in the T-cell repertoire of chronic lymphocytic leukemia: high-throughput immunoprofiling supports selection by shared antigenic elements. Leukemia. 2017;31(7):1555-1561. [DOI] [PubMed] [Google Scholar]
- 15.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573-581. [DOI] [PubMed] [Google Scholar]
- 16.Parry HM, Damery S, Hudson C, et al. . Cytomegalovirus infection does not impact on survival or time to first treatment in patients with chronic lymphocytic leukemia. Am J Hematol. 2016;91(8):776-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.te Raa GD, Pascutti MF, García-Vallejo JJ, et al. . CMV-specific CD8+ T-cell function is not impaired in chronic lymphocytic leukemia. Blood. 2014;123(5):717-724. [DOI] [PubMed] [Google Scholar]
- 18.Jadidi-Niaragh F, Ghalamfarsa G, Yousefi M, Tabrizi MH, Shokri F. Regulatory T cells in chronic lymphocytic leukemia: implication for immunotherapeutic interventions. Tumour Biol. 2013;34(4):2031-2039. [DOI] [PubMed] [Google Scholar]
- 19.Beyer M, Kochanek M, Darabi K, et al. . Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106(6):2018-2025. [DOI] [PubMed] [Google Scholar]
- 20.Skórka K, Bhattacharya N, Własiuk P, et al. . Thalidomide regulation of NF-κB proteins limits Tregs activity in chronic lymphocytic leukemia. Adv Clin Exp Med. 2014;23(1):25-32. [DOI] [PubMed] [Google Scholar]
- 21.Jitschin R, Braun M, Büttner M, et al. . CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood. 2014;124(5):750-760. [DOI] [PubMed] [Google Scholar]
- 22.Hus I, Bojarska-Junak A, Chocholska S, et al. . Th17/IL-17A might play a protective role in chronic lymphocytic leukemia immunity. PLoS One. 2013;8(11):e78091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Idler I, Giannopoulos K, Zenz T, et al. . Lenalidomide treatment of chronic lymphocytic leukaemia patients reduces regulatory T cells and induces Th17 T helper cells. Br J Haematol. 2010;148(6):948-950. [DOI] [PubMed] [Google Scholar]
- 24.Lad DP, Varma S, Varma N, Sachdeva MU, Bose P, Malhotra P. Regulatory T-cell and T-helper 17 balance in chronic lymphocytic leukemia progression and autoimmune cytopenias. Leuk Lymphoma. 2015;56(8):2424-2428. [DOI] [PubMed] [Google Scholar]
- 25.Perry C, Herishanu Y, Hazan-Halevy I, et al. . Reciprocal changes in regulatory T cells and Th17 helper cells induced by exercise in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(9):1807-1810. [DOI] [PubMed] [Google Scholar]
- 26.Nunes C, Wong R, Mason M, Fegan C, Man S, Pepper C. Expansion of a CD8(+)PD-1(+) replicative senescence phenotype in early stage CLL patients is associated with inverted CD4:CD8 ratios and disease progression. Clin Cancer Res. 2012;18(3):678-687. [DOI] [PubMed] [Google Scholar]
- 27.Göthert JR, Eisele L, Klein-Hitpass L, et al. . Expanded CD8+ T cells of murine and human CLL are driven into a senescent KLRG1+ effector memory phenotype. Cancer Immunol Immunother. 2013;62(11):1697-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monserrat J, Sánchez MA, de Paz R, et al. . Distinctive patterns of naïve/memory subset distribution and cytokine expression in CD4 T lymphocytes in ZAP-70 B-chronic lymphocytic patients. Cytometry B Clin Cytom. 2014;86(1):32-43. [DOI] [PubMed] [Google Scholar]
- 29.Zaleska J, Skorka K, Zajac M, et al. . Specific cytotoxic T-cell immune responses against autoantigens recognized by chronic lymphocytic leukaemia cells. Br J Haematol. 2016;174(4):582-590. [DOI] [PubMed] [Google Scholar]
- 30.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riches JC, Davies JK, McClanahan F, et al. . T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121(9):1612-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palma M, Gentilcore G, Heimersson K, et al. . T cells in chronic lymphocytic leukemia display dysregulated expression of immune checkpoints and activation markers. Haematologica. 2017;102(3):562-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tonino SH, van de Berg PJ, Yong SL, et al. . Expansion of effector T cells associated with decreased PD-1 expression in patients with indolent B cell lymphomas and chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(9):1785-1794. [DOI] [PubMed] [Google Scholar]
