Abstract
Plant plastids and mitochondria have dynamic proteomes. Protein homeostasis in these organelles is maintained by a proteostasis network containing protein chaperones, peptidases, and their substrate recognition factors. However, many peptidases, as well as their functional connections and substrates, are poorly characterized. This review provides a systematic insight into the organellar peptidase network in Arabidopsis thaliana. We present a compendium of known and putative Arabidopsis peptidases and inhibitors, and compare the distribution of plastid and mitochondrial peptidases to the total peptidase complement. This comparison shows striking biases, such as the (near) absence of cysteine and aspartic peptidases and peptidase inhibitors, whereas other peptidase families were exclusively organellar; reasons for such biases are discussed. A genome-wide mRNA-based coexpression data set was generated based on quality controlled and normalized public data, and used to infer additional plastid peptidases and to generate a coexpression network for 97 organellar peptidase baits (1742 genes, making 2544 edges). The graphical network includes 10 modules with specialized/enriched functions, such as mitochondrial protein maturation, thermotolerance, senescence, or enriched subcellular locations such as the thylakoid lumen or chloroplast envelope. The peptidase compendium, including the autophagy and proteosomal systems, and the annotation based on the MEROPS nomenclature of peptidase clans and families, is incorporated into the Plant Proteome Database.
INTRODUCTION
Plant plastids and mitochondria each have their complement of proteins, their proteomes. In particular, plastids are highly dynamic as they can undergo specialization during developmental transitions (e.g., from etioplast to chloroplast, or from chloroplast to chromoplast). Plastids and mitochondria require the activity of processing peptidases, proteases, and amino-peptidases (from here on all assigned as “peptidases”) for a broad range of activities, including (1) removal of presequences of nuclear-encoded organellar proteins, (2) N-terminal methionine removal of organelle-encoded proteins, (3) additional N- or C-terminal cleavages for maturation and stabilization and possibly activation of proteins, (4) removal of misfolded, damaged, or aggregated proteins, (5) removal of unwanted proteins in response to environmental or developmental transitions including senescence, and (6) metabolism of proteins as alternate respiratory substrates for stressed plants (van Wijk, 2015).
Most of the known chloroplast and mitochondrial peptidases and their direct chaperones/adaptors belong to the ATP-dependent peptidases of the AAA+ family, namely, LON, CLP, and FTSH, as well as the ATP-independent DEG peptidase family. In Arabidopsis thaliana, these four families together constitute some 61 proteins, with two proteins located in peroxisomes (LON2 and DEG15) (van Wijk, 2015; Nishimura et al., 2017). Other organellar peptidases are part of smaller families or are single gene families. In the last five years, reviews have been published for plastid peptidases (Nishimura et al., 2016, 2017), mitochondrial peptidases (Janska et al., 2010; Kwasniak et al., 2012; Mossmann et al., 2012), degradation of photosystem II (Chi et al., 2012; Yoshioka-Nishimura and Yamamoto, 2014), and specific peptidase families in plant organelles, including rhomboids and other intramembrane cleaving peptidases (Knopf and Adam, 2012; Adam, 2013; Jeyaraju et al., 2013), CLP (Nishimura and van Wijk, 2015), LON (Rigas et al., 2012, 2014), DEG (Schuhmann and Adamska, 2012; Schuhmann et al., 2012), FTSH (Liu et al., 2010; Wagner et al., 2012), and processing peptidases (Teixeira and Glaser, 2013). Recent reviews on biogenesis of plastids (Jarvis and López-Juez, 2013; Ling and Jarvis, 2015), mitochondria (Carrie et al., 2013; Welchen et al., 2014) and peroxisomes (Hu et al., 2012), and autophagy of plant organelles (Li and Vierstra, 2012; Ishida et al., 2014; Lee et al., 2014; Ren et al., 2014; Van Aken and Van Breusegem, 2015) and protein turnover in plants (Nelson and Millar, 2015) complement these reviews on organelle proteolysis.
A universal classification system for peptidases, as well as proteinaceous peptidase inhibitors, has been developed in which each protein is assigned to a family on the basis of statistically significant similarities in amino acid sequence (Rawlings et al., 2016). Peptidase families that are thought to be homologous are grouped together in a clan. This information is available through the MEROPS database (http://merops.sanger.ac.uk/). This includes peptidases for Arabidopsis as well as scattered information for other plant species; however, the information is incomplete even for Arabidopsis and cumbersome to use because of the presence of different types of Arabidopsis gene identifiers. Nevertheless, this classification system provides an excellent starting point for the search and annotation of plant peptidases (van der Hoorn, 2008; Lallemand et al., 2015; van Wijk, 2015). Furthermore, there are many excellent and in-depth studies that have assembled and annotated specific peptidase families in Arabidopsis and other plant species, such as for subtilases (Schaller et al., 2012), cysteine peptidases (Díaz-Mendoza et al., 2014), and metacaspases (Sueldo and van der Hoorn, 2017). Unfortunately, there is no simple way to extract all (putative) Arabidopsis peptidases with their gene identifiers and functional annotation. This review aims to address this and provides a comprehensive compendium for all candidate and confirmed proteolytic systems in Arabidopsis in Supplemental Data Set 1 and through integration into the Plant Proteome Database (PPDB; http://ppdb.tc.cornell.edu/). This compendium serves also as the starting point for organellar peptidase network analysis presented here. Ultimately, a community annotation effort and systematic experimental information/testing for all putative/candidate peptidases will be needed for a more complete understanding of Arabidopsis proteolytic systems.
Coexpression analysis based on large-scale mRNA expression data sets has been shown to be an excellent tool to associate genes/proteins to metabolic pathways, tissue specificity, development (e.g., senescence), or (a)biotic stress and also to suggest protein-protein interactions; this is known as the guilt-by-association (GBA) principle (Movahedi et al., 2012; Tohge and Fernie, 2012; Higashi and Saito, 2013; Rhee and Mutwil, 2014; He et al., 2016; Schaefer et al., 2017). It is less clear to what extent coexpression aids in the inference of subcellular location, but there are several genome-wide studies that showed coexpression of nuclear genes (“regulons”) encoding for plastid proteins (Mentzen and Wurtele, 2008; Leister et al., 2011). This was also demonstrated for most members of the specialized proteome located in chloroplast plastoglobules, with the exception of senescence-induced genes (Lundquist et al., 2012). In the case of nuclear genes encoding mitochondrial proteins, it appears that many genes encoding for aerobic respiration show strong coexpression, but genes encoding other mitochondrial functions generally do not (Mentzen and Wurtele, 2008; Leister et al., 2011). Hence, it seems possible to use coexpression as a tool to infer genes encoding plastid proteins and to a lesser extent for mitochondrial proteins; however, we are not aware of efforts to make such inferrals based on significance thresholds. In the second portion of this review, we identify significance thresholds for discovery of potentially new plastid peptidases based on coexpression data and consequently identify a number of additional (candidate) plastid peptidases.
In the third portion of this review, we use coexpression network analysis to associate known and newly inferred mitochondrial and plastid peptidases to biological processes, development, specific tissue types (e.g., root, pollen) and stress, and generate hypotheses about functional interactions between peptidases. Tight coexpression of peptidase subunits of the same complex within the network suggest that the network quality is high and that meaningful information about peptidase function can be extracted from such networks. This plastid and mitochondrial peptidase network can therefore serve as a resource for further exploration by the research community, including guidance for experimental determination of genetic interactions.
In the last section of this review, we evaluate the periphery of the network, including peptidases that are unconnected, and explore the network for the location of plastid biogenesis factors, involved in division, assembly, and protein modifications.
Finally, the MapMan functional annotation system within the PPDB was expanded with additional functional bins to include assignment of proteins to MEROPS families and clans; this should be an excellent resource for plant proteostasis research.
BUILDING A PLANT PEPTIDASE COMPENDIUM
Curation and Functional Assignments
We first built a comprehensive (candidate) peptidase and peptidase inhibitor gene list for Arabidopsis (Col-0) by two complementary approaches: (1) extraction from the MEROPS database of all listed sequences for Arabidopsis peptidases and peptidase inhibitors, followed by BLAST-P against the predicted Arabidopsis proteome (TAIR10) to remove redundancy and extract the current protein identifier and (2) systematic functional domain analysis for the entire predicted Arabidopsis proteome using PFAM (Finn et al., 2016) and extracting proteins with tentative peptidase domains.
Since our long-term objective is to provide a resource for functional peptidase studies and the proteostasis network, we also added nonpeptidase components of peptidases, such as the substrate adaptors CLPS1, CLPF, and AAA+ chaperones CLPC, CLPX, and CLPD that are part of the organellar CLP peptidase system (Nishimura and van Wijk, 2015). Similarly, we included the nonproteolytic lid and base of the 26S proteasome and the complete autophagy machinery following the most recent nomenclature literature (Vierstra, 2009; Avila-Ospina et al., 2014; Marshall and Vierstra, 2015) (Supplemental Data Set 1). None of these autophagy (ATG) components members have an assigned MEROPS clan and family with the exception of ATG4a (AT2G44140) and ATG4b (AT3G59950) in clan CA and family C54, functioning as cysteine peptidases in the ATG8/12 conjugation pathway (Woo et al., 2014; Zientara-Rytter and Sirko, 2016). We also included the 27 ubiquitin-specific cysteine peptidases (UBPs) involved in cleavage of ubiquitin after their synthesis or involved in removal of ubiquitin from ubiquitinated proteins (Yan et al., 2000; Isono and Nagel, 2014) following the nomenclature in Liu et al. (2008); these peptidases are assigned to clan CA, families C12 and C19 in MEROPS (Supplemental Data Set 1). Finally, we also included 44 genes encoding for peptidase inhibitors (clans IA, IC, ID, IG, IH, JD, and JE).
Another group of proteins that warrants attention are close homologs of well-studied peptidases that lack key catalytic residues involved in peptidase activity. These noncatalytic members frequently contribute to peptidase function, and in the context of generating a comprehensive Arabidopsis (and plants in general) proteostasis compendium and network, it is important to include and annotate these. For example, within the CLPP family of serine peptidases, Arabidopsis and other plants have CLPP homologs, named CLPR, that lack critical residues in the catalytic triad and are therefore likely to have no cleavage activity. Extensive investigations have demonstrated that they are integral part of a hetero-oligomeric CLP peptidase core complex (Nishimura and van Wijk, 2015). Similarly, the well-studied FTSH metallo-peptidase family (clan MA [E], family S41) has five homologs that lack the catalytic residues for peptidase function, assigned FTSHi1-5 (Kadirjan-Kalbach et al., 2012; Lu et al., 2014). Furthermore, the LON peptidase family in Arabidopsis (clan SJ, family S16) has four homologs that appear as truncated forms of LON, and we named these LON-like1-4 or LONL1-4 (Supplemental Data Set 1).
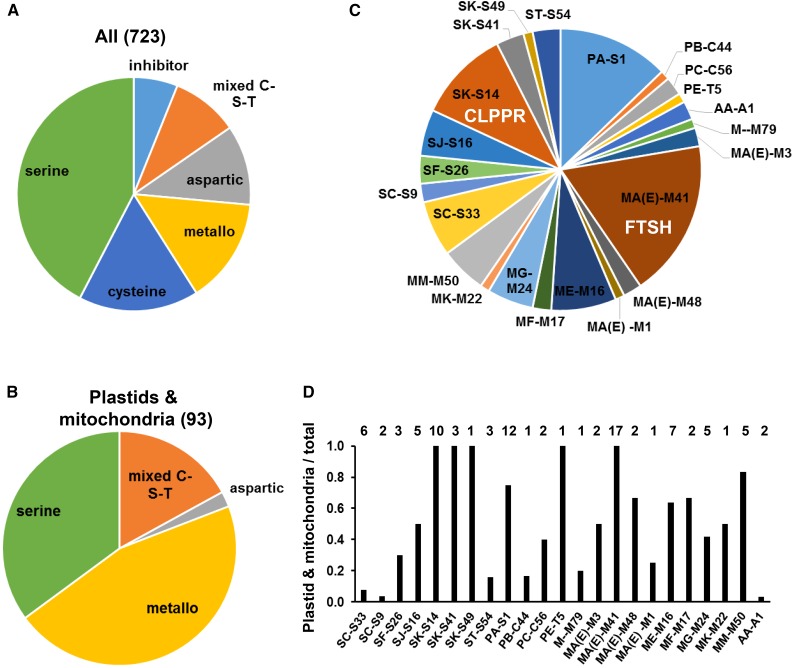
In total, we assembled 809 genes (represented by 1090 gene models) in Supplemental Data Set 1, of which 723 have a clan and family assignment (either directly from MEROPS or assigned by us). Figure 1A shows the relative distribution of these 723 proteins with assigned MEROPS clan/family. Proteins are assigned to five major groups, namely, cysteine (∼120 proteins), serine (∼306 proteins), metallo (∼105 proteins), aspartic (∼80 proteins) (Simões and Faro, 2004; Faro and Gal, 2005; Guo et al., 2013), or mixed (C-S-T) (∼68 proteins) and 44 inhibitors. We expanded the MapMan functional annotation bin system within PPDB to include detailed sub-bins for families and clans in a hierarchical manner. Following these assignments and curation of available information, in our updated MapMan annotation, all peptidases and peptidase inhibitors, as well as known auxiliary components of peptidase (systems) such as the CLP chaperones and 26S lid and base, are assigned to be within bin 29.5 “protein.degradation” (see http://ppdb.tc.cornell.edu/dbsearch/mapman.aspx and Supplemental Data Set 1). In total, 678 proteins have a primary functional assignment to this bin 29.5. If proteins with MEROPS clan/family assignments had likely functions unrelated to protein processing or turnover (based on our curation), their primary MapMan bin assignment was outside of bin 29.5 (e.g., to bin 11, lipid; bin 13, amino acids; bin 21, redox; or bin 26.57*, α/β-hydrolase family) (Supplemental Data Set 1), but some received a secondary bin assignment to 29.5. These proteins with primary bin assignment outside of 29.5 are thus suggested not to be part of the proteolysis network and have other (enzymatic) functions. An example of the curation process for one of the most complex families is discussed in the next section to illustrate our curation process. We emphasize that systematic experimental information/testing for all putative/candidate peptidases will be needed to build a truly high well-annotated peptidase compendium and a more complete understanding of Arabidopsis proteolytic systems.
Figure 1.
Distribution of All Arabidopsis and Specific Plastid and/or Mitochondrial (Putative and Confirmed) Peptidases and Peptidase Inhibitors across Families and Clans Using the MEROPS Classification System.
(A) and (B) Comparison of the relative distribution of the 723 proteins with assigned MEROPS clan/family predicted from the Arabidopsis genome (A) to those specifically located (experimentally confirmed) in plastids and/or mitochondria (B), based on Supplemental Data Set 1 (counting just one model per gene). Proteins are assigned to five major groups, namely, cysteine, serine, metallo, aspartic peptidases, or mixed (C-S-T) peptidases and peptidase inhibitors.
(C) Distribution of plastid and mitochondrial peptidases and peptidase inhibitors across the different families. Names are listed as clan family.
(D) Under- or overrepresentation of the 24 families in plastid and/or mitochondria compared with total Arabidopsis peptidase complement. Names are listed as clan family.
An Example of the Curation Process
The α/β-hydrolase (ABH) fold superfamily is found in all domains of life and serves catalytic roles such as esterases, thioesterases, lipases, epoxide hydrolases, as well as receptors and serine-dependent peptidases, and is represented by ∼638 genes in Arabidopsis (Mindrebo et al., 2016). This superfamily is one of the most challenging families when it comes to functional annotation. Based on MEROPS, we assigned 192 genes (represented by 251 gene models) within the Arabidopsis ABH superfamily to the SC clan of serine hydrolases (Supplemental Data Set 1). The SC clan has six families (S9, S10, S15, S33, and S37), and, except for S37, all families are found in plants. The SC clan includes both endopeptidases and exopeptidases (both amino- and carboxy-exopeptidases) and the active site residues Ser, Asp, and His. However, within this Arabidopsis SC clan, there are multiple members that have demonstrated functions other than peptidases, such as several methyl esterases (assigned in MEROPS to S33) (e.g., AT2G23560 and AT2G23550) (Vlot et al., 2008; Yang et al., 2008), and various carboxylesterases involved in detoxification of xenobiotic compounds (e.g., herbicides), such as CXE12 (AT3G48690) (Gershater et al., 2007; Nickel et al., 2012). Therefore, we assigned these proteins with demonstrated non-peptide functions to their most relevant bin function, but to keep track of this superfamily, they also received a secondary bin assignment to 26.57* α/β-hydrolase family. Furthermore, SC clan members without (significant) PFAM predicted S9, S10, S15, and S33 peptidase domains (E-value > 0.1 or domain not detected) or SC members with predicted S9, S10, S15, or S33 peptidase domains that have other predicted PFAM domains unrelated to proteolysis with more confident E-values (in addition the ABH domain) were given the primary bin 26.57* α/β-hydrolase family. For example, most S33 members do not appear to be peptidases, with the exception of two homologs of wheat prolyl aminopeptidase 1 (PAP1). The S15 family contains X-Pro dipeptidyl-peptidases, but MEROPS did not assign any Arabidopsis proteins to S15. To further clarify annotation of the SC clan members, we verified the E-values for these S9, S10, S15, and S33 predicted PFAM domains and assigned the proteins to their respective family based on lowest E-value; three of the MEROPS assigned S33 members were reassigned to S15 and five S9 members were reassigned to S15. We refer to more details and functional studies to the various Arabidopsis community resources, in particular TAIR (https://www.arabidopsis.org/) and ARAPORT (https://www.araport.org/).
