Abstract
Decreased clearance of potentially toxic metabolites, due to aging changes, likely plays a significant role in the accumulation of amyloid-beta peptides (Aβ) and other macromolecules in the brains of the elderly and in Alzheimer’s disease (AD). Aging is the single most important risk factor for AD development. Aβ transport receptor proteins expressed at the blood-brain barrier (BBB) are significantly altered with age: the efflux transporters LRP-1 and P-gp are reduced, whereas the influx transporter RAGE is increased. These receptors play an important role in maintaining brain biochemical homeostasis. We now report that, in a rat model of aging, gene transcription is altered in aging, as measured by Aβ receptor gene mRNA at 3, 6, 9, 12, 15, 20, 30 and 36 months. Gene mRNA expression from isolated cerebral microvessels was measured by qPCR. LRP-1 and P-gp mRNA were significantly reduced in aging, and RAGE was increased, in parallel with the changes seen in receptor protein expression. Transcriptional changes appear to play a role in aging alterations in BBB receptor expression and Aβ accumulation.
Keywords: Amyloid transport genes, LRP-1, P-gp, RAGE mRNA, blood-brain barrier, aging, Alzheimer’s disease
1. INTRODUCTION
Many macromolecular metabolites and other potentially toxic solutes are transported into and out of the central nervous system (CNS) across the blood-brain barrier (BBB). Diffusion of solutes across the BBB is severely restricted by endothelial tight junctions, unlike systemic capillaries which are permeable to most solutes (Mann et al. 1985; Zlokovic 1995; Zlokovic et al. 1985; Zlokovic et al. 1987; Mackic et al. 2002). Metabolite movement across the BBB, therefore, occurs via energy-dependent active transport at certain specific “scavenger” receptors that transport a wide range of molecules (Herz and Strickland 2001; Schmidt and Stern 2001; Sarkadi, et al. 2006).
Good markers for such transport are the amyloid-beta peptides (Aβ), which are also implicated in cognitive loss in aging and Alzheimer’s disease (AD) (Selkoe 2000; Zlokovic 2004; Hardy 2006). The efflux transport receptors for Aβ at the BBB are the low density lipoprotein receptor-related protein 1 (LRP-1) and P-glycoprotein (P-gp) (Shibata et al. 2000; Lam et al. 2001; Cirrito et al. 2005; Yamada et al. 2008). The main influx transporter is the receptor for advanced glycation end-products (RAGE aka AGER) (Vitek et al. 1994; Deane et al. 2003). Expression of these transport receptors is significantly altered in human aging and AD (Donahue et al. 2006; Miller et al. 2008; Chiu et al. 2015), aging being the single most important risk factor in developing AD (Lu et al. 2004; Yankner et al. 2008). These macromolecule transport receptors are highly conserved throughout mammalian evolution and play an important role in maintaining brain biochemical homeostasis. It also appears that the expression levels of these transporters are related. Changing expression of one transporter may alter the expression of the others (Cirrito et al. 2005). At present we know very little about how and why their expression at the BBB changes significantly with aging and AD.
In 2010 it was demonstrated that significant age-related alterations in the expression of the Aβ transport receptor proteins at the BBB in the Fischer 344/Brown-Norway (F344/BN) hybrid rat was associated with increasing concentrations of Aβ40 and Aβ42 (Silverberg et al. 2010a, 2010b). There was an early (6–9 months) and progressive decrease in LRP-1 expression and a later decrease in P-gp expression (30–36 months). RAGE expression increased between 12 and 20 months. In association with these BBB amyloid transporter alterations, brain Aβ 40 and Aβ 42 concentrations increased significantly, and measureable cognitive decline occurred between 12 and 20 months (Silverberg et al. 2010a; 2010b; Church et al. 2014). Decreased neurogenesis preceded the cognitive decline (Church et al. 2014).
This study demonstrates a relationship between BBB amyloid transporter gene mRNA expression and previously reported BBB receptor protein expression as a function of aging. Amyloid transporter gene mRNA changed with age, anticipating the BBB receptor protein expression. The data suggest that gene transcription is altered with aging by some upstream event(s) rather than a post-transcriptional, translational or direct effect on these cell surface receptors. Transcriptional alterations of gene expression may occur via modification of the gene promoter (Christensen et al. 2009) or by histone modification (Rumbaugh and Miller 2011). Histone modifications are normally seen in development and aging, usually turning on or turning off a series of related genes. However, histone acetylation and methylation have been shown to alter brain-derived neurotrophic factor expression in neurons as a function of aging and AD (Walker et al. 2013).
2. METHODS
2.1 Animals
One hundred male F344/BN hybrid rats with similar characteristics were used in this study. Sample size was based upon a power analysis and prior receptor protein studies (Silverberg et al. 2010a; 2010b). They were randomly allocated into age groups 3, 6, 9, 12, 15, 20, 30 and 36 months. These rats are relatively long-lived and suffer less from neoplasia than the more inbred strains (Turturro et al. 1999). They were obtained at each age group tested from the NIA colony. The rats were euthanized by intraperitoneal pentobarbital (125mg/kg). The brains were rapidly removed and the cerebral microvessels were separated (Yousif et al. 2007; Silverberg et al. 2010a). The cerebellum, most subcortical structures and choroid plexus tissues were also removed, leaving the cortex and hippocampus. To obtain sufficient microvessels for analysis, we used two rat brains for a single “n” at each age point, i.e., an “n” of 5 equals 10 brains. Microvessels were isolated by homogenizing cortex and hippocampus (approximately 500 mg) in Hanks’ Balanced Salt Solution (all chemicals from Sigma, St. Louis, MO, USA) (Silverberg et al. 2010a, 2010b). Briefly, we separated the microvessels by a basic mechanical separation technique with repeated centrifugation at 4°C and re-suspension of the pellets in 17.5% dextran/microvessel isolation (MVI) buffer. The resulting pellet was re-suspended in Hanks’ Balanced Salt Solution containing 1% bovine serum albumin (BSA). The homogenate was then passed through a 100 μm nylon mesh cell strainer (BD Falcon, Bedford, MA, USA). Large vessels (20–30 μm) from the fraction retained on the 100 μm nylon mesh were collected, while the filtrate was passed through a 20 μm nylon mesh. The microvessels (4–6 μm) retained by the 20 μm nylon mesh were again collected. All samples were frozen at −80°C. Only MVI’s with no glial cell membrane or neuronal membrane contamination were used for assessment of RNA expression. Microvessel purity and integrity were evaluated by Hematoxylin and Eosin (H&E) staining. These MVI preparations were free of neuronal and glial cell membranes (Silverberg et al. 2010a; 2010b).