- 34.Görgün G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115(7):1797-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsay AG, Johnson AJ, Lee AM, et al. . Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118(7):2427-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kabanova A, Sanseviero F, Candi V, et al. . Human cytotoxic T lymphocytes form dysfunctional immune synapses with b cells characterized by non-polarized lytic granule release. Cell Reports. 2016;15(1):9-18. [DOI] [PubMed] [Google Scholar]
- 37.Gaidano G, Foà R, Dalla-Favera R. Molecular pathogenesis of chronic lymphocytic leukemia. J Clin Invest. 2012;122(10):3432-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smallwood DT, Apollonio B, Willimott S, et al. . Extracellular vesicles released by CD40/IL-4-stimulated CLL cells confer altered functional properties to CD4+ T cells. Blood. 2016;128(4):542-552. [DOI] [PubMed] [Google Scholar]
- 39.Dubovsky JA, Powers JJ, Gao Y, Mariusso LF, Sotomayor EM, Pinilla-Ibarz JA. Epigenetic repolarization of T lymphocytes from chronic lymphocytic leukemia patients using 5-aza-2′-deoxycytidine. Leuk Res. 2011;35(9):1193-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubovsky JA, Wang D, Powers JJ, et al. . Restoring the functional immunogenicity of chronic lymphocytic leukemia using epigenetic modifiers. Leuk Res. 2011;35(3):394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorgun G, Ramsay AG, Holderried TA, et al. . E(mu)-TCL1 mice represent a model for immunotherapeutic reversal of chronic lymphocytic leukemia-induced T-cell dysfunction. Proc Natl Acad Sci USA. 2009;106(15):6250-6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofbauer JP, Heyder C, Denk U, et al. . Development of CLL in the TCL1 transgenic mouse model is associated with severe skewing of the T-cell compartment homologous to human CLL. Leukemia. 2011;25(9):1452-1458. [DOI] [PubMed] [Google Scholar]
- 43.McClanahan F, Riches JC, Miller S, et al. . Mechanisms of PD-L1/PD-1-mediated CD8 T-cell dysfunction in the context of aging-related immune defects in the Eµ-TCL1 CLL mouse model. Blood. 2015;126(2):212-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagnara D, Kaufman MS, Calissano C, et al. . A novel adoptive transfer model of chronic lymphocytic leukemia suggests a key role for T lymphocytes in the disease. Blood. 2011;117(20):5463-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patten PE, Ferrer G, Chen SS, et al. . Chronic lymphocytic leukemia cells diversify and differentiate in vivo via a nonclassical Th1-dependent, Bcl-6-deficient process [published online ahead of print 7 April 2016]. JCI Insight. doi:10.1172/jci.insight.86288. [DOI] [PMC free article] [PubMed]
- 46.Bichi R, Shinton SA, Martin ES, et al. . Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci USA. 2002;99(10):6955-6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gassner FJ, Zaborsky N, Catakovic K, et al. . Chronic lymphocytic leukaemia induces an exhausted T cell phenotype in the TCL1 transgenic mouse model. Br J Haematol. 2015;170(4):515-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brusa D, Serra S, Coscia M, et al. . The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica. 2013;98(6):953-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown JR, Hallek MJ, Pagel JM. Chemoimmunotherapy versus targeted treatment in chronic lymphocytic leukemia: when, how long, how much, and in which combination? Am Soc Clin Oncol Educ Book. 2016;35:e387-e398. [DOI] [PubMed] [Google Scholar]
- 50.Bachow SH, Lamanna N. Evolving strategies for the treatment of chronic lymphocytic leukemia in the upfront setting. Curr Hematol Malig Rep. 2016;11(1):61-70. [DOI] [PubMed] [Google Scholar]