Distribution of Peptidase Families across Plastids and Mitochondria
Because our immediate objective was to collect, discover, and annotate the compendium of peptidases and their regulators/adaptors within plastids and mitochondria, we curated the proteins in Supplemental Data Set 1 for known plastid and/or mitochondrial location, based on the existing information within PPDB and updates with additional experimental information from published literature (see Introduction) and online resources. Candidate and confirmed plastid and/or mitochondrial proteostasis components are listed in Supplemental Data Set 2. We note that this table also includes additional dozen proteins inferred from other information or based on coexpression, as will be discussed further in this review. Proteins were not assigned to a subcellular localization based on localization predictors alone (see Supplemental Data Set 2 for comments on assignments).
Whereas in total 67 different MEROPS families (57 when excluding non-peptidases) are reported in the total Arabidopsis data set (Supplemental Data Set 1), there are only 23 reported in plastids and mitochondria combined. Figures 1B and 1C summarize the distribution of proteins in plastids and mitochondria across the major groups of peptidases and inhibitors (Figure 1B) as well as their distribution across specific families (Figure 1C), whereas Figure 1D shows the under- or overrepresentation within these organelles. Metallo-peptidases are strongly overrepresented in plastid/mitochondria with 45% in these organelles (Figure 1B) compared with 15% on the total set (Figure 1A). By contrast, no cysteine peptidases have been experimentally identified in plastids or mitochondria, whereas they represent ∼20% of all peptidases predicted from the Arabidopsis genome (clans CA, CD, CE, CF, and CP) (Martínez et al., 2012; Díaz-Mendoza et al., 2014). Similarly, aspartic peptidases are strongly underrepresented in the organelles, but represent 14% of all predicted peptidases in Arabidopsis (clans AA and AD). There was also a lack of subtilases and peptidase inhibitors in plastids or mitochondria (see below). When considering only those proteins with clan and family assignment that are also assigned to bin 29.5 (“protein.degradation”), then the overall number of serine peptidases, and peptidases within so called mixed cysteine-serine-threonine nucleophile (C-S-T) families, decreased from 306 to 200 and from 67 to 50, respectively.
Several families are exclusively reported in the organelles, namely, MA(E)-M41 (FTSH family), SK-S14 (CLPPR family), SK-S41 (CtpA family), SK-49 (SPPA), and non-peptidase PE-T5 (Figures 1C and 1D). These are all of bacterial origin and apparently did not evolve to function outside of the organellar compartments of prokaryotic origin. Several other families are also overrepresented in the organelles, but they are not exclusively organellar, such as MA(E)-M3 (OCT, OOP, cyOP, and Zincin-like), MA(E)-M48 (STE24, OMA1, and PGM48), ME-M16 (SPP, PREP1/2, MPP, and others), MF-M17 (leucyl aminopeptidases 1,2,3), MM-M50 (RIP peptidases, including EGY1,2,3), and PA-S1 (DEG family) (Figure 1D). A few families in the organelles are strongly underrepresented, such as M-M79 (CAAX peptidases; but see below), MA(E)-M1, ST-S54 (rhomboids), SC-S9 and SC-S3 (includes prolyl aminopeptidases), and nonproteolytic PB-C44 (Figure 1D).
Lack of Cysteine Peptidases
Arabidopsis cysteine peptidases, such as the cathepsins and metacaspases, include many of the most abundant peptidases in plants with well-established functions in a variety of processes, including senescence and programmed cell death (Poret et al., 2016). Other cysteine peptidases are deubiquitinating enzymes (DUBs) and deSUMOylating enzymes (SUMO isopeptidases); neither ubiquitinylated nor SUMOylated proteins have been reported within the plastids or mitochondria, making it therefore less likely to find any of these DUBs or SUMO isopeptidases within the organelles. Cysteine peptidase activity is important for degradation of the chloroplast protein RIBULOSE 1,5-BISPHOSPHATE CARBOXYLASE OXYGENASE (RUBISCO) during senescence, but cysteine peptidase activity appears mostly confined to vesicles and the vacuole (Carrión et al., 2013, 2014). There must be a physiological or perhaps evolutionary reason why cysteine peptidases are so underrepresented or absent in mitochondria and chloroplasts. Among the cysteine peptidases, a small subset has a predicted cTP or mTP (Supplemental Data Set 1) and one of them (AT2G38025 and its homologs in other species) was identified in nonphotosynthetic plastids in maize (Zea mays), Brassica oleracea, and Arabidopsis chloroplast nucleoid samples (see PPDB). However, AT2G38025 (OTU3) showed deubiquitinase activity (Radjacommare et al., 2014), making intraplastid localization less likely, since there has been no evidence for intraplastidic (nor intramitochondrial) ubiquitination. The catalytic mechanism of cysteine peptidases involves a nucleophilic attack of peptidase bonds by a sulfhydryl group within the peptidase. We speculate that the explanation for the lack of observed cysteine peptidases in plastids and mitochondria lies in the fact that both organelles undergo strong redox changes during day-night cycles and in dependence of metabolic activity, thus posing a challenge to keep these cysteine peptidases active; a systematic analysis into this lack of plant mitochondrial and plastid cysteine peptidases is needed.
Underrepresented Aspartic Peptidases
Aspartic peptidases are widely distributed in prokaryotes and eukaryotes, and many are well studied in animals, but less so in plants (Takahashi et al., 2008; Gao et al., 2017). The aspartic peptidases in Arabidopsis are represented by clan AA with families A1 (65 proteins), A11 (8 proteins), and clan AD with family A22 (8 proteins); most have signal peptides and are sorted to the cell wall or vacuoles (Rawlings et al., 2016) (Supplemental Data Set 1). Aspartic peptidases are typically activated by acidic pH, perhaps explaining why they are so underrepresented in plastids and mitochondria. The only acidic space within the organelles is the thylakoid lumen, but (so far) no Asp peptidases have been identified within the lumenal proteome. Senescence-induced degradation of chloroplast RUBISCO involves vesicles with acidic pH (SAVs, CCVs, or RCBs), which provide a suitable environment for Asp peptidase activity, but these vesicles are not considered plastid-localized (Carrión et al., 2014; Poret et al., 2016). A few A1-type aspartic peptidases have been experimentally reported in chloroplasts of Arabidopsis (named NANA, AT3G12700) (Paparelli et al., 2012), in tobacco (Nicotiana tabacum) chloroplasts (named CND41) (Nakano et al., 1997; Kato et al., 2005) (NbS00024574g0006.1), and in Chlamydomonas reinhardtii chloroplasts (named CHLAPSIN) (Almeida et al., 2012). Tobacco CND41 was reported to be involved in degradation of RUBISCO, in particular during leaf senescence (Kato et al., 2004, 2005). The closest Arabidopsis homologs of tobacco CND41 are AT5G10770 (55% identity) and AT5G10760 (also named APOPLASTIC EDS1 DEPENDENT PROTEIN [AED1]). AT5G10770 was identified in cell wall or plasma membrane proteomes, whereas AT5G10760 was identified in the apoplast proteome or in whole leaf samples (see PPDB). The functional homolog of CND41 in Arabidopsis chloroplasts has thus not been identified and it may not exist. The functions and targets of NANA are not known and Arabidopsis NANA protein is distant from the Arabidopsis CND41 homologs. Arabidopsis NANA has been identified in mass spectrometry-based proteomics studies of nonphotosynthetic cell cultures (see PPDB; Zhang and Peck, 2011), but not in leaf chloroplast samples from Arabidopsis, maize, or rice (Oryza sativa), suggesting it has a very low abundance in leaves (see PPDB). Inspection of mRNA expression levels in public databases (e.g., the Bio-Analytic Resource for Plant Biology [BAR] at http://bar.utoronto.ca/eplant/) shows that NANA mRNA accumulates quite specifically in the hypocotyl and root in the cotyledon stage, with much lower levels elsewhere or in other developmental stages. Finally, the closest Arabidopsis homologs of Chlamydomonas CHLAPSIN are the highly abundant AT1G11910, observed in many proteomics studies but not in chloroplast proteomes, and AT1G62290 observed multiple times in vacuolar proteome studies (see PPDB). It should be noted that it was suggested that CHLAPSIN is the only aspartic peptidase encoded in the Chlamydomonas genome, unlike in higher plant species which typically have many aspartic peptidases (Almeida et al., 2012).
Lack of Subtilases
The subtilases are one of the largest families of serine peptidases in plants (clan SB, family S8; 56 subtilases and 3 truncated subtilases; Supplemental Data Set 1). They typically have N-terminal signal peptides for secretion and many are located in the cell wall or apoplast. Subtilases in plants are well known for their role in environmental interactions, including biotic stress, but are involved in many aspects of the plant life cycle (Rose et al., 2010; Schaller et al., 2012; Meyer et al., 2016; Schardon et al., 2016). The highly abundant tripeptidyl serine peptidase II (TPP2; AT4G20850) is an unusual subtilase in that it is an exopeptidase (Book et al., 2005), and it does not have a predicted signal peptide but rather a predicted chloroplast transit peptide. TPP2 likely acts downstream of the proteasome to further degrade proteolytic fragments similar as its homolog in mammalian cells (Polge et al., 2009). TPP2 was also suggested to be located in the stroma of Arabidopsis chloroplasts in a large scale proteomics study (Zybailov et al., 2008), but the very high abundance of TPP2 makes this subcellular assignment based on proteomics data alone difficult. When also considering proteomics data for the single TPP2 homolog in rice and maize using similar methodology as for Arabidopsis (see PPDB), it is likely that TPP is not a plastid protein but rather cytosolic. Consequently, none of the Arabidopsis subtilases appear to be plastidic or mitochondrial.
Lack of Peptidase Inhibitors
None of the 44 peptidase inhibitors was assigned to the organelles. There was one report of a mitochondrial proteome study in which the Kunitz-type inhibitor AT1G73260 (family I3) was reported in the mitochondria (Heazlewood et al., 2004), and the subcellular location is listed in TAIR as mitochondrial, but multiple other proteomics studies identified the peptidase in the cell wall (see PPDB). Most peptidase inhibitors either have a predicted signal peptide (for secretion) or no N-terminal targeting sequence, whereas four have a predicted mTP, but none have a cTP. However, there is no experimental support for mitochondrial location for these inhibitors; therefore, we did not assign any of these 44 peptidase inhibitors to these two organelles. The function of plant peptidase inhibitors has been reviewed for several types of inhibitors, including the multifunctional inhibitors (Grosse-Holz and van der Hoorn, 2016), the cysteine inhibitors/cystatins (I25 family) (Martínez et al., 2012; Díaz-Mendoza et al., 2014), and serpins (I4 family) (Roberts et al., 2011; Fluhr et al., 2012). Since the plastids and mitochondria have no known cysteine peptidases, it would make sense also not to have cystatins. Furthermore, plastids and mitochondria have no reported peptidases in the S1 or S8 families (Figure 1C); most of the peptidase inhibitors target these two serine peptidase families (I1, I3, I4, I13, I18, and I51), again explaining the likely lack (and lack of relevance) of these inhibitors for these organelles.
PREDICTING PLASTID AND MITOCHONDRIAL LOCALIZATIONS BASED ON COEXPRESSION AND OTHER INFERRALS
Generation of a Whole-Genome Coexpression Matrix
To predict or infer additional mitochondrial or plastid peptidases and associated nonproteolytic factors, we took advantage of the GBA principle using coexpression analysis. We generated a whole-genome coexpression network with 20,906 genes, using a public collection of 1388 GeneChip data sets (46 series, 58 experiments) from TAIR (microarray data from AtGenExpress) as the source (Obayashi et al., 2014; Aoki et al., 2016). Some series were divided into subseries according to genotypes or tissues to be treated; ME00325, ME00326, ME00327, ME00328, ME00329, ME00330, ME00338, and ME00340 were divided into shoot and root. ME00339 was divided into shoot, root, and cell suspension culture. ME00335 and ME00343 were divided into wild type and mutant. Genes were normalized based on the expression levels in the 58 experimental subseries. For each gene, the average expression value across all chips in the subseries was subtracted and all subseries were combined into one gene expression table. From the coexpression data set, Pearson correlation coefficient was used not for the selection of coexpressed genes, but to generate mutual rank for gene pairs (Obayashi and Kinoshita, 2009). RMA (robust multiarray average) normalization was applied to the 46 series in R/Bioconductor with default options. The complete genome-wide network was formatted as an edge list of correlations between Arabidopsis genes (20,906 baits) and their top 20 coexpressors (Supplemental Data Set 3A).
Inferral of Subcellular Organization Based on the GBA Principle
We annotated the baits and their coexpressors in Supplemental Data Set 3A with curated subcellular localization information from PPDB (May 2016) after removal of self-loops (i.e., the bait itself, if present) and removed obsolete gene models (Supplemental Data Set 3B; final set of 19,992 baits). PPDB has a fairly high number of subcellular or suborganellar localization types (e.g., for plastid proteins, such as thylakoid lumen; or plastid stroma) and in some cases more than one location. Whereas this is beneficial for evaluating specific functions or protein-protein interactions, for the purpose of predicting localization from coexpression analysis, a lower resolution localization assignment is more appropriate. Therefore subcellular localizations were grouped into seven categories: (1) cytosol (includes cytosol, cytoskeleton, and oil bodies), (2) mitochondrion, (3) nucleus, (4) plastid, (5) peroxisome, (6) secretory (includes endoplasmic reticulum [ER], Golgi, vacuole, cell wall, plasma membrane, and extracellular localization), and (7) unknown. For all baits, we then inferred a localization based on these seven localization categories, defined as the localization with the highest frequency among its coexpressors for each protein; we named this the TopGuess localization. Subsequent detailed analysis of the TopGuess results (data not shown) showed that coexpression of genes with genes encoding for plastid proteins is a good discovery tool for identification of new, potential plastid proteins, but it underestimates mitochondrial location.
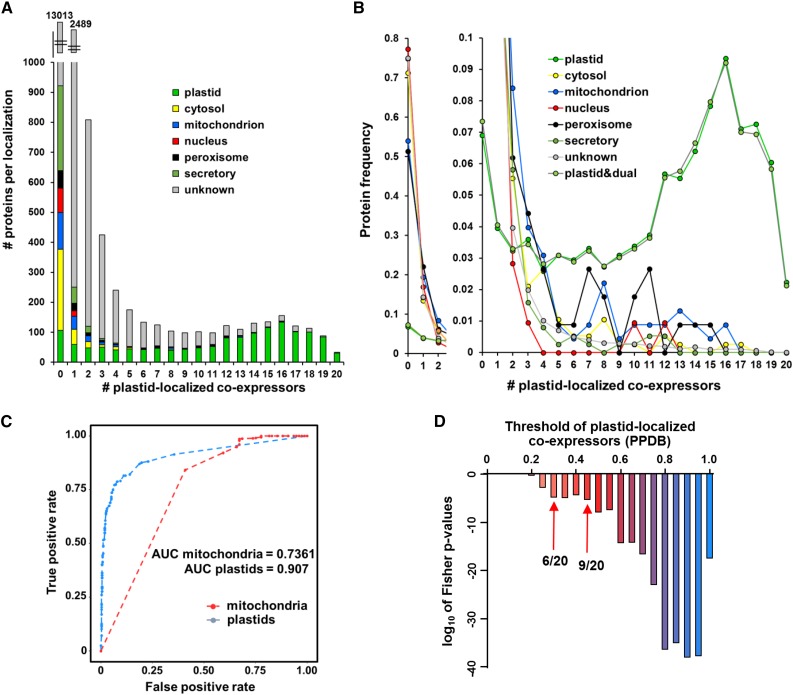
Figures 2A and 2B summarize the subcellular curated localization of proteins (either absolute number [Figure 2A] or frequency [Figure 2B] within each localization) as a function of the number of coexpressed plastid proteins. This quantitatively shows that plastid proteins have a strong propensity to coexpress with other plastid proteins. However, a significant portion of plastid proteins do not show this behavior (15% of plastid proteins have two or less plastid coexpressors out of 20); there are a variety of reasons for the lack of coexpression, including (1) incorrect plastid assignment (e.g., ASP3/YSL4 At5G11520 is likely peroxisomal; Eubel et al., 2008), (2) expression during senescence or PLAT3 AT4G39730 likely localized in the ER (Hyun et al., 2014), (3) possible metabolic regulations (e.g., for chloroplast NAD-dependent MDH AT3G47520; Yoshida and Hisabori, 2016) coexpressing with cytosolic proteins, and (4) unknown subcellular localization of coexpressors. We further note that the group of dual-targeted plastid proteins have a highly similar coexpression pattern as those plastid proteins located only in plastids (Figure 2B).