2.2 Real time RT-PCR
Samples were immediately extracted using the Aurum Total RNA Mini Kit (Bio-Rad, Hercules, CA, USA). This kit was used, per the manufacturer’s instructions, to extract the RNA. RNA concentrations were measured by a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA) and stored at −80°C until further use. 50ng of RNA were used with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) to synthesize 20μL of cDNA. The extracted RNA was reverse transcribed into cDNA and then quantified using TaqMan® real time PC 4. TaqMan® Gene Expression Assays (P/N: 4331182, Thermo Fisher Scientific, Applied Biosystems, Grand Island, NY USA) was utilized in this study. Reactions were run in an ABI 7900 Fast Sequence Detection (Life Technologies, Carlsbad, CA, USA) using an ABI suggested protocol. TaqMan probes for LRP-1, RAGE/AGER and P-gp were selected from a list of predesigned assays (Assays-on-Demand, Thermo Fisher Scientific, Applied Biosystems): LRP-1 (Cat # Rn01503901-m1), RAGE (Cat # Rn01525753-g1), P-gp (Cat # Rn01529252-g1) and β-Actin (Cat # Rn00667869_m1). All samples were run in triplicate, and all runs contained an inter-run calibrator to account for any differences between runs. Calibrators selected based on our earlier study (Pascal et al. 2011) corresponding to each gene were as follows: β-actin (β-ACT): rat cerebral cortex; LRP-1 and RAGE: rat lung; P-gp: rat liver. The same calibrator sample (3mos age group) was measured for each target gene throughout the study. All data were normalized to the housekeeping gene (endogenous control), β-ACT, in each sample before statistical analysis was performed. We employed the ΔΔCT method to calculate normalized expression of each gene sample. Briefly, all reactions were run in triplicate and the average values of Cts were used for quantification of the gene of interest and the endogenous gene for the same sample. The Ct (threshold cycle) values of target genes were normalized to an average Ct of β-ACT (ΔCT = Cttarget—Ctendo) and compared with a calibrator: ΔΔCT = ΔCtSample—ΔCtCalibrator. Relative expression (RQ) was calculated using the formula is RQ = 2−ΔΔCT.
2.3. Statistical methods
All statistical analyses were conducted using SAS software (V.9.4, SAS Institute, Cary, NC, USA). Analysis was performed on raw data using non-parametric methods such as Kruskal-Wallis (KW) and on transformed data using parametric methods such as Analysis of Variance (ANOVA). Age group comparisons for each amyloid transporter were initially analyzed for significant differences in percent mRNA expression using the Kruskal-Wallis Test (Nonparametric ANOVA). A Dunn’s test for multiple comparisons was performed to determine significant differences for all pairwise comparisons. Before performing any parametric analysis, Shapiro Wilk’s (SW) and Levene’s tests were used to assess normality and homogeneity of variances, respectively. Due to the lack of normality and unequal variation across age groups, for each of the transporters, a natural log transformation was applied to stabilize variances and to make the data more normal. Since assumptions of normality and homoscedasticity were satisfied for all log transformed transporters, one way analysis of variance (ANOVA) was used to assess differences in means across ages.
When a mean difference between ages was indicated by a one way analysis of variance, Tukey’s test for all pairwise comparisons was used to investigate significant pairwise differences between age groups. Conclusions from nonparametric and parametric methods were similar, and since parametric methods are more powerful, most of the results presented in later sections are for log transformed and parametric approaches to analysis. Significance for the overall one way analysis of variance was set at the 5% level. Tukey’s tests for all pairwise comparisons controls for experiment-wise error rate and was set up to achieve a combined type I error rate of 0.05. Regression/Correlation analysis was also used to assess associations between variables, e.g., whether expression of one receptor mRNA influences mRNA expression of other receptors.
3. RESULTS
3.1
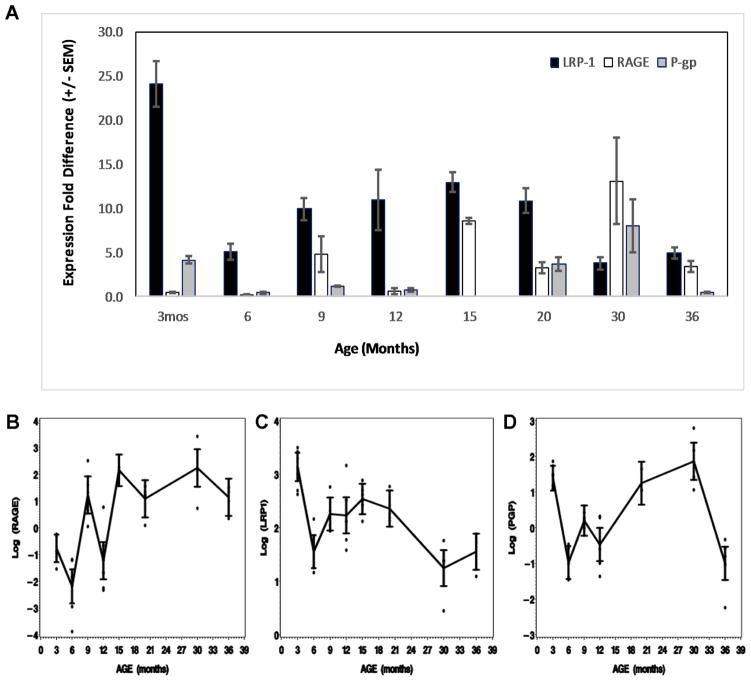
Despite rapid brain removal, separation of the BBB microvessels and BBB mRNA extraction under cold conditions, mRNA data displayed considerable within-group variance and noise between groups in the results, due, at least in part, to the fragile nature of mRNA. These wide variances and noise can be explained by the time it takes to separate the cerebral microvessels and the stresses on the microvessels by the separation procedures. However there were still significant differences between age groups for individual transporter gene mRNA expression and significant differences in the age-related trends for two transporters: LRP-1 (p=0.0001) and RAGE (p<0.0021). For lnLRP-1 and lnRAGE, p=0.0001. Because of the biphasic nature of P-gp expression, the trend line is relatively flat though there are a number of significant differences between age groups. Figure 1A shows the means and standard error of the mean (SEM) for each normalized transporter gene mRNA as a function of age group.
Figure 1.
