The Arabidopsis DHFR-TS (dihydrofolate reductase-thymidylate synthase) isoform THY3 operates as an inhibitor of its family members, thereby regulating folate and NADPH availability in cells.
Abstract
Folates (B9 vitamins) are essential cofactors in one-carbon metabolism. Since C1 transfer reactions are involved in synthesis of nucleic acids, proteins, lipids, and other biomolecules, as well as in epigenetic control, folates are vital for all living organisms. This work presents a complete study of a plant DHFR-TS (dihydrofolate reductase-thymidylate synthase) gene family that implements the penultimate step in folate biosynthesis. We demonstrate that one of the DHFR-TS isoforms (DHFR-TS3) operates as an inhibitor of its two homologs, thus regulating DHFR and TS activities and, as a consequence, folate abundance. In addition, a novel function of folate metabolism in plants is proposed, i.e., maintenance of the redox balance by contributing to NADPH production through the reaction catalyzed by methylenetetrahydrofolate dehydrogenase, thus allowing plants to cope with oxidative stress.
INTRODUCTION
Folates, also known as B9 vitamins, are vital for every living organism. The term “folate” is generic for tetrahydrofolate (THF) and its derivatives that participate in one-carbon (C1) transfer reactions, thereby being engaged in the synthesis of important biomolecules such as amino acids, nucleic acids, proteins, lipids, and pantothenate (vitamin B5). Furthermore, folates play a central role in the methyl cycle that supplies S-adenosylmethionine for methylation reactions (Scott et al., 2000; Blancquaert et al., 2010). While methanogenic and sulfate-reducing Archaea use tetrahydromethanopterin (H4MPT) and its derivative tetrahydrosarcinapterin (H4SPT) in their C1 metabolism, bacteria, eukaryotes, and other Archaea employ THF and its derivatives (van Beelen et al., 1984; Chistoserdova et al., 1998).
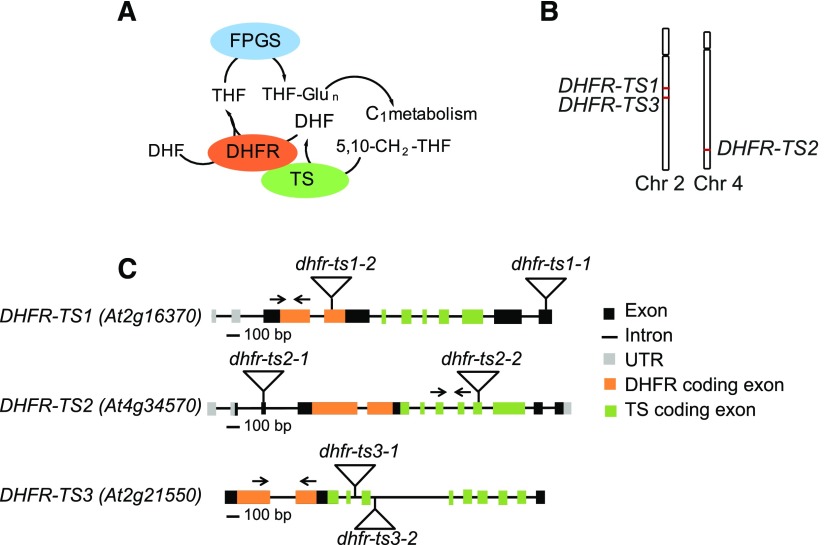
While animals fully depend on their dietary sources for the folate supply, bacteria, fungi, and plants can synthesize folates de novo. The THF molecule, assembled in mitochondria, is comprised of three moieties: a pterin ring, a para-aminobenzoic acid moiety, and a glutamate tail. In Arabidopsis thaliana, the penultimate step of THF synthesis is catalyzed by a bifunctional dihydrofolate reductase-thymidylate synthase (DHFR-TS). Apart from Arabidopsis, bifunctional DHFR-TS enzymes are present in some other plant species (Albani et al., 1985) as well as in protozoa (Ferone and Roland, 1980; Bachmann and Follmann, 1987). In such bifunctional enzymes, DHFR is located at the N terminus and catalyzes the conversion of dihydrofolate (DHF) into THF. TS is located at the C terminus and produces dTMP from dUMP, converting 5,10-methylene-THF into DHF. DHF is subsequently reduced into THF by the DHFR component of the bifunctional enzyme. Thus, bifunctional DHFR-TS enzymes couple folate with nucleotide biosynthesis (Figure 1A). Although animals cannot synthesize folates de novo, they are capable of reducing DHF into THF by the action of a monofunctional DHFR (Blancquaert et al., 2010). Due to the importance of DHFR and TS functions in cellular metabolism, one can anticipate a strict gene regulation. Expression of DHFR genes in animals is known to be regulated at the transcriptional level by repression of promoter activity by a noncoding interfering transcript (Martianov et al., 2007), at the posttranscriptional level by a miRNA binding to the 3′UTR (untranslated region) of DHFR transcript (Mishra et al., 2007), and at the translational level where DHFR protein regulates its synthesis by exerting an inhibitory influence on its translation (Ercikan et al., 1993). Mechanisms ruling DHFR expression in plants remain unreported.
Figure 1.
Organization of the Genes Encoding DHFR-TS Isoforms in Arabidopsis.
(A) Functions of bifunctional DHFR-TS.
(B) Chromosomal localization of DHFR-TS genes (red).
(C) Genomic organization of DHFR-TS genes. Triangles indicate the position of T-DNA insertions and arrows show positions of primers used for RT-qPCR.
The Arabidopsis genome encodes three DHFR-TS genes. On the one hand, the presence of the three genes might be attributed to genetic redundancy that is inherent to plant genomes. On the other hand, it might reflect an adaptation, evolving new elements that adjust plant metabolism more precisely in response to a changing environment. Finally, owing to the DHFR-TS involvement in nucleotide synthesis, one can consider possible localization of the proteins to subcellular compartments where DNA synthesis occurs, namely, the nucleus, mitochondria, and plastids. The nuclear localization of DHFR and TS proteins has previously been demonstrated in animal cells (Anderson et al., 2007; Woeller et al., 2007; Anderson and Stover, 2009), while the localization, other than mitochondrial, of their plant counterparts remains elusive. Although the biochemical processes wherein various folate derivatives take part are well known, our understanding of the physiological roles of folates and folate pathway enzymes in plants is far from being advanced. A recent study on Arabidopsis reported an interplay between folates and sucrose that modulates auxin signaling (Stokes et al., 2013). Seed reserve accumulation was also shown to be dependent on folate metabolism (Meng et al., 2014). Finally, several works illustrated a link between folate depletion and oxidative stress in animals (Cano et al., 2001; Huang et al., 2001; Ho et al., 2003; Dhitavat et al., 2005).
Generally, oxidative stress is caused by the excessive production of reactive oxygen species (ROS). The production of ROS is an unavoidable consequence of aerobic metabolism that can be enhanced by various environmental stresses. On the one hand, ROS pose a serious threat to plant cells by damaging a variety of biological molecules including DNA, proteins, and lipids. On the other hand, ROS function as important second messengers in various cellular processes. Therefore, a balance between ROS production and removal must be achieved and tightly controlled, to avoid oxidative damage while not completely eliminating ROS. A plant cell utilizes enzymatic and nonenzymatic scavenging systems to detoxify ROS (Foyer et al., 2009). GSH and ascorbate are the major cellular redox buffers that constitute the non-enzymatic antioxidative defense system.
GSH combats ROS in several ways. First, it interacts with ROS directly. Second, it protects macromolecules by the formation of adducts with reactive electrophiles or by acting as a hydride donor, yielding GSSG (Asada et al., 1994). Finally, it regenerates another important antioxidant, ascorbate, via the ascorbate-glutathione cycle (Loewus, 1999). The balance between reduced GSH and oxidized glutathione (GSSG) is tightly controlled and maintained through de novo GSH synthesis and recycling by glutathione reductase using NADPH. Besides being a glutathione reductant, NADPH is also utilized by enzymatic antioxidants known as thioredoxins. The major sources of NADPH production in plants are the photosynthetic electron transfer chain in plastids (Kramer and Evans, 2011) and the oxidative pentose phosphate pathway that takes place in the cytosol and in plastids (Kruger and von Schaewen, 2003). Mitochondria, as the actual (major) site of folate biosynthesis (Neuburger et al., 1996), contribute to a lesser extent to NADPH accumulation. NADP+ reducing functions are attributed to a number of mitochondrial enzymes (isocitrate dehydrogenase [EC 1.1.1.42], malic enzyme [EC 1.1.1.39], delta-pyrroline-5-carboxylate dehydrogenase [EC 1.5.1.12], glutamate dehydrogenase [EC 1.4.1.3], and methylenetetrahydrofolate dehydrogenase [EC 1.5.1.5]; Rasmusson and Møller, 1990; Møller and Rasmusson, 1998). The actual contribution of each of the mentioned sources to the total NADPH production in plants remains unclear to date.
We show that one Arabidopsis DHFR-TS isoform (DHFR-TS3) operates as an inhibitor of its family members DHFR-TS1 and DHFR-TS2, thereby regulating DHFR and TS activities and, as a consequence, folate availability in cells. In addition, we present evidence that the folate pathway contributes to NADPH production, thus maintaining cellular reducing power and protecting the plant against oxidative stress.
RESULTS
The Arabidopsis DHFR-TS Gene Family
The Arabidopsis genome contains three DHFR-TS genes (Figure 1C). Amino acid sequence alignments show a high degree of similarity between the three isoforms (Supplemental Figure 1 and Supplemental Table 1), with the TS domain being more conserved than the DHFR domain, as was previously reported (Lazar et al., 1993). DHFR-TS1 and DHFR-TS2 are located on chromosome 2 and chromosome 4, respectively (Figure 1B), within regions known to have undergone chromosome duplication events (Lee et al., 2013) (Supplemental Figure 2A). DHFR-TS3 is located on chromosome 2 (Figure 1B) and is most likely also a result of genome duplication (Supplemental Figure 2B). The DHFR-TS polypeptides slightly vary in size, with molecular masses for DHFR-TS1 and DHFR-TS3 of 55 and 57 kD, respectively, while being 63 kD for DHFR-TS2. The latter contains a putative transit peptide, hence the difference in molecular mass with DHFR-TS1 and DHFR-TS3.
Amino acid sequence comparison revealed that DHFR-TS1 and DHFR-TS2 share higher sequence identity than DHFR-TS3 with either of its homologs (Supplemental Table 1). The divergence of DHFR-TS3 from its family members is supported by the protein sequence analysis that revealed numerous substitutions in well conserved regions, including the folate and NADPH binding sites, as well as in the TS activity domain (Supplemental Figure 1).
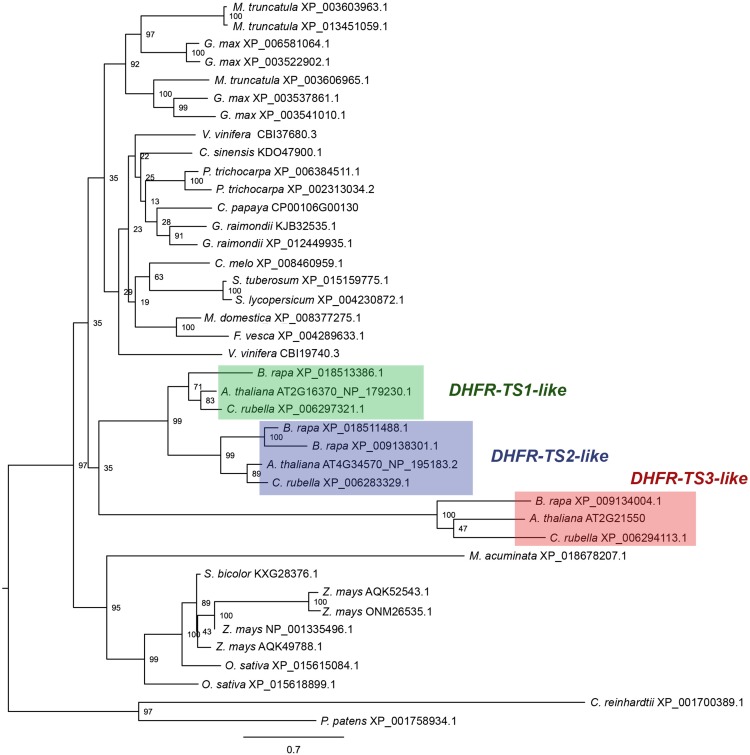
Phylogenetic analysis comparing known DHFR-TS protein sequences revealed that the number of DHFR-TS genes ranges from one to four per genome of higher plant species. Arabidopsis DHFR-TS isoforms fall into three different clades comprising putative orthologs from Brassica rapa and Capsella rubella. The analysis demonstrated that DHFR-TS1 and DHFR-TS2 fall into cognate clades, comprising putative orthologs from Brassicaceae. DHFR-TS3, on the other hand, represents a more divergent gene that is more closely related to putative orthologs from B. rapa and C. rubella than to the Arabidopsis DHFR-TS1 and DHFR-TS2 isoforms (Figure 2; Supplemental Data Set 1).
Figure 2.
Phylogenetic Analysis.
Amino acid sequence alignment was obtained using MUSCLE software (Edgar, 2004). The dendrogram was generated using RAxML (Stamatakis, 2014). The numbers at the branching points indicate the percentage of times that each branch topology was found during bootstrap analysis (n = 1000). Species names are followed by protein identifiers. The bar indicates the mean distance of 0.7 changes per amino acid residue.
The Arabidopsis DHFR-TS Isoforms Are Localized in Three Subcellular Compartments
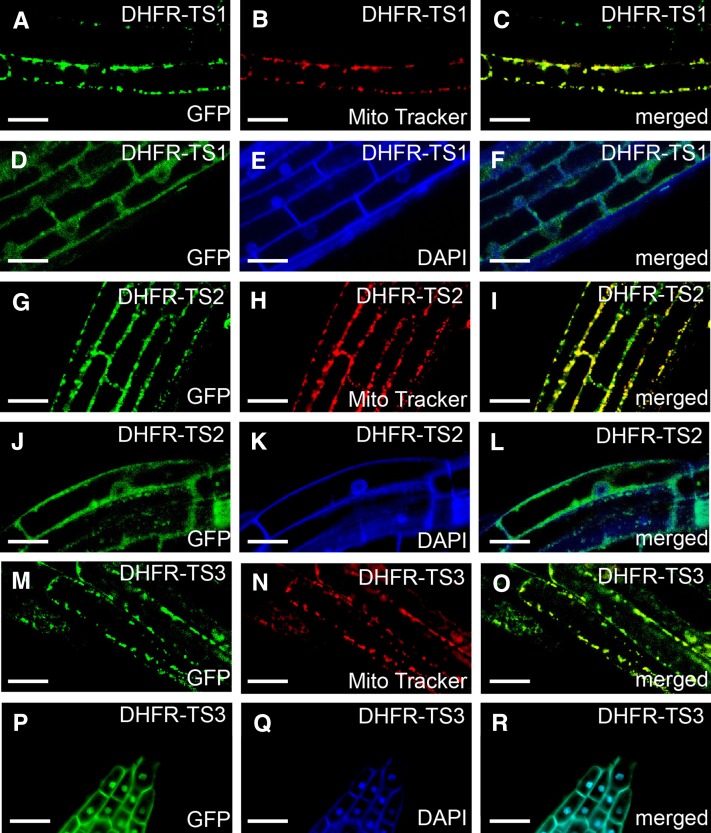
To discover potential functional differences of DHFR-TS isoforms, the subcellular localization of the DHFR-TS proteins was studied. To that end, three plant transformation vectors containing the full-length DHFR-TS coding sequences fused with GFP at the C-terminal end, driven by their native promoters, were constructed (ProDHFR-TS1:DHFR-TS1-GFP, ProDHFR-TS2:DHFR-TS2-GFP, and ProDHFR-TS3:DHFR-TS3-GFP). By means of confocal laser scanning microscopy, GFP fluorescence was observed in roots of 7-d-old ProDHFR-TS:DHFR-TS-GFP seedlings. The use of native promoters allowed detection of a root zone-specific localization of the DHFR-TS-GFP proteins (Figures 3A to 3R). In the root meristematic, transition, and elongation zones of ProDHFR-TS1:DHFR-TS1-GFP seedlings, the GFP signal was observed in both the cytosol and the nucleus, where fluorescence of the fusion protein colocalized with DAPI staining (Figures 3D to 3F). However, in the root transition and elongation zones, the DHFR-TS1-GFP fusion protein was also observed in mitochondria, as suggested by colocalization of the GFP signal with the mitochondrial probe MitoTracker Orange (Figures 3A to 3C). For the DHFR-TS2-GFP fusion, the GFP signal was found both in nuclei and in mitochondria of the meristematic and the transition zones and first cells in the elongation zone, while being confined to mitochondria further up in the elongation zone (Figures 3G to 3L). In roots of ProDHFR-TS3:DHFR-TS3-GFP seedlings, the GFP signal was mainly observed in the cytosol throughout the three developmental zones of the root. The GFP signal was only detected in the nuclei in cells of the lateral root cap (Figures 3P to 3R), while in the elongation zone, the DHFR-TS3-GFP protein was also detected in mitochondria (Figures 3M to 3O). The observed subcellular localization patterns of DHFR-TS proteins were confirmed by complementation of DHFR-TS loss-of-function mutants with the corresponding ProDHFR-TS:DHFR-TS-GFP constructs (Supplemental Figures 3 to 5).