Figure 2.
Significance Analysis of Using the Subcellular Location of Coexpressors as a Predictor for Subcellular Location for Plastids and Mitochondria.
(A) and (B) Subcellular protein localization distribution of baits as a function of the number of coexpressed plastid proteins, as either absolute number (A) or frequency (B) within each localization.
(C) and (D) Significance analysis of the GBA principle for colocalization for plastid and mitochondrial baits based on coexpression networks.
(C) ROC curves for logistic regression model for classification of mitochondrial and plastid proteins based on TopGuess. Binomial logistic regression was performed using glmnet package in R. Logistic regression model was fitted using the lasso method on coexpressor localization percentage data as independent variable and curated localization (from PPDB) as response variable (i.e., for plastid yes/no localization, for mitochondria yes/no). The independent variable was formatted as two-column matrix with plastid (or mitochondrial) coexpressor percentage and summed percentage of all other coexpressor localizations. A 10-fold cross-validation was performed to estimate minimal lambda to be used in prediction. Mitochondrial and plastid data sets with curated PPDB localizations were divided into training (75%) and test sets (25%). ROC curves and area under the curves were computed for test sets with curated localizations as true localizations and TopGuess as predictions. The minimal lambda used for the mitochondrial data set was 0.0009323943, and area under ROC curve was 0.7361. For the plastid data set, minimal lambda used was 0.001509288 and area under ROC curve was 0.907.
(D) P value distribution based on the Fischer test for different thresholds of plastid-localized coexpressors. To assess which minimum percentage of coexpressors can best serve as a threshold whether a bait can be considered to have significant percentage of plastid coexpressors, exact Fisher P values were calculated for each possible threshold (from 0/20 to 20/20). Fisher test was performed on the plastid data set with curated PPDB localizations. P values of 0.05 or 0.001 correspond to six or nine co-expressed plastid proteins, respectively, out of 20 (indicated by arrows).
Contingency matrix was designed to contain number of plastid baits with the tested number of plastid coexpressors (A), number of non-plastid baits with tested number of plastid coexpressors (B), number of plastid baits with less than given number of plastid coexpressors (C), and number of non-plastid baits with less than given number of plastid coexpressors (D).
Since our manual evaluation (above) suggests that the GBA principle appears to apply to prediction of plastid proteins, we defined statistical thresholds for coexpression as predictive for plastid localization. ROC (receiver operating characteristic) curves show that TopGuess corresponds well to true curated localization (Figure 2C). An area under ROC curve of 0.907 for plastid localization inference shows that our Top Guess approach can be applied for subcellular localization inference for plastid proteins of previously unknown localizations. To asses which minimal number of coexpressors should be used as the threshold for inferred subcellular localization, exact Fisher P values were calculated for 0/20 through 20/20 coexpressors. P value distribution across different coexpressor thresholds shows that significant P values start at 6/20 coexpressors (for corrected P value threshold of 0.05) or 9/20 (for corrected P value threshold of 0.001) for plastids (Figure 2D).
There were nearly 500 proteins with unknown location that had six or more coexpressed plastid proteins; 14 of these were part of the proteostasis proteome with a 29.5 bin assignment as listed in Supplemental Data Set 1, and we added these proteins to Supplemental Data Set 2 since they may have relevance for plastid proteostasis. These include five E3 ligases (SC-F box AT5G63780 and AT5G58580; RING-type AT1G57790, AT2G04230, and AT4G07400). The E3 ligases are unlikely to be localized within plastids since there has been no evidence for intraplastidic ubiquitination, but their coexpression with known plastid proteins suggests that they are somehow involved with the plastid life cycle or are associated with the outer envelope. For instance, they may target plastid proteins for degradation outside of the plastid, similar to what has been shown recently for three E3 RING ligases, SP1 (AT1G63900; and SP1-like AT1G59560) (Ling et al., 2012), the TPR domain AtCHIP (AT3G07370) (Shen et al., 2007a, 2007b), and PUB4 (AT2G23140) (Woodson et al., 2015). Other proteins included a cysteine peptidase inhibitor of clan IH, family i25 (AT2G40880), one aspartic peptidase AA-A1 (AT4G33490), three serine peptidases (AT5G38510, AT5G59130, and AT5G42240), Zn-metallo-protein aspartyl aminopeptidase (DAP2) (AT5G04710) within clan MH, and family M18. Serine peptidase AT5G38510 is rhomboid membrane peptidase 9 (AtRBL9) (clan ST family S54) with predicted cTP but has not been studied in plants and has not been detected in proteomics studies (see PPDB). DAP2 has a predicted cTP and was detected in leaf proteome samples.
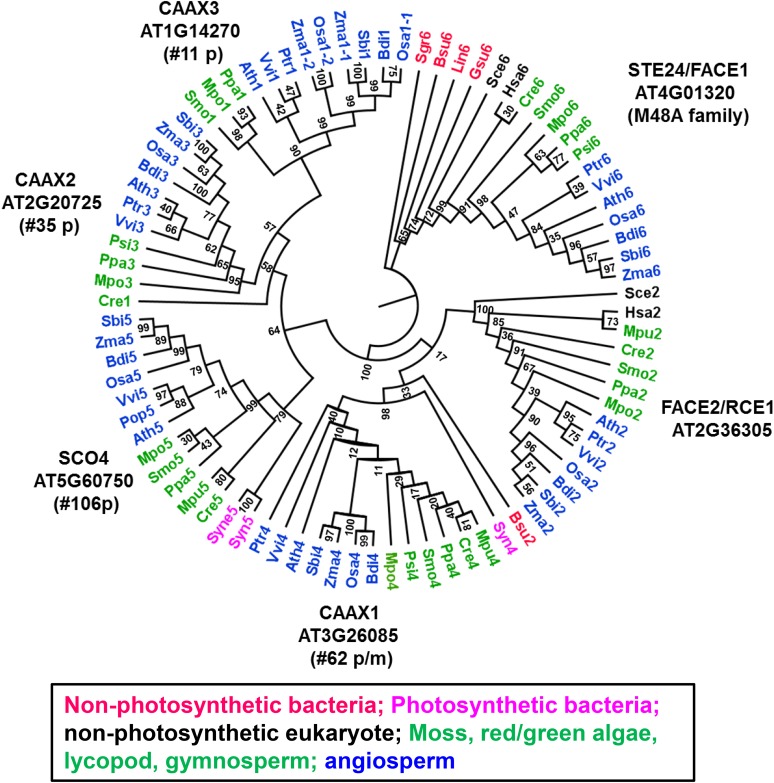
Finally, there were three CAAX metallo-peptidases of the M79 family (AT2G20725, AT1G14270, and AT3G26085). The prototype M79 peptidases typically cleave a C-terminal tripeptide from an isoprenylated protein, followed by methylation of the free carboxyl group of the isoprenyl cysteine by isoprenyl cysteine methyltransferase (ICMT); this is referred to as CAAX processing (see MEROPS). The Arabidopsis genome encodes for two additional Arabidopsis proteins in this M79 family, which are plastid-localized SNOWY COTYLEDON4 (SCO4; AT5G60750) (Albrecht-Borth et al., 2013) and plasma membrane-localized RAS-CONVERTING ENZYME1 (RCE1/FACE2; AT2G36305) (Cadiñanos et al., 2003; Bracha-Drori et al., 2008). There is no evidence that Arabidopsis SCO4 has CAAX activity, in contrast to RCE1 with demonstrated CAAX activity (Cadiñanos et al., 2003; Bracha-Drori et al., 2008). We realized that a large-scale GFP fusion analysis suggested that CAAX proteins AT2G20725 and AT1G14270 (here assigned as CAAX2 and CAAX3, respectively) are located in plastids and dual-localized in plastids and mitochondria, respectively (Ishikawa et al., 2009), but that there is no experimental localization information for AT3G26085 (assigned CAAX1). Phylogenetic analysis of the M79 family shows that the clade with RCE1/FACE2 includes nonphotosynthetic eukaryotes (e.g., yeast and human), red/green algae, and lower plants, but no prokaryotes (Figure 3). By contrast, CAAX1 and SCO4 form clades that lack nonphotosynthetic eukaryotes but include prokaryotes. This suggests that both CAAX1 and SCO4 are likely derived from a cyanobacterial ancestor, whereas CAAX2 and CAAX3 are likely derived from gene duplications in early photosynthetic eukaryotes. The phylogenetic analysis also includes ER-localized STE24/FACE1 of the M48 peptidase family (subclade M48A; Bhuiyan et al., 2016) because this has demonstrated CAAX activity in Arabidopsis as well as yeast (Cadiñanos et al., 2003; Bracha-Drori et al., 2008; Pryor et al., 2013). However, the phylogeny clearly shows that the M79 and M48 families are unrelated. We will explore these organellar M79 CAAX proteins further through coexpression network analysis below.
Figure 3.
Cladogram for the M79 Family Members across the Tree of Life.
Phylogenetic tree of the M79 family based on alignment of amino acid sequences of 68 M79 homologs from diverse archaea, prokaryotes, and eukaryotes. Additionally, as outgroup we included the M48A clade (18 sequences) with the well-characterized ER/nuclear envelope located STE24/FACE1 with demonstrated CAAX activity (Bhuiyan et al., 2016). Four M79 clades are assigned and are the FACE2/RCE1 clade, the CAAX1 clade, the SCO4 clade, and the CAAX2,3 clade. Bootstrap values are indicated at the nodes of the tree. Vitis vinifera (Vvi); Populus trichocarpa (Ptr); Arabidopsis thaliana (Ath); Zea mays (Zma); Brachypodium distachyon (Bdi); Oryza sativa (Osa); Sorghum bicolor (Sbi); Picea sitchensis (Psi); Selaginella moellendorffii (Smo); Physcomitrella patens (Ppa); Marchantia (Mpo); Cyanidioschyzon merolae (Cme); Chlamydomonas reinhardtii (Cre); Micromonas pusilla (Mpu); Synechocystis sp PCC6803 (Syne); Synechococcus sp PCC 7942 (Syn); Homo sapiens (Hsa); Saccharomyces cerevisiae (Sce); Agrobacterium tumefaciens (Atu); Nitrosomonas europaea (Neu); Escherichia coli (Eco); Sulfuricurvum kujiens (Sku); Geobacter sulfurreducens (Gsu); Streptomyces griseus (Sgr); Bacillus subtilis (Bsu); Leptospira interrogans (Lin); Leptospira biflexa (Lbi); Sulfolobus islandicus (Sis); Metallosphaera sedula (Mse); Haladaptatus paucihalophilus (Hpa); Natrinema pellirubrum (Npe). To generate a phylogenetic cladogram, the protein sequences were aligned using MUSCLE (http://www.ebi.ac.uk/Tools/msa/muscle/). The aligned sequences were exported in Clustal format and viewed in Jalview (www.jalview.org/). Sequences were then converted in PHYLIP format, and maximum likelihood phylogenetic trees were generated (1000 iterations) using the CIPRES web portal (http://www.phylo.org/) selecting the tool “RAxMLHPC blackbox.” The resulting phylogenetic tree was annotated in FigTree (http://tree.bio.ed.ac.uk/software/figtree/). RAxML bootstrap support values are shown at the nodes of the tree.
Complementary Inferral of Organellar Peptidases Based on Predicted cTP or mTP
As a complementary approach for finding new potential mitochondrial or plastid peptidases, we selected all proteins in Supplemental Data Set 1 with a predicted cTP or mTP that were not yet assigned to any subcellular location and were not detected through the coexpression analysis discussed above. These were then manually evaluated for circumstantial evidence of organellar location, while excluding peptidases of families not previously found in plastids or mitochondria. This suggested four peptidase candidates for which direct homologs in maize and rice also have predicted cTP/mTPs; these are DEG4 (AT1G65640), LON3 (AT3G05780), and rhomboid-(related) proteins (AT1G74130 and AT1G74140). DEG4 and LON3 are both in the coexpression network, but none of their direct coexpressors are plastid or mitochondria located, whereas the two rhomboids are not present in the array data set. Furthermore, there are two HSP100 chaperones CLPX2 and CLPX3 with predicted mTPs that are likely (but not formally proven to be) localized in mitochondria, together with the confirmed mitochondrial peptidase CLPP2 and chaperone CLPX1 (Halperin et al., 2001). These six proteins were added to Supplemental Data Set 2 and integrated into the coexpression network analysis below.
THE MODULAR PLASTID AND MITOCHONDRIAL COEXPRESSION NETWORK
Graphical Coexpression Network
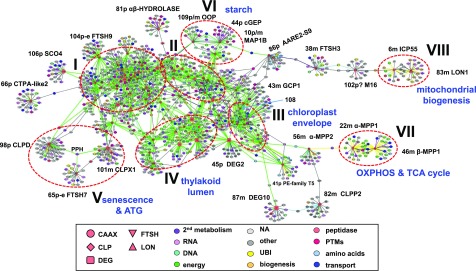
To provide insight into the possible functional roles and connectivity of organellar peptidases in Supplemental Data Set 2, we generated a graphical coexpression network with 97 (potential) plastid/mitochondrial genes serving as baits (seed genes used to build the network) (Supplemental Data Set 4A). The coexpressor data for these 97 baits were extracted from the whole-genome network edge list in Supplemental Data Set 3. The network shows a large dense central region containing the majority of plastid peptidases, a number of peptidases at the periphery of the central network (Figure 4), and several peptidases or peptidase chaperones that did not link up to this central network (Figure 5). After another round of manual annotation for function and subcellular location specifically for this gene in this network, color coding was used to highlight enriched functions (bait and coexpressor symbols) and annotated subcellular localizations (edges). To avoid cumbersome repetitions, we refer to baits and coexpressors in the network as genes and/or proteins.
Figure 4.
Graphical Display of the Central Portion of the Plastid and Mitochondrial Peptidase Coexpression Network Based on the Edge List in Supplemental Data Set 4A.
There are 97 peptidase/proteostasis baits, and the total network includes 1742 genes making 2544 edges. Color-coding of baits in the main proteolytic systems and the annotated subcellular localization, as well as annotated functions for coexpressors, are provided. The edges are colored according to the subcellular localization of the coexpressor (green, plastid; yellow, mitochondria; dark blue, dual-targeted plastid/mitochondria; light blue, cytosol; purple, secreted; gray, other). Coexpression modules (I–VIII) are indicated. Names of selected baits with their network ID and subcellular location are indicated (p, plastid; p-e, plastid envelope; m, mitochondria; p/m, dual-targeted plastid/mitochondria). Noteworthy enrichment for functional or subcellular localization in modules is indicated in blue. (A higher-resolution copy of this figure is presented as Supplemental Figure 3.)
Figure 5.
Graphical Display and mRNA Expression Patterns of Smaller Modules IX and X as Well as Unconnected Baits in the Plastid and Mitochondrial Peptidase Coexpression Network Based on the Edge List in Supplemental Data Set 4A.
Coexpression modules IX (A) and X (C). (B) shows that mRNA levels of several of the baits in module IX are strongly upregulated during heat stress. Data and image are generated from BAR. (D) shows the coexpressors of two baits just connected by one shared coexpressor (#305) and eight baits that are not connected to other baits. Color coding of baits in the main proteolytic systems and the annotated subcellular localization, as well as annotated functions for coexpressors, are provided. The edges are colored according to the subcellular localization of the coexpressor (green, plastid; yellow, mitochondria; dark blue, dual-targeted plastid/mitochondria; light blue, cytosol; purple, secreted; gray, other). Names of selected baits with their network id and subcellular location are indicated (p, plastid; p-e, plastid envelope; m, mitochondria; p/m, dual-targeted plastid/mitochondria). Noteworthy enrichment for functional or localization in modules is indicated in blue. (A higher-resolution copy of this figure is presented as Supplemental Figure 4.)