Expression of LRP-1, RAGE and P-gp with age. (A) Bar graph of untransformed LRP-1, P-gp and RAGE (means ± SEM) for each age group. (B–D) Ninety five % confidence intervals for the means of the log transformed data for each transporter at each age group. (B) LRP-1 mRNA expression trended downward from a high at 3 months to a low at 30 and 36 months (p<0.0001). (C) Significant difference was seen in mean RAGE levels across age groups (p<0.001). RAGE mean levels indicated an overall upward trend with advancing age. (D) P-gp appears to have a biphasic expression pattern with differences in mean P-gp levels across the ages (p < 0.0001). P-gp expression dropped early, recovered and then decreased again by 36 months.
Correlations between variables can give important insights into the relationship between the mRNA expression and their protein products. However, these mRNA experiments were conducted using a different set of F344/BN rats than the western blot and immunostained surface area density of receptor protein studies, though the age groups, gender and characteristics of the rats analyzed were similar. The methodology for western blots and immunostaining have been previously published (Silverberg et al. 2010a, 2010b). As the results for both parameters were from different animal sets, one cannot do correlation studies on the complete data set, but the similar trend lines for each suggest a functional correlation.
3.2. LRP-1 mRNA expression with aging
There was a marked decrease in mean LRP-1 levels after three months of age. LRP-1 mean levels rose again after the initial decrease and then decreased again to 30 months of age. The normalized value of LRP-1 at 3 months (n=8) was 24.08±2.6; at 6 months (n=6) mRNA fell to 5.06±0.9; at 9 months (n=6) it was 9.92±1.3; at 12 months (n=5) it was 11.01±3.4; at 15 months (n=7) 12.96±1.1; at 20 months (n=5) 10.86±1.4; at 30 months (n=5) LRP-1 fell to 3.77±0.7; and at 36 months (n=5), it was 4.92±0.7. Departure from normality was borderline, Shapiro-Wilk’s (SW: p-value <0.05) but heterogeneity of variances was significant at the 1% level (p-value=0.01), hence data was transformed to the log scale. Conclusions from analysis on LRP-1 were consistent when a parametric approach was used on the log transformed data or the nonparametric approach was carried out on the raw data. For consistency, subsequent results will be given for log transformed data.
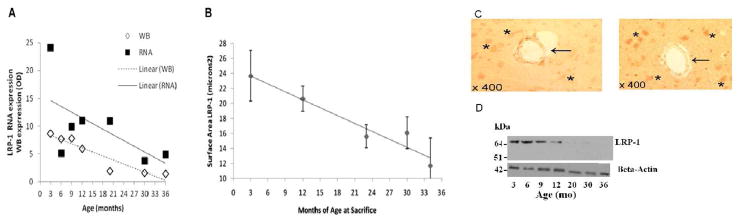
The average lnLRP-1 expression value at age 3 months was significantly higher than all ages except for 15 months. Mean lnLRP-1 was significantly lower at 6 months when compared to 9 (p-value < 0.05), 15 (p-value=0.0008) and 20 (p-value <0.03) months. At ages 9 and 12 months, lnLRP-1 was higher than at 30 months of age: 9 vs 30 months (p-value <0.002), 12 vs 30 months (p-value=0.004). lnLRP-1 at ages 15 and 20 months were significantly higher than mean lnLRP-1 at 30 and 36 months: 15 vs 30 months (p-value <0.0001), 20 vs 30 months (p-value= 0.0009), 15 vs 36 months (p-value <0.002), 20 vs 36 months (p-value <0.04). Lastly, there was no difference in mean lnLRP-1 between ages 30 and 36 months. Figure 2 shows the graphic representation of mean lnLRP-1 mRNA with age plotted along with the western blot mean measure of receptor protein at the BBB. The western blot and immunohistochemistry data comes from a separate set of F344/BN male rats at similar age groups and other characteristics and was previously published (Silverberg et al. 2010b). There was also a positive correlation (Pearson’s correlation coefficient) between the expression of LRP-1 and P-gp mRNA with aging (p<0.02).
Figure 2.
(A). Graph of mean LRP-1 mRNA (squares) and western blot optical density (OD) measurements (diamonds) of BBB protein expression. In order to plot them together on the same XY plot, the western blot OD measures were multiplied by a factor of 10. The graph shows the very close parallel between mRNA expression (solid line) and LRP-1 protein synthesis (dashed line) trend that strongly relate the two: the genetic message and the protein response as a function of aging. Note that we omitted the 15 month data as we had no western blot data to compare. (B). Mean normalized LRP-1 microvessel surface area with 95% confidence interval plotted as a function of age at sacrifice with a linear trend line. There is a significant decreasing linear trend of j0.35 Km2/mo (95% confidence interval, j0.48 to j0.23 Km2/mo; p = 0.0004). Again the trend in LRP-1 protein expression and mRNA expression is clearly downward. (C) Representative LRP-1 immunostaining at three months (left) and 34 months (right). At three months there is a robust expression of LRP-1 on the basal surface of endothelial cells (arrow), and evidence of neuronal staining (asterisks). At 34 months LRP-1 expression on endothelium is significantly reduced (arrow), whereas neuronal expression appears to be somewhat increased (asterisks). (D) Representative western blots for LRP-1 and β-Actin. The western blot OD data and the immunostaining data in figure 2 has been previously published (Silverberg et al. 2010a, 2010b).
3.3. RAGE mRNA expression with aging
RAGE mRNA expression began at a very low level at 3–6 months and trended upwards, though somewhat noisily, to a very high level at 30 months and then dropped between 30 and 36 months. At 3 months (n=9), normalized RAGE mRNA expression was 0.50±0.07; at 6 months (n=6) it was 0.16±0.05; at 9 months (n=5) it was 4.78±2; at 12 months (n=5) it was 0.61±0.4; at 15 months (n=7) it was 8.60±0.3; at 20 months (n=5) it was 3.28±0.6; at 30 months (n=5) it was 13.12±4.9 and at 36 months (n=5) it was 3.39±0.6. SW indicated non-normality in RAGE (p-value < 0.0001) and Levene’s test indicated heterogeneity of variances across age groups (p-value <0.004), hence a log transformation of the data.