Figure 3.
Subcellular Localization of DHFR-TS Isoforms in Roots of Stably Transformed ProDHFR-TS:DHFR-TS-GFP Arabidopsis Plants Using Confocal Laser Scanning Microscopy.
(A) to (F) DHFR-TS1 localization in the root elongation zone. (A) GFP fluorescence, (B) MitoTracker Orange fluorescence, (C) merged image, (D) GFP fluorescence, (E) DAPI fluorescence, and (F) merged image.
(G) to (L) DHFR-TS2 localization in the root elongation ([G] to [I]) and transition zone ([J] to [L]). (G) GFP fluorescence, (H) MitoTracker Orange fluorescence, (I) merged image, (J) GFP fluorescence, (K) DAPI fluorescence, and (L) merged image.
(M) to (O) DHFR-TS3 localization in the root elongation zone ([M] to [O]) and in the lateral root cap ([P] to [R]). (M) GFP fluorescence, (N) MitoTracker Orange fluorescence, (O) merged image, (P) GFP fluorescence, (Q) DAPI fluorescence, and (R) merged image.
These observations suggest that the DHFR-TS isoforms localize to three subcellular compartments: mitochondria, nucleus, and cytosol. In addition, the localization of the individual isoforms depends on the cell differentiation state, which is linked to the position of the cell along the root.
The Arabidopsis DHFR-TS Genes Are Differentially Expressed
To investigate the tissue-specific expression of the DHFR-TS genes, a histochemical GUS reporter assay was performed. Arabidopsis plants, stably transformed with vectors containing a DHFR-TS genomic sequence, including the promoter region and part of the coding region, followed by a GFP-GUS reporter (ProDHFR-TS1:DHFR-TS1-GFP-GUS, ProDHFR-TS2:DHFR-TS2-GFP-GUS, and ProDHFR-TS3:DHFR-TS3-GFP-GUS) were generated (Supplemental Figure 6). For each construct, five independent transformant lines were selected for GUS staining.
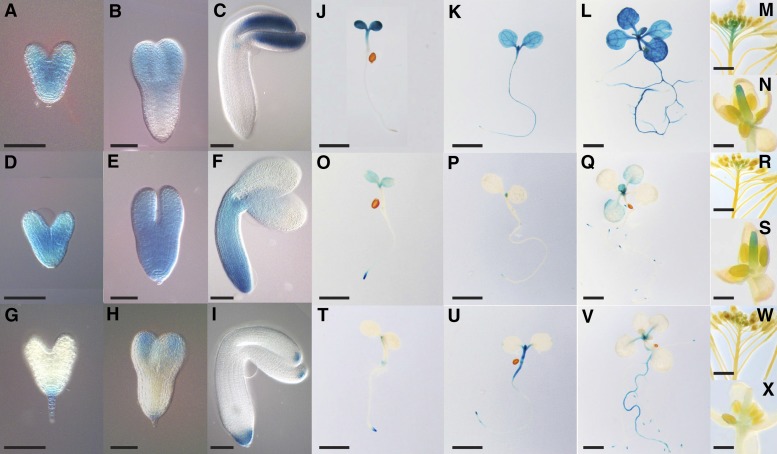
GUS activity of ProDHFR-TS1:DHFR-TS1-GFP-GUS plants was first detected in developing embryos. Strong ubiquitous GUS activity was observed at the heart stage (Figure 4A), while at the torpedo stage, it was mainly concentrated in the apical part of the embryo (Figure 4B). In the mature embryo, GUS activity was confined to the cotyledons and the shoot apex (Figure 4C). During postembryonic development, strong GUS activity appeared in the cotyledons, the apical part of the hypocotyl, and the shoot apex. While no activity was observed in the roots of 3-d-old seedlings (Figure 4J; Supplemental Figure 7), it appeared in the root tip at 5 d (Figure 4K; Supplemental Figure 7) and became ubiquitous by day 10 (Figure 4L; Supplemental Figure 7). Strong ubiquitous expression was observed in 4-week-old rosettes (Supplemental Figures 9A to 9C). In the root tip, expression was confined to the cortical and endodermal cell files (Supplemental Figure 8). During flower development, GUS activity was present in pistils (Figure 4N) and in valves of developing siliques (Figure 4M).
Figure 4.
Tissue- and Cell-Type-Specific Expression of DHFR-TS Genes in Arabidopsis.
(A) to (X) Histochemical GUS staining of ProDHFR-TS:DHFR-TS-GFP-GUS Arabidopsis lines.
(A) to (I) Expression of DHFR-TS genes during embryonic development. (A) to (C) DHFR-TS1 expression, (D) to (F) DHFR-TS2 expression, and (G) to (I) DHFR-TS3 expression. Bars = 50 µm.
(J) to (X) Expression of DHFR-TS genes during postembryonic development.
(J) to (L) DHFR-TS1 expression. (J) Three-day-old seedling, (K) 5-d-old seedling, (L) 10-d-old seedling, (M) inflorescence, and (N) flower. Bars = 2 mm in (J) to (L), 1 cm in (M), and 1 mm in (N).
(O) to (S) DHFR-TS2 expression. (O) Three-day-old seedling, (P) 5-d-old seedling, (Q) 10-d-old seedling, (R) inflorescence, and (S) flower. Bars = 2 mm in (O) to (Q), 1 cm in (R), and 1 mm in (S).
(T) to (X) DHFR-TS3 expression. (T) Three-day-old seedling, (U) 5-d-old seedling, (V) 10-d-old seedling, (W) inflorescence, and (X) flower. Bars = 2 mm in (T) to (V), 1 cm in (W), and 1 mm in (X).
DHFR-TS2 expression, revealed in ProDHFR-TS2:DHFR-TS2-GFP-GUS plants, was observed during early embryonic development, where it was ubiquitous at the heart (Figure 4D) and torpedo (Figure 4E) stages. In mature embryos, GUS activity became weaker and was restricted to the proximal parts of the cotyledons, shoot apex and the root (Figure 4F). During early postembryonic development, a weak GUS activity was observed in the cotyledons of 3-d-old seedlings, while a stronger signal could be observed in the shoot apex and the root tip (Figure 4O; Supplemental Figure 7). In the root tip, strong expression was found in the epidermis and the vasculature (Supplemental Figure 8). After 5 d, the signal disappeared from the cotyledons but was present in the shoot apex and the primary root tip, as well as emerging lateral roots (Figure 4P; Supplemental Figure 7). In 10-d-old seedlings, GUS activity was detectable in the first true leaf pair (Figure 4Q; Supplemental Figure 7), while being fairly strong and ubiquitous in rosettes of 4-week-old plants (Supplemental Figures 9D to 9F). During flower development, expression of DHFR-TS2-GFP-GUS was restricted to pistils (Figures 4R and 4S).
In ProDHFR-TS3:DHFR-TS3-GFP-GUS plants, DHFR-TS3 gene expression, though weak, was detectable in developing embryos, where it was restricted to the suspensor, the distal parts of the cotyledons and the root tip (Figures 4G to 4I). In 3-d-old seedlings, GUS activity was observed in the shoot apex, the root tip, and the collet region (Figure 4T; Supplemental Figure 7). In the root tip, DHFR-TS3 expression was confined to the lateral root cap and the columella (Supplemental Figure 8). After 5 d, the GUS signal appeared in the hypocotyl and the upper part of the root (Figure 4U; Supplemental Figure 7), while in 10-d-old seedlings, GUS activity was present throughout the primary root (Figure 4V; Supplemental Figure 7). In rosettes of 4-week-old plants, GUS activity was confined to vasculature and hydathodes (Supplemental Figures 9G to 9J). In inflorescences, expression was detected in pistils, although it was fairly weak (Figures 4W and 4X).
In summary, the above-mentioned results indicate that DHFR-TS1 is ubiquitously expressed throughout plant development. By contrast, the two other isoforms show more specific expression patterns: DHFR-TS2 is confined to the meristematic zones in the root and shoot, while DHFR-TS3 is mainly expressed in differentiated cells.
The Arabidopsis DHFR-TS Family Exhibits Functional Redundancy
To study the in vivo function of the individual DHFR-TS genes, we isolated T-DNA knockout mutants of three genes: dhfr-ts1-1 (GK_010G06), dhfr-ts1-2 (GT10472), dhfr-ts2-1 (SALK_016377), dhfr-ts2-2 (GK893CO2), dhfr-ts3-1 (SALK_114609), and dhfr-ts3-2 (SALK129194) (Figure 1C). PCR-based genotyping was employed to isolate plants that are homozygous for the mutant alleles. Real-time quantitative PCR analysis confirmed that each line was a loss-of-function mutant, lacking the corresponding DHFR-TS transcript (Supplemental Figures 10A to 10F).
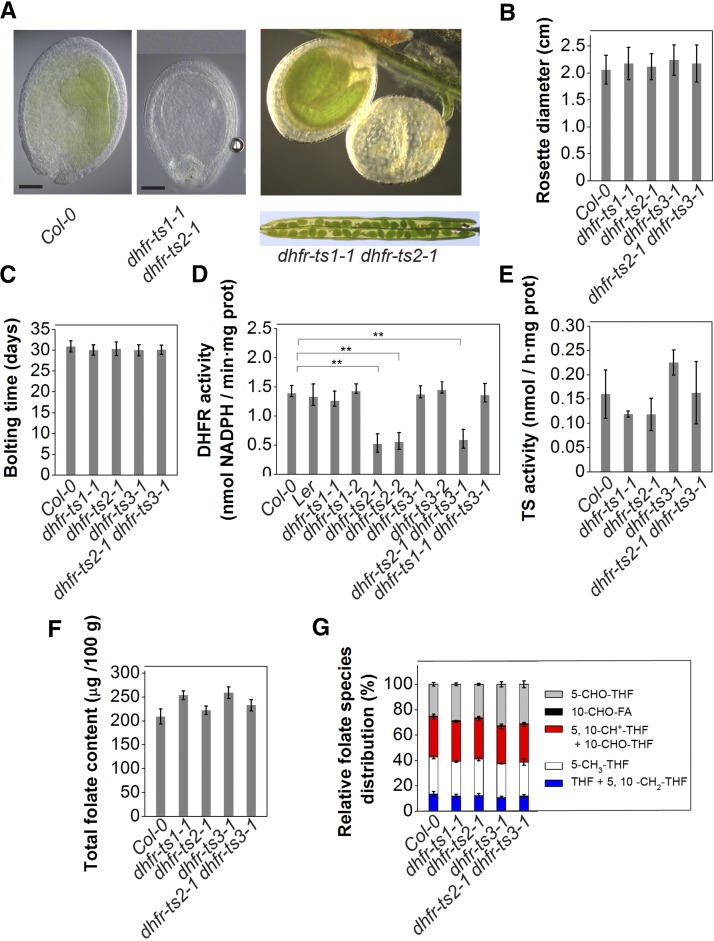
Single mutants did not show a phenotype distinct from that of wild-type plants under normal conditions (Supplemental Figure 11), suggesting functional redundancy among the DHFR-TS gene family members. To further investigate specific functions for each isoform, dhfr-ts double mutants were constructed (in all possible combinations). Mutants for DHFR-TS1, DHFR-TS2, and DHFR-TS3 were crossed pairwise to obtain heterozygous dhfr-ts1-1 dhfr-ts2-1 (+ −+ −), dhfr-ts1-1 dhfr-ts3-1 (+ −/+ −), and dhfr-ts2-1 dhfr-ts3-1 (+ −/+ −) double mutants, which were selfed. Using PCR-based genotyping of 150 individuals from each progeny, dhfr-ts1-1 dhfr-ts3-1 and dhfr-ts2-1 dhfr-ts3-1 homozygous double loss-of-function mutants could be obtained. For the dhfr-ts1-1 dhfr-ts2-1 combination, only plants homozygous for one loss-of-function allele and heterozygous for the other could be obtained (+ −/− − and − −/+ −) (Supplemental Figure 12), pointing to the sufficiency of a single functional allele of either DHFR-TS1 or DHFR-TS2 to assure normal growth and development and the inability of DHFR-TS3 to support development without its family members. The developing siliques of the dhfr-ts1-1 dhfr-ts2-1 double mutant contained aberrant seeds, appearing as albinos (Figure 5A) at a frequency that was not significantly different from the predicted 3:1 ratio (n = 162, χ2 = 0,403).
Figure 5.
Functional Characterization of Single and Double Loss-of-Function dhfr-ts Mutants.
(A) Combined loss of DHFR-TS1 and DHFR-TS2 (+ − / − −) (upper panel) activity results in embryo lethality. Dissection of dhfr-ts1-1 dhfr-ts2-1 (+ − /− −) silique revealing aborted seeds (lower panel).
(B) and (C) Rosette diameter (B) and bolting time (C) for wild type, dhfr-ts1-1, dhfr-ts2-1, dhfr-ts3-1, and dhfr-ts2-1 dhfr-ts3-1 of 2- and 4-week-old plants, respectively. Data are mean ± sd of at least 15 measurements.
(D) DHFR activity in 3-d-old dhfr-ts1-1, dhfr-ts1-2 (Ler background), dhfr-ts2-1, dhfr-ts2-2, dhfr-ts3-1, dhfr-ts3-2, dhfr-ts2-1 dhfr-ts3-1, dhfr-ts1-1 dhfr-ts3-1, and wild-type seedlings (Col-0 and Ler). DHFR activities are representative data from two independent experiments (mean of three biological replicates ± sd, each comprising >500 seedlings). DHFR activity was determined as the consumption of NADPH measured as the decrease of absorbance at 340 nm. Asterisks indicate significance by Student’s t test (*P value < 0.05, **P value < 0.01, and ***P value < 0.001).
(E) TS activity in 3-d-old dhfr-ts1-1, dhfr-ts2-1, dhfr-ts3-1, dhfr-ts2-1 dhfr-ts3-1, and wild-type seedlings. Data are mean of three biological replicates ± sd (each comprising >500 seedlings).
(F) and (G) Total folate content (F) and folate species distribution (G) in 3-d-old dhfr-ts1-1, dhfr-ts2-1, dhfr-ts3-1, dhfr-ts2-1 dhfr-ts3-1, and wild-type seedlings. Data are mean of three biological replicates ± sd (each comprising >500 seedlings).
Neither single nor double dhfr-ts loss-of-function mutants exhibited a morphological alteration of the rosette diameter (Figure 5B; Supplemental Figures 13B and 13E), the root length (Supplemental Figures 13A, 13D, and 13G) or bolting time (Figure 5C; Supplemental Figures 13C and 13F) compared with the wild type. Total DHFR and TS activities were investigated in whole 3-d-old seedlings. TS activity profiling of 3-d-old seedlings did not reveal any difference between wild-type plants and single loss-of-function mutants (Figure 5E). By contrast, DHFR activity measurements demonstrated that dhfr-ts2-1, dhfr-ts2-2, and dhfr-ts2-1 dhfr-ts3-1 loss-of-function seedlings exhibited a decrease in DHFR activity compared with the wild type (Figure 5D). To investigate whether the lowered DHFR activity in the loss-of-function mutant seedlings had an effect on folate levels, folate analysis was performed on 3-d-old seedlings. Neither total folate levels nor the distribution of the different folate species was altered in these mutants compared with those of wild-type plants (Figures 5F and 5G), underlining functional overlap among the DHFR-TS isoforms.
