Abstract
Objectives
Despite the increasing prevalence of Type 2 diabetes mellitus (T2DM) among children and adolescents, little is known about their risk of developing diabetic retinopathy (DR). We sought to identify risk factors for DR in youth with DM, to compare DR rates for youth with Type 1 diabetes mellitus (T1DM) and T2DM, and to assess whether adherence to DR screening guidelines promoted by the American Academy of Ophthalmology, American Academy of Pediatrics, and American Diabetes Association adequately capture youth with DR.
Design
Retrospective observational longitudinal cohort study.
Participants
Youth aged ≤ 21 years with newly diagnosed T1DM or T2DM enrolled in a large U.S. managed care network.
Main Outcome Measure
Hazard ratios (HR) with 95% confidence intervals (CIs) for developing DR.
Methods
In this study of youth aged ≤ 21 years with newly diagnosed T1DM or T2DM enrolled in a large U.S. managed care network who were under ophthalmic surveillance, we identified the incidence and timing of DR onset for youth with T1DM and T2DM. Kaplan-Meier survival curves assessed the timing of initial diagnosis of DR for youth with each type of diabetes. Multivariable Cox proportional hazard regression modeling identified factors associated with the hazard of developing DR. Model predictors were age and calendar year at initial diabetes mellitus diagnosis, sex, race/ethnicity, net worth, and glycosylated hemoglobin (HbA1c).
Results
Among the 2240 youth with T1DM and 1768 youth with T2DM, 20.1% and 7.2% developed DR, over a median follow-up of 3.2 and 3.1 years, respectively. Survival curves demonstrated that youth with T1DM developed DR faster than youth with T2DM (P<0.0001). For every one-point increase in HbA1c, the hazard for DR increased by 20% (HR=1.20, CI 1.06–1.35) and 30% (HR=1.30, CI 1.08–1.56) among youth with T1DM and T2DM, respectively. Current guidelines suggest ophthalmic screening begin 3–5 years after initial DM diagnosis, at which point in our study, over 18% of youth with T1DM had already had received ≥1 DR diagnosis.
Conclusions
Youth with T1DM or T2DM exhibit a considerable risk for DR and should undergo regular screenings by eye-care professionals to ensure timely DR diagnosis and limit progression to vision-threatening disease.
Introduction
The incidence of Type 1 diabetes mellitus (T1DM) and Type 2 diabetes mellitus (T2DM) is rising among children and adolescents worldwide.1–3 While in decades past, most youth with diabetes mellitus (DM) had T1DM, T2DM now accounts for up to 45% of all new DM diagnoses among adolescents, concurrent with the rise of childhood obesity.4,5
Diabetic retinopathy (DR) is a serious complication that is often asymptomatic in early stages but may progress to sight-threatening disease.6–9 Risk factors for DR in youth with T1DM include longer disease duration and the timing of puberty.10,11 Accordingly, various clinical practice guidelines for the ophthalmic screening of youth with T1DM have been developed, although medical professional societies differ in their recommended timing of monitoring. The American Academy of Ophthalmology (AAO) recommends an initial screening 5 years after T1DM onset.12 The American Diabetes Association (ADA) recommends initial screening 3–5 years after T1DM onset for patients at least 10 years of age;13 the American Academy of Pediatrics (AAP) recommends the same for patients 9 years or older.10 A recent study suggested a delay in initial ophthalmic screening until 15 years of age is acceptable.14 Optimizing DM control, as measured by glycosylated hemoglobin (HbA1c), is recommended in all these guidelines.10
The ADA and AAO recommendations for youth with T2DM—which is to screen at initial DM diagnosis— are based on limited data,15,16 as T2DM has only recently become more common among youth. Thus, it is essential to characterize the development of DR and the need for interventions among youth with T2DM to guide the creation of evidence-based practice guidelines aimed at detecting and treating DR before vision is threatened.
We evaluated the DR incidence among youth with T1DM and T2DM enrolled in a large U.S. managed-care network. We sought to: (a) identify risk factors for DR development in youth with T1DM and T2DM; (b) investigate whether DM control, as measured by HbA1c, is associated with DR development; and (c) estimate the proportion of youth with each DM type requiring laser or surgical intervention for DR. Finally, we applied the existing T1DM ophthalmic screening guidelines of the AAO, AAP, and ADA to the youth with T1DM in this dataset to assess whether delays in initial DR diagnosis would result.
Methods
Data Source
The Clinformatics DataMart database (OptumInsight, Eden Prairie, MN), a dataset which has been used previously to study ocular diseases,17–19 contains detailed records of beneficiaries in a large nationwide U.S. managed-care network. We accessed data on all beneficiaries aged 21 years or younger at their initial enrollment during January 1, 2001 through December 31, 2014. Medical claims from inpatient and outpatient health care encounters and associated ICD-9-CM diagnosis codes20 for all ocular and non-ocular conditions were available, as was information on age, sex, race/ethnicity, and household net worth. HbA1c levels were available for an enrollee subset who had this test done at an outpatient laboratory. Enrollees in the Clinformatics DataMart are very similar in sociodemographic profile to those with other types of private health insurance throughout the U.S. (Personal communication, Matthew Sulzicki, OptumInsight). Data were stripped of all protected health information prior to release from OptumInsight. The University of Michigan Institutional Review Board approved this study which involved de-identified data.