The network has a total of 2544 edges encompassing 1742 nonredundant genes, of which 35% have curated location in the plastid, 5% are in mitochondria, 2% are dually localized (30 proteins) in these two organelles, 17 proteins are in peroxisomes, with most of the rest without assigned location. Supplemental Data Set 4B provides a nonredundant list of baits and coexpressors with various annotations, including the frequency as coexpressor in the network. The dense central network is enriched for plastid proteins and can be broken up into four modules (I–IV) and four smaller modules in the periphery (V–VIII) (Figure 4). Disconnected from the central network are the two small modules IX (Figure 5A) and X (Figure 5B), and 10 other peptidases (Figure 5C). Mitochondrial peptidases are mostly in the periphery of the network, whereas plastid proteins were generally strongly connected. This is also reflected by a relatively low percentage of mitochondrial proteins in the network (4%) compared with plastid proteins (35%), and many mitochondrial peptidases are in the periphery of the network or not connected to other peptidases at all (Figures 4 and 5). Interestingly, there are several mitochondrial exceptions (modules VII and VIII). Assignment of baits and their direct coexpressors to these modules is provided in Supplemental Data Set 4A and for baits in Supplemental Data Set 2.
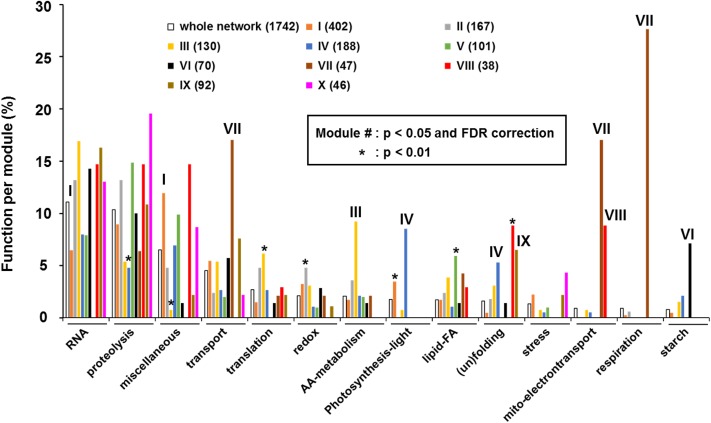
The most prevalent functions in the network were protein homeostasis (translation, folding, sorting, and proteolysis) (23%), RNA metabolism (11%), and transport (5%), whereas 23% had no assigned function. Among the mitochondrial proteins, functions were strongly biased toward protein homeostasis (32%), the tricarboxylic acid (TCA) cycle (20%), and oxidative phosphorylation (18%), in addition to RNA metabolism (10%) and transport (8%). The plastid protein members were mostly involved in protein homeostasis (28%), RNA metabolism (11%), photosynthesis (6%), and transport (4%). The relative functional distribution using 42 different functional categories was determined for the whole network and for each of the 10 modules, and significant enrichment or underrepresentation was calculated using hypergeometric distribution (Supplemental Data Set 5). The minimal threshold was P < 0.05 with false discovery rate (FDR) multiple testing correction for highest stringency, and P < 0.01 or P < 0.05 for lower stringencies (Supplemental Data Set 5).
Quality of the Network
If protein complex composition is regulated (at least in part) at the mRNA level, subunits of the same complex are expected to be coexpressed or to form part of the same coexpression module. Hence, in a biologically meaningful coexpression network, one expects subunits of complexes to be closely expressed. We therefore first probed the quality of the network using well-characterized peptidase complexes from four different subcellular locations, namely, (1) the thylakoid FTSH1,2,5,8 hexamer, (2) the lumenal DEG5/8 heterohexamer, (3) the stromal CLPPR tetradecameric protease core, and (4) the mitochondrial heteromeric α,β-MPP1 preprocessing complex.
The four FTSH1,2,5,8 are clustered in module I, with multiple direct edges between these FTSH members (1→2, 1→5, 1→8, 2→1, 2→5, 5→1, 5→2, 8→1) in support of a biologically meaningful network. Collectively these FTSH members have 100 nonredundant coexpressors (58% known plastid; most others with unknown location) that include plastid peptidase components (CLPR3, CLPC1, PREP1, SPPA, DEG1, and PGM48), as well as the E3 ring ligase SP1 involved in control of the TOC protein import machinery (Ling et al., 2012). Interestingly, seven coexpressors are involved in plastid isoprenoid metabolism: 4-hydroxy-3-methylbutyl diphosphate synthase in the methylerythritol 4-phosphate pathway, central carotenoid metabolism (PSY, PDS, and ZDS, a β-hydroxylase), PG-localized tocopherol cyclase (VTE1), as well as carotenoid cleavage enzyme CCD1, located on the cytosolic side of the chloroplast. Eleven of the coexpressors are located in the plastoglobules, including four ABC1 kinases and the peptidase PGM48, confirming our earlier observation that some of the PG functions (in particular ABC1 kinases) are transcriptionally coupled to thylakoid protein turnover (Lundquist et al., 2012; Bhuiyan et al., 2016). Together, this shows that the members of the thylakoid FTSH complex do closely coexpress together with isoprenoid metabolism (enriched at P < 0.05 with FDR correction) and plastoglobular proteins.
The stromal CLPPR complex is a well-characterized and stable tetradecameric core complex (325 kD) consisting of eight different nuclear-encoded gene products and one plastid-encoded gene product CLPP1, in one or more copies (Nishimura and van Wijk, 2015). These eight nuclear genes are closely coexpressed in the center of the network (within modules I and II) with many (32) direct edges, and a total of 114 direct nonredundant coexpressors of which 79% are known plastid or dual-targeted plastid/mitochondrial proteins. Functional distribution analysis shows a representation of other plastid peptidases (CLPC1, CLPT1, CLPT2, SPP, OOP, and CAAX), protein biogenesis (ribosomes, tRNA synthases, assembly factors, and protein sorting), and RNA metabolism, but only one protein involved in photosynthesis (stromal TRIOSEPHOSPHATE ISOMERASE-1). Interestingly, redox regulators (seven members of the plastid thioredoxin system), and amino acid metabolism and assembly factor (e.g., the SUFB,C,D complex involved in Fe-S assembly; Hu et al., 2017) are statistically significantly enriched (P < 0.01) among coexpressor functions (Supplemental Table 5). In contrast to the thylakoid FTSH complex, only three coexpressors of the CLPPR core encoded for proteins located in plastoglobuli (compared with 11 for FTSH1,2,5,8).
Finally, the two subunits of the mitochondrial MPP complex (module VIII) have 11 shared coexpressors and stand out in that 62% coexpressors are mitochondrial; TCA cycle and mitochondrial electron transport components are strongly overrepresented (47% of all coexpressors) (Figure 4). This is highly consistent with the observation that the plant mitochondrial MPP complex is part of the bc1 complex in the mitochondrial inner membrane, and the catalytic site is located in the β subunit of the MPP complex (Teixeira and Glaser, 2013).
We therefore conclude that the network reveals biologically relevant relationships and hence should provide a great tool for discovery of peptidase function. In the following sections we explore connectivity between peptidases and evaluate functional enrichment of coexpressors for the 10 modules. Figure 6 compares this functional distribution across modules and the whole network, showing those functions which were among the top five highest scoring functions in at least one of the modules; significantly enriched or underrepresented functions are marked, with thresholds as indicated. The functional distribution showed dramatic differences between modules, as will be discussed below.
Figure 6.
Functional Enrichment Analysis for the Modules I to X of the Coexpression Network Shown in Figures 4 and 5.
Coexpressors were assigned one out of 42 different functional categories. The bar diagram compares this functional distribution across modules and the whole network, showing those functions that were among the top five highest scoring functions in at least one of the modules. The enrichment analysis showed dramatic differences between modules. Significant overrepresentation or underrepresentation using hypergeometric distribution. The minimal threshold was P < 0.01 or P < 0.05 without or with FDR multiple testing correction for higher stringency as indicated (see Supplemental Data Set 5 for all frequencies and relative distributions and P values).
Modules of the Coexpression Network
Module I
This is a dense network with over 20 plastid peptidases, including several CLP components (S1, F, C1, R1, R3, and R4), the thylakoid FTSH1-2-5-8 complex, CAAX1,2, two of the uncharacterized plastid metallo M50 peptidases, LON-like 1 and 3, envelope FTSH9, PGM48, SPP, stress-induced thylakoid SPPA, and glycyl metallo-aminopeptidase (M1) (MPA1). The dually targeted PREP1 metalloprotease (#59 in the network graph; Supplemental Data Sets 4A and 4B) is assigned to both modules I and II, whereas CLPD is peripheral to module I, in the small module V. Module I contains ∼400 nonredundant genes (54% plastid-localized) making just over 700 edges. Compared with the whole network, module I is overrepresented (at P < 0.01) in genes involved in plastid isoprenoid metabolism, plastid protein assembly, photosynthetic light reactions, and plastid division and positioning, whereas genes involved in RNA metabolism are strongly (P < 0.05 and FRD correction) underrepresented.
Module II
This network includes 11 peptidases comprising the rest of the plastid CLP system (R2, P3, P4, P5, P6, T1, and T2), dual-localized thylakoid lumen and inner envelope PLSP1 (one of the three members of the THYLAKOID PROCESSING PEPTIDASE FAMILY), FTSHi3 (one of the five inactive plastid FTSHi members), and dual-localized plastid/mitochondrial MAP1D and PREP1. This module contains 168 nonredundant genes (81% plastid-localized of which 6% also mitochondrial) making 292 edges. This module is enriched (P < 0.01) for redox regulators, protein sorting, as well as plastid division and positioning.
Module III
Module III contains five plastid peptidases or non-peptidase homologs that are likely envelope-localized, namely, the other (nonproteolytic) FTSHi subunits (FTSHi1, 2, 4, and 5) and envelope FTSH12, as well as stromal PREP2 that is also localized to the mitochondrial matrix. This module contains 130 nonredundant genes (71% plastid-localized of which 8% also mitochondrial) making 196 edges. The module is enriched for proteins involved in plastid translation, as well as plastid division and positioning. However, especially striking is the strong enrichment in genes involved in amino acid metabolism (Figure 6), but without particular enrichment for a specific amino acid or amino acid family. Interestingly, the putative 3-hydroquinate synthase (AT3G28760) was a coexpressor with each FTSHi1, 2, 4, and 5 (Supplemental Data Set 4), but the function of this protein has not been established. Instead AT5G66120 is the canonical hydroquinate synthase that carries out the second step in the chorismate synthesis pathway (Bischoff et al., 1996) but is not part of the peptidase coexpression network, and instead coexpresses with a DAHP synthase in the same chorismate pathway. Both AT5G66120 and AT3G28760 have a high confidence DHQ-synthase PFAM domain, but they exhibit low sequence identity. Functional analysis of AT3G28760 might also shed light on the role of the FTSHi family.
Module IV
Module IV contains 10 plastid (putative) proteostasis factors: CAAX3, LON-like 2, EGY2, RBL8, four out the six thylakoid lumenal peptidases in the network (the lumenal DEG5/8 complex, D1 protein C-terminal peptidase CTPA, and its homolog CTPA-like-1), thylakoid AT5G27290, a homolog of thylakoid VIRESCENT3 (VIR3; AT1G56180), and a α/β-hydrolase. We note that it is not clear that AT5G27290 or VIR3 itself are actual metallo-peptidases as suggested by Qi et al. (2016), since they lack homology to other peptidases and are not assigned to a clan/family in MEROPS. However, they do have a typical Zn binding domain (as also demonstrated experimentally for VIR3; Qi et al., 2016), but this is not unique to metallo-proteases. The other (nonpeptidase) lumenal proteins (mostly isomerases and biogenesis factors) are strongly enriched in this module (31 edges). Just two other lumenal peptidases are not in this module; these are PLSP1 in module II and lumenal CTPA-like2 peripherally attached to module II. Module IV contains 188 nonredundant genes (72% plastid-localized) making 271 edges. The module stands out for the high enrichment in genes encoding for the photosynthetic transport system and (un)folding due to the presence of multiple thylakoid lumenal isomerases (Figure 6).
Connections between Modules I to IV
A few peptidases are centrally positioned between modules. The abundant plastid stromal M24 aminopeptidase 2 (APP2; #51) with unknown function connects modules I and IV. All but two of its coexpressors encode for plastid-localized proteins and include genes involved in plastid biogenesis and metabolism, including lumenal DEG8 in module IV. Envelope-localized FTSH11 (#100) connects modules I, II, and III, whereas aspartic peptidase DAP2 (#78) with as yet unproven subcellular location connects modules II, III, and IV. DAP2 and FTSH11 are direct mutual coexpressors and they share four coexpressors with diverse functions. DAP2 coexpressors are enriched in RNA metabolism (∼33% of coexpressors). Mitochondrial inner membrane glycopeptidase GCP1 (#43) connects module II and III and was initially assigned a member of M22 metallo-peptidase family. GCP1 is essential for embryogenesis; however, peptidase activity could not be confirmed for this Arabidopsis protein or it homologs in bacteria and nonphotosynthetic eukaryotes (Haussuehl et al., 2009). Interestingly, GCP1 coexpressors are strongly (>25%) enriched for translation components, including five dual-targeted mitochondrial/plastid tRNA synthases as well as plastid RNA metabolism. GCP1 is a functional homolog of KAE1 or YGJD, which have recently been shown to be involved in the biosynthesis of N6-threonylcarbamoyl adenosine, a universal modification found in tRNAs that read codons beginning with adenine (El Yacoubi et al., 2011; Srinivasan et al., 2011). Indeed, such a function for GCP1 would be highly consistent with the tight coexpression to t-RNA synthases. We therefore changed its bin assignment to 29.2 protein synthesis, instead of 29.5 protein degradation (Supplemental Data Set 2). GCP1 has one homolog in Arabidopsis (GCP2; AT4G22720) (Haussuehl et al., 2009) that appears localized in the cytosol, presumably carrying this tRNA modifying function outside of the organelles. Immunoblotting suggested that GCP1 was specific to mitochondria and absent in plastids (Haussuehl et al., 2009). It remains to be determined if this function is also required within plastids, which we suspect it is because this function is required in apicoplasts (Mallari et al., 2014). Plastid stromal DEG2 (#45) connects module III and IV and shares two coexpressors with DAP2 (AT3G29185 and AT3G62000; both encoding for plastid proteins). Over 80% of the 30 DEG2 coexpressors encode for plastid proteins, including nine ribosomal subunits and three proteins involved in RNA metabolism. This suggests that stromal DEG2 plays a particular role in early plastid biogenesis. Previous loss-of-function Arabidopsis DEG2 mutants showed only a weak phenotype, and it was proposed that DEG2 is required for normal plant development, including the chloroplast life cycle, and in the degradation of Lhcb6 (Luciński et al., 2011) and the D1 protein (Huesgen et al., 2006).
Module V
This small module is peripheral to module I and contains plastid envelope FTSH7 and a plastid and mitochondrial CLP chaperones CLPD and CLPX1, respectively. This module contains 101 nonredundant genes of which only 8% encode for known plastid-localized proteins. Public mRNA expression profiles in BAR show that CLPX1, FTSH7, and CLPD are all highly expressed during senescence (in rosette leaves, sepals, petals, and cauline leaves). The low percentage of plastid or mitochondrial localized proteins in this module is consistent with upregulation during senescence. Mitochondrial CLPX1 and envelope FTSH7 make an indirect connection to each other via an uncharacterized homolog of mitochondrial ICP55 (AT4G29490; #1304), and both with CLPD via pheophytin pheophorbide hydrolase (pheophytinase) (PPH; #1306) a key enzyme in chlorophyll degradation (Schelbert et al., 2009). Whereas none of the ATG components are located within plastids (or mitochondria), four ATG components (P13K, ATG4B, ATG1A, and ATG7) were found within this module V as coexpressors of either CLPX1 or FTSH7. P13K and ATG1 are part of the same kinase complex, while ATG4B and ATG7 are both part of the ATG8/12 lipidation step (Zientara-Rytter and Sirko, 2016). We note that these are the only ATG members in the whole network, making their presence within the small module V of potential functional significance and supporting the idea that autophagy is partially involved in removal of chloroplast content during senescence (Ishida et al., 2014). Finally, this module is enriched in lipid and fatty acid metabolism (Figure 6), in particular lipid degradation, which is consistent with membrane breakdown during senescence. It should be noted that several genes in this module are also upregulated in response to osmotic stress, in particular CLPD and PPH.