Altogether, these results demonstrate a high degree of functional redundancy within the Arabidopsis DHFR-TS family, while suggesting an important role for DHFR-TS1 and DHFR-TS2 during embryogenesis and in overall development and for DHFR-TS2 in maintaining high DHFR activity during seedling development.
DHFR-TS3 Overexpressors Have Reduced Folate Levels and a Delay in Development
To gain further insight into the in vivo role of the DHFR-TS genes, the effect of DHFR-TS overexpression was studied. Therefore, Arabidopsis plants were stably transformed with vectors in which the DHFR-TS coding sequences were driven by the constitutive 35S promoter (Pro35S:DHFR-TS1, Pro35S:DHFR-TS2, and Pro35S:DHFR-TS3). Plants of the F4 generation of homozygous single T-DNA insertion lines, showing overexpression of the respective DHFR-TS isoforms, were used (Supplemental Figures 14A to 14D).
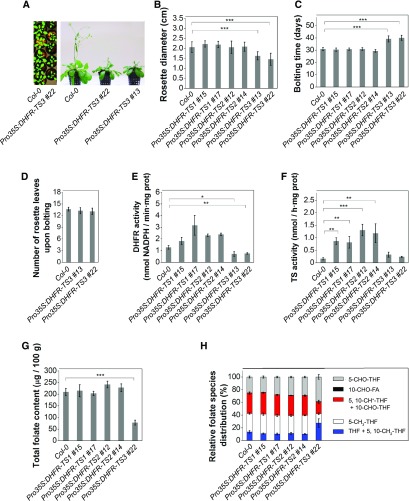
Morphological analysis revealed that while DHFR-TS1 and DHFR-TS2 overexpressors showed no phenotypic difference from wild-type plants in the morphological traits studied (rosette diameter [Figure 6B], root length [Supplemental Figure 13H], and bolting time [Figure 6C]), DHFR-TS3 overexpressors exhibited a retardation in development (Figure 6A). DHFR-TS3 overexpressors had smaller rosettes at day 12 after germination and exhibited delayed bolting (Figure 6C), although having the same number of rosette leaves upon bolting compared with wild-type plants (Figure 6D). The delay in development was the only observed difference between DHFR-TS3 overexpressors and wild-type plants.
Figure 6.
Functional Characterization of DHFR-TS Gain-of-Function Lines.
(A) Pro35S:DHFR-TS3 demonstrate a delay in development, 3-week-old (left) and 5-week-old (right) plants.
(B) Rosette diameter of 2-week-old plants. Data are mean of at least 15 measurements ± sd.
(C) and (D) Bolting time of the wild type, Pro35S:DHFR-TS1, Pro35S:DHFR-TS2, and Pro35S:DHFR-TS3 gain-of-function lines (C) and number of leaves upon bolting in wild-type and Pro35S:DHFR-TS3 gain-of-function plants (D). Data are mean of at least 15 measurements ± sd.
(E) DHFR activity in 3-d-old Pro35S:DHFR-TS1, Pro35S:DHFR-TS2, and Pro35S:DHFR-TS3 gain-of-function and wild-type seedlings. DHFR activities are representative data from two independent experiments (mean of three biological replicates ± sd, n > 500 seedlings). DHFR activity was determined as the consumption of NADPH measured as the decrease of absorbance at 340 nm.
(F) TS activity in 3-d-old Pro35S:DHFR-TS1, Pro35S:DHFR-TS2, and Pro35S:DHFR-TS3 gain-of-function and wild-type seedlings. Data are mean of three biological replicates ± sd (n > 500 seedlings).
(G) and (H) Total folate content (G) and folate species distribution (H) in 3-d-old Pro35S:DHFR-TS1, Pro35S:DHFR-TS2, and Pro35S:DHFR-TS3 gain-of-function and wild-type seedlings. Data are mean of three biological replicates ± sd (n > 500 seedlings).
Asterisks indicate significant differences with the wild type, as assessed by Student’s t test (*P value < 0.05, **P value < 0.01, and ***P value < 0.001).
To determine whether the delay in development was caused by alterations in DHFR or TS activities, enzyme activity assays were conducted on 3-d-old seedlings. While DHFR-TS1 and DHFR-TS2 overexpressors had elevated DHFR and TS activities, DHFR-TS3 overexpressors did not exhibit an increase in DHFR and TS activities; moreover, their DHFR activity was significantly lower than that of the wild type (Figures 6E and 6F).
To address whether the developmental delay was accompanied by alterations in total folate level or in folate species distribution, liquid chromatography-tandem mass spectrometry folate profiling was conducted on 3-d-old seedlings. DHFR-TS1 and DHFR-TS2 overexpressors demonstrated a total folate abundance and folate species distribution comparable to those of wild-type seedlings. By contrast, DHFR-TS3 overexpressors exhibited lower total folate levels (Figure 6G; Supplemental Figure 15A), a decrease in the 5-methyl-THF and the combined 5,10-methenyl-THF and 10-formyl-THF fractions, and an increase in the combined THF and 5,10-methylene-THF fraction (Figure 6H; Supplemental Figure 15B).
These data indicate that DHFR-TS3 overexpression results in a developmental delay and reduced growth, accompanied by a decrease in DHFR activity and total folate content, and alterations in the distribution of different folate species, suggesting an inhibitory role of DHFR-TS3 on the other DHFR-TS isoforms.
DHFR-TS3 Does Not Show DHFR and TS Enzymatic Activities
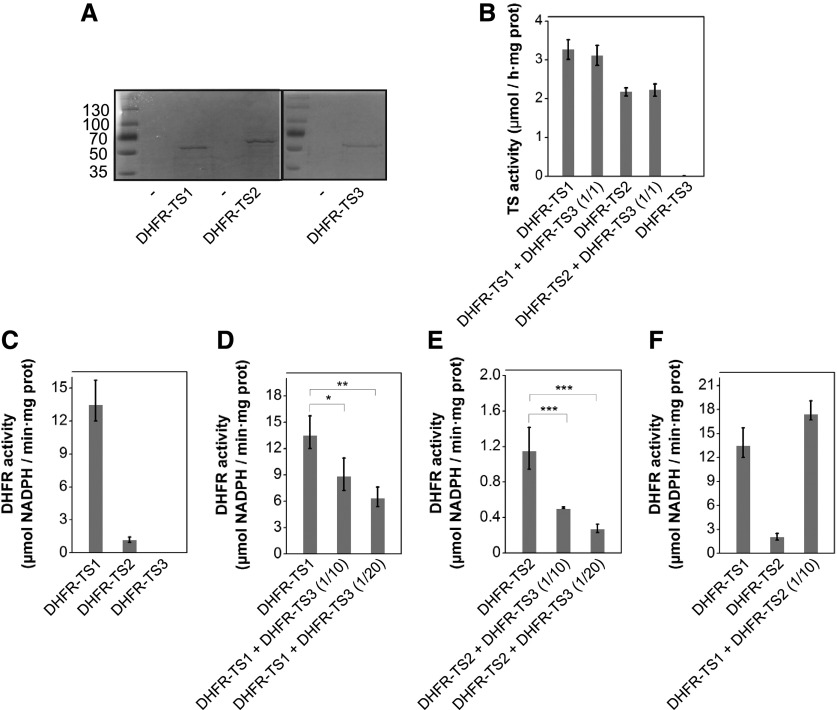
To further elucidate the specific roles of the DHFR-TS isoforms, DHFR and TS activities were investigated on purified DHFR-TS proteins. To do so, the DHFR-TS isoforms were expressed in Escherichia coli and purified via an N-terminal 6xHis tag (Figure 7A). First, DHFR and TS activities of individual DHFR-TS recombinant proteins were measured. DHFR activity could not be detected in the DHFR-TS3 purified fraction, while DHFR-TS1 and DHFR-TS2 preparations demonstrated detectable DHFR activity levels, with the DHFR activity of DHFR-TS1 being considerably higher than that of DHFR-TS2 (Figure 7C; Supplemental Figure 16A). Similar results were obtained for TS activity: In the DHFR-TS3 purified protein fraction, TS activity was below the limit of detection, while DHFR-TS1 and DHFR-TS2 showed high TS activities, again with a conspicuously higher TS activity for DHFR-TS1 (Figure 7B).
Figure 7.
Functional Analysis of DHFR-TS Isoforms in Vitro.
(A) SDS PAGE of Ni-NTA-purified recombinant 6xHis-DHFR-TS1, -DHFR-TS2, and -DHFR-TS3 proteins heterologously expressed in E. coli.
(B) TS activities of Ni-NTA-purified DHFR-TS1, DHFR-TS2, and DHFR-TS3 protein fractions and their mixtures (DHFR-TS1 + DHFR-TS3 and DHFR-TS2 + DHFR-TS3) with indicated protein molar ratios. TS activities (mean ± sd) are representative data from two independent experiments; each protein preparation was assayed at least three times with three technical replicates.
(C) to (F) DHFR activities of Ni-NTA-purified DHFR-TS1, DHFR-TS2, and DHFR-TS3 proteins. Molar ratios of protein mixtures are indicated in brackets. DHFR activity was determined as the consumption of NADPH measured as the decrease of absorbance at 340 nm. DHFR activities (mean ± sd) are representative data from two independent experiments; each experiment included three biological and three technical replicates. An amount of storage buffer equal to the DHFR-TS3 protein volume used was added to individual DHFR-TS1 and DHFR-TS2 protein fractions. Asterisks indicate significance by Student’s t test (*P value < 0.05, **P value < 0.01, and ***P value < 0.001).
(C) DHFR activity of equimolar amounts of DHFR-TS1, DHFR-TS2, and DHFR-TS3 purified proteins.
(D) Inhibition of DHFR activity of DHFR-TS1 by addition of DHFR-TS3.
(E) Inhibition of DHFR activity of DHFR-TS2 by addition of DHFR-TS3.
(F) DHFR activity of DHFR-TS1, DHFR-TS2, and their mixture.
To address whether DHFR-TS3 plays an inhibitory role on the other DHFR-TS proteins, DHFR and TS activities were measured in mixtures of DHFR-TS recombinant proteins DHFR-TS1 + DHFR-TS3 and DHFR-TS2 + DHFR-TS3 and subsequently compared with the individual DHFR and TS activities of DHFR-TS1 and DHFR-TS2. The TS activity assay did not reveal any difference between individual DHFR-TS1 and DHFR-TS2 protein fractions and those mixed with DHFR-TS3 in equimolar ratios (Figure 7B). By contrast, DHFR activities of DHFR-TS1 and DHFR-TS2 protein fractions were significantly lowered by the addition of an excess of DHFR-TS3 protein (1/10 and 1/20 ratios for DHFR-TS1/DHFR-TS3 and DHFR-TS2/DHFR-TS3 were tested) (Figures 7D and 7E; Supplemental Figures 16B and 16C). DHFR activity of the DHFR-TS1/DHFR-TS2 mixture at 1/10 ratio did not significantly differ from the measured individual DHFR-TS1 DHFR activity (Figure 7F; Supplemental Figure 16D). The possibility of inhibition of DHFR-TS1 and DHFR-TS2 DHFR activities by buffer components was excluded (Figures 7C to 7F; Supplemental Figures 16A to 16D). Since the abundance of DHFR-TS1 and DHFR-TS2 transcripts was not altered in the DHFR-TS3-OE background (Supplemental Figures 17A and 17B), we could also eliminate the possibility of inhibition of DHFR-TS1 or DHFR-TS2 (or both) by DHFR-TS3 at the RNA level.
In summary, these results suggest that DHFR-TS3 inhibits DHFR activity of its homologs at the protein level.
DHFR-TS3 Overexpression Increases Sensitivity to Hydroxyurea
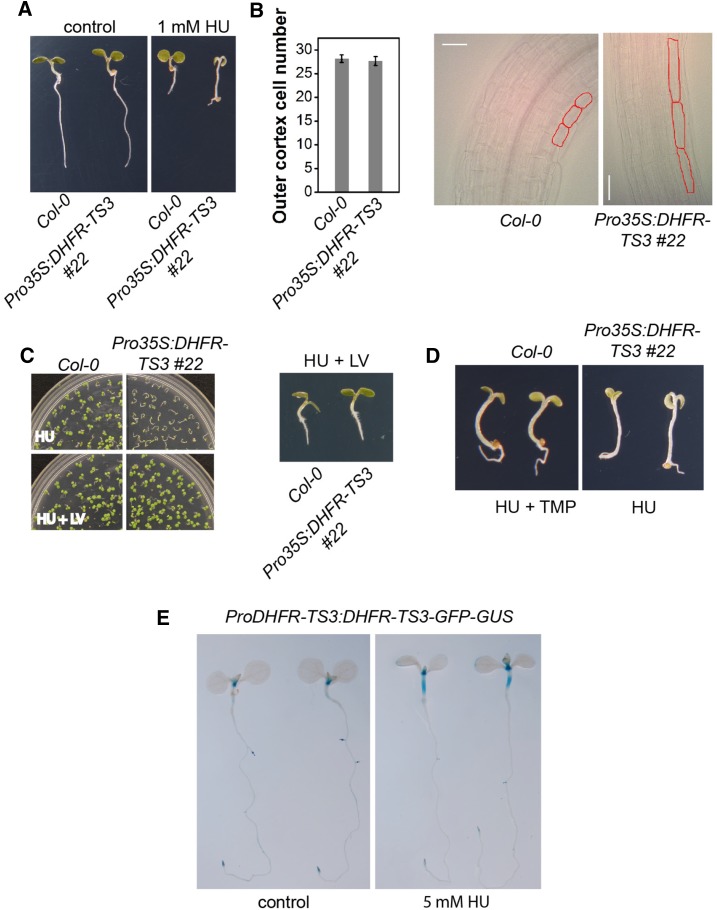
Since DHFR-TS3 overexpression caused a significant decrease in both DHFR activity and total folate levels, it was first studied whether the overexpression was associated with alterations in stress responses. Based on the notion that maintaining dNTP synthesis is important for survival upon DNA damage (Elledge et al., 1992; Desany et al., 1998) and on the assumption that Arabidopsis nuclear-localized DHFR-TS proteins (this study) might, like their monofunctional animal counterparts, take part in the formation of the replitase complex, which integrates dNTP synthesis and DNA replication (Prem veer Reddy and Pardee, 1980), we investigated whether DHFR-TS3 overexpression alters a genotoxic stress response. Therefore, DHFR-TS3 overexpressing seedlings (Pro35S:DHFR-TS3) were grown on media supplemented with 1 mM hydroxyurea (HU), which is known to impose replication stress (Wang and Liu, 2006; Saban and Bujak, 2009). Wild-type seedlings grown on HU-containing medium exhibited smaller cotyledons, shorter roots, and shorter hypocotyls than those grown on control medium (Figure 8A). These morphological features are indicative of cessation of the mitotic cell cycle through the initiation of DNA damage-induced cell cycle checkpoints triggered by depletion of the dNTP pool and DNA damage (Melo and Toczyski, 2002; Culligan et al., 2004). Unlike the other tested genotypes, Pro35S:DHFR-TS3 exhibited a hypersensitive phenotype upon HU supplementation (Figure 8A; Supplemental Figure 18C). Although the sizes of Pro35S:DHFR-TS3 cotyledons and roots were significantly reduced, the hypocotyls were much longer than those of wild-type seedlings grown on the same medium. Examination of the Pro35S:DHFR-TS3 hypocotyls with differential interference contrast (DIC) microscopy revealed a strong elongation of hypocotyl cells (Figure 8B). To test whether cell division contributed to the increase in hypocotyl length, the number of hypocotyl outer cortical cells of 4-d-old wild-type and Pro35S:DHFR-TS3 seedlings was counted after growth on medium supplemented with 1 mM HU. The number of cells in Pro35S:DHFR-TS3 did not increase upon HU supplementation compared with wild-type seedlings (Figure 8B). A strong correlation between cell size and ploidy was substantiated by numerous studies (Satina and Blakeslee, 1941; Melaragno et al., 1993; Gendreau et al., 1998). To test whether the elongation of hypocotyl cells of Pro35S:DHFR-TS3 seedlings grown on 1 mM HU was accompanied by an increase in ploidy level, a ploidy distribution analysis was conducted. The analysis revealed no difference between DHFR-TS3-overexpressing seedlings and the wild type (Supplemental Figures 19A to 19D).
