SUMMARY
We hypothesize that breast cancer susceptibility stems from interactions between difficult-to-modify cultural and dietary habits and aging processes that are modifiable. We propose a pathway to prevention that uses human organotypic systems that recapitulate hallmarks of aging in situ in order to better understand and to modulate the biological consequences of aging in breast.
BODY
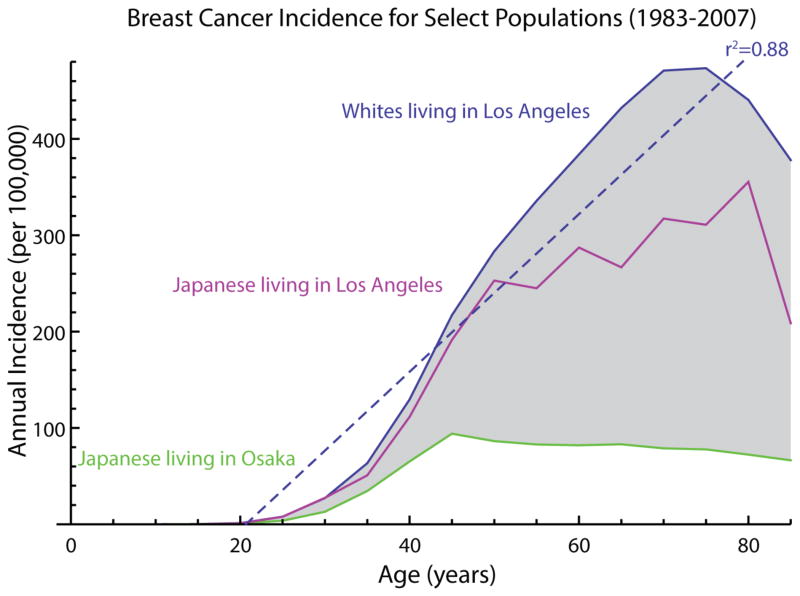
With modern strides against infectious disease and child mortality, over 90% of all human deaths are aging-associated. Aging is the most important risk factor for most human diseases - so much so that it is often not even considered a risk factor in the usual sense. The importance of aging to modern death and disease practically goes without saying, but we understate its importance to public health at our peril. For United States women, the correlation coefficient between chronological age and breast cancer incidence was 0.88 in 1990 (Figure 1), and in the same period, breast cancer was the number three cause of death for women over age 55. Indeed, 75% of new breast cancer diagnoses are made in women over 55. The incidence and age distributions vary across borders, e.g. between women in the U.S. and Japan, and among immigrants, suggesting that (i) cultural, environmental, and dietary differences modifies cancer susceptibility and (ii) most age-related breast cancer should be preventable. Age-related breast cancers are not due only to accumulation of somatic mutations1. We hypothesize that breast cancer susceptibility stems from interactions between aging processes and difficult-to-modify cultural and dietary habits. To prevent the disease we must understand and modify the consequences of aging in the breast context, but studying aging and cancer susceptibility in humans presents challenges.
Figure 1.
Breast cancer incidence is highly correlated with aging, and the age-dependent component of breast cancer incidence varies highly, even between genetically matched groups living in different countries. This genetically independent variability suggests that the majority of breast cancer may often be preventable (e.g., 77% of the total breast cancer incidence, shown in grey, for whites living in Los Angeles).
The first obstacle is that aging in model organisms do not necessarily model aging in humans. Organisms with short lifespans are convenient for laboratory research, but organisms with short lifespans are the least likely to show signs of aging that correlate with those experienced by humans2. On the molecular level, many aging-associated defects such as methylcytosine deamination3 or collagen degradation4 do not apply to evolutionarily distant organisms. Even fundamentals like body temperature are not conserved across model organisms. Furthermore, the most common aging-relevant measurement from non-human models is lifespan, which neglects human-health-specific correlates, Organisms such as yeast, fish, and worms do not even possess many aging-relevant organs, such as the breast.
A second obstacle to studying aging is inherent to studying preventive medicine as opposed to curative medicine. Important ethical guidelines establish strict limits on experiments performed on humans. Even in dire situations, cancer patients may only receive some experimental therapies after failing accepted standard of care therapies. Preventive medicine, by its very nature, is not sufficiently dire to justify such experiments. Furthermore, it is fundamentally challenging to prove that, when disease does not occur, that its non-occurrence was due to a given intervention administered years prior. Success stories in preventive epidemiology, such as smoking cessation for lung cancer5 or sunlight protection for skin cancer6, required hundreds of thousands of person-years before drawing useful associations. Most of these successes also required large numbers of people to already engage in the behavior of interest. And when preventive epidemiology has imposed experimental conditions, such as the DASH diet study on hypertension7, the studies sought results within a few years. Primary cancer prevention studies using conventional means could take decades.