Module VI
Module VI contains just three highly connected peptidases, namely, dual-localized plastid/mitochondrial OOP and MAP1B, as well as stromal cGEP. This module contains 70 nonredundant genes (59% encode for confirmed plastid-localized proteins) making 81 edges. Surprising is the relative enrichment in starch metabolism (7% compared with 0.8% for the whole network; Figure 6), in particular genes encoding for starch degradation coexpressing with OOP and cGEP, as well as enrichment for plastid division and positioning.
Module VII
This module contains 47 nonredundant genes (64% mitochondria-localized) making 58 edges. The α- and β-subunits of the mitochondrial processing peptidase (MPP) (Murcha et al., 2014) show a striking coexpression with other mitochondrial proteins, as well as to each other (Figure 4). MPP in yeast and humans is a soluble complex in the matrix, while in plants it is integrated into the inner membrane cytochrome bc1 complex (complex III) of the respiratory chain (Brumme et al., 1998; Glaser and Dessi, 1999). The mitochondrial coexpressors are mostly involved in the TCA cycle and the electron transport chain (in particular the bc1 complex, cytochrome c1, and the ATP-synthase, as well as several mitochondrial metabolite transporters, such as adenine nucleotide carrier [ADNT1], phosphate transporter PHT3-1/PIC1, voltage-dependent ion channel [VDAC2], ADP/ATP carrier protein 1 [AAC1], adenine nucleotide carrier [ADNT1], and phosphate transporter [PHT3-1 or PIC1]). Given the role of MPP in pre-protein processing it is surprising that none of the coexpressors are members of the mitochondrial protein import machinery (e.g., TIM and TOM components) (Murcha et al., 2014).
Module VIII
Another very interesting case is the direct and tight connection between ICP55 (Carrie et al., 2015; Huang et al., 2015) and LON1 (Janska et al., 2010; Solheim et al., 2012), two well-studied mitochondrial peptidases involved in mitochondrial biogenesis (Figure 4). This module contains 34 nonredundant genes (35% mitochondria-localized) making 40 edges. Similar to yeast mitochondrial ICP55 (Vögtle et al., 2009), plant mitochondrial ICP55 removes unstable amino acids from the N termini of mitochondrial proteins after MPP cleavage (Carrie et al., 2015; Huang et al., 2015). Proteome analysis of Arabidopsis null mutants of LON1 suggested that LON1 functions as a general household peptidase, removing dysfunctional and aggregated proteins (Rigas et al., 2009; Solheim et al., 2012; Li et al., 2017). Among the coexpressors of ICP55 and LON1 are 10 other confirmed mitochondrial proteins, several of which have a function related to biogenesis, such as chaperone HSP60 and cochaperone GRPE-1, ribosome recycling factor, and RNA editing and processing factors. These coexpressors are in line with the function of LON1 as household/biogenesis peptidase. Cytosolic methionine amino-peptidase MAP2A involved in the removal of N-terminal methionine of proteins synthesized in the cytosol including mitochondrial proteins, also coexpressed with ICP55, in concordance with the fact that MAP2A operates immediately upstream of ICP55 (OCT mitochondrial peptidase, also involved in protein maturation by cleaving eight amino acids from the N terminus [Carrie et al., 2015], was not present in the array data set). However, whereas the MPP coexpressors are strongly enriched in TCA cycle and mitochondrial electron transport components, this is not the case for the LON1-ICP55 module, likely reflecting that MPP is part of the bc1 complex. Mitochondrial electron transport and (un)folding are both significantly enriched functions in this module (Figure 6).
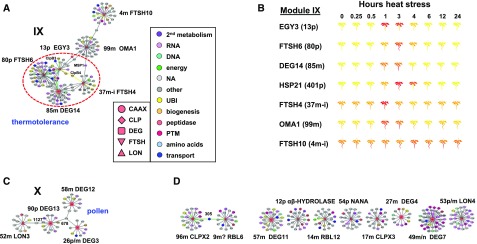
Module IX
Disconnected from the central network is a small network (module IX) with four mitochondrial (DEG14, FTSH4, FTSH10, and OMA1) and two plastid peptidases (FTSH6 and EGY3). In particular, DEG14, FTSH6, EGY3, and FTSH4 are tightly connected (Figure 5A). This module has 92 proteins making 115 edges and only 8% are plastid- or mitochondrial-localized, with most proteins lacking an annotated location. Functional enrichment analysis shows that protein (un)folding is highly overrepresented (Figure 6). Plastid HSP21 is a direct coexpressor of FTSH6, EGY3, and DEG14. FTSH6 is involved in heat stress and thermo-memory through regulation of cpHSP21 abundance (Sedaghatmehr et al., 2016), and DEG14, the direct homolog of mammalian and yeast mitochondrial PARK13, is also involved in heat stress response in mitochondria and confers thermotolerance (Basak et al., 2014). Investigation of public mRNA data sets shows that expression of six of the peptidases in this module are strongly heat induced (Figure 5B). Although not specifically reported in the literature, plastid EGY3, a coexpressor of both FTSH6 and DEG14, is also strongly heat induced, in particular after 1 to 3 h of heat stress, similar to FTSH6, DEG14, and cpHSP21 (Figure 5B). However, it was observed that other (likely) metallo-peptidases also contribute to degradation of cpHSP21 following the recovery phase after heat stress (Sedaghatmehr et al., 2016). We note that 39 organellar peptidases were specifically verified for mRNA upregulation by heat treatment, but only FTSH6 was enhanced (Sedaghatmehr et al., 2016). Neither plastid EGY3 nor mitochondrial DEG14 were among these 39 genes; hence, the metallopeptidase EGY3 is a good candidate for the complementary cpHSP21 degradation activity. Inspection of the supplemental information in this study shows that EGY3 is in fact among the top 15 most heat-induced proteins (Sedaghatmehr et al., 2016), very much in line with module IX in our network. Mitochondrial FTSH4, located in the intermembrane space, shared three coexpressors with mitochondrial DEG14 (a potential cochaperone of Hsp90/Hsp70 of the carboxylate clamp TPR protein, MAPKK FUSCA5 of the COP9 signalosome, and a protein of unknown function), and one with EGY3, namely, mitochondrial chaperone/unfoldase CLPB4. FTSH4 (with its active site facing the intermembrane space) has been studied quite extensively in Arabidopsis and loss-of-function mutants show accumulation of abnormal enlarged mitochondria, decreased activity/accumulation of OXPHOS, reduced mitochondrial cardiolipin content, and increased levels of H2O2 (Gibala et al., 2009; Kicia et al., 2010). Because mitochondria are critical in early cell differentiation, disruption of mitochondrial function and overaccumulation of ROS results in defects in the shoot apical meristem (Dolzblasz et al., 2016) and flower development, including sepals (Hong et al., 2016). Finally, the more distant OMA1 (expressed quite specifically in late stage embryos and dry seed (see BAR) also has a heat-induced expression, albeit less pronounced, whereas the very distant mitochondrial FTSH10 is not heat induced (Figure 5B). Interestingly, the heat-induced peptidases are also induced by osmotic stress (after 24 h), but not other abiotic stresses such as cold, salt, drought, UV, wounding, or oxidative stress.
Functional analysis of the 90 nonredundant coexpressors of the four heat-induced peptidases EGY3, FTSH4, FTSH6, and DEG14 show enrichment of chaperones (plastid CLPB3 and cpHSP21, mitochondrial CLPB4, HSP20, and HSP70, and three cytosolic HSP70 and the CC-TPR type cochaperones), as well as two heat shock transcription factors (AT3G63350 and AT5G03720). Similar to cpHSP21, cytosolic ascorbate peroxidase (APX2) and cytosolic/nuclear GTPase DGR1-3 (AT1G72660) also coexpressed with three of the four heat-induced peptidases. It can thus be concluded that we discovered a local network of heat stress response peptidases.
Module X
This module contains plastid/mitochondrial DEG3, DEG13 (tentative plastid localization), mitochondrial LON3, and mitochondrial DEG12. DEG3, 12, and 13 are connected through a glycosyl hydrolase (AT1G33220; #678) with preferential and high expression in pollen (Figure 5C). Consistently, DEG12 and DEG3 also have strong pollen expression. Module X contains 46 nonredundant genes making 49 edges and there are no obvious coexpressors that encode for mitochondrial (or plastid) proteins. Proteolysis factors are enriched (P < 0.05) in this module representing nearly 20% of all gene functions (Figure 6); this includes four F-box and two Ring E3 ligases, as well as two cysteine peptidases. Furthermore, enrichment of enzymes involved in cell wall metabolism (P < 0.01; Supplemental Data Set 5), including multiple β-galactosidase and a variety of glycosyl hydrolases, is consistent with functions in pollen biology since cell wall growth and expansion is such a critical process in pollen tube growth.
CONNECTIVITY AND DISTRIBUTION OF PEPTIDASE FAMILIES WITHIN THE NETWORK
Plastid Peptidases at the Periphery or Disconnected from the Central Network
Only one plastid peptidase, NANA (Paparelli et al., 2012), is completely disconnected from other peptidases. NANA is the only known plastid aspartic peptidase and none of its coexpressors have a known plastid location; in fact many have predicted ER signal peptides (Supplemental Data Set 4A). Its coexpressors include four extraplastidic peroxidases and two jacalin lectins, CLAVATA44, a papain-like cysteine peptidase, and others with no obvious functional relation to plastids. Inspection of public mRNA data at BAR shows that NANA shows maximum expression in the hypocotyl and root of the cotyledon stage seedling. Many of NANA’s coexpressors also show maximal accumulation in root tissue. Whereas immunoblotting suggested that NANA is plastid-localized (Paparelli et al., 2012), the GFP fusion fluorescence analysis was carried out with the GFP moiety at the N terminus of the protein, which is problematic given that this would interfere with subcellular sorting. Therefore, further investigation of its subcellular location (perhaps it is not plastidic) and its role in roots seems warranted.
Three (putative) plastid peptidases are connected only through one coexpressor to the central dense network: CTPA-like2 (#66) connected via #186 to FTSH8 in module I, an S9 α/β-hydrolase (#81) connected to module II, and plastid membrane SCO4 protease (#106) with two connections (Albrecht-Borth et al., 2013) to the central, dense network. The S9 α/β-hydrolase is likely involved in the demethylation of prenylated proteins and has consequently also been named prenylcysteine methylesterase (PCME1) (Deem et al., 2006; Lan et al., 2010). Upon inspection of all available data, PCME1 does not seem to be plastid-localized, but rather located in Golgi and ER, explaining why it is peripheral to the rest of the network and has very few plastid-localized coexpressors. The M79 SCO4 plastid membrane peptidase makes a direct edge to the stromal processing peptidase (SPP) and stromal CpNifS/NSF2 involved in Fe-S assembly in module I. We will discuss SCO4 and other M79 peptidases in a later section on the CAAX M79 family.
CTPA-like2 (#66; AT3g57680) is one of the two homologs of lumenal peptidase CTPA (At4g17740, #71 in module IV) with demonstrated exclusive role in C-terminal processing of the plastid-encoded D1 protein in PSII (Che et al., 2013). Given that CTPA is uniquely responsible for D1 processing, the functions of CTPA-like1 (#95; AT5G46390 in module IV similar as CTPA) and CTPA-like2 remain to be determined. CTPA-like1 has not been studied in higher plants, whereas a loss-of-function mutant in Arabidopsis CTPA-like2 did not show a phenotype under normal growth conditions, but the mutant was suggested to be somewhat more sensitive to high light intensity (Yin et al., 2008). Both CTPA and CTPA-like1 coexpress mostly with other plastid proteins (85 and 68%, respectively, including several thylakoid lumenal proteins), whereas only two (8%) coexpressors of CTPA-like2 have a plastid location. Expression profiles of CTPA and CTPA-like1 are comparable, in contrast to CTPA-like2, which has pronounced expression in senescing tissue but also after osmotic stress, similar as other senescence-induced proteostasis genes, likely explaining why plastid proteins are underrepresented among its coexpressors. Given that CTPA-like2 has a clear cTP and predicted lTP, we suggest that CTPA-like2 is also a lumenal plastid protein. CTPA and CTPA-like1 coexpress with plastid-localized NDH subunits, assembly and biogenesis factors, and in the case of CTPA also the proteases lumenal DEG5, thylakoid EGY2, and Rhomboid 10/12. We anticipate that CTPA-like1 cleaves a lumenal region of a thylakoid protein other than D1. Cyanobacteria also have three CTPA homologs, assigned CTPA, B, and C (Jansèn et al., 2003; Komenda et al., 2007). Whereas cyanobacterial CTPA (crystallized at 1.8 Å; Liao et al., 2000) has been shown to be responsible for C-terminal processing of the D1 protein (Anbudurai et al., 1994; Shestakov et al., 1994; Roose and Pakrasi, 2004), the function for the two homologs is unclear.
Finally, three plastid peptidases, namely, FTSH6, EGY3, and DEG13, are part of small disconnected subnetworks (modules IX and X) together with dual-localized or mitochondrial peptidases, as discussed above.
Lack of Connectivity within the Mitochondrial CLP System Points to Functional Specialization
The CLP system in mitochondria consists of a homotetrameric CLPP2 peptidase core (Peltier et al., 2004) and CLPX chaperones (Halperin et al., 2001). CLPP2 and CLPX1 have been detected with good sequence coverage by mass spectrometry in multiple proteomics studies, but CLPX2 and CLPX3 are far less abundant in these studies (see PPDB). CLPP2 is indirectly and peripherally connected to module III, through nodes with mitochondrial DEG10 (via #1573, ribosomal protein) and α-MPP2 (via #1208, likely a mitochondrial ribosomal protein) (Figure 4). CLPX1 connects peripherally to module I, forming a small module (V) with plastidic FTSH7 and CLPD, as discussed above, whereas CLPX2 is disconnected from the network, making just one indirect connection (via 305) to rhomboid RBL6 with putative mitochondrial location. CLPX3 is completely disconnected from the network (Figure 5D). To better understand this lack of connectivity within the mitochondrial CLP system, we evaluated mRNA expression patterns for each mitochondrial CLP gene in BAR. None of the four CLP members were particularly affected by stress factors, but they showed dramatic differences across development and organ: CLPP2 and CLPX2 expressed in leaves, flowers, siliques, and seeds, whereas CLPX1 expressed mostly in senescing tissue (leaves, petals, sepals, and cauline leaves) and CLPX3 showed nearly exclusive expression in pollen. Interestingly, transcriptional analysis of the four stages of pollen development (uninucleate microphore, bicellular, tricellular, and mature pollen) showed that CLPX1 was expressed constitutively, whereas CLPP2, CLPX3, and CLPX2 showed peak expression in early, mid, and late pollen development, respectively. More than 50% of the CLPP2 coexpressors are ribosomal proteins and translation factors, with the remaining mostly with unknown function; this suggests that CLPP2 is involved in early mitochondrial biogenesis. Many of the coexpressors of CLPX2 and X3 do not have a defined function nor an assigned subcellular location.
Distribution of Mitochondrial FTSH Members across the Network
Mitochondria possess two matrix-facing inner envelope FTSH members, FTSH3 and 10, each assembling with prohibitins in high mass complexes, similar to the homologs in yeast (Piechota et al., 2010). In the same study, it was suggested that FTSH3/10 also accumulate as heteromers. However, they do not coexpress in our network; in fact, FTSH10 has one shared coexpressor with inner mitochondrial membrane OMA1 (module IX) (Figure 5A), whereas FTSH3 shares two coexpressors with an uncharacterized M16 peptidase AT5G56730 (the same family as SPP, MPP1/2, and PREP1/2) and one with uncharacterized S9 peptidase AARE2/AHLP, the ortholog of S9 tyrosine amino peptidase in daikon radish; Tsuji et al., 2011) (Figure 4). Mitochondria possess a third FTSH member, FTSH4, with the active site facing the intramembrane space. FTSH4 has been the focus of multiple independent studies in Arabidopsis that indicated that loss of FTSH4 results in impaired mitochondrial electron transport and reduced cardiolipin content in the inner membrane and cardiopilin vesicles at the periphery of mitochondria, resulting in accumulation of ROS species, triggering a range of developmental phenotypes and perhaps mitophagy (Gibala et al., 2009; Kicia et al., 2010; Janska et al., 2010; Kmiec et al., 2012; Dolzblasz et al., 2016; Hong et al., 2016). FTSH4 is in module IX and makes multiple indirect connections to mitochondrial DEG14 and EGY3 (through mitochondrial unfoldase/chaperone CLPB4) and to OMA1 through a P-loop nucleoside hydrolase (AT4G27680), the homolog of yeast MITOCHONDRIAL SORTING PROTEIN1 (MSP1). MSP1 has not been studied in plants, but in yeast (and its homolog ATAD1 in humans) it detects and extracts mistargeted tail-anchored membrane proteins in mitochondria and peroxisome (Chen et al., 2014; Okreglak and Walter, 2014). Module IX is associated with thermotolerance as will be discussed in the next section below.