Figure 8.
Pro35S:DHFR-TS3 Gain-of-Function Plants Show Elevated Sensitivity to HU and TMP.
(A) Representative phenotypes of wild-type and Pro35S:DHFR-TS3 seedlings grown for 4 d on control and 1 mM HU-containing media.
(B) DIC microscopy images of 4-d-old Pro35S:DHFR-TS3 and wild-type seedlings grown on 1 mM HU; outer cortex cells are outlined in red. The graph represents the number of outer cortex cells of wild-type and Pro35S:DHFR-TS3 4-d-old seedlings grown on 1 mM HU. Data are mean of at least 20 measurements ± sd.
(C) Administration of 500 µM folinic acid (LV) restores wild-type phenotype on HU. Upper panel: Wild-type and Pro35S:DHFR-TS3 grown on 1 mM HU (upper picture) and 1 mM HU + 500 µM folinic acid (lower picture) containing media for 6 d. Lower panel: Representative phenotypes of wild-type and Pro35S:DHFR-TS3 seedlings grown for 6 d on medium containing 1 mM HU and 500 µM folinic acid.
(D) Comparison of representative phenotypes of 4-d-old wild-type and Pro35S:DHFR-TS3 seedlings grown on 1 mM HU + 30 µM TMP and 1 mM HU containing media, respectively.
(E) GUS activity in ProDHFR-TS3:DHFR-TS3-GFP-GUS seedlings grown on MS/2 for 7 d and transferred to control (left) or 5 mM HU containing liquid MS/2 (right) for 5 h.
Since DHFR-TS3 overexpression affected folate levels, it was hypothesized that folate depletion could cause the observed HU hypersensitivity. To test this, we investigated whether supplementation with folinic acid (leucovorin [LV]), a folate derivative (5-formyl-THF), could rescue Pro35S:DHFR-TS3 seedlings treated with HU. When wild-type and Pro35S:DHFR-TS3 seedlings were grown on medium supplemented with both 1 mM HU and 500 µM folinic acid (LV), the Pro35S:DHFR-TS3 hypersensitive phenotype could indeed be rescued (Figure 8C). To support the assumption that a lack of DHFR activity could cause the observed Pro35S:DHFR-TS3 phenotype, wild-type plants were grown on medium supplemented with 1 mM HU and 30 µM trimethoprim (TMP). The latter acts as an inhibitor of DHFR activity, consequently leading to a decrease in folate abundance (Bushby and Hitchings, 1968; Assaraf, 2007). Wild-type plants subjected to both replication stress (HU) and to the DHFR inhibitor (TMP) displayed the same phenotypical traits as Pro35S:DHFR-TS3 seedlings grown on medium with HU alone (Figure 8D). To determine whether DHFR-TS3 is expressed in the hypocotyl upon HU treatment, 5 mM HU was administered to 7-d-old ProDHFR-TS3:DHFR-TS3-GFP-GUS seedlings for 5 h. Indeed, the DHFR-TS3 promoter was activated in the hypocotyl upon HU supplementation (Figure 8E). The same treatment did not affect the activity of DHFR-TS1 and DHFR-TS2 promoters (Supplemental Figure 20).
Altogether, these results demonstrate that the HU hypersensitivity of Pro35S:DHFR-TS3 plants is caused by the lowered DHFR activity and decreased folate abundance, suggesting an important role for folate metabolism in response to stress imposed by HU.
DHFR-TS3 Gain-of-Function Plants Display Elevated ROS Abundance
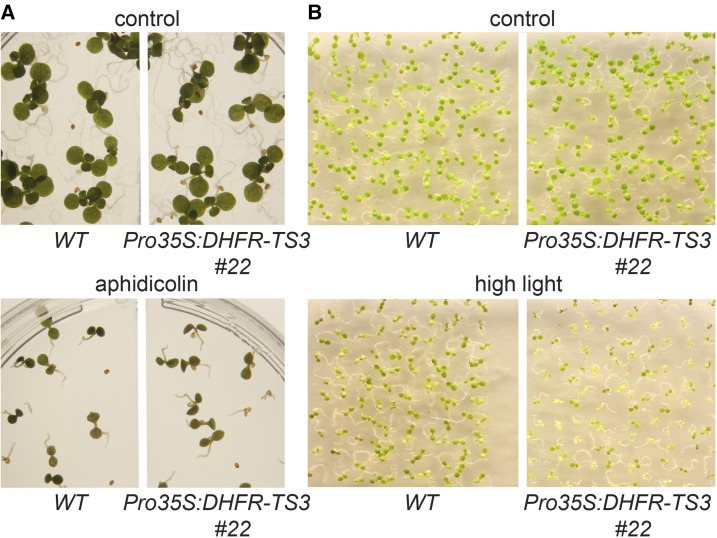
Besides being an inhibitor of the replication fork progression, HU is also known to cause oxidative stress due to its inhibitory effect on ROS detoxifying enzymes (Chen and Asada, 1990; Juul et al., 2010; Yi et al., 2014a). To further understand the HU-dependent hypersensitivity of Pro35S:DHFR-TS3 plants, the growth of Pro35S:DHFR-TS3 seedlings was compared with that of the wild type upon exposure to the double strand break inducer aphidicolin and to high-light stress. Aphidicolin was reported to induce replication stress by inhibiting DNA polymerase (Sala et al., 1980), while high-light stress is known to enhance ROS production and cause oxidative damage (Niyogi, 1999). Pro35S:DHFR-TS3 plants did not differ from the wild type in their sensitivity to aphidicolin (Figure 9A), while being more vulnerable to high-light stress (Figure 9B).
Figure 9.
Sensitivity of Pro35S:DHFR-TS3 and Wild-Type Seedlings to Aphidicolin and High-Light Treatment.
(A) Seedlings were grown for 5 d on control MS/2 medium and MS/2 medium supplemented with 10 µg/mL aphidicolin.
(B) Seedlings were subjected to high-light treatment (1100 µmol m−2 s−1) for 8 h per day, 5 d after germination.
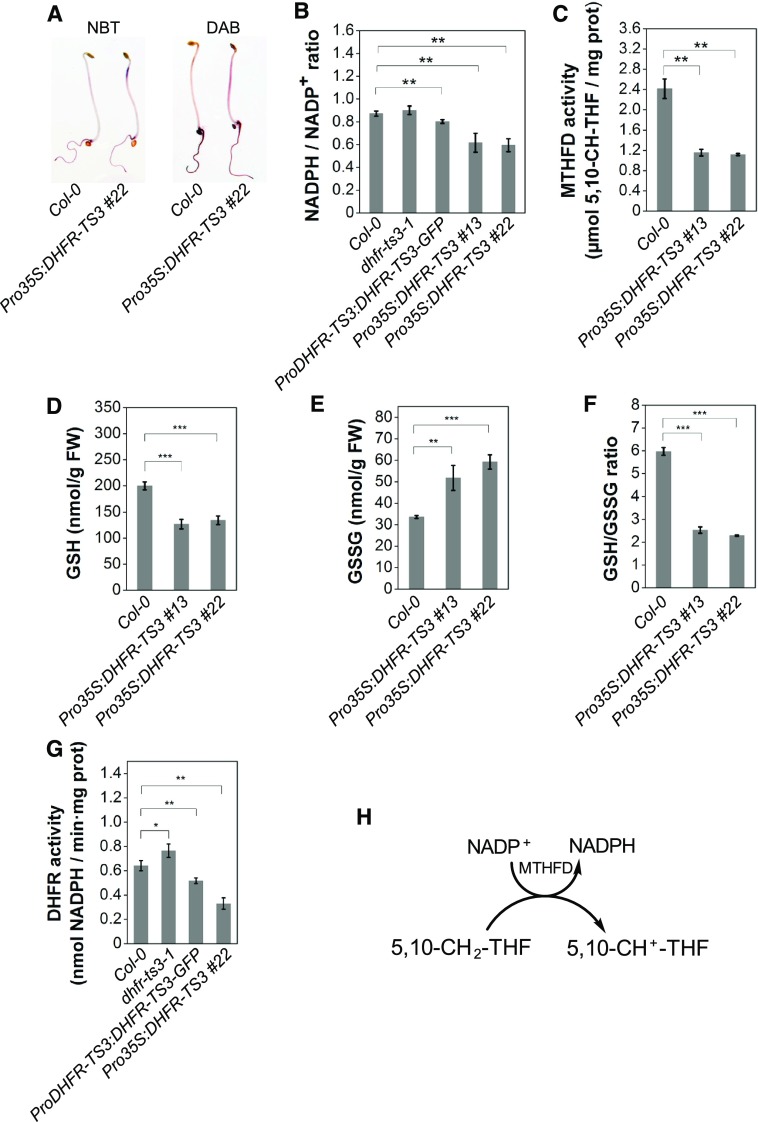
To elucidate the sensitivity of Pro35S:DHFR-TS3 plants to oxidative stress, the level of two ROS was visualized in 3-d-old dark-grown seedlings by diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) staining procedures that detect hydrogen peroxide (H2O2) and superoxide radicals (O2·−), respectively. Due to their lower staining intensity, allowing detection of subtle differences in ROS abundance, dark-grown seedlings were chosen over light-grown ones. Seedlings were grown in darkness on both HU-containing and control media. As shown in Supplemental Figure 21C, Pro35S:DHFR-TS3 seedlings grown on HU exhibited higher H2O2 and O2·− contents than wild-type seedlings. Slightly higher levels of both ROS were also detected in Pro35S:DHFR-TS3 seedlings, compared with those of the wild type on control medium (Figure 10A; Supplemental Figures 21A and 21B).
Figure 10.
Pro35S:DHFR-TS3 Gain-of-Function Plants Exhibit Elevated Vulnerability to Oxidative Stress.
(A) NBT and DAB staining of Pro35S:DHFR-TS3 and wild-type etiolated seedlings grown on control and 1 mM HU-containing media for 2 and 3 d, respectively.
(B) Measurement of the NADPH/NADP+ ratio in roots of 10-d-old Pro35S:DHFR-TS3 and wild-type seedlings. The NADPH/NADP+ ratio is the mean ± sd of ratios from four independent experiments. Every experiment included three biological replicates for each line (n > 150 seedlings).
(C) Measurement of MTHFD activity of 3-d-old Pro35S:DHFR-TS3 and wild-type seedlings. MTHFD activities are representative data from three independent experiments. Every experiment included three biological replicates for each line (n > 500 seedlings).
(D) to (F) From left to right: Measurement of GSH (D) and GSSG (E) content, and GSH/GSSG ratio (F) of 3-d-old seedlings. GSH and GSSG contents (mean ± sd) are representative data from three independent experiments. Every experiment included three biological replicates for each line (n > 500 seedlings). The GSH/GSSG ratio was calculated for each independent experiment and the presented ratio is the mean of these ratios ± sd.
(G) DHFR activity in roots of 10-d-old dhfr-ts3-1, ProDHFR-TS3:DHFR-TS3-GFP, Pro35S:DHFR-TS3 #22, and wild-type seedlings. DHFR activities are representative data from two independent experiments (mean of three biological replicates ± sd, each comprising >500 seedlings). Asterisks indicate significance by Student’s t test (*P value < 0.05, **P value < 0.01, and ***P value < 0.001).
(H) The production of NADPH resulting from the conversion of 5,10-methylene-THF into 5,10-methenyl-THF catalyzed by MTHFD.
These data indicate that the hypersensitivity of Pro35S:DHFR-TS3 plants to HU was caused by oxidative damage resulting from high ROS accumulation, rather than by replication stress.
DHFR-TS3 Negatively Affects Reducing Power
The balance between NADPH and NADP+ is considered to reflect the redox state of a cell (Rasmusson and Møller, 1990; Møller and Rasmusson, 1998). In animal cells, the folate metabolic pathway was previously reported to participate in defense against oxidative stress by contributing to NADPH production, via the conversion of 5,10-methylene-THF to 5,10-methenyl-THF catalyzed by 5,10-methylene-THF dehydrogenase (MTHFD) (Figure 10H) (Fan et al., 2014). To determine whether DHFR-TS3 overexpression could affect the redox balance by interfering with NADPH production, the NADPH/NADP+ ratio of Pro35S:DHFR-TS3 (strong overexpressors; Supplemental Figure 14D) and ProDHFR-TS3:DHFR-TS3-GFP (weak overexpressors; Supplemental Figure 14D) plants was determined and compared with that of the wild type. As demonstrated in Figure 10B, the NADPH/NADP+ ratio was decreased in Pro35S:DHFR-TS3 and, though to a lesser extent, in ProDHFR-TS3:DHFR-TS3-GFP plants compared with the wild type. In addition, analysis of folate species distribution suggested that the conversion of 5,10-methylene THF to 5,10-methenyl-THF was considerably hampered in DHFR-TS3-overexpressing plants (Figure 6H; Supplemental Figure 15B). To test whether an alteration of MTHFD activity could account for the observed impairment, MTHFD activity of Pro35S:DHFR-TS3 plants was measured and compared with that of the wild type. As expected, MTHFD activity of DHFR-TS3 overexpressing plants was significantly lower than that of wild-type plants (Figure 10C; Supplemental Figures 22A and 22B).
NADPH is used in the reduction of one of the most important ROS scavengers, glutathione. This reduction is catalyzed by glutathione reductase that converts oxidized dimeric glutathione (GSSG) into its reduced form (GSH). A decrease of NADPH abundance was shown to negatively affect the GSSG to GSH conversion and consequently lower the GSH:GSSG ratio (Fan et al., 2014). To test whether the GSSG to GSH conversion was affected by the lowered NADPH abundance in Pro35S:DHFR-TS3 plants, reduced and oxidized glutathione forms were measured in 3-d-old seedlings, revealing a significantly lower GSH:GSSG ratio in Pro35S:DHFR-TS3 compared with the wild type (Figures 10D to 10F; Supplemental Figures 22C to 22F).
These results suggest that Pro35S:DHFR-TS3 plants might be vulnerable to the oxidative stress due to a reduced GSH recycling efficiency caused by the lowered NADPH production resulting from a decreased activity of MTHFD.
DISCUSSION
This study provides new insights into plant folate metabolism. First, it underscores the notion that folate abundance affects plant development and reveals its regulation through adjustment of DHFR activity. Second, our work draws a parallel with a recently discovered role of folate metabolism in animals (Fan et al., 2014; Lewis et al., 2014; Piskounova et al., 2015; Ducker et al., 2016) in the modulation of cellular redox state by contributing to NADPH production, thus allowing a plant to cope with oxidative stress. It is possible that the role of folates in redox metabolism might be common not only for animals and plants but represent a conserved feature of organismal metabolism throughout evolution.
DHFR-TS Family Members Demonstrate Redundant and Specific Functions during Arabidopsis Development
The crucial roles of the DHFR-TS gene family in folate biosynthesis and thymidylate production suggest a high degree of redundancy between the family members. The redundancy between DHFR-TS family members was inferred from their overlapping subcellular localization (mitochondria, cytosol, and nucleus) and tissue-specific expression patterns (Figures 3 and 4). In agreement with this notion, single loss-of-function T-DNA insertion mutants grown under normal conditions showed neither obvious growth phenotypes (Figures 5B and 5C; Supplemental Figures 11 and 13A to 13G) nor any alterations in folate content (Figures 5F and 5G).
