TO THE EDITOR:
Large granular lymphocytic (LGL) leukemia and myelodysplastic syndromes (MDSs) both typically present with unexplained cytopenias, yet these diseases are pathobiologically distinct and associated with substantial differences in prognosis and therapy. LGL leukemias, including both T-cell LGL leukemia and chronic lymphoproliferative disorder of natural killer (NK) cells, are clonal lymphoid disorders characterized by phenotypically abnormal cytotoxic T or NK cells and a typically indolent clinical course. In contrast, MDSs are clonal disorders of hematopoietic stem cells defined by ineffective hematopoiesis, morphologic dysplasia, and an elevated risk of acute myeloid leukemia.
Despite these differences, an accurate diagnosis can be challenging, in part because both diseases exhibit clinicopathologic overlap with reactive conditions, and because the lesional cells in both entities can lack obvious morphologic abnormalities.1,2 In addition, although it is well-established that the initial evaluation of cytopenias should include consideration for both entities, these principles do not always translate into practice due to nonuniform diagnostic methodology and interprovider variability across institutions. Thus, objective markers that promote increased uniformity in the diagnostic approach to cytopenias are needed. Recently, activating STAT3 mutations, primarily within the SH2 domain, have been identified in 40% to 70% of LGL leukemia.3-8 Although STAT3 mutations are not required for a diagnosis of LGL leukemia, detection of the mutation should prompt additional evaluation for the disease if unsuspected. In this article, we report the frequency and type of STAT3 mutations within our patient population and assess the impact of this information on diagnosis, particularly in the setting of a suspected MDS. We find that inclusion of STAT3 on a standard next-generation sequencing (NGS) gene panel ensures objective and systematic evaluation for cryptic or unsuspected LGL leukemia.
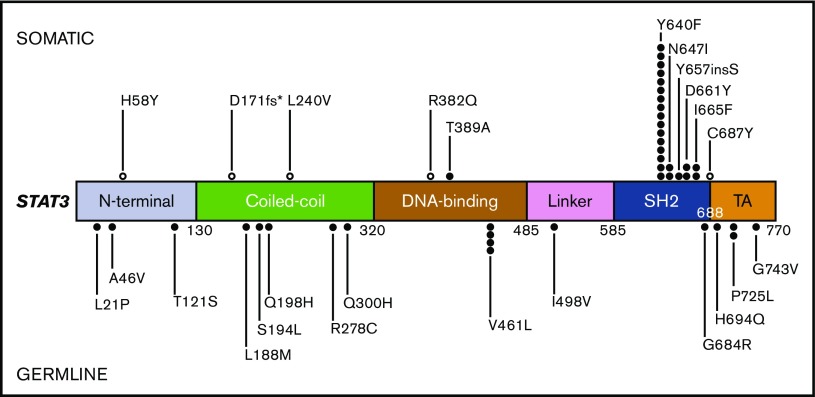
Between 1 January 2015 and 31 October 2016, 4244 peripheral blood or bone marrow samples from 3088 unique patients evaluated at our institutions were analyzed with a custom 95-gene, amplicon-based panel comprised of genes recurrently mutated in hematologic malignancies (supplemental Table 1).9 Indications for testing included known or suspected myeloid neoplasm or acute leukemia (57%), unexplained cytopenias/cytoses (18%), known or suspected lymphoproliferative disorders (17%) or unspecified (7%). The panel includes exons 2-17 and 21- 23 of STAT3 (NM_139276). We identified 45 patients with 47 candidate STAT3 mutations (Figure 1). Eighteen variants were classified as presumed germ line single nucleotide polymorphisms (SNPs), although 3 variants were not found in SNP databases and may represent very low frequency/private SNPs or potential novel disease-associated mutations (Figure 1; supplemental Table 2). An additional 23 variants, from 23 patients, were localized within the SH2 domain and included previously documented somatic variants in LGL leukemia (Y640F, n = 16; N647I, n = 2; and D661Y, n = 2) as well as novel variants (657insS, n = 1; and I665F, n = 2), all of which map to the STAT3 dimerization interface. The remaining 6 were classified as somatic non–SH2 domain variants. One occurred in a patient with LGL leukemia who had a concomitant STAT3 SH2 domain mutation (Y640F) (Table 1). The other 5 mutations were identified in disparate clinical scenarios, suggesting that some non–SH2 domain STAT3 mutations are not associated with LGL leukemia but could have a pathogenic role in other hematologic malignancies (supplemental Table 2). Institutional review board approval was granted for the retrospective review of charts and pathology data.