Study Participants
Eligible participants were age ≤21 years at plan enrollment, continuously enrolled in the medical plan for 3 years or more, and had at least 2 DM diagnoses (ICD-9-CM codes 250.xx or 362.01–362.07) on separate dates. Individuals who never filled a prescription for insulin or an oral hypoglycemic agent were excluded. To help exclude nonincident DM cases, the first DM diagnosis must have occurred at least 12 months after plan enrollment. Only youth with 1 or more ophthalmologist- or optometrist-performed examinations after the initial DM diagnosis were included. Youth lacking information on race/ethnicity or household net worth were also excluded.
Diabetes Type: Classification
Enrollees were classified with T1DM or T2DM based on a previously validated algorithm.21 Briefly, children younger than 10 years at their first DM diagnosis were considered to have T1DM. Among youth 10 years or older, those who were prescribed only insulin in the 730 days after initial diagnosis were also considered to have T1DM. The remaining individuals were classified as having T2DM. In this group, patients must have filled an oral hypoglycemic (e.g., metformin, sulfonylureas) prescription, with or without a concurrent insulin prescription, within 730 days of their initial diagnosis. This algorithm had a sensitivity and specificity of 98.6% and 78.2% for detecting T1DM, and 83.2% and 97.5% for T2DM, among youth in a Canadian study.21
Outcome
The primary outcome was DR development, diagnosed by an optometrist or ophthalmologist and coded appropriately (ICD-9-CM or 250.50–250.53 or 362.01–362.07). The billing codes capture patients with nonproliferative DR (362.03–362.06), proliferative DR (362.02), and diabetic macular edema (362.07). Patients with only 250.50–250.53 or 362.01 were considered to have nonspecific DR. Current Procedural Terminology (CPT) billing codes were used to determine whether patients underwent interventions for DR including panretinal photocoagulation (CPT 67228), focal laser (CPT 67210), or intravitreal injection (CPT 67028).
Analysis
Data analyses were performed using SAS software, version 9.4 (SAS, Inc., Cary, NC); Kaplan-Meier curves were created using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA). Characteristics of the study population were summarized using medians and interquartile ranges (IQRs) for continuous variables and frequencies and percentages for categorical variables.
Retinopathy incidence, risk factors
DR incidence was calculated as the number of youth with newly diagnosed DR per thousand person-years of follow-up. Kaplan-Meier survival curves assessed the timing from first DM diagnosis to initial DR diagnosis in youth with T1DM and T2DM; groups were compared using the log-rank test. Multivariable Cox proportional hazard regression modeling evaluated the extent to which sociodemographic factors affected the hazard for DR for youth with each DM type. Model predictors were age, sex, race/ethnicity, household net worth, and calendar year at initial DM diagnosis (e.g., 2008, 2009, etc.).
Hemoglobin A1c
For patients who had one or more HbA1c tests performed at an outpatient laboratory, the first value at least 6 months after initial DM diagnosis was analyzed. This allowed for initiation of treatment and initial stabilization of DM. The test must have also been performed before the initial DR diagnosis. The distribution of HbA1c values was evaluated by medians and IQRs. The Wilcoxon rank-sum test compared the distributions between groups (T1DM vs. T2DM, with DR vs. without DR). Additional Cox proportional hazard models were constructed to evaluate HbA1c as a predictor for DR development among youth with T1DM and T2DM. Model covariates included age at first DM diagnosis, sex, race/ethnicity, household worth, and calendar year of initial DM diagnosis. Cox models were left-truncated, because to be eligible for the outcome, a patient’s HbA1c laboratory values must have preceded her initial DR diagnosis.
Diagnostic timing under current screening guidelines
Using Kaplan-Meier survival analysis estimates, we calculated the percentage of youth with DM who developed DR and would have had a delayed DR diagnosis if existing AAO, ADA, or AAP screening guidelines were followed.
Results
Patient Characteristics
Among the 2240 eligible youth with newly diagnosed T1DM and 1768 with newly diagnosed T2DM, the median age at DM onset in those with T1DM and T2DM were 12 and 18 years, respectively, and the median follow-up after initial DM diagnosis was 3.2 years and 3.1 years, respectively. The maximum follow-up time was 13.0 and 12.7 years for youth with T1DM and T2DM, respectively, resulting in a maximum age of 34 years at the end of follow-up. The majority of youth with T2DM were female (83.0%). Of those with T1DM, 85.1% were white, 7.0% black, and 5.9% Latino. Of those with T2DM, 72.3% were white, 11.7% black, and 12.1% Latino (Table 1).