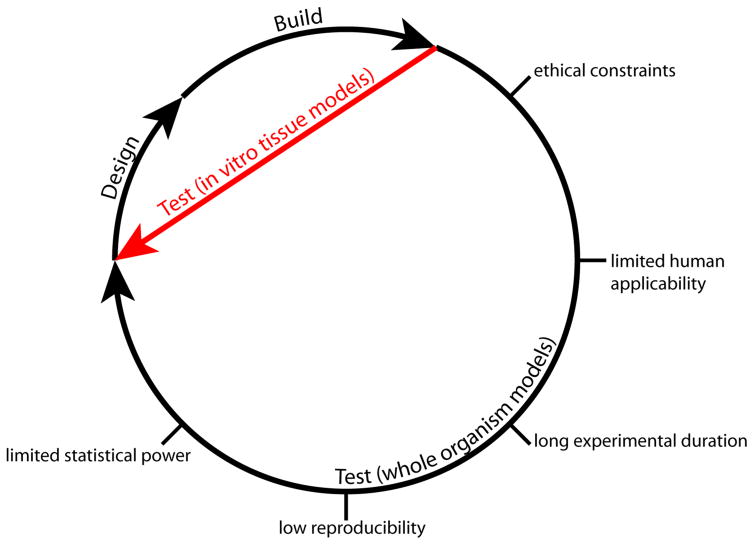
A third obstacle to studying aging is that it simply takes too long. An enduring paradigm in engineering is the design-build-test cycle - the faster one can iterate through this cycle, the faster one can converge on a solution to a problem. In programming, an iteration through this cycle may be as brief as several minutes. In biomedical research, an iteration may take years or even decades. As biomedical researchers, we are certainly capable of design, and we are reasonably effective at building, but we are stymied when it comes to testing. Non-human model systems of preclinical studies have limited predictive value, and human clinical trials are slow and expensive. These factors break the cycle between testing and design (Figure 2).
Figure 2.
Developing a research idea into a clinical outcome requires iterating through design-build-test cycles. In vitro human tissue models afford the opportunity to hasten the testing phase of the cycle, substituting slow animal experiments with fast organotypic culture experiments.
Despite these challenges, researchers press on - and indeed we must, for the economic and societal costs of aging are great. Because cancers are tissue-specific diseases, we assume that aging also has tissue specific consequences. Thus, we propose a tractable approach to overcome these obstacles is an in vitro human approach that takes advantage of the primary cell growth and organotypic culture systems developed mainly for breast cancer research. We propose that the pathway to prevention comprises four main steps.
First, we must identify the phenotypes and molecular, cell, and tissue-level mechanisms associated with cancer susceptibility. Phenotypes of healthy-appearing breast tissue in very high-risk women are being revealed by combinations of in situ analysis, cell biology, and clinical correlations8. Primary human epithelial cell culture systems are laying bare molecular and functional consequences of chronological aging that make women susceptible to breast cancer9. Approaches such as these simultaneously catalog the changes that precede cancer and provide experimental systems to assess interventions.
Second, we must design and build experimental systems that can recapitulate these molecular phenotypes, and we must do so in a manner whereby we can produce credible results - this means statistically robust systems that are manipulable and iterable. Instead of conducting experiments on people, we can reconstitute the relevant tissues from people. Pared-down organoids derived from primary mammary epithelial cells recapitulate characteristic structural and functional features of breast tissue that reveal moleuclar-functional relationships that are relevant to aging breast in vivo10. We can give disease to these tissues, attempt to prevent its spread, and even attempt to cure it entirely. This concept has been growing for some time in the cancer and regenerative medicine fields, where organoids are produced in vitro to mimic various tissues, including breast, prostate, pancreas, liver, and kidney. Stem cell, tissue engineering, and bioprinting research share a common vision for in vitro human research. Generating minimal tissues, organoids, from human cells is an approach that can bridge the testing gap. Stem cells have been used to reconstitute whole mammary organoids11, methods have been developed to produce large arrays of defined multicomponent organoids12, and defined extracellular matrices have been formulated to control the behavior of organoids13. The mammary gland in particular has been well-studied in terms of organoid behavior. Minimal tissues enable in vitro assays that we could not feasibly do on whole organisms: longitudinal biopsy, microscopy, genetic modification, and omics analysis. Large sample sizes allow for statistically robust experiment designs that enable detection of nuanced emergent phenotypes.