- 51.Brown JR, Byrd JC, Coutre SE, et al. . Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lannutti BJ, Meadows SA, Herman SE, et al. . CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong S, Guinn D, Dubovsky JA, et al. . IPI-145 antagonizes intrinsic and extrinsic survival signals in chronic lymphocytic leukemia cells. Blood. 2014;124(24):3583-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madanat YF, Smith MR, Almasan A, Hill BT. Idelalisib therapy of indolent B-cell malignancies: chronic lymphocytic leukemia and small lymphocytic or follicular lymphomas. Blood Lymphat Cancer. 2016;6:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrientos JC, Kaur M, Mark A, et al. . Outcomes of patients with chronic lymphocytic leukemia (CLL) after idelalisib therapy discontinuation [abstract]. Blood. 2015;126(23). Abstract 4155. [Google Scholar]
- 56.Lampson BL, Kasar SN, Matos TR, et al. . Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128(2):195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flinn I, Patel MR, Maris MB, Matous J, Cherry M, Berdeja JG. An open-label, phase Ib study of duvelisib (IPI-145) in combination with bendamustine, rituximab, or bendamustine/rituximab in select subjects with lymphoma or chronic lymphocytic leukemia [abstract]. Blood. 2014;124(21). Abstract 4422. [Google Scholar]
- 58.Brown JR, Davids MS, Rodon J, et al. . Phase I trial of the pan-PI3K inhibitor pilaralisib (SAR245408/XL147) in patients with chronic lymphocytic leukemia (CLL) or relapsed/refractory lymphoma. Clin Cancer Res. 2015;21(14):3160-3169. [DOI] [PubMed] [Google Scholar]
- 59.Herman SE, Gordon AL, Wagner AJ, et al. . Phosphatidylinositol 3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matos TR, Lampson BL, Hirakawa M, et al. . Altered expression of functional proteins in CD4 regulatory T cells during therapy with idelalisib [abstract]. Blood. 2015;126(23). Abstract 1735. [Google Scholar]
- 61.Burris HA, Patel MR, Brander DM, et al. . TGR-1202, a novel once daily PI3Kd inhibitor, demonstrates clinical activity with a favorable safety profile, lacking hepatotoxicity, in patients with chronic lymphocytic leukemia and B-cell lymphoma [abstract]. Blood. 2014;124(21). Abstract 1984. [Google Scholar]
- 62.Burris HA, Flinn IW, Lunning M, et al. . Long-term follow-up of the PI3Kd inhibitor TGR-1202 to demonstrate a differentiated safety profile and high response rates in CLL and NHL: Integrated-analysis of TGR-1202 monotherapy and combined with ublituximab. Presented at 2016 American Society of Clinical Oncology. 3-7 June 2016. Chicago IL. [Google Scholar]
- 63.Maharaj KPJ. Modulation of T cell compartment in a preclinical CLL murine model by a selective PI3K delta inhibitor, TGR-1202 [abstract]. Blood. 2016;128(22). Abstract 3236. [Google Scholar]
- 64.Maharaj KPJ. Differential regulation of human T-cells by TGR-1202, a novel PI3Kδ inhibitor. Presented at 2016 American Association for Cancer Research Annual Meeting. 17 April 2016. New Orleans LA. [Google Scholar]
- 65.Deng C, Lipstein MR, Scotto L, et al. . Silencing c-Myc translation as a therapeutic strategy through targeting PI3Kδ and CK1ε in hematological malignancies. Blood. 2017;129(1):88-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garçon F, Okkenhaug K. PI3Kδ promotes CD4(+) T-cell interactions with antigen-presenting cells by increasing LFA-1 binding to ICAM-1. Immunol Cell Biol. 2016;94(5):486-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu Rev Immunol. 2013;31:675-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patton DT, Garden OA, Pearce WP, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177(10):6598-602. [DOI] [PubMed] [Google Scholar]
- 69.Steinbach EC, Kobayashi T, Russo SM, et al. . Innate PI3K p110δ regulates Th1/Th17 development and microbiota-dependent colitis. J Immunol. 2014;192(8):3958-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu D, Zhang T, Marshall AJ, Okkenhaug K, Vanhaesebroeck B, Uzonna JE. The p110delta isoform of phosphatidylinositol 3-kinase controls susceptibility to Leishmania major by regulating expansion and tissue homing of regulatory T cells. J Immunol. 2009;183(3):1921-1933. [DOI] [PubMed] [Google Scholar]