Network Distribution and Lack of Connectivity of the Mitochondrial DEG Proteins
Mitochondria contain four DEG proteins (DEG6, DEG11, DEG12, and DEG14) and share two DEG proteins (DEG3 and DEG10) with plastids, whereas DEG4 is a candidate mitochondrial protein and, based on experimental data, DEG7 has been suggested to be either plastid (Sun et al., 2010) or in mitochondria and nucleus (Tanz et al., 2014). DEG6, a truncated protein, is not in the coexpression data set. We already mentioned the plastid/mitochondrial dual targeted DEG3 (in module X) and DEG10 (connected to CLPP2), DEG14 in module IX and DEG12 in module X. Neither DEG4, 7, or 11 is connected to other organellar peptidases (Figure 5C). Coexpressors of DEG4 seem to mostly encode for nonorganellar proteins involved in plant development and protein degradation, including transcription factors, F-box E3 ligases, an aspartic peptidase, and translationally controlled tumor protein 2 (TCTP2) (Toscano-Morales et al., 2015). Coexpressors of DEG7 have a broad range of functions and none have confirmed mitochondrial or plastid location. Coexpressors of DEG11 show overrepresentation of players involved in RNA metabolism (24%), as well as four F-box E3 ligases. Only one coexpressor encodes for a confirmed mitochondrial protein, a PPR protein with DYW domain (REME2) involved in mitochondrial RNA editing (Bentolila et al., 2013). Comparison of expression profiles during development or abiotic stress from BAR shows that all three disconnected DEG genes are expressed in roots, as well as many aboveground tissues, and that expression levels of DEG7 are much (>10- to 30-fold) higher than DEG4 and 11.
Integration of Plastid Division Machinery in the Network
In lineages of algae with just a single or a few chloroplasts, expression of known nucleus-encoded chloroplast division genes and stability of proteins are coupled to the host cell cycle; the regulation of both expression and the degradation of most nuclear-encoded chloroplast division proteins is apparently sufficient to inhibit chloroplast division in cells during the G1 phase and late M phase of the cell cycle (Miyagishima et al., 2012). In higher plants, cells have many chloroplasts and the pool of plastid division proteins in higher plants appears quite stable, with division proteins recruited when plastid division is triggered as needed (see Discussion in Pedroza-Garcia et al., 2016). Indeed, inspection of the mRNA levels of plastid division genes in public database (e.g., BAR) shows that they are relatively constant across leaf development, which is surprising because plastid division stops once leaves have matured. Nine components of the plastid division machinery (ARC3, ARC6, CRL, FTSZ1,2, GC1, MINE, MCD, and DVD2) are found as coexpressors in the peptidase network. These are direct coexpressors of envelope-localized FTSH/FTSHi members (FTSH9/12 and FTSHi1/5), the CLP peptidase core (CLPR2,4 and CLPP4,5,6), CLPS1, MAP1B, EGY2, lumenal DEG5/8, and APP2. Interesting coexpression patterns were observed for stroma-localized MINE (ARC12), which coexpressed with four CLPPR genes, and inner envelope ARC6, which coexpressed with envelope FTSH12, FTSHi1 (ARC1), and FTSHi5. It is important to remember that the FTSHi proteins are noncatalytic peptidase homologs, and our network analysis corroborates the role FTSHi family in the plastid division process at the envelope.
Assembly Factors in the Network
Peptidase activity is likely integrated with protein chaperones, isomerases, DnaJ or J-domain proteins, and other assembly factors, together forming the proteostasis network (Powers and Balch, 2013; Trösch et al., 2015; van Wijk, 2015; Balchin et al., 2016). Our coexpression network included 78 of such factors, most with assigned plastid location (56 proteins) and mitochondria location (9 proteins), with the remainder of unknown location. The chaperones included general chaperones (CPN60/20/10, HSP70, and HSP90), small sHSPs, and the unfoldases CLPB3 (plastid) and CLPB4 (mitochondria), the CLPC/D and CLPX chaperones of the plastid and mitochondrial CLP proteases, respectively. Additionally, the network contained isomerases and FKBPs (most of them in the lumen in module IV), assembly factors involved in Fe-S cluster assembly, or assembly of other chloroplast and mitochondrial electron transport complexes. Finally, there were a surprising number of plastid proteins of unknown function with DnaJ cysteine repeats or just J-domains. Some of these factors made edges to four, five, or even six other genes, whereas other coexpressed with just one of the peptidases (Supplemental Data Set 4A). In absolute terms, most edges for these factors were found in module I (52 edges), followed by module IV (33 edges), but when normalized to the total number of edges per module, modules IV (enriched for lumenal proteins) and IX (thermo-tolerance) exhibited the highest proportion (12% and 15%, respectively).
Posttranslational Protein Modifiers in the Network
Another part of the organelle protein homeostasis network are nonpeptidase enzymes involved in protein modifications, such as N-terminal acetylation, N-terminal methionine deformylation, methionine sulfoxide repair, lysine acetylation, methylation of arginine, lysine, or N-terminal amines, and (de)phosphorylation (Friso and van Wijk, 2015). Indeed, many of the known organellar players are part of the coexpression network. Here, we highlight some key examples. Dual-targeted plastid/mitochondrial peptide deformylase PDF1B (#280) involved in removing of the formyl-group of the N-terminal methionine of plastid- and mitochondrial-encoded proteins (Giglione and Meinnel, 2001) coexpressed in module II with dual-targeted methionine aminopeptidase MAP1D. The coexpression with MAP1D is consistent with them acting in series on newly synthesized proteins. Three methionine sulfoxide reductases (Rouhier et al., 2006; Tarrago et al., 2009), namely, plastid-localized MSRA4 (#1249), MSRB1 (#188), and MSS4-like (#688) with unknown location, were found in the coexpression network. In particular, the well-characterized MSRB1 was tightly connected to the peptidase network in module I, coexpressing with the thylakoid peptidases FTSH1,5,8, and light-stressed induced SPPA, plastoglobular PGM48, and stromal PREP1, as well as an uncharacterized stromal αβ-hydrolase. MSRA4 also coexpressed with PREP1.
N-terminal acetylation has emerged as a common modification in plants, including chloroplasts (Dinh et al., 2015; Rowland et al., 2015). N-terminal acetylation can act as a degradation signal in a novel branch of the N-end rule pathway (Lee et al., 2016), and in plants it has been shown to be involved in resistance to drought and immune response (Aksnes et al., 2016; Hosp et al., 2017). It is clear that N-terminal acetylation can affect proteins in several different ways (Aksnes et al., 2016). Acetylation can occur spontaneously, driven by high levels of acetyl-CoA, or performed by enzymes of the NAT family, often as a cotranslational process. There are seven potential NAT proteins with predicted plastid location (Dinh et al., 2015); four of these were identified in the coexpression network (AT1G24040, AT1G32070, AT4G28030, and AT4G19985). Based on proteomics information, we assigned AT1G32070 and AT4G28030 to chloroplast stroma, and based on its homology to a plastid rice protein (see PPDB), it is likely that AT1G24040 is plastid-localized as well. AT1G24040 and AT1G32070 each coexpressed with three peptidases in modules I and II, respectively. The recently identified plastid NATG (NAA70; AT2G39000) (Dinh et al., 2015) was not part of our proteostasis network. Furthermore, the catalytic subunit of the cytosolic NATA complex (AT5G13780) (Linster et al., 2015) coexpressed with mitochondrial CLPP2, and NAT AT4G19985 coexpressed with EGY2 and a rhomboid in module IV. The well-characterized mitochondrial deacetylase sirtuin 2 (SRT2; #729) (König et al., 2014) coexpressed with light stress-induced thylakoid SPPA in module I. Collectively, this suggests that acetylation and proteolysis are well integrated within plastids but their functional relationships remain to be determined.
Finally, multiple protein kinases and phosphatases were part of the network, including the well-characterized thylakoid state transition kinase STN7, PSII core phosphatase PBCP, as well as chloroplast sensor kinase protein (CSK). CSK and STN7 both coexpressed with thylakoid lumen DEG1 (module I), whereas PBCP coexpressed with plastid CAAX3 and LON-like 2 in module IV.
The CAAX M79 Family
The four CAAX M79 peptidases in the organelles have no known function, except that loss of SCO4 function results in increased sensitivity to excess light (Albrecht-Borth et al., 2013). To better visualize the distribution of the CAAX members across the coexpression network and infer potential suborganellar location and function, we generated a simplified network showing how these CAAX proteins directly or indirectly connect to other peptidases (Supplemental Figure 1). CAAX1 and 2 are in module I, whereas SCO4 is peripheral to module I, making a direct edge to stromal SPP. By contrast, CAAX3 is deeply integrated in module IV (enriched for thylakoid lumenal proteins; Figure 4) together with lumenal DEG5/8, CTPA and CTPA-like1, and other integral membrane peptidases EGY2 and rhomboid 8/10. Direct coexpressors of CAAX3 are highly enriched for plastid proteins (88%) and include three enzymes involved in chlorophyll synthesis, four lumenal proteins involved in NDH biogenesis or protein isomerization, and thylakoid EGY2 peptidase. We speculate that CAAX3 is localized to the thylakoid membranes. CAAX1 and CAAX2 are both in module I and ∼50% of their coexpressors encode for plastid proteins, whereas the location for most others is unknown. We did not observe any particular functional enrichment of coexpressors for either CAAX1 or CAAX2, in part because nearly half of the coexpressors have no assigned function. Only 30% of the SCO4 coexpressors have known plastid location, and in fact only 25% have a predicted cTP/mTP, which is very low compared with coexpressors of CAAX1,2,3 (68, 73, and 92%, respectively).
Public mRNA expression profiles of these four CAAX genes in BAR showed little response to abiotic stress treatment (data not shown), and public mRNA data indicate that all four genes expressed in most tissues throughout development. However, there are some developmental differences, e.g., low expression of CAAX3 in senescing leaves, relatively more pronounced expression of SCO4 in seeds, and lower expression levels of CAAX2 and CAAX3 in flowers compared with CAAX1 and SCO4. We previously determined that mature integral thylakoid proteins have on average an ∼50% lower cysteine content and lower pI value (6.9 compared with 9.2) compared with integral inner envelope proteins (Sun et al., 2004). After removal of the predicted transit peptides, SCO4 has a very basic pI (8.6), whereas CAAX1,2,3 have (somewhat) acidic pI values (respectively 6.3, 6.7, and 4.8). The cysteine content of CAAX 1 and CAAX3 is low (two and one cysteine, respectively), but that of SCO4 and CAA2 relatively high (6 and 10 cysteines). On this basis and the network analysis (e.g., CAAX3 in module IV enriched for thylakoid lumen proteins), we speculate that CAAX1 and CAAX3 are thylakoid proteins, whereas CAAX2 and SCO4 are located in inner envelopes. CAAX3 could perhaps also be localized to mitochondria based on the large-scale GFP study (Ishikawa et al., 2009) and mRNA accumulation in pollen (pollen has a high mitochondrial content).
An Extended Network Including E3 Ligases
The genome-wide coexpression analysis and cTP prediction suggested a dozen additional (candidate) peptidases and components of the UPS, as discussed earlier. We extended our network with these genes, including 5 E3 ligases (baits #24, 34, 67, 103, and 107), resulting in a network with 109 baits and 7794 edges (Supplemental Data Set 4A and Supplemental Figure 2). We will not further consider baits #36 and #48 since upon further inspection as they appear to have other functions (Supplemental Data Set 2).
In particular the coexpressors of E3 F-box ligase VFB3 (#67) and E3-RING ligase STRF1/ATL69 (#103) were enriched for RNA metabolism and clustered together outside the central network between modules III and IV. E3-RING ligase SHA1 (#107), required for postembryonic shoot apical meristem maintenance (Sonoda et al., 2007), aspartic peptidase (#75), and subtilase SBT4.11 (#105) were enriched for starch metabolism (mostly starch degradation enzymes) and clustered together in module VI with peptidases cGEP, OOP, and MAP1B. In total, these three genes coexpress with 13 nonredundant starch metabolism enzymes, making a total of 18 edges. This strong functional enrichment is surprising, and it is not clear how this should be interpreted. However, it has been reported previously that autophagy appears involved in remobilizing starch (Wang et al., 2013, 2016); conversely, blocking starch degradation triggers chloroplast degradation (Stettler et al., 2009).
Coexpressors of E3-F-box ligase (#24), peripheral to the central network, were enriched to genes encoding for components of the photosynthetic electron transport chain. Three baits, E3-F-box (#34), cysteine protease inhibitor cystatin (#42), and the rhomboid RBL9 (#88), fell within module I, whereas SCPL42 (#91) fell in module III, enriched in envelope proteins. It remains to be determined if and how these proteins contribute to the plastid life cycle and/or function.
CONCLUSIONS AND OUTLOOK
This review provides a compendium for all putative peptidases and auxiliary factors in Arabidopsis and their (tentative) assignments to plastids and mitochondria, and includes all proteins that have an assigned clan and family in the MEROPS database. The PPDB now incorporates this annotation using an extended hierarchical MapMan bin classification. The new bins for peptidases can be used to extract proteins in different clans and/or families rapidly and also helps rapid classification of peptidases or proteins with peptidase(-like) domains. This review also provides insight and statistical support for the use of coexpression data to infer plastid-subcellular localization; it confirms previous reports of the notion that plastid proteins often coexpress with other plastid proteins, but the same holds true only for a more limited set of mitochondrial proteins. A detailed, well-annotated, coexpression network and its analysis is described for plastid and mitochondrial peptidases and associated factors, as well as components of the proteostasis network that highly coexpress with plastid proteins, such as several E3 ligases. Multiple coexpression modules were detected and where possible associated with specific processes (e.g., thermotolerance) and (sub)organellar locations (e.g., thylakoid lumen and chloroplast envelope). This organellar modular proteostasis network provides a strategic foundation for experimental testing of the role of proteolysis in biogenesis, differentiation, and metabolic functions associated with plastids and mitochondria.
Supplemental Data
Supplemental Figure 1. The minimal subnetwork (direct neighborhood) of the four organellar CAAX M79 proteins within the complete peptidase network.
Supplemental Figure 2. Graphical display of the extended coexpression network of plastid and mitochondrial peptidases and 12 genes for which its coexpressors are enriched in genes encoding for plastid proteins.
Supplemental Figure 3. Higher-resolution copy of Figure 4.
Supplemental Figure 4. Higher-resolution copy of Figure 5.
Supplemental Data Set 1. Putative and confirmed peptidases, as well as associated nonproteolytic proteins (such as adaptors and peptidase peptidase-specific chaperones), as well as MEROPS clan family members without peptidase functions (total 809 genes, represented by 1090 gene models).
Supplemental Data Set 2. Confirmed and candidate peptidases, non-peptidases with assigned MEROPS clan and family, and associated factors in plastids and/or mitochondria, as well as proteostasis components that coexpress with plastid proteins (total 122 proteins).
Supplemental Data Set 3. Arabidopsis genome-wide coexpression network and localization enrichment analysis (cytosol, mitochondrion, nucleus, peroxisome, plastid, secretory, and unknown).
Supplemental Data Set 4. Arabidopsis plastid and mitochondrial coexpression network information for baits and coexpressors with extended annotations.
Supplemental Data Set 5. Functional distribution and enrichment analysis of the 10 modules and the whole network using 42 different functional categories.
Acknowledgments
This work was supported by a grant from the National Science Foundation, Division of Molecular and Cellular Biosciences (MCB #1614629 to K.J.v.W.) and by European Social Fund Grant HR.3.2.01-0095 to K.M. We thank other members of the K.J.v.W. lab for discussions and feedback on the manuscript.
AUTHOR CONTRIBUTIONS
K.M. constructed the coexpression graphs, did the GBA analysis, and provided statistics for the functional enrichment of the network. Q.S. extracted sequences from MEROPS, carried out the BLASTP and PFAM analysis, and did all PPDB construction and management. N.H.B. carried out the phylogeny for the M79 family and provided insight on peptidase families and general feedback on the manuscript. S.K., V.K., and D.W. provided the edge list for the coexpression analysis and provided feedback on the manuscript. K.M. and K.J.v.W. wrote the article. K.J.v.W. obtained the funding for this project, recruited the coauthors, was responsible for protein annotation, curation, and network interpretations, and provided general oversight.