Cytosolic localization of DHFR-TS1 was suggested by analysis of the Arabidopsis cytosolic proteome (Ito et al., 2011). The mitochondrial and cytosolic localization of other folate biosynthesis enzymes was previously described (Ravanel et al., 2001), whereas nuclear localization of enzymes involved in this pathway remained unreported in plants. Although folates are present in the cytosolic-nuclear fraction (Gambonnet et al., 2001), neither the localization of DHFR/TS proteins nor their corresponding enzymatic activities were previously assigned to plant nuclei. In mammals, DHFR and TS proteins were observed in the nucleus (Prem veer Reddy and Pardee, 1980; Noguchi et al., 1983), as part of the replitase complex that assembles when cells enter the S-phase, produces deoxynucleoside triphosphates, and delivers them to DNA polymerase, which also resides in the complex (Prem veer Reddy and Pardee, 1980; Reddy and Fager, 1993). Furthermore, in human cell lines, DHFR and TS were reported to be shuttled from the cytosol to the nucleus upon sumoylation (Anderson et al., 2007; Woeller et al., 2007; Anderson and Stover, 2009). Thus, it is tempting to speculate that in plant cells, the subcellular localization of DHFR-TS isoforms can also alter according to the developmental state or in response to environmental cues. The results open a new perspective for the study of the role of DHFR-TS proteins in the plant cell nucleus.
The differential expression of DHFR-TS genes and the analysis of double mutants suggested that each DHFR-TS homolog fulfills specific functions during Arabidopsis development. DHFR-TS1 was ubiquitously expressed in the shoot throughout plant development. The abundant expression of DHFR-TS1 and DHFR-TS2 in the embryo as well as the absolute necessity of at least one functional allele of either one of these genes in embryo development suggested their essential and redundant functions during embryogenesis. The necessity of folate biosynthesis during plant embryogenesis was previously reported (Ishikawa et al., 2003; Mehrshahi et al., 2010). Being in accordance with the idea of functional redundancy between DHFR-TS1 and DHFR-TS2, the lack of any phenotypic defects in DHFR-TS1 loss-of-function plants suggested that DHFR-TS2 can fully substitute for the loss of DHFR-TS1. In agreement with this notion, DHFR-TS2 expression was elevated in dhfr-ts1 mutants (Supplemental Figures 10C and 10D) and DHFR-TS2 expression was highly prevalent in dividing cells, which is in agreement with previous findings in mammals, where elevated expression of DHFR and TS was found in tumor cells (Blakley, 1969), as well as in plants, where DHFR-TS was shown to be highly expressed in root tips and developing kernels of maize (Zea mays; Cox et al., 1999). In support of this notion, DHFR-TS2 expression diminished as cotyledon cells switched from the division to expansion mode during postembryonic development and appeared in emerging leaves, which enlarge mainly by cell division during primary morphogenesis (Figures 4O to 4Q). These data are in agreement with the notion that folate biosynthesis is highly active in dividing tissues (Jabrin et al., 2003). An important role for DHFR-TS2 during early postembryonic development was inferred from an elevated sensitivity of dhfr-ts2-1 and dhfr-ts2-2 seedlings to the DHFR inhibitor TMP (Supplemental Figures 23E, 24C, and 24D) and their lower DHFR activity (Figure 5D). Among all tested mutant genotypes, such sensitivity to TMP was demonstrated only by mutants bearing the dhfr-ts2 allele. This points to a significant contribution of DHFR-TS2 to the total DHFR activity during early postembryonic development. Interestingly, dhfr-ts1 mutant seedlings did not demonstrate any decrease in DHFR activity (Figure 5D), pointing to different roles of DHFR-TS1 and DHFR-TS2 during postembryonic development. In agreement with this notion, DHFR-TS1 expression was not elevated in dhfr-ts2 seedlings (Supplemental Figure 10B), suggesting that DHFR-TS1 cannot fully substitute for the loss of DHFR-TS2 during postembryonic development.
In contrast to DHFR-TS1 and DHFR-TS2, expression of DHFR-TS3 was mainly restricted to the differentiation zones, such as the suspensor (Figure 4G), the first part of the embryo that undergoes differentiation (D’Amato, 1984; Raghavan, 1986) or root cap cells (Supplemental Figure 8C), which, as shown by studies on maize primary roots (Barlow, 1974), are characterized by a highly differentiated state. The activity of the DHFR-TS3 promoter in leaf hydathodes is another example of DHFR-TS3 expression in differentiated cell types (Supplemental Figure 9J). Contradicting the commonly accepted notion that folate biosynthesis in plants occurs mainly in dividing tissues and photosynthetic leaves (Jabrin et al., 2003), the prevalence of DHFR-TS3 expression in differentiated regions suggested a specific role implemented by this gene. In accordance with this distinct expression pattern, only DHFR-TS3 is unable to support development without its family members.
DHFR-TS3 Inhibits Its Homologs, Thereby Fine-Tuning the Folate Level during Development
Several lines of evidence substantiate an inhibitory activity of DHFR-TS3 toward its homologs. First, overexpression of DHFR-TS3 in Pro35S:DHFR-TS3 and ProDHFR-TS3:DHFR-TS3 plants resulted in a decrease of DHFR activity, while its disruption in dhfr-ts3-1 plants caused an elevation of DHFR activity (Figures 6E and 10G). Second, plants overexpressing DHFR-TS3 demonstrated lowered total folate content (Figure 6G; Supplemental Figure 15B). Finally, we demonstrated that DHFR-TS3 lacks both DHFR and TS activities and inhibits DHFR activities of both homologs in vitro. Moreover, DHFR-TS3 is unable to support development without its homologs, as inferred from the lethality of dhfr-ts1-1 dhfr-ts2-1 double mutants. Two mechanisms can be proposed to explain this inhibition. The first involves a heterodimerization of the inhibitory and enzymatically inactive isoform with its active homologs, which may either affect the enzymatic activity of the bound proteins, their subcellular localization, or an interaction with a third partner. The latter two cases might be plausible scenarios if the subcellular localization of DHFR-TS proteins in plants is dependent on SUMOylation as it is in the case with their animal counterparts (Anderson et al., 2007; Woeller et al., 2007; Anderson and Stover, 2009). Homodimerization of DHFR-TS proteins in plants was previously reported (Cella and Parisi, 1993), whereas formation of heterodimers has not been demonstrated thus far. Since DHFR-TS3 does not show substantial alterations in the DHF binding sites (Supplemental Figure 1), a second possible mechanism of inhibition may involve DHFR substrate sequestration by DHFR-TS3.
Our data also suggest that the inhibition of DHFR-TS homologs by DHFR-TS3 observed in DHFR-TS3-OE plants can reflect the natural situation. First, DHFR-TS3 is coexpressed with its homologs at every developmental stage: in the embryonic root tip (with DHFR-TS1 at the heart stage and DHFR-TS2 throughout embryo development), in distal parts of cotyledons (with DHFR-TS1 throughout embryo development), in the shoot apex (with DHFR-TS1 and DHFR-TS2 throughout development), in the hypocotyl (with DHFR-TS1), in the primary root (with DHFR-TS1), in rosettes (with DHFR-TS1 and DHFR-TS2), and in inflorescences (with DHFR-TS1 and DHFR-TS2).
Second, DHFR-TS3 shares subcellular localization with its homologs: with DHFR-TS1 in the cytosol in cells of the root transition zone, with DHFR-TS1 in the cytosol of cells in the elongation zone, and with DHFR-TS2 in the mitochondria of cells in the elongation zone. Finally, weak overexpression of DHFR-TS3 driven by its native promoter reduced DHFR activity, while the loss of DHFR-TS3 function resulted in an increase of DHFR activity in roots of 10-d-old seedlings (Figure 10G). The failure to detect elevation of DHFR activity in whole 3-d-old dhfr-ts3 mutants (Figure 5D) could be ascribed to the low expression of DHFR-TS3 at this stage of development (Figure 4T). However, in roots of 10-d-old seedlings, where DHFR-TS3 expression is abundant (Figure 4V), DHFR activity of dhfr-ts3 mutants is increased in comparison with that of the wild type (Figure 10G). These data provide an additional confirmation of DHFR-TS3 inhibitory function in vivo.
It is possible that the above-described subcellular and tissue-specific spatio-temporal overlap of DHFR-TS3 with either DHFR-TS1 or DHFR-TS2 might tune DHFR and TS activities to suit particular developmental and differentiation states of a cell. The prevalence of DHFR-TS3 transcripts in highly differentiated regions may be indicative of the importance of lowering DHFR and TS activities for progression or maintenance of differentiation. In support of an implication in development, Pro35S:DHFR-TS3 plants, which exhibited a disturbed folate metabolism, also demonstrated a developmental delay, reflected in the delay in flowering time while retaining the same number of leaves upon flowering (Figure 6D). Being in accordance with the previous studies reporting a severe inhibition of plant development and cessation of basic metabolic processes in response to folate depletion by DHFR inhibitors (Loizeau et al., 2008; Navarrete et al., 2013), these results further reinforce the notion of the importance of folate metabolism for implementation of developmental programs (Srivastava et al., 2011; Reyes-Hernández et al., 2014). The importance of folate metabolism in development was initially demonstrated in animal systems (Blom et al., 2006). The regulation of folate abundance presented in this work might enable plants to integrate de novo folate biosynthesis with other metabolic processes in order to execute developmental programs and respond to the challenges of a changing environment.
Interestingly, DHFR-TS3-OE also demonstrated an altered distribution of folate species, having a lower 5-methyl-THF and 5,10-methenyl-THF content and elevated 5,10-methylene-THF levels (Figure 6H; Supplemental Figure 15B). 5-Methyl-THF donates its methyl group to convert homocysteine into methionine in the methyl cycle, while 5,10-methylene-THF is involved in thymidylate synthesis (Blancquaert et al., 2010). Similar results were obtained in Arabidopsis cells depleted in folates by application of methotrexate, another potent inhibitor of DHFR (Loizeau et al., 2008) and in human colon epithelial cells grown in folate-deficient conditions (Hayashi et al., 2007). Presumably, DHFR-TS3-OE plants, being limited in folates, preserve DNA synthesis at the expense of methylation reactions.
Regulation of protein function by inactive isoforms occurs in different eukaryotes. Thus, inactive 1-aminocyclopropane-1-carboxylate synthase isoforms regulate ethylene biosynthesis in Arabidopsis by binding their active family members (Tsuchisaka et al., 2009). Naturally occurring truncated isoforms of human alfa1A-adrenoceptors (Cogé et al., 1999), activin-like kinase 4 (Zhou et al., 2000), human interleukin-1 receptor-associated kinases (Hardy and O’Neill, 2004), and rat neurotrophin (Yacoubian and Lo, 2000) inhibit activities of their full-length counterparts, having a role in mammalian pathologies or development.
Lowered DHFR Activity and Disturbed Folate Metabolism Hamper Survival upon Oxidative Stress Inflicted by HU
Up-regulation of dNTP synthesis was reported to be essential for survival upon DNA damage and replication stress (Elledge et al., 1992; Desany et al., 1998). HU inhibits ribonucleotide reductase, thereby depleting the dNTP pool and causing a slowdown of the replication fork progression and a diminished activation of replication origins (Wang and Liu, 2006; Saban and Bujak, 2009). The severe reduction of growth upon HU treatment is indicative of cessation of the mitotic cell cycle through the initiation of DNA damage-induced cell cycle checkpoints (Melo and Toczyski, 2002; Culligan et al., 2004). Since the activity of DHFR-TS proteins providing DHFR and TS activities needed for thymidylate synthesis is inhibited in Pro35S:DHFR-TS3 plants, the HU-hypersensitive response of Pro35S:DHFR-TS3 plants was attributed to the impaired ability to survive under replication stress. In support of this notion, DHFR activity was shown to be important for survival upon HU treatment, as wild-type plants being impaired in DHFR activity by TMP and grown on HU were able to mimic the HU hypersensitivity of Pro35S:DHFR-TS3 plants. Furthermore, the reversion of the hypersensitivity by supplementation with folinic acid (LV) pointed toward the necessity of maintenance of folate level to withstand stress imposed by HU.
Since an increase in cell size is often accompanied by an increase in DNA ploidy level (Satina and Blakeslee, 1941; Melaragno et al., 1993; Gendreau et al., 1998), which is attained by switching from the mitotic cell cycle to the endocycle (Sugimoto-Shirasu et al., 2002; Castellano et al., 2004; Vlieghe et al., 2005), the elongation of the hypocotyl of HU-treated Pro35S:DHFR-TS3 plants was first assumed to be accompanied by an increase in ploidy level. However, this was not the case, thereby providing additional evidence for the notion that an increase in ploidy level is not always a prerequisite for cell growth, as was reported in several other studies (De Veylder et al., 2001, 2002; Beemster et al., 2002; Schnittger et al., 2003). The restriction of cell elongation in hypocotyl cells in response to HU treatment was in accordance with the activation of the DHFR-TS3 promoter exclusively in hypocotyls upon HU treatment (Figure 8E). In contrast to the hypersensitive phenotype of Pro35S:DHFR-TS3 plants to HU, wild-type-like sensitivity was observed upon exposure to aphidicolin, which induces replication stress by inhibiting DNA polymerase (Sala et al., 1980), refuting the hypothesis of a link with replication stress. Although both compounds induce replication stress, HU is known to inflict oxidative stress by inhibiting ROS-detoxifying enzymes, while aphidicolin is not (Chen and Asada, 1990; Juul et al., 2010; Yi et al., 2014b).
Plant Folate Metabolism Mediates NADPH Production, Thereby Contributing to Oxidative Stress Tolerance
Pro35S:DHFR-TS3 plants displayed elevated vulnerability to high-light treatments, which are known to induce oxidative damage (Niyogi, 1999; Rossel et al., 2002; Müller-Moulé et al., 2004), confirming that the hypersensitive phenotype of Pro35S:DHFR-TS3 seedlings on HU was likely caused by oxidative stress and not by replication stress (Figure 9). This notion was further supported by NBT and DAB staining, revealing an elevated ROS abundance in dark-grown Pro35S:DHFR-TS3 seedlings (Figure 10A; Supplemental Figure 21). Moreover, Pro35S:DHFR-TS3 seedlings exhibited a lowered NADPH/NADP+ ratio, which is indicative of reduced oxidative stress scavenging (Møller and Rasmusson, 1998). As elevated ROS levels were reported to be necessary for pollen tube (Potocký et al., 2007) and root hair (Foreman et al., 2003) growth, as well as for cell expansion during leaf growth (Rodríguez et al., 2002), the elongation of hypocotyl cells of DHFR-TS3-OE seedlings upon HU treatment could be attributed to the elevated ROS levels.
Our study revealed that the hypersensitivity of Pro35S:DHFR-TS3 seedlings to oxidative stress was accompanied by a lowered MTHFD activity (Figure 10C; Supplemental Figures 22A and 22B). This decrease in MTHFD activity was in line with the accumulation of 5,10-methylene-THF together with a decreased share of 5,10-methenyl-THF in Pro35S:DHFR-TS3 plants (Figure 6H; Supplemental Figure 15B). It is possible that a similar regulation in response to changes in folate level is also inherent to other enzymes of C1 metabolism.
The lower NADPH abundance in Pro35S:DHFR-TS3 plants resulted in a diminished GSH:GSSG ratio (Figure 10F), which is indicative of a hampered glutathione reduction. Since glutathione is considered a primary ROS scavenger (Foyer et al., 2009), one can assume that the decrease in reduced glutathione caused the observed accumulation of ROS in Pro35S:DHFR-TS3 plants (Figure 10A). These results parallel the recent finding in animal cells revealing the crucial function of folate metabolism in generating reducing power through NADPH production (Fan et al., 2014; Lewis et al., 2014; Piskounova et al., 2015; Ducker et al., 2016).
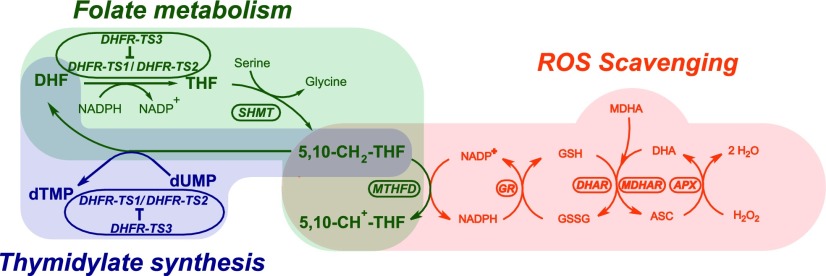
Our findings suggest that plant folate metabolism, like its animal counterpart, is linked to ROS homeostasis through NADPH production coupled with the 5,10-methylene-THF to 5,10-methenyl-THF conversion catalyzed by MTHFD. Furthermore, this study implies that the availability of 5,10-methylene-THF is influenced by the step controlled by DHFR enzymes. DHFR-TS3, being a regulator of its homologs, probably fine-tunes the flux of NADP+ in folate-mediated C1 metabolism, thereby contributing to NADPH formation and subsequent ROS scavenging through the Asada-Halliwell pathway (Figure 11).