Figure 1.
Linear depiction of the protein domains of the STAT3 gene and the location of each variant detected within our cohort. Variants predicted to be somatic are positioned above the gene diagram, and variants predicted to be germ line are positioned below. Open circles represent somatic STAT3 mutations potentially associated with conditions or diseases other than LGL leukemia.
Table 1.
Clinicopathologic characteristics of 23 patients with STAT3 SH2 domain variants
| Age, y | Sex | NGS indication | Specimen | All somatic variants detected on NGS panel (VAF %) | T-cell clonality* | Splenomegaly | WBCs, ×109/L | Neutrophil count, ×109/L | Lymphocyte count, ×109/L | Hgb, g/dL | Platelets, ×109/L | Follow-up, mo† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 | F | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (13.8) | Yes | No | 1.16 | 0.31 | 0.6 | 14.6 | 106 | 14.7 |
| 55 | F | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (14.9)‡ | Yes | No | 3.27 | 0.97 | 1.81 | 14.0 | 212 | 4.2 |
| 51 | F | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (19.4) | Yes | Yes | 4.6 | 1.15 | 3.04 | 11.4 | 187 | 13.0 |
| 69 | F | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (2.3) | Yes | No | 3.23 | 1.36 | 1.54 | 9.5 | 51 | 24.5 |
| STAT3 c.1165T>C p.T389A (10.7) | ||||||||||||
| 29 | M | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (25.2) | Yes | Yes | 5.88 | 1.3 | 3.87 | 15.2 | 145 | 0 |
| 61 | M | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (3.4) | Yes | Yes | 0.9 | 0.24 | 0.5 | 11.4 | 95 | 0 |
| 71 | F | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (32.7) | Yes | No | 16.02 | 1.97 | 12.75 | 13.2 | 225 | 12.1 |
| 45 | F | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (35.7) | Yes | No | 10.78 | 1.29 | 7.76 | 12.8 | 255 | 13.1 |
| BRAF c.1781A>G p.D594G (4.7) | ||||||||||||
| 49 | M | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (4.2) | Yes | No | 2.04 | 0.33 | 1 | 13.8 | 225 | 20.1 |
| 36 | F | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (5.2) | Yes | No | 1.13 | 0.57 | 0.49 | 12.2 | 121 | 7.0 |
| 60 | F | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (5.6) | Yes | No | 4.44 | 2.26 | 1.63 | 14.3 | 156 | 0 |
| 51 | F | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (5.7) | Yes | Yes | 2.9 | 2.38 | 0.47 | 13.2 | 167 | 25.7 |
| 33 | M | LGL leukemia | PB | STAT3 c.1919A>T p.Y640F (6.2) | Yes | No | 3.97 | 2.03 | 1.54 | 15.9 | 154 | 6.1 |
| 74 | F | LGL leukemia | PB | STAT3 c.1940A>T p.N647I (14.1)§ | ND | No | 8.11 | 3.91 | 3.37 | 8.9 | 472 | 0 |
| CXCR4 NM_003467 c.702G>T p.K234N (16.3) | ||||||||||||
| 70 | M | LGL leukemia | PB | STAT3 c.1940A>T p.N647I (19.3) | I¶ | Yes | 9.52 | 2.19 | 6.56 | 11.7 | 160 | 0.7 |
| 91 | F | LGL leukemia | PB | STAT3 c.1981G>T p.D661Y (18.3) | Yes | No | 9.15 | 2.65 | 5.5 | 9.8 | 223 | 12.3 |
| SETD2 c.1366C>T p.R456* (12.0) | ||||||||||||
| TET2 c.1999_2000insTG p.H667fs* (15.7) | ||||||||||||
| 83 | M | LGL leukemia | PB | STAT3 c.1981G>T p.D661Y (22.7) | Yes | No | 2.8 | 0.45 | 1.79 | 12.1 | 145 | 13.4 |
| SMC3 NM_005445 c.763C>T p.Q255* (10.0) | ||||||||||||
| 34 | F | LGL leukemia | PB | STAT3 c.1993T>A p.I665F (9.0) | Yes¶ | No | 3.09 | 1.19 | 1.68 | 12.8 | 158 | 13.2 |
| 29 | M | LGL leukemia | PB | STAT3 c.1993T>A p.I665F (9.7) | Yes | No | 4.23 | 1.27 | 2.42 | 14.5 | 153 | 18.5 |