- 71.Pearce VQ, Bouabe H, MacQueen AR, Carbonaro V, Okkenhaug K. PI3Kδ regulates the magnitude of CD8+ T cell responses after challenge with Listeria monocytogenes. J Immunol. 2015;195(7):3206-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. . Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102(27):9571-9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin AL, Schwartz MD, Jameson SC, Shimizu Y. Selective regulation of CD8 effector T cell migration by the p110 gamma isoform of phosphatidylinositol 3-kinase. J Immunol. 2008;180(4):2081-2088. [DOI] [PubMed] [Google Scholar]
- 74.Winkler DG, Faia KL, DiNitto JP, et al. . PI3K-δ and PI3K-γ inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013;20(11):1364-1374. [DOI] [PubMed] [Google Scholar]
- 75.Webb LM, Vigorito E, Wymann MP, Hirsch E, Turner M. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005;175(5):2783-2787. [DOI] [PubMed] [Google Scholar]
- 76.Ji H, Rintelen F, Waltzinger C, et al. . Inactivation of PI3Kgamma and PI3Kdelta distorts T-cell development and causes multiple organ inflammation. Blood. 2007;110(8):2940-2947. [DOI] [PubMed] [Google Scholar]
- 77.Zwang NA, Zhang R, Germana S, et al. . Selective sparing of human Tregs by pharmacologic inhibitors of the phosphatidylinositol 3-kinase and MEK pathways. Am J Transplant. 2016;16(9):2624-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abu-Eid R, Samara RN, Ozbun L, et al. . Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer Immunol Res. 2014;2(11):1080-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ali K, Soond DR, Pineiro R, et al. . Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510(7505):407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marshall NA, Galvin KC, Corcoran AM, Boon L, Higgs R, Mills KH. Immunotherapy with PI3K inhibitor and Toll-like receptor agonist induces IFN-γ+IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer Res. 2012;72(3):581-591. [DOI] [PubMed] [Google Scholar]
- 81.Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, Berg LJ. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem. 2000;275(3):2219-2230. [DOI] [PubMed] [Google Scholar]
- 82.Gomez-Rodriguez J, Wohlfert EA, Handon R, et al. . Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J Exp Med. 2014;211(3):529-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dubovsky JA, Beckwith KA, Natarajan G, et al. . Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niemann CU, Herman SE, Maric I, et al. . Disruption of in vivo chronic lymphocytic leukemia tumor-microenvironment interactions by ibrutinib--findings from an investigator-initiated phase II study. Clin Cancer Res. 2016;22(7):1572-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yin Q, Sivina M, Robins H, et al. . Ibrutinib therapy increases T cell repertoire diversity in patients with chronic lymphocytic leukemia. J Immunol. 2017;198(4):1740-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen SS, Chang BY, Chang S, et al. . BTK inhibition results in impaired CXCR4 chemokine receptor surface expression, signaling and function in chronic lymphocytic leukemia. Leukemia. 2016;30(4):833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sagiv-Barfi I, Kohrt HEK, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci USA. 2015;112(9):E966-E972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sagiv-Barfi I, Kohrt HE, Burckhardt L, Czerwinski DK, Levy R. Ibrutinib enhances the antitumor immune response induced by intratumoral injection of a TLR9 ligand in mouse lymphoma. Blood. 2015;125(13):2079-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Natarajan G, Terrazas C, Oghumu S, et al. . Ibrutinib enhances IL-17 response by modulating the function of bone marrow derived dendritic cells. OncoImmunology. 2015;5(1):e1057385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stiff A, Trikha P, Wesolowski R, et al. . Myeloid-derived suppressor cells express Bruton’s tyrosine kinase and can be depleted in tumor-bearing hosts by ibrutinib treatment. Cancer Res. 2016;76(8):2125-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Byrd JC, Harrington B, O’Brien S, et al. . Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herman SEM, Montraveta A, Niemann CU, et al. . The Bruton tyrosine kinase (BTK) inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic leukemia. Clin Cancer Res. 2017;23(11):2831-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]