Figure 11.
Regulation of Redox Balance by DHFR-TS3 and Coupling of Folate Metabolism with ROS Scavenging and Thymidylate Synthesis.
DHFR-TS3 inhibits DHFR-TS1 and DHFR-TS2, thereby lowering the folate level and causing a decrease in the conversion of 5,10-methylene-THF to 5,10-methenyl-THF, catalyzed by MTHFD. This results in a lower NADPH production leading to a decrease in GSH recycling rate through the glutathione-ascorbate cycle (Asada-Halliwell pathway) and, consequently, to an increased ROS abundance. dTMP, deoxythymidine monophosphate; dUMP, deoxyuridine monophosphate; 5,10-CH2-THF, 5,10-methylene tetrahydrofolate; 5,10-CH+-THF, 5,10-methenyl tetrahydrofolate; DHA, dehydroascorbate; ASC, ascorbate; MDHA, monodehydroascorbate; SHMT, serine hydroxymethyltransferase; GR, glutathione reductase; DHAR, dehydroascorbate reductase; MDHAR, monodehydroascorbate reductase; APX, ascorbate peroxidase.
In animal cells, it was demonstrated that folate metabolism contributes to NADPH production mainly in mitochondria through methylenetetrahydrofolate dehydrogenase 2, methylenetetrahydrofolate dehydrogenase 2-like (MTHFD2 and MTHFD2L), and 10-formyltetrahydrofolate dehydrogenase (ALDH1L2), while in the cytosol, C1 pathway fluxes are running through cytosolic MTHFD1 in the opposite, NADPH-consuming direction (Lewis et al., 2014; Piskounova et al., 2015; Ducker et al., 2016). Predictions of the subcellular localization of MTHFD and the known localization of glutathione reductase suggest that the reduction of glutathione can potentially take place in mitochondria, plastids, and cytosol in plant cells (Christensen and MacKenzie, 2006). The mitochondrial and cytosolic localization of DHFR-TS proteins in Arabidopsis, demonstrated in this study, indicates that folate metabolism can contribute to NADPH production needed for glutathione reduction in those compartments.
The suggested mechanism of adjustment of the redox balance through modulating DHFR activity by DHFR-TS3 is probably common for Brassicaceae, as putative orthologs of DHFR-TS3 are found in other species from this family (Figure 2). Originating from a very recent gene duplication event, DHFR-TS3-like genes appear to have diverged roughly twice as much in ∼47 million years of species radiation within the Brassicaceae family (Hohmann et al., 2015) than DHFR-TS1- and DHFR-TS2-like genes have done since the emergence of land plants 450 million years ago. This indicates that Arabidopsis DHFR-TS3 is no longer under selective pressure, hence the lack of DHFR and TS catalytic activities. The lack of DHFR-TS3 orthologs in other plant species suggests that other mechanisms of regulation might operate. It is possible that, like their animal counterparts, plant DHFR-TS genes are controlled at various levels (Ercikan et al., 1993; Martianov et al., 2007; Mishra et al., 2007).
Folate Metabolism Establishes Important Interactions with Other Metabolic Pathways
Folate-mediated C1 reactions are tightly linked to nucleotide and lipid biosynthesis (Hanson and Roje, 2001). The link with nucleotide synthesis is established by 10-formyl-THF and 5,10-methylene-THF derivatives that take part in the production of purines and thymidylate, respectively. Besides its role in nucleotide biosynthesis, 5,10-methylene-THF is also implicated in the synthesis of fatty acids, through the formation of pantothenate. The possible contribution of folate metabolism to lipid synthesis, which is highly dependent on NADPH (Ohlrogge and Jaworski, 1997), establishes a stronger link between these two processes. Moreover, folates are key factors in methylation reactions, another crucial element of lipid metabolism. This study demonstrating the role of folate metabolism in the regulation of redox balance and development is therefore underscoring the paramount importance of folates in regulating basic metabolic processes and optimal cellular function.
METHODS
Plant Growth Conditions
Arabidopsis thaliana (ecotype Columbia) plants were grown in a growth chamber at 22°C and 150 µmol m−2 s−1 under a 16-h-light/8-h-dark cycle. Half-strength Murashige and Skoog medium (Duchefa) was used for growing seeds and for treatments. Nicotiana tabacum plants were grown in a growth chamber at 22°C and 80 µmol m−2 s−1 under a 16-h-light/8-h-dark cycle. Arabidopsis seeds were sterilized in 5% NaOCl and 0.05% Tween 20 solution for 15 min and imbibed at 4°C for 2 d before sowing. Seed germination was scored when the radicle protrusion through the seed coat was visible.
Plant Materials
dhfr-ts1-1 (GK_010G06; Col-0 background) and dhfr-ts2-2 (GK_893CO2; Col-0 background) were purchased from the GABI-Kat collection (Kleinboelting et al., 2012); dhfr-ts2-1 (SALK_016377; Col-0 background), dhfr-ts3-1 (SALK_114609; Col-0 background), and dhfr-ts3-2 (SALK_129194; Col-0 background) were purchased from the NASC; and dhfr-ts1-2 line (GT10477; Ler background) was purchased from Cold Spring Harbor Laboratory (gene trap collection). Plants homozygous for the T-DNA insertion were identified by PCR-based genotyping with gene-specific primers and T-DNA left border-specific primers as illustrated in Supplemental Figure 12. The primers used were 32 – 26_rev and 25_forw – 26-rev for dhfr-ts1-1, 27_forw – 31 and 27_forw – 28_rev for dhfr-ts2-1, 31 – 3_rev and 29_forw – 30_rev for dhfr-ts3-1, 41_forw – 42_rev and 42_rev – 47 for dhfr-ts1-2, 43_forw – 44_rev and 43_forw – 32 for dhfr-ts2-2, and 45_forw – 46_rev and 45_forw – 31 for dhfr-ts3-2 (Supplemental Table 1). Wild-type Col-0 and Ler were used as a control.
Phylogenetic Analysis
Amino acid sequences were obtained using P-BLAST. Full-length amino acid sequences were aligned using MUSCLE software (Edgar, 2004). The phylogenetic relationship was inferred using the maximum likelihood method implemented in RaxML (Stamatakis, 2014). The maximum likelihood tree was evaluated with 1000 bootstrap replicates.
Plasmid Vector Construction and Transgenic Plant Production
All constructs used in this study were generated using the Gateway technology (Invitrogen). The PCR-amplified fragments were first cloned into pDONR221 vector through BP recombination reactions to obtain Entry clones that were confirmed by sequencing. The Entry clones were recombined with destination vectors to yield final binary vectors for plant transformation, which were also verified by sequencing. The vectors were then introduced in Arabidopsis via Agrobacterium tumefaciens-mediated floral dip transformation, as described previously (Clough and Bent, 1998). Genetic analysis confirmed single locus integration (75% resistant to 25% sensitive in the T2 generation). Transgenic lines were used in the homozygous T3 generation. All primers used can be found in Supplemental Table 1.
For Pro35S:DHFR-TS1, Pro35S:DHFR-TS2, and Pro35S:DHFR-TS3, full-length coding regions of DHFR-TS1, DHFR-TS2, and DHFR-TS3 were amplified from Arabidopsis cDNAs with high-fidelity polymerase using the following combinations of primers: 1_forw – 2_rev for DHFR-TS1, 3_forw – 4_rev for DHFR-TS2, and 5_forw – 6_rev for DHFR-TS3. The obtained fragments were cloned into pH7WG2,0 vector to yield destination vectors, where DHFR-TS coding DNA sequences (CDSs) are placed under control of the CaMV 35S promoter. Transgenic plants bearing these vectors were selected for resistance to hygromycin B.
For ProDHFR-TS1:GFP-GUS, 1429 bp upstream (comprising the promoter region and 5′UTR) and 63 bp (a part of exon 1, encoding 21 amino acids) downstream of the start codon were amplified from Columbia genomic DNA with high-fidelity polymerase using 10_forw and 11_rev primers; for ProDHFR-TS2:GFP-GUS, 1152 bp upstream (comprising promoter region and 5′UTR) and 712 bp (exon 1, exon 2, a part of exon 3 and introns 1 and 2, encoding 21 amino acids) downstream of the start codon were amplified using 12_forw and 13_rev primers, while for ProDHFR-TS3:GFP-GUS, 1991 bp upstream (comprising promoter region and 5′UTR) and 63 bp downstream of the start codon (a part of exon 1, encoding 21 amino acids) were amplified using 14_forw and 15_rev primers. The amplified fragments were cloned into pKGWFS7, where in the respective parts of the protein-coding region of DHFR-TS genes are fused in frame with a combined GFP-GUS reporter and driven by DHFR-TS promoters. Transgenic plants carrying these binary vectors were selected for kanamycin resistance. The schematic representation of the constructs can be found in Supplemental Figure 6.
For ProDHFR-TS1:DHFR-TS1-GFP, the DHFR-TS1 promoter and 5′UTR containing region (1429 bp upstream of the start codon) was amplified using high-fidelity polymerase with 10_forw and 16_rev, while the DHFR-TS1 CDS was amplified with 19_forw and 20_rev primers. The two fragments were fused by PCR reaction using high-fidelity polymerase and cloned into pGWB4 (Nakagawa et al., 2007) to obtain a construct wherein the DHFR-TS1 CDS fused in frame to GFP is driven by the DHFR-TS1 promoter. ProDHFR-TS2:DHFR-TS2-GFP was generated likewise using 1152-bp DHFR-TS2 promoter and 5′UTR containing genomic region, amplified with 12_forw – 17_rev primer combination, and DHFR-TS2 CDS was amplified with 21_forw – 22_rev primer combination. To generate ProDHFR-TS3:DHFR-TS3-GFP, the genomic region containing DHFR-TS3 promoter and 5′UTR (1991 bp upstream of the start codon) was amplified with 14_forw – 18_rev primer combination, and DHFR-TS3 CDS was amplified using 23_forw – 24_rev primer combination. The transgenic plants bearing these genetic constructs were selected for resistance to hygromycin B.
For DHFR-TS1-His6, DHFR-TS2-His6, and DHFR-TS3-His6, DHFR-TS1, DHFR-TS2, and DHFR-TS3 CDSs lacking stop codons, respectively, were cloned into pDEST17 to yield constructs wherein CDSs of DHFR-TS genes are fused in frame with a 6xHis tag.
Nucleic Acid Isolation and qPCR Analysis
Total RNA was prepared from 3-d-old seedlings grown on half-strength Murashige and Skoog medium using an RNeasy kit (Qiagen). Subsequently, RNA was treated with amplification grade DNase I (Invitrogen). cDNA was synthesized using a SuperScript II first-strand synthesis kit (KAPA Biosystems). RT-qPCR was performed using a Bio-Rad real-time PCR system. The comparative threshold cycle method was used to determine relative gene expression, with the expression of Actin2 (ACT2) serving as an internal control. Data represent mean values and standard deviations from three biological replicates, with three technical replicates each. The primers for real-time PCR analysis are presented in Supplemental Table 1.
Subcellular Localization Analysis and Confocal Laser Scanning Microscopy
For the analysis of the subcellular localization of DHFR-TS proteins, 7-d-old seedlings of transgenic Arabidopsis lines bearing ProDHFR-TS:DHFR-TS-GFP constructs were stained with 250 nM Mito Tracker Orange or with 4′,6-diamidino-2-phenylindole (DAPI) for 15 min. Root cells of the stained plants were observed with an APO 40×/1.25 objective of a confocal microscope (Nikon Eclipse TE2000-S). GFP fluorescence was detected with excitation at 488 nm and emission at 525 nm, fluorescence from Mito Tracker Orange staining was detected with excitation at 543 nm and emission at 615 nm, and fluorescence from DAPI staining was detected with excitation at 405 nm and emission at 450 nm. Images were acquired with the NIS Elements 4.10 Nikon confocal software. To minimize the crossover between the partially overlapping emission spectra when Mito Tracker Orange or DAPI staining were combined with GFP, the sequential scanning mode was used.
DIC Microscopy
Arabidopsis embryos and roots cleared in a mixture of chloral hydrate, glycerol, and water (8:1:2, w/v/v) were observed under a Carl Zeiss Stemi SV11 microscope equipped with Nomarski optics (differential interference contrast).
Determination of Folate Levels
Approximately 200 mg of 3-d-old seedlings was used for folate level determination by liquid chromatography with tandem mass spectrometry detection as described (Storozhenko et al., 2007; De Brouwer et al., 2010). The data represent the mean of three biological (each comprising >500 seedlings) and three technical replicates ±sd.
Expression of Recombinant DHFR-TS Proteins in Escherichia coli
E. coli (Rosetta-gami) cells containing pDEST17_DHFR-TS constructs were inoculated into 150 mL LB medium at 28°C. When cultures reached a density of 0.3 to 0.4 (OD600), protein expression was induced by adding 1 mM IPTG and 0.2% l-arabinose. After further incubation for 5 h at 19°C, cells were harvested by centrifugation at 4000g for 15 min at 4°C. The pellets were resuspended in 5 mL of lysis solution (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, 0.5% Triton X-100, 1 mM PMSF, 5% glycerol, and 1 mg/mL lysozyme, DNase). The suspensions were kept on ice for 30 min and centrifuged at 10,000g for 10 min at 4°C. Soluble 6xHis-tagged DHFR-TS proteins were mixed with 1 mL of Ni-NTA Agarose superflow resin (Qiagen) for 4 h at 4°C, centrifuged at 1000g for 1 min at 4°C. Subsequently, the resins were washed five times with washing solution (50 mM NaH2PO4, 300 mM NaCl, 0.5% Triton X-100, 5% glycerol, and 20 mM imidazole, pH 8.0), and the recombinant proteins were eluted in 3 mL of elution buffer (50 mM NaH2PO4, 300 mM NaCl, 0.1% Triton X-100, 2.5% glycerol, and 100 mM imidazole, pH 8.0). The proteins were further purified on Amicon Ultra-4 30 K centrifugal filter units (Merck Millipore) to concentrate the eluate volume to 2 mL and to exchange the elution buffer for a storage buffer (50 mM Tris, pH 8.5, 1 mM DTT, 1 mM EDTA, and 15% glycerol), snap-frozen in liquid nitrogen, and stored at –80°C.
Histochemical GUS Staining
For GUS activity analysis, seedlings and embryos were incubated in 5-bromo-4-chloro-3-indolyl-β- d-glucuronide (X-Gluc) staining buffer solution [0.1 M phosphate buffer, pH 7.0, 10 mM EDTA, 0.1% Triton X-100, 1 mM K3Fe(CN)6, 1 mM K4Fe(CN)6, and 2 mM X-Gluc] at 37°C for 3 to 5 h in darkness. The staining solution was further replaced by 70% ethanol to remove chlorophyll.
Detection of ROS
Staining for hydrogen peroxide with DAB and for superoxide with NBT was performed according to He et al. (2012). For DAB staining, seedlings were incubated in 2 mg/mL of 3,3′-diaminobenzidine tetrahydrochloride dehydrate (Sigma-Aldrich), pH 3.8, for 6 h. For the NBT staining, seedlings were incubated in 2 mM NBT (Sigma-Aldrich) in 20 mM phosphate buffer for 2 h.
TS Activity Measurement
Measurements of TS activity in both crude plant extracts and purified recombinant DHFR-TS preparations were performed using a radioactive assay with tritium-labeled deoxyuridine 5′-monophosphate as described previously for peripheral blood mononuclear cells (Pluim et al., 2013). To prepare crude extracts, 3-d-old seedlings were ground in liquid nitrogen, homogenized in RM solution (Pluim et al., 2013), incubated for 20 min on ice, and centrifuged at 10,000g for 15 min at 4°C. Supernatants were transferred to clean tubes for immediate determination of total protein concentration by Bradford assay (Bradford, 1976) and TS activity (TSA) measurement. In TSA reactions, 50 µg of total protein for crude extracts and 0.5 pmols (as the lowest protein input) for purified recombinant DHFR-TS preparations were used. The data represent the mean of three biological (each comprising >500 seedlings) and three technical replicates ±sd.