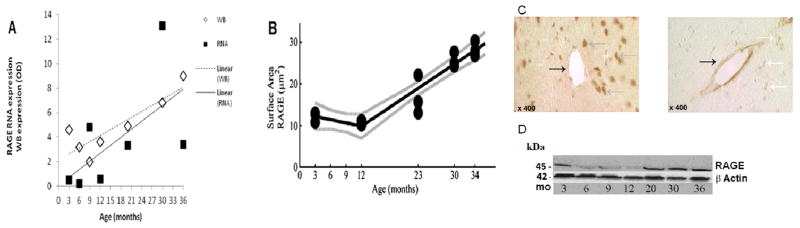
In making comparisons of the means of the log transformed data, Tukey’s test indicated strong significant differences between the age group at 3 months with 15 and 30 months; age group 6 months with 9, 15, 20, 30 and 36 months; at 12 months with 15 and 30 months, all with a p-value <0.0001. Other significant differences existed between ages 3 and 6, 9, 20 and 36 months; 9 and 12 months; 12 with 20 and 36 months, all with p-values <0.003. It is interesting to note that the mean of the log transformed RAGE expression in the higher age groups (15 months and older) did not show any statistical differences (p–values >0.3). Figure 3 shows the parallel expressions of RAGE mRNA and receptor protein expression at the BBB. The western blot and immunohistochemistry data comes from a separate set of F344/BN male rats at similar age groups and other characteristics and was previously published (Silverberg et al. 2010a). There was also significant correlation between RAGE and P-gp expression (p-value<0.03). The raw data also showed a correlation (p-value=0.02).
Figure 3.
(A) RAGE mRNA expression (squares) plotted with RAGE protein expression (diamonds) measured by western blot. Western blot OD x10. There is a general upward trend in both mRNA and protein beginning between 12 and 15 months. (B) Immunohistochemistry surface area shows no significant decrease from 3 to 12 months followed by a substantial increase in RAGE expression up to 34 months (p < 0.0001). (C) RAGE immunostaining at three months (left), is primarily neuronal (gray arrow) in distribution, and much less staining is seen in microvessels (black arrow). At 30 months (right), microvessel RAGE immunoreactivity (black arrow) is increased whereas neuronal staining (white arrow) is less evident. (D) Representative western blots for RAGE and β-Actin proteins. The western blot OD data and the immunostaining data in figure 3 have been previously published (Silverberg et al. 2010a, 2010b).
3.4. P-gp mRNA expression with age
P-gp mRNA expression initially decreased from a high level at 3 months, and then rose again to 30 months followed by a marked decrease at 36 months. At 3 months P-gp mRNA expression was 4.18±0.4 (n=9); at 6 months (n=5), it fell to 0.41±0.08; at 9 months (n=6) it was 1.23±0.08; at 12 months (n=5) it was 0.78±0.2; at 20 months (n=3) it was 3.63±0.8; at 30 months (n=4) it rose to 8.02±3.03 and at 36 months (n=5) it fell to 0.44±0.1.
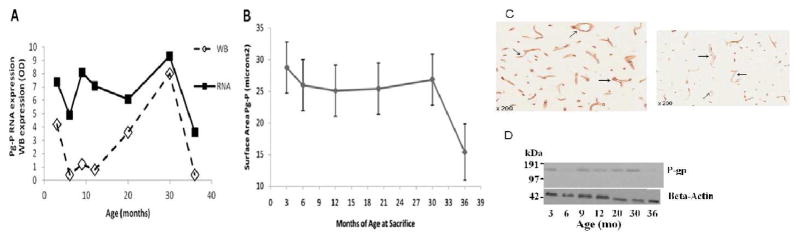
A log transformation for P-gp was justified on the basis of non-normality (SW p-value<0.0001) and heterogeneity of variances (Levene’s, p-value=0.001). At 3 months, the mean of the log transformed data showed a sharp decrease to 6 months (p<0.0001) and an overall increase from 6 to 30 months and then sharp decrease to 36 months (p<0.0001). Significant increased differences were seen between 6 and 9 months (p-value<0.02), and between 6 months and 20 and 30 months (p-value<0.0001). There was a significant increase from 9 to 30 months (p-value=0.0005) and then a decrease at 36 months (p value<0.0001). An increase in lnP-gp expression was observed from 12 months to 20 months (p-value=0.0015) and a further increase at 30 months (p-value<0.0001). Both 20 and 30 months showed a marked decrease to 36 months (p-value<0.0001). This biphasic expression of P-gp is also seen in the BBB P-gp receptor protein expression, as shown in Figure 4. The western blot and immunohistochemistry data comes from a separate set of F344/BN male rats at similar age groups and other characteristics and was previously published (Silverberg et al. 2010b).
Figure 4.
(A) Graph of mean P-gp mRNA and mean western blot measure of P-gp protein expression at the BBB. Western blot OD x10. Note the similar biphasic pattern of expression for both the P-gp mRNA and protein. (B) Mean normalized P-gp microvessel surface area staining with 95% confidence interval plotted with means joined as a function of age at sacrifice; 36-month-old rats were significantly lower than all other ages (adjusted p G 0.05 for all comparisons). No other age groups differed significantly (adjusted p Q 0.05). P-gp mRNA did correlate with P-gp receptor protein expression (Pearson correlation of means) r= 0.69, p<0.05. (C) Expression of endothelial P-gp from three (left) to 36 months (right). At three months there is abundant capillary P-gp staining evident (arrows). At 36 months there is a significant decrease in endothelial P-gp staining. (D) Representative western blots for P-gp and β-Actin proteins. The western blot OD data and the immunostaining data in figure 4 have been previously published (Silverberg et al. 2010a, 2010b).
4. DISCUSSION
Clearance of potentially noxious metabolites, such as Aβ, via the BBB receptors LRP-1, P-gp and RAGE, and the cerebrospinal fluid (CSF) circulation is critical to maintaining brain homeostasis. Failure to clear such macromolecular solutes because of age-related changes in BBB transporter expression appears to lead to the accumulation of these substances, e.g., Aβ, in the brain (Selkoe 2000; Silverberg et al. 2010a, 2010b; Zlokovic et al. 2010; Pascale et al. 2011; Chiu et al. 2012, 2015). As aging is the single most important risk factor for developing AD (NIH 2002; Lu et al. 2004; Yankner et al. 2008), and the accumulation of Aβ is felt to be central to AD pathogenesis (Selkoe 2000; Hardy 2006; Querfurth and LaFerla 2010), it is of more than passing interest to understand the mechanisms involved in the expression alterations of the Aβ transport receptors at the brain barriers that occur in the non-demented elderly as well as in the AD population (Arriagada et al. 1992; Davis et al. 1999).