Table 1.
Characteristics of Study Sample
| Diabetes Mellitus | ||||
|---|---|---|---|---|
| T1DM N=2240 |
T2DM N=1768 |
|||
|
|
||||
| Median (25 percentile, 75th percentile) | Median (25th percentile, 75th percentile | |||
|
|
||||
| Age at initial diabetes diagnosis (years) | 12 | (8,15) | 18 | (16, 21) |
| Years of follow-up after initial diabetes diagnosis | 3.2 | (1.8, 5.4) | 3.1 | (1.9, 4.9) |
| HbA1ca | 6.6 | (5.6, 8.1) | 5.6 | (5.4, 6.4) |
|
|
||||
| N | % | N | % | |
|
|
||||
| Female | 1066 | 47.6% | 1468 | 83.0% |
| Race | ||||
| White | 1907 | 85.1% | 1278 | 72.3% |
| Black | 157 | 7.0% | 206 | 11.7% |
| Latino | 131 | 5.9% | 214 | 12.1% |
| Asian | 45 | 2.1% | 70 | 4.0% |
| Household Net Worth | ||||
| <$25,000 | 227 | 10.1% | 350 | 15.4% |
| $25,000–$149,999 | 490 | 7.5% | 411 | 10.8% |
| $150,000–$249,999 | 359 | 11.2% | 235 | 12.0% |
| $250,000–$499,999 | 641 | 51.9% | 403 | 43.8% |
| ≥$500,000 | 523 | 19.3% | 369 | 18.1% |
| Diabetic Retinopathy | 451 | 20.1% | 127 | 7.2% |
| Proliferative Diabetic Retinopathy | 12 | 0.5% | 1 | 0.1% |
| Nonproliferative Diabetic Retinopathy | 21 | 1.0% | 6 | 0.3% |
| Diabetic Macular Edemab | 5 | 0.2% | 0 | 0.0% |
| Nonspecific diabetic retinopathy | 418 | 18.7% | 120 | 6.8% |
T1DM=Type 1 diabetes mellitus. T2DM = Type 2 diabetes mellitus. HbA1c = Glycosylated hemoglobin
HbA1c was available only for a subset of patients (N=594 for T1DM, N=389 for T2DM)
Some patients with diabetic macular edema also had other codes for diabetic retinopathy
Retinopathy incidence and risk factors
Overall, 578 youth (14.4%) received a DR diagnosis. The percentages of youth with T1DM and T2DM receiving a DR diagnosis were 20.1% and 7.2%, respectively. The DR incidence rates for youth with T1DM and T2DM were 52.3 and 19.6 cases per 1000 person-years, respectively. Thirteen youth were diagnosed with proliferative DR, of whom 12 had T1DM (Table 1). Of these persons with proliferative DR, the age at initial proliferative DR diagnosis ranged from 6 to 31 years. Diabetic macular edema was diagnosed in 5 persons, all with T1DM, with ages ranging from 15–29 years old at the time of initial diagnosis of diabetic macular edema. Of all the youth with diabetic macular edema and/or proliferative DR (total N=15), the median age at initial DM diagnosis was 18 years (IQR 10–21), and the median duration of DM at the time of initial DR diagnosis was 2.0 years (IQR 0.8–5.6 years). The median age at initial diagnosis of PDR or DME was 23 years (IQR 14–25). The remainder of those with DR diagnoses had nonproliferative retinopathy or had less specific DR codes. No patients with T1DM or T2DM underwent panretinal photocoagulation, focal laser, or intravitreal injections. Youth with T1DM developed DR sooner than those with T2DM did (P<0.0001, log-rank test). Kaplan-Meier survival analysis estimated that at 6 years’ follow-up, 27.6% and 8.6% of those with T1DM and T2DM, respectively, were diagnosed with DR (Figure 1). At 8 years’ follow-up, 31.2% and 10.3%, respectively, had a diagnosis of DR. In those with DR, the median age at initial DR diagnosis was 14.2 (IQR 10.6–18.2) years for T1DM and 20.4 (IQR 16.2–23.2) years for T2DM.
Figure 1. Time to development of diabetic retinopathy among youth with Type 1 and Type 2 diabetes mellitus.