Third, we must identify the means to safely reduce cancer susceptibility within the confines of in vitro human systems. In vitro cell culture experiments are often brief and performed under highly mitogenic conditions. Neither is appropriate for a cancer prevention experiment. Instead, long-term culture conditions should be found wherein cells thrive but are minimally replicative, just as they are in the human body. Although highly mitogenic conditions are convenient for getting cells to divide and encourage malignant and invasive behavior, minimally mitogenic conditions are more relevant. The ideal experiment is a format wherein healthy organoids gradually develop cancer over a measurable period, such that “disease progression” becomes a meaningful term across the course of the experiment. This is not a new concept14, just a minimally practiced one. Moreover, we should not limit ourselves to small molecule interventions. There are a finite number of dosable small molecules with cost-effective synthetic routes, and only the rarest among these will have sufficiently few side-effects for healthy patients to tolerate prophylactic dosage across their lifespans. Genetic and cell-based solutions, wearable devices, and life style modification are all worthy of consideration.
Fourth, we must translate these findings to clinical trials. Even if great strides can be made with in vitro human systems, these findings must be connected to more established pre-clinical systems. One approach to do this is to phenocopy and reconstitute the disease-drug relationships seen in biomedically relevant mice, such as humanized mice, to build a body of evidence demonstrating parallels between the systems. STAT1-KO mice15 are a potential candidate, as they have incidence mechanics and tumor subtype/histology that suggest it may be the only pre-clinical mouse model of age-related breast cancer. Once enough parallels are demonstrated between current standard-of-evidence preclinical models and in vitro human tissue models, it may be reasonable to jump directly from in vitro models to phase I clinical trials. The long-term goal is to make in vitro human models a new standard of evidence for preclinical studies, a standard that is cheaper, faster, and more relevant than existing systems.
When we consider preventing or curing cancer, we typically envision a goal that is careers or lifetimes away. This is at least due to our broken design cycle as it is to the inherent biological complexity of the problem. But this may be a problem we can fix because, at this moment of history, our tools are many: combinational chemistry, personal- and population-level sequencing, immune therapy, gene therapy, advanced computational analysis, statistical learning, and automated laboratory workflows. More biomedical research papers are published in a month than were once published in years. Although the challenges are great, our capacity to overcome now exceeds what we have ever had before. We should seriously consider that the substantial prevention of human cancer may be possible within the spans of our careers, and we should strategize accordingly.
Acknowledgments
We thank the breast cancer patient advocates involved with this work, Susan Samson of the UCSF Breast Advocate Core and Sandy Preto of Notes4Hope.org, and funding from the National Institutes of Aging (NIA R01AG040081), the Era of Hope Scholar Award from the Congressionally Directed Medical Research Program’s Breast Cancer Research Program (BC141351).
References
- 1.LaBarge MA, Mora-Blanco EL, Samson S, Miyano M. Breast Cancer beyond the Age of Mutation. Gerontology. 2015;62:434–442. doi: 10.1159/000441030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JI, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson VJ, Johnson TE, Hammen RF. Caenorhabditis elegans DNA does not contain 5-methylcytosine at any time during development or aging. Nucleic Acids Res. 1986;14:6711–6719. doi: 10.1093/nar/14.16.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyer DG, et al. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White C. Research on smoking and lung cancer: a landmark in the history of chronic disease epidemiology. Yale J Biol Med. 1990;63:29–46. [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 7.SVETKEY LP, et al. The DASH Diet, Sodium Intake and Blood Pressure Trial (DASH-Sodium): Rationale and Design. J Am Diet Assoc. 1999;99:S96–S104. doi: 10.1016/s0002-8223(99)00423-x. [DOI] [PubMed] [Google Scholar]
- 8.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. 2015;15:248–254. doi: 10.1038/nrc3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garbe JC, et al. Accumulation of Multipotent Progenitors with a Basal Differentiation Bias during Aging of Human Mammary Epithelia. Cancer Res. 2012;72:3687–3701. doi: 10.1158/0008-5472.CAN-12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelissier FA, et al. Age-Related Dysfunction in Mechanotransduction Impairs Differentiation of Human Mammary Epithelial Progenitors. Cell Rep. 2014;7:1926–1939. doi: 10.1016/j.celrep.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villadsen R, et al. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol. 2007;177:87–101. doi: 10.1083/jcb.200611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todhunter ME, et al. Programmed synthesis of three-dimensional tissues. Nat Methods. 2015;12:975–981. doi: 10.1038/nmeth.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranga A, et al. 3D niche microarrays for systems-level analyses of cell fate. Nat Commun. 2014;5:4324. doi: 10.1038/ncomms5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SHT, Albert L. Cytologic and Functional Characterization of Single Cell Clones Isolated from the Krebs-2 and Ehrlich Ascites Tumors2. 1958 doi: 10.1093/jnci/21.1.77. [DOI] [PubMed] [Google Scholar]
- 15.Chan SR, et al. STAT1-deficient mice spontaneously develop estrogen receptor α-positive luminal mammary carcinomas. Breast Cancer Res. 2012;14:R16. doi: 10.1186/bcr3100. [DOI] [PMC free article] [PubMed] [Google Scholar]