LRP-1, RAGE and P-gp are the main transporters of Aβ and a wide variety of other macromolecules at the BBB (Ambudkar et al. 1999; Herz and Strickland 2001; Schmidt et al. 2001; Deane et al. 2003; Zlokovic 2004). We have shown that the expression of these scavenger transport receptor proteins is significantly impacted by age in the accepted rat model of human aging, the F344/BN hybrid (Church et al. 2014): LRP-1 and P-gp are significantly down-regulated whereas RAGE is significantly up-regulated with age (Silverberg et al. 2010a, 2010b). Aging, AD and hydrocephalus of the elderly (NPH) also decrease CSF production and turnover in both rats and humans (Chiu et al. 2012, 2015; Silverberg et al. 2001, 2002, 2003). Although there is evidence that Aβ transport at the blood-CSF barrier (choroid plexus) favors Aβ clearance from the CSF, it is not sufficient to decrease Aβ accumulation with age (Ghersi-Egea et al. 1996; Pascale et al. 2011; Silverberg et al. 2010a). Analysis of mRNA expression of LRP-1, P-gp and RAGE gives an insight into how these age-related alterations occur. Although there was variability in the mRNA measurement results, it appears that transcriptional events play a major role in the BBB receptor protein changes in the rat model of aging.
LRP-1 receptor protein at the BBB is expressed primarily on pericytes, the BBB layer abutting the interstitial fluid (Daneman et al. 2010). The LRP-1 receptor gene promoter has been previously characterized (Kutt et al. 1989). LRP-1 antisense blockade of LRP-1 function at the BBB reduced Aβ clearance, increased Aβ brain levels and impaired learning and memory in mice (Jaeger et al. 2009). LRP receptor overexpression decreased Aβ deposition and increased Aβ clearance (Kim et al. 2009). In AD patients and mouse models of AD, overexpression of serum response factor and myocardin in cerebral vascular smooth muscle cells generates an Aβ non-clearing vascular smooth muscle cell phenotype through transactivation of sterol regulatory element binding protein-2, which downregulates LRP-1 (Bell et al. 2009). Also acting through LRP1, reduced expression of PICALM (rs541458, an endocytosis gene) in AD and murine brain endothelium correlated with Aβ pathology and cognitive impairment. Moreover, PICALM deficiency diminished Aβ clearance across the murine BBB and accelerated Aβ pathology (Zhao et al. 2015). Interestingly, reduced levels of LRP-1 expression and increased RAGE expression could be reversed. Under conditions of hypoxia and hypoglycemia in tissue culture LRP-1 mRNA expression was decreased and RAGE increased, similar to what is seen in aging and AD, and these effects could be partially reversed by a ginko biloba extract (Yan et al. 2008). In hypoxic vascular smooth muscle cells LRP-1 regulation was mediated by promoter modification and was reflected in LRP-1 mRNA expression (Castellano et al. 2011). In human studies, it has been shown that LRP-1 promoter polymorphisms play a significant role in LRP-1 expression in brain vessels (Glaser et al. 2004). Our measurements of LRP-1 mRNA show an overall trend of decreased mRNA expression that is reflected in our LRP-1 measures of receptor protein expression at the BBB.
In the CNS, P-gp is expressed only on endothelial cells of the BBB and plays a functional part in denying certain pharmaceuticals and other potentially noxious solutes access to the brain. In human brain tissue P-gp expression at the BBB is age and AD dependent and is related, through aging, to amyloid accumulation (Chiu et al. 2015). P-gp mRNA also tracks protein expression in human brains. In several multidrug resistant (MDR) leukemic cell lines increased MDR transcription and P-gp expression are associated with promoter demethylation (Moreira et al. 2014). It appears that activation of P-gp protein is preceded by an increase in P-gp mRNA which is also associated with activation of the nuclear factor kappa B (NF-kB) (Zhang et al. 2014; Fan et al. 2015). In isolated rat and human brain microvessel endothelium, vitamin D3-liganded vitamin D receptor increases P-gp mRNA expression and increases P-gp transporter expression (Durk et al. 2012). It has been shown that fetal expression of P-gp is very high and falls progressively with age in the post-natal period. This increased expression is associated with increased transforming growth factor beta 1 (TGF-B1) activity (Baello et al. 2014). It is not known whether the early decrease in P-gp expression in adult aging is also associated with TGF-B1 levels. It has also been demonstrated in capillaries affected by amyloid angiopathy that decreased expression of P-gp receptor protein is associated with decreased P-gp mRNA expression (Carrano et al. 2014). In our study, the biphasic nature of age-related P-gp protein expression seen in both rats and humans is related to a similar biphasic expression of P-gp mRNA in the rat model (Chiu et al. 2015).
RAGE is expressed on the endothelial cells of the BBB and is known to be an influx transporter of Aβ along with a number of glycated proteins and other metabolic end-products (Vitek et al. 1994; Schmidt et al. 1994; 1998; 2001). Of the three primary Aβ transport receptors, it is the only one to be up-regulated with advancing age. In this study, we found that RAGE mRNA was increased at the BBB with aging up until 36 months of age when the accumulation of Aβ42 appears to have significantly reduced all Aβ transporter mRNA (Brenn et al. 2011). Of interest, anesthesia (Sevoflurane) also decreases LRP-1 mRNA and increases RAGE mRNA (Liu et al. 2013). It has also been shown on human autopsy brain tissue that increased RAGE expression is associated with RAGE promoter demethylation (Tohgi et al. 1999).
In this study, we show that Aβ transporter mRNA expression at the BBB is markedly altered with age. LRP-1 and P-gp are decreased significantly and RAGE mRNA is increased. Age-related alterations in one transporter influences the expression of the others. However, at the end of the F344/BN rat lifespan, all of the Aβ transporter mRNAs are reduced in expression. In a prior study, this reduction was induced by subcutaneous infusion of Aβ42 (Brenn et al. 2011). The changes we show in mRNA expression are paralleled by similar changes in receptor protein expression seen in two prior publications (Silverberg et al. 2010a; 2010b). The data indicate that the alterations in receptor protein expression at the BBB are, at least in part, transcriptional in nature. Of the many signaling pathways and epigenetic factors that impinge upon these Aβ transporter genes, it will be critical to discover which ones are related to these aging changes and how can they be returned to an earlier rate of expression (Li et al. 1997; Tohgi et al. 1999; Kang et al. 2000; Herz 2001; Vogelgesang et al. 2002; Liu et al. 2007; Pilorget et al. 2007; Hong et al. 2009; Liu et al. 2009; Chouliaras et al. 2010). This study, as well as other human and animal studies, helps to explain the marginal responses or actual failure of recent intravenous Aβ antibody (e.g., Solanezumab, Bapineuzumab, Crenezumab) infusion clinical trials. These trials appear to depend upon the Aβ antibodies enhancing efflux transport of Aβ across the BBB (Prins and Scheltens, 2013; Castello, et al. 2014; Toyn, 2015; Godyn, et al. 2016; Bouter, et al. 2015; Fuller et al. 2015). Alternatively the poor performance of Aβ antibody therapy may be the multi-morbidity of a number of pathologies seen in the brains of the AD and non-demented elderly, e.g., Lewy body disease, various tauopathies and cerebrovascular disease (Dugger et al, 2014; Attems et al. 2013; Echavarri et al. 2012). Targeting Aβ only may not be an adequate solution for such patients.