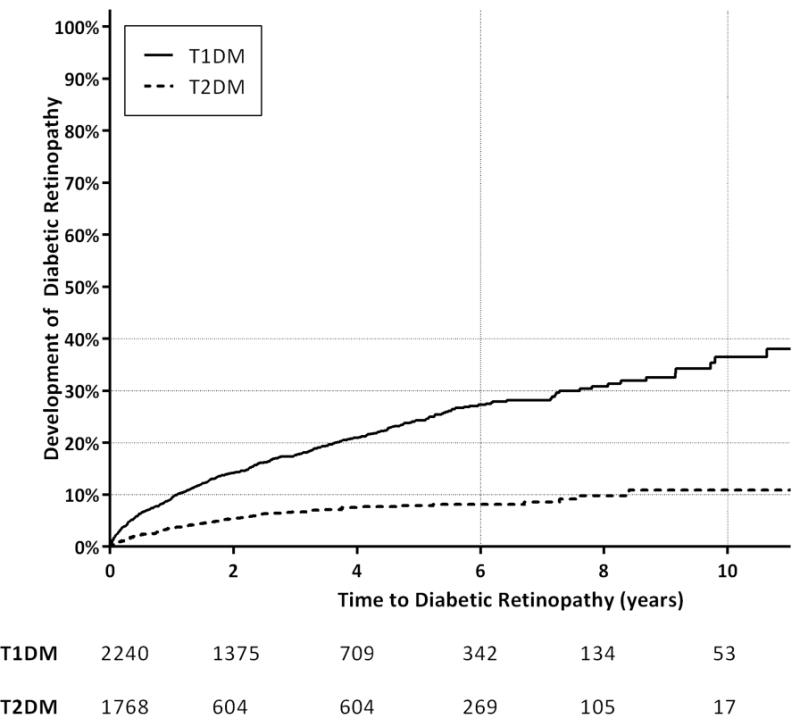
Kaplan-Meier plot depicts the number of years to development of diabetic retinopathy from initial diagnosis of diabetes for youth with Type 1 diabetes mellitus (T1DM) and Type 2 diabetes mellitus (T2DM) (p<0.0001, Log-Rank). The table below the figure shows the number of individuals at risk for development of retinopathy at corresponding time points on the horizontal time axis.
Among youth with T1DM, for each 1-year increase in age at initial DM diagnosis, the hazard for DR increased by 4.6% (HR 1.05, CI 1.03–1.07). Race, sex, and household worth were not associated with DR development (P>0.05 for all). Among youth with T2DM, males had a 122% increased hazard for DR development compared to females (HR 2.22, CI 1.52–3.25). Those in the highest household net worth category (≥$500,000) had a 52% decreased hazard for development of DR (HR 0.48, CI 0.25–0.90) compared to those with the lowest net worth level (<$25,000). Other sociodemographic factors were not statistically significant (Table 2).
Table 2.
Hazard ratios for development of diabetic retinopathy
| T1DM | T2DM | |||
|---|---|---|---|---|
|
| ||||
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-Value | |
| Age at initial DM diagnosis (per year increase) | 1.05 (1.03–1.07) | <0.001 | 1.04 (1.00–1.09) | 0.08 |
| Male (Reference=Female) | 0.88 (0.73–1.06) | 0.19 | 2.22 (1.52–3.25) | <0.0001 |
| Calendar year of initial DM diagnosis Race (Reference=White) | 0.96 (0.93–0.99) | 0.004 | 1.01 (0.95–1.07) | 0.86 |
| Latino | 1.21 (0.84–1.74) | 0.31 | 0.97 (0.56–1.66) | 0.90 |
| Black | 0.83 (0.56–1.23) | 0.35 | 1.54 (0.96–2.48) | 0.08 |
| Asian | 0.82 (0.39–1.74) | 0.61 | 1.15 (0.47–2.86) | 0.76 |
| Household Net Worth (Reference=<$25,000) | ||||
| $25,000–$149,999 | 0.86 (0.56–1.2) | 0.42 | 1.04 (0.64–1.70) | 0.87 |
| $150,000–$249,999 | 1.10 (0.76–1.60) | 0.62 | 0.64 (0.33–1.23) | 0.18 |
| $250,000–$499,999 | 0.89 (0.62–1.24) | 0.46 | 0.86 (0.52–1.47) | 0.61 |
| ≥$500,000 | 1.21 (0.86–1.70) | 0.27 | 0.48 (0.25–0.90) | 0.02 |
T1DM = Type 1 diabetes mellitus. T2DM = Type 2 diabetes mellitus. DM = diabetes mellitus
CI = confidence interval
Glycosylated hemoglobin as a risk factor for retinopathy development
Glycosylated hemoglobin values at least 6 months after initial DM diagnosis and before DR diagnosis were available for 774 (19.4%) of the participants (T1DM N=385, T2DM N=389). The median HbA1c among youth with T1DM was higher than that with T2DM [7.6 (IQR 6.6–8.8) vs. 5.6 (IQR 5.4–6.4), respectively] (p<0.0001, Wilcoxon rank sum), indicating those with T1DM had poorer disease control. Youth with DR had a higher median HbA1c value (7.5, IQR 6.5–8.9) than youth without DR (6.4, IQR 5.6–8.0) (p<0.0001, Wilcoxon rank sum). For every 1-point increase in HbA1c, the DR hazard increased by 20% (HR 1.20, CI 1.06–1.35) among those with T1DM and by 30% (HR 1.30, CI 1.08–1.56) among those with T2DM, after adjustment for sex, age at and year of initial DM diagnosis, race, and household net worth. Those with T1DM continued to have increased hazard of developing DR compared to those with T2DM (HR 2.00, CI 1.11–3.60) after adjustment for HbA1c as well as other model covariates.