| 69 | M | Reported MDS | BM | STAT3 c.1971_1971insAAG p.657insS (22.7) | Yes¶ | No | 2.48 | 1.17 | 1.02 | 8.0 | 349 | 24.7 |
| 75 | M | Reported MDS | PB | STAT3 c.1919A>T p.Y640F (18.9) | Yes | No | 2.02 | 0.28 | 1.18 | 14.4 | 220 | 8.9 |
| 75 | M | Reported MDS | PB | STAT3 c.1919A>T p.Y640F (6.9) | Yes¶ | Yes | 8.99 | 3.38 | 4.46 | 10.0 | 249 | 16.1 |
| 76 | F | Reported MDS | BM | STAT3 c.1919A>T p.Y640F (12.9) | Yes | Yes | 1.11 | 0.13 | 0.83 | 8.6 | 87 | 1.3 |
BM, bone marrow; F, female; Hgb, hemoglobin; M, male; ND, no data; I, indeterminate; NGS, next-generation sequencing; PB, peripheral blood; WBCs, white blood cells.
T-cell clonality determined by BIOMED-2 TCR-γ clonality assay.
All patients alive with disease at follow-up.
Concomitant rheumatoid arthritis.
Also germ line STAT3 NM_139276 c.832C>T p.R278C (VAF 48.2%).
NK cell–like immunophenotyped (sCD3-CD16+).
The clinicopathologic characteristics of the 23 patients with STAT3 SH2 domain variants are presented in Table 1. Nineteen had known or suspected LGL leukemia at the time of NGS evaluation, based on the presence of expanded populations of clonal, surface CD3+CD8+CD57+ T cells (n = 16); of surface CD3–CD16+ NK cells (n = 2); or a clonal T-cell population with an atypical immunophenotype (surface CD3+CD4–CD8–CD57–) but with a clinical course compatible with LGL leukemia (n = 1).
The remaining 4 patients with STAT3 SH2 domain variants were 69 to 76 years of age and initially were referred to our institution with a diagnosis of an MDS based on cytopenias (Table 1) and reported unilineage erythroid dysplasia (n = 2), ring sideroblasts (n = 1), or other unspecified marrow findings (n = 1). By report, flow cytometry demonstrated an inverted CD4:CD8 ratio in peripheral blood in 1 patient and an atypical lymphoid population with an NK-like phenotype in the bone marrow of another, but these were not incorporated into the reported final diagnosis. All 4 cases reportedly demonstrated a normal karyotype; an MDS fluorescence in situ hybridization panel performed in 1 case showed no abnormalities, and a commercial myeloid neoplasm–based gene panel performed in another case reported no somatic variants.
On presentation to our institution, peripheral blood or bone marrow was submitted for routine gene panel sequencing to identify MDS-specific gene mutations and facilitate accurate risk stratification.10-15 Despite the reported histories of MDSs, no MDS-associated mutations were identified. In contrast, each sample demonstrated an isolated somatic STAT3 SH2 domain mutation (Table 1). This discovery prompted NK/T-cell flow cytometric analysis of peripheral blood, which in all 4 cases revealed either an expanded population of T-cell LGLs (n = 2: 30% surface CD3+CD4–CD8+CD2+CD5dimCD7+CD16–CD56–CD57+CD94dimTCR-α/β+; 19% surface CD3+CD4–CD8dimCD2+CD5dimCD7–CD16–CD56–CD57+CD94dimTCR-α/β+) or NK-like LGLs (n = 2: 27% surface CD3–CD4–CD8–CD2+CD5dimCD7dimCD16 subset CD56–CD57 subset CD94+; 71% surface CD3–CD4–CD8dimCD2+CD5–CD7dimCD16+CD56–CD57–CD94dim). Clonal T-cell receptor gene rearrangements were detected in each case, including those with an NK-like immunophenotype.16 As a result of these findings, all 4 patients were diagnosed with LGL leukemia.