Highlights.
Three rat blood-brain barrier amyloid transporter mRNA measures at ages 3 –36 months are presented.
The altered expressions of the amyloid transport receptors are related to gene transcription.
LRP-1 and P-gp mRNAs are reduced and RAGE is increased, most likely due to promoter modifications.
Transporter alterations may explain why clinical trials of amyloid antibodies in AD have failed.
Acknowledgments
Funding sources: NIH grant 1RO1 AG027910-01 and the Saunders Family Fund at the Neurosurgery Foundation at Brown University.
Funding for this study was provided by NIH grant 1RO1 AG027910-01A1. Capital equipment purchases were provided by the Saunders Family Fund and the Rae and Jerry Richter AD Research Fund at the Neurosurgical Foundation, Rhode Island Hospital and The Warren Alpert Medical School, Brown University.
Footnotes
6. CONFLICT OF INTEREST
GDS is a co-founder and CEO of CSFRefresh, Inc., a new startup company developing a novel biomedical device to improve CSF clearance of macromolecules from the brain. CSFRefresh provided no financial support to this study and has no connection with the laboratory or the data in this article. None of the other authors or their relatives has any conflict of or competing interest that would lead to financial gain with the publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- Arriagada P, Marzloff K, Hyman B. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology. 1992;42:1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- Attems J, Jellinger K. Neuropathological correlates of cerebral multimorbidity. Curr Alzheimer Res. 2013;10:569–577. doi: 10.2174/15672050113109990002. [DOI] [PubMed] [Google Scholar]
- Baello S, Iqbal M, Bloise E, Javam M, Gibb W, Matthews SG. TGF-β1 regulation of multidrug resistance P-glycoprotein in the developing male blood-brain barrier. Endocrinology. 2014;155:475–484. doi: 10.1210/en.2013-1472. [DOI] [PubMed] [Google Scholar]
- Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, Streb JW, Guo H, Rubio A, Van Nostrand W, Miano JM, Zlokovic BV. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouter Y, Lopez Noguerola JS, Tucholla P, Crespi GA, Parker MW, Wiltfang J, Miles LA, Bayer TA. Abeta targets of the biosimilar antibodies of Bapineuzumab, Crenezumab, Solanezumab in comparison to an antibody against N-truncated Abeta in sporadic Alzheimer disease cases and mouse models. Acta Neuropathol. 2015;130:713–729. doi: 10.1007/s00401-015-1489-x. [DOI] [PubMed] [Google Scholar]
- Brenn A, Grube M, Peters Ml, Fischer A, Jedlitschky G, Kroemer HK, Warzok RW, Vogelgesang S. Beta-amyloid downregulates MDR1-P-glycoprotein (Abcb1) expression at the blood-brain barrier in mice. Int J Alzheimers Dis. 2011;2011:690121. doi: 10.4061/2011/690121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano A, Snkhchyan H, Kooij G, van der Pol S, van Horssen J, Veerhuis R, Hoozemans J, Rozemuller A, de Vries HE. ATP-binding cassette transporters P-glycoprotein and breast cancer related protein are reduced in capillary cerebral amyloid angiopathy. Neurobiol Aging. 2014;35:565–575. doi: 10.1016/j.neurobiolaging.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Castellano J, Aledo R, Sendra J, Costales P, Juan-Babot O, Badimon L, Llorente-Cortés V. Hypoxia stimulates low-density lipoprotein receptor-related protein-1 expression through hypoxia-inducible factor-1α in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31:1411–420. doi: 10.1161/ATVBAHA.111.225490. [DOI] [PubMed] [Google Scholar]
- Castello MA, Jeppson JD, Soriano S. Moving beyond anti-amyloid therapy for the prevention and treatment of Alzheimer’s disease. BMC Neurol. 2014:169. doi: 10.1186/s12883-014-0169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Miller MC, Caralopoulos IN, Worden MS, Brinker T, Gordon ZN, Johanson CE, Silverberg GD. Te. Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids Barriers CNS. 2012;9:1–8. doi: 10.1186/2045-8118-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Miller MC, Monahan R, Osgood DP, Stopa EG, Silverberg GD. P-glycoprotein expression and amyloid accumulation in human aging and AD. Neurobiol Aging. 2015;36:2475–2482. doi: 10.1016/j.neurobiolaging.2015.05.020. [DOI] [PubMed] [Google Scholar]
- Christensen BC, Houseman EA, Marsit CJ, Zheng S, Wrensch MR, Wiemels JL, Nelson HH, Karagas MR, Padbury JF, Bueno R, Sugarbaker DJ, Yeh RF, Wiencke JK, Kelsey KT. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 2009;5:e1000602. doi: 10.1371/journal.pgen.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouliaras L, Rutten BP, Kenis G, Peerbooms O, Visser PJ, Verhey F, van Os J, Steinbusch HW, van den Hove DL. Epigenetic regulation in the pathophysiology of Alzheimer’s disease. Prog Neurobiol. 2010;90:498–510. doi: 10.1016/j.pneurobio.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Church RM, Miller MC, Freestone D, Chiu C, Osgood DP, Machan JT, Messier AA, Johanson CE, Silverberg GD. Amyloid-beta accumulation, neurogenesis, behavior, and the age of rats. Behav Neurosci. 2014;128:523–536. doi: 10.1037/a0036433. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood–brain barrier Increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres B. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One. 2010;5:e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Schmitt F, Wekstein D, Markesbery W. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Donahue JE, Flaherty SL, Johanson CE, Duncan JA, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol (Ber) 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- Dugger BN, Hentz JG, Adler CH, Sabbagh MN, Shill HA, Jacobson S, Caviness JN, Belden C, Driver-Dunckley E, Davis KJ, Sue LI, Beach TG. Clinicopathological outcomes of prospectively followed normal elderly brain bank volunteers. J Neuropathol Exp Neurol. 2014;73:244–252. doi: 10.1097/NEN.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durk MR, Chan GN, Campos CR, Peart JC, Chow EC, Lee E, Cannon RE, Bendayan R, Miller DS, Pang KS. 1α,25-Dihydroxyvitamin D3-liganded vitamin D receptor increases expression and transport activity of P-glycoprotein in isolated rat brain capillaries and human and rat brain microvessel endothelial cells. J Neurochem. 2012;123:944–953. doi: 10.1111/jnc.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echavarri C, Burgmans S, Caballero MC, Garcia-Bragado F, Verhey FR, Uylings HB. Co-occurrence of different pathologies in dementia: implications for dementia diagnosis. J Alzheimers Dis. 2012;30:909–917. doi: 10.3233/JAD-2012-111400. [DOI] [PubMed] [Google Scholar]