Diagnostic delay under current screening guidelines
The AAO guidelines advocate waiting until 5 years after initial T1DM diagnosis to screen for DR, regardless of patient age. According to Kaplan-Meier survival analysis, 24.7% of youth with T1DM developed DR by 5 years after initial DM diagnosis and thus would have experienced a delayed DR diagnosis under these guidelines. A recent study involving T1DM suggests that waiting until 15 years of age, or 5 years after DM onset,14 for initial DR screening would be acceptable; this age requirement would cause even further diagnostic delays. Under guidelines by the AAP and the ADA, screening should occur 3–5 years after initial DM diagnosis in patients aged at least 9 years or 10 years, respectively. According to Kaplan-Meier estimates, by 3 years after DM diagnosis, at least 18.0% of youth with T1DM developed DR and therefore would receive a delayed DR diagnosis with AAP–ADA screening guidelines.
Discussion
In this study of youth in a large U.S. managed-care network, over 20% of youth with T1DM and 7% with T2DM, with over a median of 3 years of follow-up, received a diagnosis of DR. Youth with T1DM had nearly a threefold-increased incidence and prevalence of DR, compared with youth with T2DM. For each year of age older a child was at initial DM diagnosis, the risk for developing DR rose among those with T1DM. Higher household net worth and female sex appeared protective against DR among those with T2DM. Every one-point increase in HbA1c increased the hazard of developing DR by 20–30% among those with T1DM and T2DM. These results highlight that DR may be more common than previously suspected in youth with DM, and youth with poor glycemic control may especially benefit from undergoing screening for DR sooner than the current clinical practice guidelines recommend.
Previously reported rates of DR among children and adolescents have varied from as low as 0% to over 50% for youth with T1DM and T2DM,14,22–26 in settings as diverse as Sweden,22 southern India,24 the U.S.,14,26 and Canada.25 Direct comparison of the present analysis with these earlier studies is challenging because they differ in participant age-range, sample size, local standards of DM care, follow-up duration, glycemic control, and DR-assessment method. A pilot study of the U.S. SEARCH for Diabetes in Youth cohort reported that among 265 persons with DM diagnosed before 20 years of age, 17% of those with T1DM and 42% with T2DM developed DR on fundus photography over a median follow-up of 6.8 years. That study had few participants with T2DM and their glycemic control was, on average, poorer than in our study cohort.23 In a Canadian population-based cohort, aged 1–18 years, 13.8 % of the 1011 youth with T1DM and 11.7% of the 342 youth with T2DM had billing code-documented DR after a median follow-up of 4.4 years and 6.7 years, respectively.25 Among the 517 participants in the TODAY clinical trial—the largest study of DR among youth with T2DM—the prevalence of DR on fundus photography after a mean DM duration of 4.9 years was 13.7%.26 A recent retrospective chart review of 338 children with T1DM with a median DM duration of 4.9 years and 32 children with T2DM with a median DM duration of 2.0 years did not observe any DR based on clinical records.14
Our sample size is larger than all these prior studies, exceeding 2200 youth with T1DM and 1700 youth with T2DM. Our 20.1% reported rate of DR in youth with T1DM is similar to other studies’, although our 7.2% incidence rate among youth with T2DM is slightly lower than in past studies. However, most prior studies included few youth with T2DM and had a longer follow-up period than ours. Whereas many other studies used fundus photography for DR screening, we used claims data, requiring participants to seek care to receive a diagnosis and for clinicians to properly diagnose and code for DR.
Similar to prior studies, HbA1c levels in our study were higher among patients with DR than among those without DR.22–24,26–28 We found that higher HbA1c values is associated with increased hazard of DR, consistent with previous literature among youth with T1DM6,22,23 and T2DM26. While we also found that males with T2DM were at increased risk for DR compared with females, previous studies reporting sex-related differences in the development of DR have only been in youth with T1DM and have been inconsistent, noting either increased DR among females29,30 or among males,31 postulating hormonal differences during puberty as a possible explanatory factor. Increasing risk of DR among patients who were diagnosed with T1DM at older ages has also been reported previously and postulated to be related to increased risk associated with puberty.32 We also found that youth with T2DM from households with higher net worth had decreased development of DR, similar to previous literature on socioeconomic risk factors for DR which reported that persons of lower affluence levels were at greater risk for DR both for adults33 and youth34 with T2DM and T1DM.35 This may be related to lifestyle factors such as diet, exercise, and smoking.