References
- Adam Z. (2013). Emerging roles for diverse intramembrane proteases in plant biology. Biochim. Biophys. Acta 1828: 2933–2936. [DOI] [PubMed] [Google Scholar]
- Aksnes H., Drazic A., Marie M., Arnesen T. (2016). First things first: vital protein marks by N-terminal acetyltransferases. Trends Biochem. Sci. 41: 746–760. [DOI] [PubMed] [Google Scholar]
- Albrecht-Borth V., Kauss D., Fan D., Hu Y., Collinge D., Marri S., Liebers M., Apel K., Pfannschmidt T., Chow W.S., Pogson B.J. (2013). A novel proteinase, SNOWY COTYLEDON4, is required for photosynthetic acclimation to higher light intensities in Arabidopsis. Plant Physiol. 163: 732–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida C.M., Pereira C., da Costa D.S., Pereira S., Pissarra J., Simões I., Faro C. (2012). Chlapsin, a chloroplastidial aspartic proteinase from the green algae Chlamydomonas reinhardtii. Planta 236: 283–296. [DOI] [PubMed] [Google Scholar]
- Anbudurai P.R., Mor T.S., Ohad I., Shestakov S.V., Pakrasi H.B. (1994). The ctpA gene encodes the C-terminal processing protease for the D1 protein of the photosystem II reaction center complex. Proc. Natl. Acad. Sci. USA 91: 8082–8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y., Okamura Y., Tadaka S., Kinoshita K., Obayashi T. (2016). ATTED-II in 2016: A plant coexpression database towards lineage-specific coexpression. Plant Cell Physiol. 57: e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Ospina L., Moison M., Yoshimoto K., Masclaux-Daubresse C. (2014). Autophagy, plant senescence, and nutrient recycling. J. Exp. Bot. 65: 3799–3811. [DOI] [PubMed] [Google Scholar]
- Balchin D., Hayer-Hartl M., Hartl F.U. (2016). In vivo aspects of protein folding and quality control. Science 353: aac4354. [DOI] [PubMed] [Google Scholar]
- Basak I., Pal R., Patil K.S., Dunne A., Ho H.P., Lee S., Peiris D., Maple-Grødem J., Odell M., Chang E.J., Larsen J.P., Møller S.G. (2014). Arabidopsis AtPARK13, which confers thermotolerance, targets misfolded proteins. J. Biol. Chem. 289: 14458–14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila S., Babina A.M., Germain A., Hanson M.R. (2013). Quantitative trait locus mapping identifies REME2, a PPR-DYW protein required for editing of specific C targets in Arabidopsis mitochondria. RNA Biol. 10: 1520–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan N.H., Friso G., Rowland E., Majsec K., van Wijk K.J. (2016). The plastoglobule-localized metallopeptidase PGM48 is a positive regulator of senescence in Arabidopsis thaliana. Plant Cell 28: 3020–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff M., Rösler J., Raesecke H.R., Görlach J., Amrhein N., Schmid J. (1996). Cloning of a cDNA encoding a 3-dehydroquinate synthase from a higher plant, and analysis of the organ-specific and elicitor-induced expression of the corresponding gene. Plant Mol. Biol. 31: 69–76. [DOI] [PubMed] [Google Scholar]
- Book A.J., Yang P., Scalf M., Smith L.M., Vierstra R.D. (2005). Tripeptidyl peptidase II. An oligomeric protease complex from Arabidopsis. Plant Physiol. 138: 1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha-Drori K., Shichrur K., Lubetzky T.C., Yalovsky S. (2008). Functional analysis of Arabidopsis postprenylation CaaX processing enzymes and their function in subcellular protein targeting. Plant Physiol. 148: 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumme S., Kruft V., Schmitz U.K., Braun H.P. (1998). New insights into the co-evolution of cytochrome c reductase and the mitochondrial processing peptidase. J. Biol. Chem. 273: 13143–13149. [DOI] [PubMed] [Google Scholar]
- Cadiñanos J., Varela I., Mandel D.A., Schmidt W.K., Díaz-Perales A., López-Otín C., Freije J.M. (2003). AtFACE-2, a functional prenylated protein protease from Arabidopsis thaliana related to mammalian Ras-converting enzymes. J. Biol. Chem. 278: 42091–42097. [DOI] [PubMed] [Google Scholar]
- Carrie C., Venne A.S., Zahedi R.P., Soll J. (2015). Identification of cleavage sites and substrate proteins for two mitochondrial intermediate peptidases in Arabidopsis thaliana. J. Exp. Bot. 66: 2691–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie C., Murcha M.W., Giraud E., Ng S., Zhang M.F., Narsai R., Whelan J. (2013). How do plants make mitochondria? Planta 237: 429–439. [DOI] [PubMed] [Google Scholar]
- Carrión C.A., Martínez D.E., Costa M.L., Guiamet J.J. (2014). Senescence-associated vacuoles, a specific lytic compartment for degradation of chloroplast proteins? Plants (Basel) 3: 498–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión C.A., Costa M.L., Martínez D.E., Mohr C., Humbeck K., Guiamet J.J. (2013). In vivo inhibition of cysteine proteases provides evidence for the involvement of ‘senescence-associated vacuoles’ in chloroplast protein degradation during dark-induced senescence of tobacco leaves. J. Exp. Bot. 64: 4967–4980. [DOI] [PubMed] [Google Scholar]
- Che Y., Fu A., Hou X., McDonald K., Buchanan B.B., Huang W., Luan S. (2013). C-terminal processing of reaction center protein D1 is essential for the function and assembly of photosystem II in Arabidopsis. Proc. Natl. Acad. Sci. USA 110: 16247–16252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Umanah G.K., Dephoure N., Andrabi S.A., Gygi S.P., Dawson T.M., Dawson V.L., Rutter J. (2014). Msp1/ATAD1 maintains mitochondrial function by facilitating the degradation of mislocalized tail-anchored proteins. EMBO J. 33: 1548–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W., Sun X., Zhang L. (2012). The roles of chloroplast proteases in the biogenesis and maintenance of photosystem II. Biochim. Biophys. Acta 1817: 239–246. [DOI] [PubMed] [Google Scholar]
- Deem A.K., Bultema R.L., Crowell D.N. (2006). Prenylcysteine methylesterase in Arabidopsis thaliana. Gene 380: 159–166. [DOI] [PubMed] [Google Scholar]
- Díaz-Mendoza M., Velasco-Arroyo B., González-Melendi P., Martínez M., Díaz I. (2014). C1A cysteine protease-cystatin interactions in leaf senescence. J. Exp. Bot. 65: 3825–3833. [DOI] [PubMed] [Google Scholar]
- Dinh T.V., Bienvenut W.V., Linster E., Feldman-Salit A., Jung V.A., Meinnel T., Hell R., Giglione C., Wirtz M. (2015). Molecular identification and functional characterization of the first Nα-acetyltransferase in plastids by global acetylome profiling. Proteomics 15: 2426–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzblasz A., Smakowska E., Gola E.M., Sokołowska K., Kicia M., Janska H. (2016). The mitochondrial protease AtFTSH4 safeguards Arabidopsis shoot apical meristem function. Sci. Rep. 6: 28315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi B., Hatin I., Deutsch C., Kahveci T., Rousset J.P., Iwata-Reuyl D., Murzin A.G., de Crécy-Lagard V. (2011). A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J. 30: 882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubel H., Meyer E.H., Taylor N.L., Bussell J.D., O’Toole N., Heazlewood J.L., Castleden I., Small I.D., Smith S.M., Millar A.H. (2008). Novel proteins, putative membrane transporters, and an integrated metabolic network are revealed by quantitative proteomic analysis of Arabidopsis cell culture peroxisomes. Plant Physiol. 148: 1809–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faro C., Gal S. (2005). Aspartic proteinase content of the Arabidopsis genome. Curr. Protein Pept. Sci. 6: 493–500. [DOI] [PubMed] [Google Scholar]
- Finn R.D., et al. (2016). The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44: D279–D285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Lampl N., Roberts T.H. (2012). Serpin protease inhibitors in plant biology. Physiol. Plant. 145: 95–102. [DOI] [PubMed] [Google Scholar]
- Friso G., van Wijk K.J. (2015). Posttranslational protein modifications in plant metabolism. Plant Physiol. 169: 1469–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Zhang Y., Wang W., Zhao K., Liu C., Bai L., Li R., Guo Y. (2017). Two membrane-anchored aspartic proteases contribute to pollen and ovule development. Plant Physiol. 173: 219–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershater M.C., Cummins I., Edwards R. (2007). Role of a carboxylesterase in herbicide bioactivation in Arabidopsis thaliana. J. Biol. Chem. 282: 21460–21466. [DOI] [PubMed] [Google Scholar]
- Gibala M., Kicia M., Sakamoto W., Gola E.M., Kubrakiewicz J., Smakowska E., Janska H. (2009). The lack of mitochondrial AtFtsH4 protease alters Arabidopsis leaf morphology at the late stage of rosette development under short-day photoperiod. Plant J. 59: 685–699. [DOI] [PubMed] [Google Scholar]
- Giglione C., Meinnel T. (2001). Organellar peptide deformylases: universality of the N-terminal methionine cleavage mechanism. Trends Plant Sci. 6: 566–572. [DOI] [PubMed] [Google Scholar]
- Glaser E., Dessi P. (1999). Integration of the mitochondrial-processing peptidase into the cytochrome bc1 complex in plants. J. Bioenerg. Biomembr. 31: 259–274. [DOI] [PubMed] [Google Scholar]
- Grosse-Holz F.M., van der Hoorn R.A. (2016). Juggling jobs: roles and mechanisms of multifunctional protease inhibitors in plants. New Phytol. 210: 794–807. [DOI] [PubMed] [Google Scholar]
- Guo R., Xu X., Carole B., Li X., Gao M., Zheng Y., Wang X. (2013). Genome-wide identification, evolutionary and expression analysis of the aspartic protease gene superfamily in grape. BMC Genomics 14: 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin T., Zheng B., Itzhaki H., Clarke A.K., Adam Z. (2001). Plant mitochondria contain proteolytic and regulatory subunits of the ATP-dependent Clp protease. Plant Mol. Biol. 45: 461–468. [DOI] [PubMed] [Google Scholar]
- Haussuehl K., Huesgen P.F., Meier M., Dessi P., Glaser E., Adamski J., Adamska I. (2009). Eukaryotic GCP1 is a conserved mitochondrial protein required for progression of embryo development beyond the globular stage in Arabidopsis thaliana. Biochem. J. 423: 333–341. [DOI] [PubMed] [Google Scholar]
- He F., Yoo S., Wang D., Kumari S., Gerstein M., Ware D., Maslov S. (2016). Large-scale atlas of microarray data reveals the distinct expression landscape of different tissues in Arabidopsis. Plant J. 86: 472–480. [DOI] [PubMed] [Google Scholar]
- Heazlewood J.L., Tonti-Filippini J.S., Gout A.M., Day D.A., Whelan J., Millar A.H. (2004). Experimental analysis of the Arabidopsis mitochondrial proteome highlights signaling and regulatory components, provides assessment of targeting prediction programs, and indicates plant-specific mitochondrial proteins. Plant Cell 16: 241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Saito K. (2013). Network analysis for gene discovery in plant-specialized metabolism. Plant Cell Environ. 36: 1597–1606. [DOI] [PubMed] [Google Scholar]
- Hong L., et al. (2016). Variable cell growth yields reproducible organ development through spatiotemporal averaging. Dev. Cell 38: 15–32. [DOI] [PubMed] [Google Scholar]
- Hosp F., Lassowskat I., Santoro V., De Vleesschauwer D., Fliegner D., Redestig H., Mann M., Christian S., Hannah M.A., Finkemeier I. (2017). Lysine acetylation in mitochondria: From inventory to function. Mitochondrion 33: 58–71. [DOI] [PubMed] [Google Scholar]
- Hu J., Baker A., Bartel B., Linka N., Mullen R.T., Reumann S., Zolman B.K. (2012). Plant peroxisomes: biogenesis and function. Plant Cell 24: 2279–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Kato Y., Sumida A., Tanaka A., Tanaka R. (2017). The SUFBC2 D complex is required for the biogenesis of all major classes of plastid Fe-S proteins. Plant J. 90: 235–248. [DOI] [PubMed] [Google Scholar]
- Huang S., Nelson C.J., Li L., Taylor N.L., Ströher E., Peteriet J., Millar A.H. (2015). INTERMEDIATE CLEAVAGE PEPTIDASE55 modifies enzyme amino termini and alters protein stability in Arabidopsis mitochondria. Plant Physiol. 168: 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesgen P.F., Schuhmann H., Adamska I. (2006). Photodamaged D1 protein is degraded in Arabidopsis mutants lacking the Deg2 protease. FEBS Lett. 580: 6929–6932. [DOI] [PubMed] [Google Scholar]
- Hyun T.K., van der Graaff E., Albacete A., Eom S.H., Großkinsky D.K., Böhm H., Janschek U., Rim Y., Ali W.W., Kim S.Y., Roitsch T. (2014). The Arabidopsis PLAT domain protein1 is critically involved in abiotic stress tolerance. PLoS One 9: e112946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H., Izumi M., Wada S., Makino A. (2014). Roles of autophagy in chloroplast recycling. Biochim. Biophys. Acta 1837: 512–521. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Fujiwara M., Sonoike K., Sato N. (2009). Orthogenomics of photosynthetic organisms: bioinformatic and experimental analysis of chloroplast proteins of endosymbiont origin in Arabidopsis and their counterparts in Synechocystis. Plant Cell Physiol. 50: 773–788. [DOI] [PubMed] [Google Scholar]
- Isono E., Nagel M.K. (2014). Deubiquitylating enzymes and their emerging role in plant biology. Front. Plant Sci. 5: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansèn T., Kidron H., Soitamo A., Salminen T., Mäenpää P. (2003). Transcriptional regulation and structural modelling of the Synechocystis sp. PCC 6803 carboxyl-terminal endoprotease family. FEMS Microbiol. Lett. 228: 121–128. [DOI] [PubMed] [Google Scholar]
- Janska H., Piechota J., Kwasniak M. (2010). ATP-dependent proteases in biogenesis and maintenance of plant mitochondria. Biochim. Biophys. Acta 1797: 1071–1075. [DOI] [PubMed] [Google Scholar]
- Jarvis P., López-Juez E. (2013). Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 14: 787–802. [DOI] [PubMed] [Google Scholar]
- Jeyaraju D.V., Sood A., Laforce-Lavoie A., Pellegrini L. (2013). Rhomboid proteases in mitochondria and plastids: keeping organelles in shape. Biochim. Biophys. Acta 1833: 371–380. [DOI] [PubMed] [Google Scholar]
- Kadirjan-Kalbach D.K., Yoder D.W., Ruckle M.E., Larkin R.M., Osteryoung K.W. (2012). FtsHi1/ARC1 is an essential gene in Arabidopsis that links chloroplast biogenesis and division. Plant J. 72: 856–867. [DOI] [PubMed] [Google Scholar]
- Kato Y., Yamamoto Y., Murakami S., Sato F. (2005). Post-translational regulation of CND41 protease activity in senescent tobacco leaves. Planta 222: 643–651. [DOI] [PubMed] [Google Scholar]
- Kato Y., Murakami S., Yamamoto Y., Chatani H., Kondo Y., Nakano T., Yokota A., Sato F. (2004). The DNA-binding protease, CND41, and the degradation of ribulose-1,5-bisphosphate carboxylase/oxygenase in senescent leaves of tobacco. Planta 220: 97–104. [DOI] [PubMed] [Google Scholar]
- Kicia M., Gola E.M., Janska H. (2010). Mitochondrial protease AtFtsH4 protects ageing Arabidopsis rosettes against oxidative damage under short-day photoperiod. Plant Signal. Behav. 5: 126–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec B., Urantowka A., Lech M., Janska H. (2012). Two-step processing of AtFtsH4 precursor by mitochondrial processing peptidase in Arabidopsis thaliana. Mol. Plant 5: 1417–1419. [DOI] [PubMed] [Google Scholar]
- Knopf R.R., Adam Z. (2012). Rhomboid proteases in plants - still in square one? Physiol. Plant. 145: 41–51. [DOI] [PubMed] [Google Scholar]
- Komenda J., Kuviková S., Granvogl B., Eichacker L.A., Diner B.A., Nixon P.J. (2007). Cleavage after residue Ala352 in the C-terminal extension is an early step in the maturation of the D1 subunit of Photosystem II in Synechocystis PCC 6803. Biochim. Biophys. Acta 1767: 829–837. [DOI] [PubMed] [Google Scholar]
- König A.C., et al. (2014). The Arabidopsis class II sirtuin is a lysine deacetylase and interacts with mitochondrial energy metabolism. Plant Physiol. 164: 1401–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwasniak M., Pogorzelec L., Migdal I., Smakowska E., Janska H. (2012). Proteolytic system of plant mitochondria. Physiol. Plant. 145: 187–195. [DOI] [PubMed] [Google Scholar]