- Fan X, Chai L, Zhang H, Wang Y, Zhang B, Gao X. Borneol depresses P-glycoprotein function by a NF-κB signaling mediated mechanism in a blood brain barrier in vitro model. Int J Mol Sci. 2015;16:27576–27588. doi: 10.3390/ijms161126051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller JP, Stavenhagen JB, Christensen S, Kartberg F, Glennie MJ, Teeling JL. Comparing the efficacy and neuroinflammatory potential of three anti-abeta antibodies. Acta Neuropathol. 2015;130:699–711. doi: 10.1007/s00401-015-1484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghersi-Egea JF, Gorevic PD, Ghiso J, Frangione B, Patlak CS, Fenstermacher JD. Fate of cerebrospinal fluid-borne amyloid beta-peptide: rapid clearance into blood and appreciable accumulation by cerebral arteries. J Neurochem. 1996;67:880–883. doi: 10.1046/j.1471-4159.1996.67020880.x. [DOI] [PubMed] [Google Scholar]
- Gläser C, Schulz S, Handschug K, Huse K, Birkenmeier G. Genetic and functional characteristics of the human in vivo LRP1/A2MR receptor suggested as a risk marker for Alzheimer’s disease and other complex (degenerative) diseases. Neurosci Res. 2004;50:85–101. doi: 10.1016/j.neures.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Godyń J, Jończyk J, Panek D, Malawska B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol Rep. 2016;68:127–138. doi: 10.1016/j.pharep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Herz J. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 2001;29:571–581. doi: 10.1016/s0896-6273(01)00234-3. [DOI] [PubMed] [Google Scholar]
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Liu LP, Liao JM, Wang TS, Ye FY, Wu J, Wang YY, Wang Y, Li YQ, Long Y, Xia YZ. Downregulation of LRP1 [correction of LPR1] at the blood-brain barrier in streptozotocin-induced diabetic mice. Neuropharmacology. 2009;56:1054–1059. doi: 10.1016/j.neuropharm.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Hwang MC, Farr SA, Murphy MP, Fleegal-DeMotta MA, Lynch JL, Robinson SM, Niehoff ML, Johnson SN, Kumar VB, Banks WA. Testing the neurovascular hypothesis of Alzheimer’s disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. J Alzheimers Dis. 2009;17:553–570. doi: 10.3233/JAD-2009-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Pietrzik CU, Baum L, Chevallier N, Merriam DE, Kounnas MZ, Wagner SL, Troncoso JC, Kawas CH, Katzman R, Koo EH. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest. 2000;106:1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Castellano JM, Jiang H, Basak JM, Parsadanian M, Pham V, Mason SM, Paul SM, Holtzman DM. Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular A beta clearance. Neuron. 2009;64:632–644. doi: 10.1016/j.neuron.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kütt H, Herz J, Stanley KK. Structure of the low-density lipoprotein receptor-related protein (LRP) promoter. Biochim Biophys Acta. 1989;1009:229–236. doi: 10.1016/0167-4781(89)90107-3. [DOI] [PubMed] [Google Scholar]
- Lam FC, Liu R, Lu P, Shapiro AB, Renoir JM, Sharom FJ, Reiner PB. beta-Amyloid efflux mediated by P-glycoprotein. J Neurochem. 2001;76:1121–1128. doi: 10.1046/j.1471-4159.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end-products. J Biol Chem. 1997;272:16498–16506. doi: 10.1074/jbc.272.26.16498. [DOI] [PubMed] [Google Scholar]
- Liu LP, Hong H, Liao JM, Wang TS, Wu J, Chen SS, Li YQ, Long Y, Xia YZ. Upregulation of RAGE at the blood-brain barrier in streptozotocin-induced diabetic mice. Synapse. 2009;63:636–642. doi: 10.1002/syn.20644. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, Cole SL, Herz J, Muglia L, Bu G. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gao M, Ma L, Zhang L, Pan N. Sevoflurane alters the expression of receptors and enzymes involved in Aβ clearance in rats. Acta Anaesthesiol Scand. 2013;57:903–910. doi: 10.1111/aas.12098. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao S, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Mackic JB, Bading J, Ghiso J, Walker L, Wisniewski T, Frangione B, Zlokovic BV. Circulating amyloid-beta peptide crosses the blood-brain barrier in aged monkeys and contributes to Alzheimer’s disease lesions. Vascul Pharmacol. 2002;38:303–13. doi: 10.1016/s1537-1891(02)00198-2. [DOI] [PubMed] [Google Scholar]
- Mann GE, Zlokovic BV, Yudilevich DL. Evidence for a lactate transport system in the sarcolemmal membrane of the perfused rabbit heart: kinetics of unidirectional influx, carrier specificity and effects of glucagon. Biochim Biophys Acta. 1985;819:241–248. doi: 10.1016/0005-2736(85)90179-8. [DOI] [PubMed] [Google Scholar]
- Miller MC, Tavares R, Johanson CE, Hovanesian V, Donahue JE, Gonzalez L, Silverberg GD, Stopa EG. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease. Brain Res. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira MA, Bagni C, de Pinho MB, Mac-Cormick TM, dos Santos Mota M, Pinto-Silva FE, Daflon-Yunes N, Rumjanek VM. Changes in gene expression profile in two multidrug resistant cell lines derived from a same drug sensitive cell line. Leuk Res. 2014;38:983–987. doi: 10.1016/j.leukres.2014.06.001. [DOI] [PubMed] [Google Scholar]
- National Institute of Aging. Alzheimer’s Disease Unraveling the Mystery. US Department of Health and Human Services, National Institutes of Health; NIH Publication Number 2002;02–3782. [Google Scholar]
- Pascale CL, Miller MC, Chiu C, Boylan M, Caralopoulos IN, Gonzalez L, Johanson CE, Silverberg GD. Amyloid-beta transporter expression at the blood-CSF barrier is age dependent. Fluids Barriers CNS. 2011 Jul 8;8:21. doi: 10.1186/2045-8118-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilorget A, Demeule M, Barakat S, Marvaldi J, Luis J, Béliveau R. Regulation of P-glycoprotein function by sphingosine kinase-1 in brain endothelial cells. J Neurochem. 2007;100:1203–1210. doi: 10.1111/j.1471-4159.2006.04295.x. [DOI] [PubMed] [Google Scholar]