Our analysis shows that waiting 3–5 years after initial T1DM diagnosis to screen for DR, as present guidelines advocate, would have delayed the diagnosis of ocular disease in 18% of patients by 3 years and 25% by 5 years. These estimates of delayed DR diagnoses are conservative, since waiting to screen until 9 or 10 years of age, as AAP and ADA guidelines recommend, would further delay patients’ initial DR diagnosis. As the T2DM incidence rate among youth has risen, the ADA and AAO have recommended screening youth with T2DM at their initial DM diagnosis based on limited data and extrapolation from adult guidelines.15 Although affected youth with T2DM in our study developed DR more slowly than those with T1DM did, our study supports screening youth with T2DM for DR at patients’ first DM diagnosis, similar to current recommendations for adults.13
No patient with DR in our study had claims data evidence of receiving common treatments for DR such as focal or panretinal laser photocoagulation or intravitreal injection of anti-vascular endothelial growth factor agents. This finding comports with those reported in previous studies indicating that DR requiring laser treatment rarely occurs in children’s first 10 years with T1DM.36,37 The median length of follow-up after initial DM diagnosis was 3 years in our population, and the few patients diagnosed with proliferative DR or macular edema may have been managed conservatively, refused treatment, or either the diagnosis or procedure billing code may have been incorrect. However, after decades with the disease, the proportion of patients with T1DM requiring laser treatment climbs to over 60%.36–38 Detecting DR in youth relatively early in its course, before vision is threatened or interventions are required, can be beneficial, as providers can increase the monitoring intensity, improve glycemic control, and coordinate care among eye-care providers, pediatricians, and endocrinologists to avert or delay poor long-term visual outcomes and increase vigilance regarding non-ocular complications of DM.
As more adolescents receive DM diagnoses and need to undergo screening for DR, researchers will need to develop novel strategies for DR screening that are cost-effective and cause no undue burden to the child, parent, or health care provider. The use of telemedicine with nonmydriatic fundus photography29 in pediatricians’ and primary-care providers’ offices may be a viable mechanism to screen large numbers of youth for DR.39,40
This study has limitations. Caution must be exercised in generalizing these findings to youth with other forms of health insurance such as Medicaid or whose families are uninsured. Although we relied on a validated algorithm to determine enrollees’ T1DM–T2DM status,21 some cases may have been misclassified. We found a greater female predominance among youth with T2DM than has been previously reported in the literature (typically 61% to 65% female), which may be due to differences in characteristics of the study samples.25,26,41,42 Despite efforts to ensure that the study population included only incident DM cases by requiring 12 months in the plan without prior initial DM diagnosis, some of these youth may have had pre-existing DM, especially among those with T2DM. Thus, we may be underestimating the time from disease onset to first recorded DR diagnosis and overestimating the numbers of youth with DR whose diagnosis may be delayed by following current guidelines. Since this study included only patients visiting eye-care providers, the DR rates among those not seeking ophthalmic care remain unknown, and we may be under- or overestimating the true DR incidence due to referral bias. Determining the presence of macular edema and proliferative DR relied upon claims data, which may underestimate the prevalence of these conditions due the possibility that clinicians may instead code with nonspecific DR codes for patients with these manifestations. In addition, billing code errors may also contribute to misclassification errors in determining presence and type of DR, though a recent study validating billing codes in common ophthalmic disorders (including proliferative DR) found a 97% accuracy rate compared to medical record documentation.43 Clinical data such as visual acuity, retinal examination findings, or ophthalmologic imaging and testing were unavailable, and verification of DR presence or severity was infeasible. Youth were likely seen by eye care providers who had varying levels of experience and expertise in diagnosing DR and some may have had access to diagnostic equipment to facilitate diagnosis of DR while others may not have had access to such equipment. HbA1c values were unavailable for some patients (some youth may have not undergone this testing and others may have had the testing done as a point of care test in the clinic rather than at an outpatient lab); those with HbA1c measurements may not represent the glycemic control of the entire cohort, and the included HbA1c measurement may not accurately represent overall glycemic control of the individual.
Conclusions
Counter to previous beliefs, DR among youth with T1DM and T2DM is fairly common. Since early detection is key to preventing irreversible retinal damage and preserving sight, we propose screening youth with T1DM and T2DM for DR early in the disease course to limit delays in DR detection and maximize opportunities to improve glycemic control, thus limiting DR progression.
Acknowledgments
Funding Sources: Research to Prevent Blindness Physician Scientist Award (JDS, TWG), W.K. Kellogg Foundation; Juvenile Diabetes Research Foundation, R01EY20582 and DP3DK094292, the Taubman Institute (TWG); P30DK020572 Michigan Diabetes Research Center (WHH)
The funding organizations had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Association for Research in Vision and Ophthalmology; Denver, CO, May 4, 2015
No conflicting relationship exists for any author.