Notably, LGL proliferations and frank LGL leukemia can co-occur with MDSs,17-19 and screening for STAT3 mutations in patients with MDS has been shown to facilitate detection of subclinical LGL clones.5 Therefore, identification of STAT3 mutations and a subsequent diagnosis of LGL leukemia does not exclude the possibility of a concomitant MDS. However, recent findings suggest a high negative predictive value for underlying myeloid neoplasms in patients who have neither karyotypic abnormalities nor mutations in typical driver genes,15 as was observed in these 4 patients. Therefore, we re-reviewed the bone marrow biopsy specimens for these 4 patients and found that none met the diagnostic criteria for an MDS. Overall, these cases underscore the challenges of evaluating older patients presenting with cytopenias and highlight the value of targeted NGS in correctly diagnosing an MDS or LGL leukemia.
As the accessibility of routine NGS testing increases, many institutions are incorporating mutational profiling into the clinical evaluation of myeloid neoplasms, in part to facilitate the diagnosis of cases with ambiguous morphologic features.20-22 These clinical myeloid NGS panels are often designed with a disease-restricted focus, with content limited to genes recurrently mutated in myeloid disease. However, our data suggest that it is useful to systematically deploy more comprehensive gene panels that include genes that are mutated in other diseases sharing clinical features with myeloid neoplasms, such as LGL leukemia, hairy cell leukemia, and paroxysmal nocturnal hemoglobinuria. STAT3 mutations are not entirely specific for LGL leukemia given that they have been identified at low frequency in other hematopoietic and nonhematopoietic malignancies; therefore, the identification of these mutations requires careful clinicopathologic correlation.23 Due to technical constraints, our current panel does not include the entirety of the STAT3 gene SH2 domain or STAT5B, which has since been found to be mutated in a smaller proportion (2%) of patients with LGL leukemia and may be enriched in the less common CD4+ T cell-LGL subtype.24,25 The inclusion of these and other targets identified by ongoing gene discovery efforts will further improve the clinical utility of gene panel sequencing approaches.
In summary, systematic STAT3 mutation testing in patients with known or suspected hematologic malignancies identified unsuspected LGL leukemia in patients with unexplained cytopenias who were initially, but incorrectly, diagnosed with MDS. As the use of clinical NGS panels becomes routine in the evaluation of patients with known or suspected myeloid malignancies, we advocate for the inclusion of STAT3 in such panels to clarify ambiguous phenotypes and avert the consequences of misdiagnosis or diagnostic delay.
Supplementary Material
The full-text version of this article contains a data supplement.
Authorship
Acknowledgment: The authors thank Xiang Xu for his protein modeling expertise.
Contribution: E.A.M., J.C.A., C.J.G., and R.C.L. were responsible for the conception and design of the study; E.A.M., M.N.L., D.J.D., D.P.S., R.M.S., and F.C.K. were responsible for the acquisition of data; E.A.M. and R.C.L. interpreted and analyzed data; E.A.M. wrote the manuscript; and all authors critically revised the article and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elizabeth A. Morgan, Department of Pathology, Brigham and Women’s Hospital, Amory 3, 75 Francis St, Boston, MA 02115; e-mail: eamorgan@bwh.harvard.edu.