DHFR Activity Measurement
DHFR activity was determined spectrophotometrically (using a SmartSpec Plus spectrophotometer; Bio-Rad) by measuring the oxidation of NADPH at 340 nm, as described previously (Neuburger et al., 1996). The reaction solution contained 50 mM phosphate buffer (pH 7.4), 5 mM 2-mercaptoethanol, 1 mM DTT, 0.2 mM NADPH, and 10 µM 7,8-dihydrofolic acid (Schircks Laboratories). To prepare crude extracts, 3-d-old seedlings were ground in liquid nitrogen and homogenized in the extraction buffer composed of 100 mM phosphate buffer (pH 7.4), 5% glycerol, 1 mM PMSF, 5 mM 2-mercaptoethanol. The homogenates were centrifuged at 4000g for 30 min at 4°C. The supernatants were concentrated using Amicon Pro centrifugal filters (10,000 NMWL; Merck Millipore). In DHFR reactions of a final volume of 0.5 mL, 1 mg of total protein for crude extracts and 0.5 pmols (as the lowest protein input) for purified recombinant DHFR-TS preparations were used. The total protein concentration was measured by the Bradford assay (Bradford, 1976). DHFR activity data presented are representative results from two independent experiments and the mean of three biological (each comprising >500 seedlings) and three technical replicates ±sd.
MTHFD Activity Measurement
Total MTHFD activity was measured as described (Tan et al., 1977). The (6R,S)-5,10-methylene-5,6,7,8-tetrahydrofolic acid was purchased from Schircks Laboratories. For the assay, 200 mg of 3-d-old seedlings was ground in liquid nitrogen and homogenized in 1 mL of the reaction buffer (Tan et al., 1977). The homogenate was centrifuged twice at 10,000g at 4°C for 10 min. Subsequently, 100 μL of the supernatant (crude extract) was added to 900 μL of the reaction buffer (Tan et al., 1977). MTHFD activities are representative data from three independent experiments. Every experiment included three biological (each comprising >500 seedlings) and three technical replicates for each line.
NADPH/NADP+ Ratio Measurement
The NADPH/NADP+ ratio was measured exactly as described by Kolbe et al. (2005). NADPH/NADP+ ratio is the mean ± sd of ratios from four independent experiments. Every experiment included three biological (each comprising >500 seedlings) and three technical replicates for each line.
Glutathione Measurement
GSH and GSSG content was measured as described previously (Queval and Noctor, 2007). Absorbance was measured with a SmartSpec Plus spectrophotometer (Bio-Rad). GSH and GSSG contents (mean ± sd) are representative data from three independent experiments. Every experiment included three biological replicates per line (each comprising >500 seedlings). The GSH/GSSG ratio was calculated for each independent experiment and the presented ratio reflects the mean thereof ± sd.
Ploidy Analysis
For flow cytometry analysis, whole seedlings were chopped with a razor blade in 600 μL of a buffer containing 45 mM MgCl2, 30 mM sodium citrate, 20 mM 3-morpholinopropane-1-sulfonic acid, and 0.1% Triton, pH 7.0 (Galbraith et al., 1991). The obtained suspension was filtered through a 50-µm filter. Two milliliters of DAPI from a stock of 2 µg/mL (dissolved in 0.5 M NaH2PO4) was added to the filtrate. The mixture was read by the PA RV70 Flow Cytometer (Partec).
TMP and HU Treatments
For long-term treatments, seeds were plated on control MS/2 media or on MS/2 media supplemented with 30 µM TMP (Trimethoprim; Sigma-Aldrich), 1 mM HU (Sigma-Aldrich), or both. For short-term treatments, 3-d-old seedlings were cultured in liquid MS/2 media supplemented with 5 mM HU for 5 h.
Aphidicolin and High-Light Treatments
For aphidicolin treatment, seeds were plated on control MS/2 medium or MS/2 medium supplemented with 10 µg/mL aphidicolin (Sigma-Aldrich).
For high-light treatment, seedlings were grown at 22°C and 80 µmol m−2 s−1 under a 16-h-light/8-h-dark cycle and subjected daily to high light, ∼1100 µmol m−2 s−1, for 8 h using 4000K LED (Cree LED components; 400–700 nm). The temperature of seedlings was registered using a K-type thermocouple (Labfacility) and remained within the range of 21 to 22°C.
Statistical Analysis
Statistically significant differences were determined using Student’s t test. Sampling sizes are presented in each figure legend.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: DHFR-TS1 (At2g16370), DHFR-TS2 (At4g34570), DHFR-TS3 (At2g21550), and ACT2 (At3g18780).
Supplemental Data
Supplemental Figure 1. Amino acid sequence alignment for Arabidopsis DHFR-TS isoforms.
Supplemental Figure 2. Gene duplication events.
Supplemental Figure 3. Complementation of the dhfr-ts1-1 dhfr-ts2-1 (+−/ − −) double mutant with ProDHFR-TS1:DHFR-TS1-GFP.
Supplemental Figure 4. Complementation of dhfr-ts1-1 dhfr-ts2-1 (+ − / − −) mutant with ProDHFR-TS2:DHFR-TS2-GFP.
Supplemental Figure 5. Complementation of dhfr-ts1-1 dhfr-ts3-1 plants with ProDHFR-TS3:DHFR-TS3-GFP.
Supplemental Figure 6. Schematic view of ProDHFR-TS:DHFR-TS-GFP-GUS constructs.
Supplemental Figure 7. Tissue-specific expression of DHFR-TS genes in Arabidopsis.
Supplemental Figure 8. Cell-type-specific expression of DHFR-TS genes in Arabidopsis roots.
Supplemental Figure 9. Expression of DHFR-TS genes in rosettes of 4-week-old Arabidopsis plants.
Supplemental Figure 10. Expression of DHFR-TS genes in dhfr-ts loss-of-function mutants.
Supplemental Figure 11. Morphological phenotypes of wild-type, DHFR-TS gain-of-function, and DHFR-TS loss-of-function plants.
Supplemental Figure 12. Genotyping of dhfr-ts1-1 dhfr-ts2-1 double mutant.
Supplemental Figure 13. Root length of DHFR-TS loss-of-function and gain-of-function lines.
Supplemental Figure 14. Expression of DHFR-TS genes in transgenic plants harboring Pro35S:DHFR-TS.
Supplemental Figure 15. Total folate content and folate species distribution in Pro35S:DHFR-TS3 plants.
Supplemental Figure 16. DHFR activities of Ni-NTA-purified DHFR-TS1, DHFR-TS2, and DHFR-TS3 proteins.
Supplemental Figure 17. Expression of DHFR-TS1 and DHFR-TS2 in Pro35S:DHFR-TS3 plants.
Supplemental Figure 18. Sensitivity of DHFR-TS loss-of-function and gain-of-function lines to 1 mM HU.
Supplemental Figure 19. Ploidy distribution analysis.
Supplemental Figure 20. Histochemical analysis of GUS activity in 7-d-old ProDHFR-TS1:DHFR-TS1-GFP-GUS and ProDHFR-TS2:DHFR-TS2-GFP-GUS seedlings.
Supplemental Figure 21. DAB and NBT staining of etiolated Pro35S:DHFR-TS3 and wild-type seedlings.
Supplemental Figure 22. Measurements of MTHFD activity and glutathione content in Pro35S:DHFR-TS3 seedlings.
Supplemental Figure 23. Sensitivity of DHFR-TS loss-of-function and gain-of-function lines to 50 µM TMP.
Supplemental Figure 24. Sensitivity of DHFR-TS loss-of-function lines to 100 mM HU and 30 µM TMP.
Supplemental Table 1. Percent identity matrix for amino acid sequences of Arabidopsis DHFR-TS isoforms.
Supplemental Table 2. Primers used in this study.
Supplemental Data Set 1. Alignments used to generate the phylogeny in Figure 2.
Acknowledgments
D.V.D.S. acknowledges financial support from Ghent University (Bijzonder Onderzoeksfonds BOF2009-G0A-004) and the Research Foundation-Flanders (FWO project 3G012609). D.B. is indebted to the Research Foundation-Flanders for a postdoctoral fellowship. We thank Marek Malek for the construction of Pro35S:DHFR-TS vectors and preliminary subcellular localization experiments. V.G. and D.V.D.S. thank Ronnie Viane for helpful suggestions and Bram Van de Poel for critical reading of the manuscript.
AUTHOR CONTRIBUTIONS
V.G. conducted all experiments (with the exception of folate measurements; Figures 5D and 6C; Supplemental Figure 16), designed experiments, analyzed and interpreted data, and wrote the manuscript. J.D.L. helped with genetic analysis of transgenic lines. J.V.D. performed folate analyses. D.P. performed TS activity analyses. C.M. conducted preliminary DHFR activity analysis. A.C. conducted preliminary GSH/GSSG content measurements. O.L. helped with ploidy analysis. F.R. conducted preliminary DHFR activity analysis. J.H.M.S. helped with TS activity measurements. D.B. provided helpful comments and helped V.G. with writing by commenting on the draft manuscript. C.P.S. helped with folate data analysis. D.V.D.S. coordinated the project, designed experiments, interpreted data, and wrote the manuscript. All authors commented on the manuscript.
References
- Albani D., Parisi B., Carbonera D., Cella R. (1985). Dihydrofolate reductase from Daucus carota cell suspension cultures: purification, molecular and kinetic characterization. Plant Mol. Biol. 5: 363–372. [DOI] [PubMed] [Google Scholar]
- Anderson D.D., Stover P.J. (2009). SHMT1 and SHMT2 are functionally redundant in nuclear de novo thymidylate biosynthesis. PLoS One 4: e5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.D., Woeller C.F., Stover P.J. (2007). Small ubiquitin-like modifier-1 (SUMO-1) modification of thymidylate synthase and dihydrofolate reductase. Clin. Chem. Lab. Med. 45: 1760–1763. [DOI] [PubMed] [Google Scholar]
- Asada K., Foyer C., Mullineaux P. (1994). Production and action of active oxygen species in photosynthetic tissues. In Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants, Foyer C.H., Mullineaux P.M., eds (Boca Raton, FL: CRC Press; ), pp. 77–104. [Google Scholar]
- Assaraf Y.G. (2007). Molecular basis of antifolate resistance. Cancer Metastasis Rev. 26: 153–181. [DOI] [PubMed] [Google Scholar]
- Bachmann B., Follmann H. (1987). Deoxyribonucleotide biosynthesis in green algae: characterization of thymidylate synthase-dihydrofolate reductase in Scenedesmus obliquus. Arch. Biochem. Biophys. 256: 244–252. [DOI] [PubMed] [Google Scholar]
- Barlow P. (1974). Regeneration of the cap of primary roots of Zea mays. New Phytol. 73: 937–954. [Google Scholar]
- Beemster G.T., De Vusser K., De Tavernier E., De Bock K., Inzé D. (2002). Variation in growth rate between Arabidopsis ecotypes is correlated with cell division and A-type cyclin-dependent kinase activity. Plant Physiol. 129: 854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakley R.L. (1969). Chemical and physical properties of pterins and folate derivatives. Biochemistry of Folic Acid and Related Pteridines, Blakley R.L., ed (New York: Wiley; ), pp. 58–105. [Google Scholar]
- Blancquaert D., Storozhenko S., Loizeau K., De Steur H., De Brouwer V., Viaene J., Ravanel S., Rébeillé F., Lambert W., Van Der Straeten D. (2010). Folates and folic acid: from fundamental research toward sustainable health. Crit. Rev. Plant Sci. 29: 14–35. [Google Scholar]
- Blom H.J., Shaw G.M., den Heijer M., Finnell R.H. (2006). Neural tube defects and folate: case far from closed. Nat. Rev. Neurosci. 7: 724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Bushby S.R., Hitchings G.H. (1968). Trimethoprim, a sulphonamide potentiator. Br. Pharmacol. Chemother. 33: 72–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano M.J., Ayala A., Murillo M.L., Carreras O. (2001). Protective effect of folic acid against oxidative stress produced in 21-day postpartum rats by maternal-ethanol chronic consumption during pregnancy and lactation period. Free Radic. Res. 34: 1–8. [DOI] [PubMed] [Google Scholar]
- Cella R., Parisi B. (1993). Dihydrofolate reductase and thymidylate synthase in plants: an open problem. Physiol. Plant. 88: 509–521. [Google Scholar]
- Chen G.-X., Asada K. (1990). Hydroxyurea and p-aminophenol are the suicide inhibitors of ascorbate peroxidase. J. Biol. Chem. 265: 2775–2781. [PubMed] [Google Scholar]
- Chistoserdova L., Vorholt J.A., Thauer R.K., Lidstrom M.E. (1998). C1 transfer enzymes and coenzymes linking methylotrophic bacteria and methanogenic Archaea. Science 281: 99–102. [DOI] [PubMed] [Google Scholar]
- Christensen K.E., MacKenzie R.E. (2006). Mitochondrial one-carbon metabolism is adapted to the specific needs of yeast, plants and mammals. BioEssays 28: 595–605. [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Cox K., Robertson D., Fites R. (1999). Mapping and expression of a bifunctional thymidylate synthase, dihydrofolate reductase gene from maize. Plant Mol. Biol. 41: 733–739. [DOI] [PubMed] [Google Scholar]
- Culligan K., Tissier A., Britt A. (2004). ATR regulates a G2-phase cell-cycle checkpoint in Arabidopsis thaliana. Plant Cell 16: 1091–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato F. (1984). Role of Polyploidy in Reproductive Organs and Tissues. In Embryology of Angiosperms, Johri B., ed (Berlin, Heidelberg: Springer; ), pp. 519–566. [Google Scholar]
- De Brouwer V., Storozhenko S., Stove C.P., Van Daele J., Van Der Straeten D., Lambert W.E. (2010). Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) for the sensitive determination of folates in rice. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878: 509–513. [DOI] [PubMed] [Google Scholar]
- De Veylder L., Beeckman T., Beemster G.T., Krols L., Terras F., Landrieu I., van der Schueren E., Maes S., Naudts M., Inzé D. (2001). Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13: 1653–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L., Beeckman T., Beemster G.T., de Almeida Engler J., Ormenese S., Maes S., Naudts M., Van Der Schueren E., Jacqmard A., Engler G., Inzé D. (2002). Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa-DPa transcription factor. EMBO J. 21: 1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano Mdel.M., Boniotti M.B., Caro E., Schnittger A., Gutierrez C. (2004). DNA replication licensing affects cell proliferation or endoreplication in a cell type-specific manner. Plant Cell 16: 2380–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desany B.A., Alcasabas A.A., Bachant J.B., Elledge S.J. (1998). Recovery from DNA replicational stress is the essential function of the S-phase checkpoint pathway. Genes Dev. 12: 2956–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhitavat S., Ortiz D., Rogers E., Rivera E., Shea T.B. (2005). Folate, vitamin E, and acetyl-L-carnitine provide synergistic protection against oxidative stress resulting from exposure of human neuroblastoma cells to amyloid-beta. Brain Res. 1061: 114–117. [DOI] [PubMed] [Google Scholar]
- Ducker G.S., Chen L., Morscher R.J., Ghergurovich J.M., Esposito M., Teng X., Kang Y., Rabinowitz J.D. (2016). Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 23: 1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S.J., Zhou Z., Allen J.B. (1992). Ribonucleotide reductase: regulation, regulation, regulation. Trends Biochem. Sci. 17: 119–123. [DOI] [PubMed] [Google Scholar]
- Ercikan E., Banerjee D., Waltham M., Schnieders B., Scotto K.W., Bertino J.R. (1993). Translational regulation of the synthesis of dihydrofolate reductase. In Chemistry and Biology of Pteridines and Folates. (Boston: Springer), pp. 537–540. [DOI] [PubMed] [Google Scholar]
- Fan J., Ye J., Kamphorst J.J., Shlomi T., Thompson C.B., Rabinowitz J.D. (2014). Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510: 298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferone R., Roland S. (1980). Dihydrofolate reductase: thymidylate synthase, a bifunctional polypeptide from Crithidia fasciculata. Proc. Natl. Acad. Sci. USA 77: 5802–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J., Demidchik V., Bothwell J.H., Mylona P., Miedema H., Torres M.A., Linstead P., Costa S., Brownlee C., Jones J.D., Davies J.M., Dolan L. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446. [DOI] [PubMed] [Google Scholar]