References
- 1.Green A, Patterson CC, Group on behalf of the ETS Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia. 2001;44:B3–B8. doi: 10.1007/pl00002950. [DOI] [PubMed] [Google Scholar]
- 2.Lin W-H, Wang M-C, Wang W-M, et al. Incidence of and Mortality from Type I Diabetes in Taiwan From 1999 through 2010: A Nationwide Cohort Study. PLoS ONE. 2014;9:e86172. doi: 10.1371/journal.pone.0086172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day C. The rising tide of type 2 diabetes. Br J Diabetes Vasc Dis. 2001;1:37–43. [Google Scholar]
- 4.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 5.D’Adamo E, Caprio S. Type 2 Diabetes in Youth: Epidemiology and Pathophysiology. Diabetes Care. 2011;34:S161–S165. doi: 10.2337/dc11-s212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broe R. Early risk stratification in pediatric type 1 diabetes. Acta Ophthalmol (Copenh) 2015;93(1):1–19. doi: 10.1111/aos.12702. Thesis. [DOI] [PubMed] [Google Scholar]
- 7.Porta M, Allione A. Diabetic retinopathy and its relevance to paediatric age. An update. Pediatr Endocrinol Rev PER. 2004;1:404–411. [PubMed] [Google Scholar]
- 8.Thomas RL, Dunstan FD, Luzio SD, et al. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br J Ophthalmol. 2015;99:64–68. doi: 10.1136/bjophthalmol-2013-304017. [DOI] [PubMed] [Google Scholar]
- 9.Lian JX, Gangwani RA, McGhee SM, et al. Systematic screening for diabetic retinopathy (DR) in Hong Kong: prevalence of DR and visual impairment among diabetic population. Br J Ophthalmol. 2015 doi: 10.1136/bjophthalmol-2015-307382. [DOI] [PubMed] [Google Scholar]
- 10.Lueder GT, Silverstein J. Screening for Retinopathy in the Pediatric Patient With Type 1 Diabetes Mellitus. Pediatrics. 2005;116:270–273. doi: 10.1542/peds.2005-0875. [DOI] [PubMed] [Google Scholar]
- 11.Cho YH, Craig ME, Donaghue KC. Puberty as an accelerator for diabetes complications. Pediatr Diabetes. 2014;15:18–26. doi: 10.1111/pedi.12112. [DOI] [PubMed] [Google Scholar]
- 12.Anon. Screening for Retinopathy in the Pediatric Patient with Type 1 Diabetes Mellitus - Reaffirmed. 2014 doi: 10.1542/peds.2005-0875. Available at: http://www.aao.org/clinical-statement/screening-retinopathy-in-pediatric-patient-with-ty-2 [Accessed March 14, 2016] [DOI] [PubMed]
- 13.American Diabetes Association. Diabetic Retinopathy. Diabetes Care. 2002;25:s90–s93. [Google Scholar]
- 14.Geloneck MM, Forbes BJ, Shaffer J, et al. Ocular Complications in Children with Diabetes Mellitus. Ophthalmology. 2015 doi: 10.1016/j.ophtha.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Standards of Medical Care in Diabetes–2010. Diabetes Care. 2009;33:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Academy of Ophthalmology. Diabetic Retinopathy PPP - 2014. Available at: http://one.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp–2014 [Accessed August 22, 2015]
- 17.Newman-Casey PA, Talwar N, Nan B, et al. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology. 2011;118:1318–1326. doi: 10.1016/j.ophtha.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderbeek BL, Zacks DN, Talwar N, et al. Racial differences in age-related macular degeneration rates in the United States: a longitudinal analysis of a managed care network. Am J Ophthalmol. 2011;152:273–282.e3. doi: 10.1016/j.ajo.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein JD, Kim DS, Niziol LM, et al. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011;118:1031–1037. doi: 10.1016/j.ophtha.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ICD-9-CM for Physicians. International Classification of Diseases 9th Revision Clinical Modification. Salt Lake City, UT: Contexo Media; 2006. [Google Scholar]
- 21.Vanderloo SE, Johnson JA, Reimer K, et al. Validation of classification algorithms for childhood diabetes identified from administrative data. Pediatr Diabetes. 2012;13:229–234. doi: 10.1111/j.1399-5448.2011.00795.x. [DOI] [PubMed] [Google Scholar]
- 22.Henricsson M, Nystrom L, Blohme G, et al. The incidence of retinopathy 10 years after diagnosis in young adult people with diabetes: Results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS) Diabetes Care. 2003;26:349–54. doi: 10.2337/diacare.26.2.349. [DOI] [PubMed] [Google Scholar]
- 23.Mayer-Davis EJ, Davis C, Saadine J, et al. Diabetic retinopathy in the SEARCH for Diabetes in Youth Cohort: a pilot study. Diabet Med. 2012;29:1148–1152. doi: 10.1111/j.1464-5491.2012.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajalakshmi R, Amutha A, Ranjani H, et al. Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset type 1 and type 2 diabetes. J Diabetes Complications. 2014;28:291–297. doi: 10.1016/j.jdiacomp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Dart AB, Martens PJ, Rigatto C, et al. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care. 2014;37:436–443. doi: 10.2337/dc13-0954. [DOI] [PubMed] [Google Scholar]