References
- 1.Steensma DP. Dysplasia has A differential diagnosis: distinguishing genuine myelodysplastic syndromes (MDS) from mimics, imitators, copycats and impostors. Curr Hematol Malig Rep. 2012;7(4):310-320. [DOI] [PubMed] [Google Scholar]
- 2.Neff JL, Howard MT, Morice WG. Distinguishing T-cell large granular lymphocytic leukemia from reactive conditions: laboratory tools and challenges in their use. Surg Pathol Clin. 2013;6(4):631-639. [DOI] [PubMed] [Google Scholar]
- 3.Koskela HL, Eldfors S, Ellonen P, et al. . Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366(20):1905-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerez A, Clemente MJ, Makishima H, et al. . STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood. 2012;120(15):3048-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jerez A, Clemente MJ, Makishima H, et al. . STAT3 mutations indicate the presence of subclinical T-cell clones in a subset of aplastic anemia and myelodysplastic syndrome patients. Blood. 2013;122(14):2453-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasan A, Kern W, Grossmann V, Haferlach C, Haferlach T, Schnittger S. STAT3 mutations are highly specific for large granular lymphocytic leukemia. Leukemia. 2013;27(7):1598-1600. [DOI] [PubMed] [Google Scholar]
- 7.Kristensen T, Larsen M, Rewes A, Frederiksen H, Thomassen M, Møller MB. Clinical relevance of sensitive and quantitative STAT3 mutation analysis using next-generation sequencing in T-cell large granular lymphocytic leukemia. J Mol Diagn. 2014;16(4):382-392. [DOI] [PubMed] [Google Scholar]
- 8.Andersson E, Kuusanmäki H, Bortoluzzi S, et al. . Activating somatic mutations outside the SH2-domain of STAT3 in LGL leukemia. Leukemia. 2016;30(5):1204-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kluk MJ, Lindsley RC, Aster JC, et al. . Validation and implementation of a custom next-generation sequencing clinical assay for hematologic malignancies. J Mol Diagn. 2016;18(4):507-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bejar R, Stevenson K, Abdel-Wahab O, et al. . Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364(26):2496-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papaemmanuil E, Gerstung M, Malcovati L, et al. ; Chronic Myeloid Disorders Working Group of the International Cancer Genome Consortium. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616-3627, quiz 3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haferlach T, Nagata Y, Grossmann V, et al. . Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28(2):241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bejar R, Stevenson KE, Caughey B, et al. . Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J Clin Oncol. 2014;32(25):2691-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsley RC, Saber W, Mar BG, et al. . Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376(6):536-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malcovati L, Gallì A, Travaglino E, et al. . Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129(25):3371-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gattazzo C, Teramo A, Passeri F, et al. . Detection of monoclonal T populations in patients with KIR-restricted chronic lymphoproliferative disorder of NK cells. Haematologica. 2014;99(12):1826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunthararajah Y, Molldrem JL, Rivera M, et al. . Coincident myelodysplastic syndrome and T-cell large granular lymphocytic disease: clinical and pathophysiological features. Br J Haematol. 2001;112(1):195-200. [DOI] [PubMed] [Google Scholar]
- 18.Huh YO, Medeiros LJ, Ravandi F, Konoplev S, Jorgensen JL, Miranda RN. T-cell large granular lymphocyte leukemia associated with myelodysplastic syndrome: a clinicopathologic study of nine cases. Am J Clin Pathol. 2009;131(3):347-356. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Sokol L, Bennett JM, Moscinski LC, List A, Zhang L. T-cell large granular lymphocyte proliferation in myelodysplastic syndromes: clinicopathological features and prognostic significance. Leuk Res. 2016;43:18-23. [DOI] [PubMed] [Google Scholar]
- 20.Kuo FC, Dong F. Next-generation sequencing-based panel testing for myeloid neoplasms. Curr Hematol Malig Rep. 2015;10(2):104-111. [DOI] [PubMed] [Google Scholar]
- 21.McKerrell T, Moreno T, Ponstingl H, et al. . Development and validation of a comprehensive genomic diagnostic tool for myeloid malignancies. Blood. 2016;128(1):e1-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartels S, Schipper E, Hasemeier B, Kreipe H, Lehmann U. Routine clinical mutation profiling using next generation sequencing and a customized gene panel improves diagnostic precision in myeloid neoplasms. Oncotarget. 2016;7(21):30084-30093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajala HL, Porkka K, Maciejewski JP, Loughran TP Jr, Mustjoki S. Uncovering the pathogenesis of large granular lymphocytic leukemia-novel STAT3 and STAT5b mutations. Ann Med. 2014;46(3):114-122. [DOI] [PubMed] [Google Scholar]
- 24.Andersson EI, Tanahashi T, Sekiguchi N, et al. . High incidence of activating STAT5B mutations in CD4-positive T-cell large granular lymphocyte leukemia. Blood. 2016;128(20):2465-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajala HL, Eldfors S, Kuusanmäki H, et al. . Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood. 2013;121(22):4541-4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.