- Foyer C.H., Bloom A.J., Queval G., Noctor G. (2009). Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu. Rev. Plant Biol. 60: 455–484. [DOI] [PubMed] [Google Scholar]
- Cogé F., Guenin S.P., Renouard-Try A., Rique H., Ouvry C., Fabry N., Beauverger P., Nicolas J.P., Galizzi J.P., Boutin J.A., Canet E. (1999). Truncated isoforms inhibit [3H]prazosin binding and cellular trafficking of native human α1A-adrenoceptors. Biochem. J. 343: 231–239. [PMC free article] [PubMed] [Google Scholar]
- Galbraith D.W., Harkins K.R., Knapp S. (1991). Systemic endopolyploidy in Arabidopsis thaliana. Plant Physiol. 96: 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambonnet B., Jabrin S., Ravanel S., Karan M., Douce R., Rebeille F. (2001). Folate distribution during higher plant development. J. Sci. Food Agric. 81: 835–841. [Google Scholar]
- Gendreau E., Höfte H., Grandjean O., Brown S., Traas J. (1998). Phytochrome controls the number of endoreduplication cycles in the Arabidopsis thaliana hypocotyl. Plant J. 13: 221–230. [DOI] [PubMed] [Google Scholar]
- Hanson A.D., Roje S. (2001). One-carbon metabolism in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52: 119–137. [DOI] [PubMed] [Google Scholar]
- Hardy M.P., O’Neill L.A. (2004). The murine IRAK2 gene encodes four alternatively spliced isoforms, two of which are inhibitory. J. Biol. Chem. 279: 27699–27708. [DOI] [PubMed] [Google Scholar]
- Hayashi I., Sohn K.-J., Stempak J.M., Croxford R., Kim Y.-I. (2007). Folate deficiency induces cell-specific changes in the steady-state transcript levels of genes involved in folate metabolism and 1-carbon transfer reactions in human colonic epithelial cells. J. Nutr. 137: 607–613. [DOI] [PubMed] [Google Scholar]
- He J., Duan Y., Hua D., Fan G., Wang L., Liu Y., Chen Z., Han L., Qu L.-J., Gong Z. (2012). DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell 24: 1815–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P.I., Ashline D., Dhitavat S., Ortiz D., Collins S.C., Shea T.B., Rogers E. (2003). Folate deprivation induces neurodegeneration: roles of oxidative stress and increased homocysteine. Neurobiol. Dis. 14: 32–42. [DOI] [PubMed] [Google Scholar]
- Hohmann N., Wolf E.M., Lysak M.A., Koch M.A. (2015). A time-calibrated road map of Brassicaceae species radiation and evolutionary history. Plant Cell 27: 2770–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R.-F.S., Hsu Y.-C., Lin H.-L., Yang F.L. (2001). Folate depletion and elevated plasma homocysteine promote oxidative stress in rat livers. J. Nutr. 131: 33–38. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Machida C., Yoshioka Y., Kitano H., Machida Y. (2003). The GLOBULAR ARREST1 gene, which is involved in the biosynthesis of folates, is essential for embryogenesis in Arabidopsis thaliana. Plant J. 33: 235–244. [DOI] [PubMed] [Google Scholar]
- Ito J., Batth T.S., Petzold C.J., Redding-Johanson A.M., Mukhopadhyay A., Verboom R., Meyer E.H., Millar A.H., Heazlewood J.L. (2011). Analysis of the Arabidopsis cytosolic proteome highlights subcellular partitioning of central plant metabolism. J. Proteome Res. 10: 1571–1582. [DOI] [PubMed] [Google Scholar]
- Jabrin S., Ravanel S., Gambonnet B., Douce R., Rébeillé F. (2003). One-carbon metabolism in plants. Regulation of tetrahydrofolate synthesis during germination and seedling development. Plant Physiol. 131: 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul T., Malolepszy A., Dybkaer K., Kidmose R., Rasmussen J.T., Andersen G.R., Johnsen H.E., Jørgensen J.-E., Andersen S.U. (2010). The in vivo toxicity of hydroxyurea depends on its direct target catalase. J. Biol. Chem. 285: 21411–21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinboelting N., Huep G., Kloetgen A., Viehoever P., Weisshaar B. (2012). GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Res. 40: D1211–D1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe A., Tiessen A., Schluepmann H., Paul M., Ulrich S., Geigenberger P. (2005). Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc. Natl. Acad. Sci. USA 102: 11118–11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer D.M., Evans J.R. (2011). The importance of energy balance in improving photosynthetic productivity. Plant Physiol. 155: 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger N.J., von Schaewen A. (2003). The oxidative pentose phosphate pathway: structure and organisation. Curr. Opin. Plant Biol. 6: 236–246. [DOI] [PubMed] [Google Scholar]
- Lazar G., Zhang H., Goodman H.M. (1993). The origin of the bifunctional dihydrofolate reductase-thymidylate synthase isogenes of Arabidopsis thaliana. Plant J. 3: 657–668. [DOI] [PubMed] [Google Scholar]
- Lee T.-H., Tang H., Wang X., Paterson A.H. (2013). PGDD: a database of gene and genome duplication in plants. Nucleic Acids Res. 41: D1152–D1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C.A., Parker S.J., Fiske B.P., McCloskey D., Gui D.Y., Green C.R., Vokes N.I., Feist A.M., Vander Heiden M.G., Metallo C.M. (2014). Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewus F.A. (1999). Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry 52: 193–210. [Google Scholar]
- Loizeau K., De Brouwer V., Gambonnet B., Yu A., Renou J.-P., Van Der Straeten D., Lambert W.E., Rébeillé F., Ravanel S. (2008). A genome-wide and metabolic analysis determined the adaptive response of Arabidopsis cells to folate depletion induced by methotrexate. Plant Physiol. 148: 2083–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martianov I., Ramadass A., Serra Barros A., Chow N., Akoulitchev A. (2007). Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature 445: 666–670. [DOI] [PubMed] [Google Scholar]
- Mehrshahi P., et al. (2010). Functional analysis of folate polyglutamylation and its essential role in plant metabolism and development. Plant J. 64: 267–279. [DOI] [PubMed] [Google Scholar]
- Melaragno J.E., Mehrotra B., Coleman A.W. (1993). Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 5: 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo J., Toczyski D. (2002). A unified view of the DNA-damage checkpoint. Curr. Opin. Cell Biol. 14: 237–245. [DOI] [PubMed] [Google Scholar]
- Meng H., Jiang L., Xu B., Guo W., Li J., Zhu X., Qi X., Duan L., Meng X., Fan Y., Zhang C. (2014). Arabidopsis plastidial folylpolyglutamate synthetase is required for seed reserve accumulation and seedling establishment in darkness. PLoS One 9: e101905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P.J., Humeniuk R., Mishra P.J., Longo-Sorbello G.S., Banerjee D., Bertino J.R. (2007). A miR-24 microRNA binding-site polymorphism in dihydrofolate reductase gene leads to methotrexate resistance. Proc. Natl. Acad. Sci. USA 104: 13513–13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller I.M., Rasmusson A.G. (1998). The role of NADP in the mitochondrial matrix. Trends Plant Sci. 3: 21–27. [Google Scholar]
- Müller-Moulé P., Golan T., Niyogi K.K. (2004). Ascorbate-deficient mutants of Arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol. 134: 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Kurose T., Hino T., Tanaka K., Kawamukai M., Niwa Y., Toyooka K., Matsuoka K., Jinbo T., Kimura T. (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104: 34–41. [DOI] [PubMed] [Google Scholar]
- Navarrete O., Van Daele J., Stove C., Lambert W., Storozhenko S., Van Der Straeten D. (2013). Isolation and characterisation of an antifolate insensitive (afi1) mutant of Arabidopsis thaliana. Plant Biol (Stuttg) 15: 37–44. [DOI] [PubMed] [Google Scholar]
- Neuburger M., Rébeillé F., Jourdain A., Nakamura S., Douce R. (1996). Mitochondria are a major site for folate and thymidylate synthesis in plants. J. Biol. Chem. 271: 9466–9472. [DOI] [PubMed] [Google Scholar]
- Niyogi K.K. (1999). Photoprotection revisited: genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 333–359. [DOI] [PubMed] [Google Scholar]
- Noguchi H., Prem veer Reddy G., Pardee A.B. (1983). Rapid incorporation of label from ribonucleoside disphosphates into DNA by a cell-free high molecular weight fraction from animal cell nuclei. Cell 32: 443–451. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J.B., Jaworski J.G. (1997). Regulation of fatty acid synthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48: 109–136. [DOI] [PubMed] [Google Scholar]
- Piskounova E., Agathocleous M., Murphy M.M., Hu Z., Huddlestun S.E., Zhao Z., Leitch A.M., Johnson T.M., DeBerardinis R.J., Morrison S.J. (2015). Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluim D., Schilders K.A., Jacobs B.A., Vaartjes D., Beijnen J.H., Schellens J.H. (2013). Pharmacodynamic assay of thymidylate synthase activity in peripheral blood mononuclear cells. Anal. Bioanal. Chem. 405: 2495–2503. [DOI] [PubMed] [Google Scholar]
- Potocký M., Jones M.A., Bezvoda R., Smirnoff N., Zárský V. (2007). Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 174: 742–751. [DOI] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A.B. (1980). Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc. Natl. Acad. Sci. USA 77: 3312–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval G., Noctor G. (2007). A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: Application to redox profiling during Arabidopsis rosette development. Anal. Biochem. 363: 58–69. [DOI] [PubMed] [Google Scholar]
- Raghavan V. (1986). Embryogenesis in Angiosperms: A Developmental and Experimental Study. (Cambridge, UK: Cambridge: University Press; ). [Google Scholar]
- Rasmusson A.G., Møller I.M. (1990). NADP-utilizing enzymes in the matrix of plant mitochondria. Plant Physiol. 94: 1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanel S., Cherest H., Jabrin S., Grunwald D., Surdin-Kerjan Y., Douce R., Rébeillé F. (2001). Tetrahydrofolate biosynthesis in plants: molecular and functional characterization of dihydrofolate synthetase and three isoforms of folylpolyglutamate synthetase in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 98: 15360–15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy G.P., Fager R.S. (1993). Replitase: a complex integrating dNTP synthesis and DNA replication. Crit. Rev. Eukaryot. Gene Expr. 3: 255–277. [PubMed] [Google Scholar]
- Reyes-Hernández B.J., Srivastava A.C., Ugartechea-Chirino Y., Shishkova S., Ramos-Parra P.A., Lira-Ruan V., Díaz de la Garza R.I., Dong G., Moon J.C., Blancaflor E.B., Dubrovsky J.G. (2014). The root indeterminacy-to-determinacy developmental switch is operated through a folate-dependent pathway in Arabidopsis thaliana. New Phytol. 202: 1223–1236. [DOI] [PubMed] [Google Scholar]
- Rodríguez A.A., Grunberg K.A., Taleisnik E.L. (2002). Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol. 129: 1627–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossel J.B., Wilson I.W., Pogson B.J. (2002). Global changes in gene expression in response to high light in Arabidopsis. Plant Physiol. 130: 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban N., Bujak M. (2009). Hydroxyurea and hydroxamic acid derivatives as antitumor drugs. Cancer Chemother. Pharmacol. 64: 213–221. [DOI] [PubMed] [Google Scholar]
- Sala F., Parisi B., Burroni D., Amileni A.R., Pedrali-Noy G., Spadari S. (1980). Specific and reversible inhibition by aphidicolin in the α-like DNA polymerase of plant cells. FEBS Lett. 117: 93–98. [DOI] [PubMed] [Google Scholar]
- Satina S., Blakeslee A. (1941). Periclinal chimeras in Datura stramonium in relation to development of leaf and flower. Am. J. Bot. 28: 862–871. [Google Scholar]
- Schnittger A., Weinl C., Bouyer D., Schöbinger U., Hülskamp M. (2003). Misexpression of the cyclin-dependent kinase inhibitor ICK1/KRP1 in single-celled Arabidopsis trichomes reduces endoreduplication and cell size and induces cell death. Plant Cell 15: 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J., Rébeillé F., Fletcher J. (2000). Folic acid and folates: the feasibility for nutritional enhancement in plant foods. J. Sci. Food Agric. 80: 795–824. [Google Scholar]
- Srivastava A.C., Ramos-Parra P.A., Bedair M., Robledo-Hernández A.L., Tang Y., Sumner L.W., Díaz de la Garza R.I., Blancaflor E.B. (2011). The folylpolyglutamate synthetase plastidial isoform is required for postembryonic root development in Arabidopsis. Plant Physiol. 155: 1237–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes M.E., Chattopadhyay A., Wilkins O., Nambara E., Campbell M.M. (2013). Interplay between sucrose and folate modulates auxin signaling in Arabidopsis. Plant Physiol. 162: 1552–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storozhenko S., De Brouwer V., Volckaert M., Navarrete O., Blancquaert D., Zhang G.-F., Lambert W., Van Der Straeten D. (2007). Folate fortification of rice by metabolic engineering. Nat. Biotechnol. 25: 1277–1279. [DOI] [PubMed] [Google Scholar]
- Sugimoto-Shirasu K., Stacey N.J., Corsar J., Roberts K., McCann M.C. (2002). DNA topoisomerase VI is essential for endoreduplication in Arabidopsis. Curr. Biol. 12: 1782–1786. [DOI] [PubMed] [Google Scholar]
- Tan L.U., Drury E.J., MacKenzie R.E. (1977). Methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase-formyltetrahydrofolate synthetase. A multifunctional protein from porcine liver. J. Biol. Chem. 252: 1117–1122. [PubMed] [Google Scholar]
- Tsuchisaka A., Yu G., Jin H., Alonso J.M., Ecker J.R., Zhang X., Gao S., Theologis A. (2009). A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 183: 979–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beelen P., Labro J.F.A., Keltjens J.T., Geerts W.J., Vogels G.D., Laarhoven W.H., Guijt W., Haasnoot C.A.G. (1984). Derivatives of methanopterin, a coenzyme involved in methanogenesis. Eur. J. Biochem. 139: 359–365. [DOI] [PubMed] [Google Scholar]
- Vlieghe K., Boudolf V., Beemster G.T., Maes S., Magyar Z., Atanassova A., de Almeida Engler J., De Groodt R., Inzé D., De Veylder L. (2005). The DP-E2F-like gene DEL1 controls the endocycle in Arabidopsis thaliana. Curr. Biol. 15: 59–63. [DOI] [PubMed] [Google Scholar]
- Wang C., Liu Z. (2006). Arabidopsis ribonucleotide reductases are critical for cell cycle progression, DNA damage repair, and plant development. Plant Cell 18: 350–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeller C.F., Anderson D.D., Szebenyi D.M., Stover P.J. (2007). Evidence for small ubiquitin-like modifier-dependent nuclear import of the thymidylate biosynthesis pathway. J. Biol. Chem. 282: 17623–17631. [DOI] [PubMed] [Google Scholar]
- Yacoubian T.A., Lo D.C. (2000). Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat. Neurosci. 3: 342–349. [DOI] [PubMed] [Google Scholar]
- Yi D., et al. (2014). The Arabidopsis SIAMESE-RELATED cyclin-dependent kinase inhibitors SMR5 and SMR7 regulate the DNA damage checkpoint in response to reactive oxygen species. Plant Cell 26: 296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Sun H., Danila D.C., Johnson S.R., Sigai D.P., Zhang X., Klibanski A. (2000). Truncated activin type I receptor Alk4 isoforms are dominant negative receptors inhibiting activin signaling. Mol. Endocrinol. 14: 2066–2075. [DOI] [PubMed] [Google Scholar]