- 26.TODAY Study Group. Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care. 2013;36:1772–1774. doi: 10.2337/dc12-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: Risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156–163. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Krzentowski G, Albert A, Lefebvre PJ. Risk of Developing Retinopathy in Diabetes Control and Complications Trial Type 1 Diabetic Patients With Good or Poor Metabolic Control. Diabetes Care. 2001;24:1275–1279. doi: 10.2337/diacare.24.7.1275. [DOI] [PubMed] [Google Scholar]
- 29.Minuto N, Emmanuele V, Vannati M, et al. Retinopathy screening in patients with type 1 diabetes diagnosed in young age using a non-mydriatic digital stereoscopic retinal imaging. J Endocrinol Invest. 2012;35:389–394. doi: 10.3275/8016. [DOI] [PubMed] [Google Scholar]
- 30.Benitez-Aguirre P, Craig ME, Cass HG, et al. Sex Differences in Retinal Microvasculature Through Puberty In Type 1 Diabetes: Are Girls at Greater Risk of Diabetic Microvascular Complications? Sex Differences in Retinal Vascular Geometry. Invest Ophthalmol Vis Sci. 2015;56:571–577. doi: 10.1167/iovs.14-15147. [DOI] [PubMed] [Google Scholar]
- 31.Harjutsalo V, Maric C, Forsblom C, et al. Sex-related differences in the long-term risk of microvascular complications by age at onset of type 1 diabetes. Diabetologia. 2011;54:1992–1999. doi: 10.1007/s00125-011-2144-2. [DOI] [PubMed] [Google Scholar]
- 32.Forga L, Goñi MJ, Ibáñez B, et al. Influence of Age at Diagnosis and Time-Dependent Risk Factors on the Development of Diabetic Retinopathy in Patients with Type 1 Diabetes. J Diabetes Res. 2016;2016 doi: 10.1155/2016/9898309. Available at: http://www-ncbi-nlm-nih-gov.proxy.lib.umich.edu/pmc/articles/PMC4861784/ [Accessed June 25, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao X, Li J, Zhu X, et al. Association between socioeconomic status and metabolic control and diabetes complications: a cross-sectional nationwide study in Chinese adults with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15 doi: 10.1186/s12933-016-0376-7. Available at: http://www-ncbi-nlm-nih-gov.proxy.lib.umich.edu/pmc/articles/PMC4822246/ [Accessed June 25, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Downie E, Craig ME, Hing S, et al. Continued Reduction in the Prevalence of Retinopathy in Adolescents With Type 1 Diabetes: Role of insulin therapy and glycemic control. Diabetes Care. 2011;34:2368. doi: 10.2337/dc11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Low L, Law JP, Hodson J, et al. Impact of socioeconomic deprivation on the development of diabetic retinopathy: a population-based, cross-sectional and longitudinal study over 12 years. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-007290. Available at: http://www-ncbi-nlm-nih-gov.proxy.lib.umich.edu/pmc/articles/PMC4401835/ [Accessed June 25, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kytö JP, Harjutsalo V, Forsblom C, et al. Decline in the cumulative incidence of severe diabetic retinopathy in patients with type 1 diabetes. Diabetes Care. 2011;34:2005–2007. doi: 10.2337/dc10-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sano H, Nishimura R, Asao K, et al. Blindness and laser photocoagulation in patients with childhood-onset type 1 diabetes in Japan. Br J Ophthalmol. 2009;93:726–730. doi: 10.1136/bjo.2008.149534. [DOI] [PubMed] [Google Scholar]
- 38.Harvey JN, Allagoa B. The long-term renal and retinal outcome of childhood-onset Type 1 diabetes. Diabet Med J Br Diabet Assoc. 2004;21:26–31. doi: 10.1046/j.1464-5491.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- 39.Kolomeyer AM, Nayak NV, Simon MA, et al. Feasibility of retinal screening in a pediatric population with type 1 diabetes mellitus. J Pediatr Ophthalmol Strabismus. 2014;51:299–306. doi: 10.3928/01913913-20140709-01. [DOI] [PubMed] [Google Scholar]
- 40.Tapley JL, McGwin G, Jr, et al. Feasibility and efficacy of diabetic retinopathy screening among youth with diabetes in a pediatric endocrinology clinic: a cross-sectional study. Diabetol Metab Syndr. 2015;7 doi: 10.1186/s13098-015-0054-z. Available at: http://www-ncbi-nlm-nih-gov.proxy.lib.umich.edu/pmc/articles/PMC4487844/ [Accessed May 31, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benhalima K, Wilmot E, Khunti K, et al. Type 2 diabetes in younger adults: Clinical characteristics, diabetes-related complications and management of risk factors. Prim Care Diabetes. 2011;5:57–62. doi: 10.1016/j.pcd.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muir KW, Gupta C, Gill P, Stein JD. Accuracy of International Classification of Disease (ICD-9-CM) Billing Codes for Common Ophthalmologic Conditions. JAMA Ophthalmol. 2013;131:119–120. doi: 10.1001/jamaophthalmol.2013.577. [DOI] [PMC free article] [PubMed] [Google Scholar]