- Prins ND, Scheltens P. Treating Alzheimer’s disease with monoclonal antibodies: current status and outlook for the future. Alzheimers Res Ther. 2013;5:56. [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Eng J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Miller CA. Epigenetic changes in the brain: measuring global histone modifications. Methods Mol Biol. 2011;670:263–274. doi: 10.1007/978-1-60761-744-0_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B, Homolya L, Szakács G, Váradi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Hasu M, Popov D, Zhang JH, Chen J, Yan SD, Brett J, Cao R, Kuwabara K, Costache G. Receptor for advanced glycation end products (AGEs) has a central role in vessel wall interactions and gene activation in response to circulating AGE proteins. Proc Natl Acad Sci USA. 1994;91:8807–8811. doi: 10.1073/pnas.91.19.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Wautier J-L, Stern D. RAGE: A receptor with a taste for multiple ligands and varied pathophysiologic states. In: O’Malley BW, editor. Hormones and Signaling. Amsterdam, Holland: Elsevier Inc; 1998. pp. 41–63. [Google Scholar]
- Schmidt AM, Stern DM. Receptor for age (RAGE) is a gene within the major histocompatibility class lll region: implications for host response mechanisms in homeostasis and chronic disease. Front Biosci. 2001;6:D1151–1160. doi: 10.2741/schmidt. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann NY Acad Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-β1–40 peptide from brain by LDL receptor-related protein-1 at the blood–brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, Rubenstein E, Possin K, Saul TA. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology. 2001;57:1763–1766. doi: 10.1212/wnl.57.10.1763. [DOI] [PubMed] [Google Scholar]
- Silverberg GD, Huhn S, Jaffe RA, Chang SD, Saul T, Heit G, Von Essen A, Rubenstein E. Downregulation of cerebrospinal fluid production in patients with chronic hydrocephalus. J Neurosurg. 2002;97:1271–1275. doi: 10.3171/jns.2002.97.6.1271. [DOI] [PubMed] [Google Scholar]
- Silverberg GD, Mayo M, Saul T, Rubenstein E, McGuire D. Alzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol. 2003;2:506–511. doi: 10.1016/s1474-4422(03)00487-3. [DOI] [PubMed] [Google Scholar]
- Silverberg GD, Miller MC, Messier AA, Majmudar S, Machan JT, Donahue JE, Stopa EG, Johanson CE. Amyloid deposition and influx transporter expression at the blood-brain barrier increase in normal aging. J Neuropathol Exp Neurol. 2010;69:98–108. doi: 10.1097/NEN.0b013e3181c8ad2f. [DOI] [PubMed] [Google Scholar]
- Silverberg GD, Messier AA, Miller MC, Machan JT, Majmudar S, Stopa EG, Donahue JE, Johanson CE. Amyloid efflux transporter expression at the blood-brain barrier declines in normal aging. J Neuropathol Exp Neurol. 2010;69:1034–1043. doi: 10.1097/NEN.0b013e3181f46e25. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Utsugisawa K, Nagane Y, Yoshimura M, Ukitsu M, Genda Y. Decrease with age in methylcytosines in the promoter region of receptor for advanced glycation end products (RAGE) gene in autopsy human cortex. Brain Res Mol Brain Res. 1999;65:124–128. doi: 10.1016/s0169-328x(98)00351-9. [DOI] [PubMed] [Google Scholar]
- Toyn J. What lessons can be learned from failed Alzheimer’s disease trials? Expert Rev Clin Pharmacol. 2015;8:267–269. doi: 10.1586/17512433.2015.1034690. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Vitek MP, Battacharaya K, Glendening JM, Stopa E, Vlassara H, Bucala R, Manogue K, Cerami A. Advanced glycation end products contribute to amyloidosis in Alzheimer’s disease. Proc Natl Acad Sci USA. 1994;91:4766–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelgesang S, Cascorbi I, Schroeder E, Pahnke J, Kroemer HK, Siegmund W, Kunert-Keil C, Walker LC, Warzok RW. Deposition of Alzheimer’s beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogen. 2002;12:535–541. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- Walker MP, LaFerla FM, Oddo SS, Brewer GJ. Reversible epigenetic histone modifications and Bdnf expression in neurons with aging and from a mouse model of Alzheimer’s disease. Age (Dordr) 2013;35:519–531. doi: 10.1007/s11357-011-9375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Hashimoto T, Yabuki C, Nagae Y, Tachikawa M, Strickland DK, Liu Q, Bu G, Basak JM, Holtzman DM, Ohtsuki S, Terasaki T, Iwatsubo T. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid beta peptides in an in vitro model of the blood-brain barrier cells. J Biol Chem. 2008;283:34554–34562. doi: 10.1074/jbc.M801487200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan FL, Zheng Y, Zhao FD. Effects of ginkgo biloba extract EGb761 on expression of RAGE and LRP-1 in cerebral microvascular endothelial cells under chronic hypoxia and hypoglycemia. Acta Neuropathol. 2008;116:529–535. doi: 10.1007/s00401-008-0435-6. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- Yousif S, Marie-Claire C, Roux F, Scherrmann JM, Declèves X. Expression of drug transporters at the blood–brain barrier using an optimized isolated rat brain microvessel strategy. Brain Res. 2007;1134:1–11. doi: 10.1016/j.brainres.2006.11.089. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang M, Sun B, Li Y, Xu P, Liu C, Liu L, Liu X. Hyperammonemia enhances the function and expression of P-glycoprotein and Mrp2 at the blood-brain barrier through NF-κB. J Neurochem. 2014;131:791–802. doi: 10.1111/jnc.12944. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, Owens NC, Rege SV, Si G, Ahuja A, Zhu D, Miller CA, Schneider JA, Maeda M, Maeda T, Sugawara T, Ichida JK, Zlokovic BV. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat Neurosci. 2015;18:978–987. doi: 10.1038/nn.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucine-enkephalin, D-alanine2-D-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336:125–132. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- Zloković BV, Lipovac MN, Begley DJ, Davson H, Rakić L. Transport of leucine-enkephalin across the blood-brain barrier in the perfused guinea pig brain. J Neurochem. 1987;49:310–315. doi: 10.1111/j.1471-4159.1987.tb03431.x. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm Res. 1995;12:1395–1406. doi: 10.1023/a:1016254514167. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Clearing amyloid through the blood-brain barrier. J Neurochem. 2004;89:807–811. doi: 10.1111/j.1471-4159.2004.02385.x. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J Neurochem. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]