- Lallemand J., Bouché F., Desiron C., Stautemas J., de Lemos Esteves F., Périlleux C., Tocquin P. (2015). Extracellular peptidase hunting for improvement of protein production in plant cells and roots. Front. Plant Sci. 6: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P., Li W., Wang H., Ma W. (2010). Characterization, sub-cellular localization and expression profiling of the isoprenylcysteine methylesterase gene family in Arabidopsis thaliana. BMC Plant Biol. 10: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.N., Kim J., Chung T. (2014). Degradation of plant peroxisomes by autophagy. Front. Plant Sci. 5: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.E., Heo J.E., Kim J.M., Hwang C.S. (2016). N-terminal acetylation-targeted N-end rule proteolytic system: The Ac/N-end rule pathway. Mol. Cells 39: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister D., Wang X., Haberer G., Mayer K.F., Kleine T. (2011). Intracompartmental and intercompartmental transcriptional networks coordinate the expression of genes for organellar functions. Plant Physiol. 157: 386–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Vierstra R.D. (2012). Autophagy: a multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci. 17: 526–537. [DOI] [PubMed] [Google Scholar]
- Li L., Nelson C., Fenske R., Trosch J., Pruzinska A., Millar A.H., Huang S. (2017). Changes in specific protein degradation rates in Arabidopsis thaliana reveal multiple roles of Lon1 in mitochondrial protein homeostasis. Plant J. 89: 458–471. [DOI] [PubMed] [Google Scholar]
- Liao D.I., Qian J., Chisholm D.A., Jordan D.B., Diner B.A. (2000). Crystal structures of the photosystem II D1 C-terminal processing protease. Nat. Struct. Biol. 7: 749–753. [DOI] [PubMed] [Google Scholar]
- Ling Q., Jarvis P. (2015). Functions of plastid protein import and the ubiquitin-proteasome system in plastid development. Biochim. Biophys. Acta 1847: 939–948. [DOI] [PubMed] [Google Scholar]
- Ling Q., Huang W., Baldwin A., Jarvis P. (2012). Chloroplast biogenesis is regulated by direct action of the ubiquitin-proteasome system. Science 338: 655–659. [DOI] [PubMed] [Google Scholar]
- Linster E., et al. (2015). Downregulation of N-terminal acetylation triggers ABA-mediated drought responses in Arabidopsis. Nat. Commun. 6: 7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yu F., Rodermel S. (2010). Arabidopsis chloroplast FtsH, var2 and suppressors of var2 leaf variegation: a review. J. Integr. Plant Biol. 52: 750–761. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang F., Zhang H., He H., Ma L., Deng X.W. (2008). Functional characterization of the Arabidopsis ubiquitin-specific protease gene family reveals specific role and redundancy of individual members in development. Plant J. 55: 844–856. [DOI] [PubMed] [Google Scholar]
- Lu X., Zhang D., Li S., Su Y., Liang Q., Meng H., Shen S., Fan Y., Liu C., Zhang C. (2014). FtsHi4 is essential for embryogenesis due to its influence on chloroplast development in Arabidopsis. PLoS One 9: e99741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciński R., Misztal L., Samardakiewicz S., Jackowski G. (2011). The thylakoid protease Deg2 is involved in stress-related degradation of the photosystem II light-harvesting protein Lhcb6 in Arabidopsis thaliana. New Phytol. 192: 74–86. [DOI] [PubMed] [Google Scholar]
- Lundquist P.K., Poliakov A., Bhuiyan N.H., Zybailov B., Sun Q., van Wijk K.J. (2012). The functional network of the Arabidopsis plastoglobule proteome based on quantitative proteomics and genome-wide coexpression analysis. Plant Physiol. 158: 1172–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallari J.P., Oksman A., Vaupel B., Goldberg D.E. (2014). Kinase-associated endopeptidase 1 (Kae1) participates in an atypical ribosome-associated complex in the apicoplast of Plasmodium falciparum. J. Biol. Chem. 289: 30025–30039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R.S., Vierstra R.D. (2015). Eat or be eaten: The autophagic plight of inactive 26S proteasomes. Autophagy 11: 1927–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez M., Cambra I., González-Melendi P., Santamaría M.E., Díaz I. (2012). C1A cysteine-proteases and their inhibitors in plants. Physiol. Plant. 145: 85–94. [DOI] [PubMed] [Google Scholar]
- Mentzen W.I., Wurtele E.S. (2008). Regulon organization of Arabidopsis. BMC Plant Biol. 8: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Huttenlocher F., Cedzich A., Procopio S., Stroeder J., Pau-Roblot C., Lequart-Pillon M., Pelloux J., Stintzi A., Schaller A. (2016). The subtilisin-like protease SBT3 contributes to insect resistance in tomato. J. Exp. Bot. 67: 4325–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindrebo J.T., Nartey C.M., Seto Y., Burkart M.D., Noel J.P. (2016). Unveiling the functional diversity of the alpha/beta hydrolase superfamily in the plant kingdom. Curr. Opin. Struct. Biol. 41: 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishima S.Y., Suzuki K., Okazaki K., Kabeya Y. (2012). Expression of the nucleus-encoded chloroplast division genes and proteins regulated by the algal cell cycle. Mol. Biol. Evol. 29: 2957–2970. [DOI] [PubMed] [Google Scholar]
- Mossmann D., Meisinger C., Vögtle F.N. (2012). Processing of mitochondrial presequences. Biochim. Biophys. Acta 1819: 1098–1106. [DOI] [PubMed] [Google Scholar]
- Movahedi S., Van Bel M., Heyndrickx K.S., Vandepoele K. (2012). Comparative co-expression analysis in plant biology. Plant Cell Environ. 35: 1787–1798. [DOI] [PubMed] [Google Scholar]
- Murcha M.W., Kmiec B., Kubiszewski-Jakubiak S., Teixeira P.F., Glaser E., Whelan J. (2014). Protein import into plant mitochondria: signals, machinery, processing, and regulation. J. Exp. Bot. 65: 6301–6335. [DOI] [PubMed] [Google Scholar]
- Nakano T., Murakami S., Shoji T., Yoshida S., Yamada Y., Sato F. (1997). A novel protein with DNA binding activity from tobacco chloroplast nucleoids. Plant Cell 9: 1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.J., Millar A.H. (2015). Protein turnover in plant biology. Nat. Plants 1: 15017. [DOI] [PubMed] [Google Scholar]
- Nickel S., Kaschani F., Colby T., van der Hoorn R.A., Kaiser M. (2012). A para-nitrophenol phosphonate probe labels distinct serine hydrolases of Arabidopsis. Bioorg. Med. Chem. 20: 601–606. [DOI] [PubMed] [Google Scholar]
- Nishimura K., van Wijk K.J. (2015). Organization, function and substrates of the essential Clp protease system in plastids. Biochim. Biophys. Acta 1847: 915–930. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Kato Y., Sakamoto W. (2016). Chloroplast proteases: Updates on proteolysis within and across suborganellar compartments. Plant Physiol. 171: 2280–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K., Kato Y., Sakamoto W. (2017). Essentials of proteolytic machineries in chloroplasts. Mol. Plant 10: 4–19. [DOI] [PubMed] [Google Scholar]
- Obayashi T., Kinoshita K. (2009). Rank of correlation coefficient as a comparable measure for biological significance of gene coexpression. DNA Res. 16: 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi T., Okamura Y., Ito S., Tadaka S., Aoki Y., Shirota M., Kinoshita K. (2014). ATTED-II in 2014: evaluation of gene coexpression in agriculturally important plants. Plant Cell Physiol. 55: e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okreglak V., Walter P. (2014). The conserved AAA-ATPase Msp1 confers organelle specificity to tail-anchored proteins. Proc. Natl. Acad. Sci. USA 111: 8019–8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paparelli E., et al. (2012). Misexpression of a chloroplast aspartyl protease leads to severe growth defects and alters carbohydrate metabolism in Arabidopsis. Plant Physiol. 160: 1237–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroza-Garcia J.A., Domenichini S., Bergounioux C., Benhamed M., Raynaud C. (2016). Chloroplasts around the plant cell cycle. Curr. Opin. Plant Biol. 34: 107–113. [DOI] [PubMed] [Google Scholar]
- Peltier J.B., Ripoll D.R., Friso G., Rudella A., Cai Y., Ytterberg J., Giacomelli L., Pillardy J., van Wijk K.J. (2004). Clp protease complexes from photosynthetic and non-photosynthetic plastids and mitochondria of plants, their predicted three-dimensional structures, and functional implications. J. Biol. Chem. 279: 4768–4781. [DOI] [PubMed] [Google Scholar]
- Piechota J., Kolodziejczak M., Juszczak I., Sakamoto W., Janska H. (2010). Identification and characterization of high molecular weight complexes formed by matrix AAA proteases and prohibitins in mitochondria of Arabidopsis thaliana. J. Biol. Chem. 285: 12512–12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polge C., Jaquinod M., Holzer F., Bourguignon J., Walling L., Brouquisse R. (2009). Evidence for the existence in Arabidopsis thaliana of the proteasome proteolytic pathway: Activation in response to cadmium. J. Biol. Chem. 284: 35412–35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poret M., Chandrasekar B., van der Hoorn R.A., Avice J.C. (2016). Characterization of senescence-associated protease activities involved in the efficient protein remobilization during leaf senescence of winter oilseed rape. Plant Sci. 246: 139–153. [DOI] [PubMed] [Google Scholar]
- Powers E.T., Balch W.E. (2013). Diversity in the origins of proteostasis networks--a driver for protein function in evolution. Nat. Rev. Mol. Cell Biol. 14: 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor E.E. Jr., Horanyi P.S., Clark K.M., Fedoriw N., Connelly S.M., Koszelak-Rosenblum M., Zhu G., Malkowski M.G., Wiener M.C., Dumont M.E. (2013). Structure of the integral membrane protein CAAX protease Ste24p. Science 339: 1600–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y., Liu X., Liang S., Wang R., Li Y., Zhao J., Shao J., An L., Yu F. (2016). A putative chloroplast thylakoid metalloprotease VIRESCENT3 regulates chloroplast development in Arabidopsis thaliana. J. Biol. Chem. 291: 3319–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radjacommare R., Usharani R., Kuo C.H., Fu H. (2014). Distinct phylogenetic relationships and biochemical properties of Arabidopsis ovarian tumor-related deubiquitinases support their functional differentiation. Front. Plant Sci. 5: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings N.D., Barrett A.J., Finn R. (2016). Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 44: D343–D350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C., Liu J., Gong Q. (2014). Functions of autophagy in plant carbon and nitrogen metabolism. Front. Plant Sci. 5: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S.Y., Mutwil M. (2014). Towards revealing the functions of all genes in plants. Trends Plant Sci. 19: 212–221. [DOI] [PubMed] [Google Scholar]
- Rigas S., Daras G., Tsitsekian D., Hatzopoulos P. (2012). The multifaceted role of Lon proteolysis in seedling establishment and maintenance of plant organelle function: living from protein destruction. Physiol. Plant. 145: 215–223. [DOI] [PubMed] [Google Scholar]
- Rigas S., Daras G., Tsitsekian D., Alatzas A., Hatzopoulos P. (2014). Evolution and significance of the Lon gene family in Arabidopsis organelle biogenesis and energy metabolism. Front. Plant Sci. 5: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas S., Daras G., Laxa M., Marathias N., Fasseas C., Sweetlove L.J., Hatzopoulos P. (2009). Role of Lon1 protease in post-germinative growth and maintenance of mitochondrial function in Arabidopsis thaliana. New Phytol. 181: 588–600. [DOI] [PubMed] [Google Scholar]
- Roberts T.H., Ahn J.W., Lampl N., Fluhr R. (2011). Plants and the study of serpin biology. Methods Enzymol. 499: 347–366. [DOI] [PubMed] [Google Scholar]
- Roose J.L., Pakrasi H.B. (2004). Evidence that D1 processing is required for manganese binding and extrinsic protein assembly into photosystem II. J. Biol. Chem. 279: 45417–45422. [DOI] [PubMed] [Google Scholar]
- Rose R., Schaller A., Ottmann C. (2010). Structural features of plant subtilases. Plant Signal. Behav. 5: 180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhier N., Vieira Dos Santos C., Tarrago L., Rey P. (2006). Plant methionine sulfoxide reductase A and B multigenic families. Photosynth. Res. 89: 247–262. [DOI] [PubMed] [Google Scholar]
- Rowland E., Kim J., Bhuiyan N.H., van Wijk K.J. (2015). The Arabidopsis chloroplast stromal N-terminome: Complexities of amino-terminal protein maturation and stability. Plant Physiol. 169: 1881–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer R.J., Michno J.M., Myers C.L. (2017). Unraveling gene function in agricultural species using gene co-expression networks. Biochim. Biophys. Acta 1860: 53–63. [DOI] [PubMed] [Google Scholar]
- Schaller A., Stintzi A., Graff L. (2012). Subtilases - versatile tools for protein turnover, plant development, and interactions with the environment. Physiol. Plant. 145: 52–66. [DOI] [PubMed] [Google Scholar]
- Schardon K., Hohl M., Graff L., Pfannstiel J., Schulze W., Stintzi A., Schaller A. (2016). Precursor processing for plant peptide hormone maturation by subtilisin-like serine proteinases. Science 354: 1594–1597. [DOI] [PubMed] [Google Scholar]
- Schelbert S., Aubry S., Burla B., Agne B., Kessler F., Krupinska K., Hörtensteiner S. (2009). Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 21: 767–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann H., Adamska I. (2012). Deg proteases and their role in protein quality control and processing in different subcellular compartments of the plant cell. Physiol. Plant. 145: 224–234. [DOI] [PubMed] [Google Scholar]
- Schuhmann H., Huesgen P.F., Adamska I. (2012). The family of Deg/HtrA proteases in plants. BMC Plant Biol. 12: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghatmehr M., Mueller-Roeber B., Balazadeh S. (2016). The plastid metalloprotease FtsH6 and small heat shock protein HSP21 jointly regulate thermomemory in Arabidopsis. Nat. Commun. 7: 12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G., Adam Z., Zhang H. (2007a). The E3 ligase AtCHIP ubiquitylates FtsH1, a component of the chloroplast FtsH protease, and affects protein degradation in chloroplasts. Plant J. 52: 309–321. [DOI] [PubMed] [Google Scholar]
- Shen G., Yan J., Pasapula V., Luo J., He C., Clarke A.K., Zhang H. (2007b). The chloroplast protease subunit ClpP4 is a substrate of the E3 ligase AtCHIP and plays an important role in chloroplast function. Plant J. 49: 228–237. [DOI] [PubMed] [Google Scholar]
- Shestakov S.V., Anbudurai P.R., Stanbekova G.E., Gadzhiev A., Lind L.K., Pakrasi H.B. (1994). Molecular cloning and characterization of the ctpA gene encoding a carboxyl-terminal processing protease. Analysis of a spontaneous photosystem II-deficient mutant strain of the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 269: 19354–19359. [PubMed] [Google Scholar]
- Simões I., Faro C. (2004). Structure and function of plant aspartic proteinases. Eur. J. Biochem. 11: 2067–2075. [DOI] [PubMed] [Google Scholar]
- Solheim C., Li L., Hatzopoulos P., Millar A.H. (2012). Loss of Lon1 in Arabidopsis changes the mitochondrial proteome leading to altered metabolite profiles and growth retardation without an accumulation of oxidative damage. Plant Physiol. 160: 1187–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y., Yao S.G., Sako K., Sato T., Kato W., Ohto M.A., Ichikawa T., Matsui M., Yamaguchi J., Ikeda A. (2007). SHA1, a novel RING finger protein, functions in shoot apical meristem maintenance in Arabidopsis. Plant J. 50: 586–596. [DOI] [PubMed] [Google Scholar]
- Srinivasan M., Mehta P., Yu Y., Prugar E., Koonin E.V., Karzai A.W., Sternglanz R. (2011). The highly conserved KEOPS/EKC complex is essential for a universal tRNA modification, t6A. EMBO J. 30: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler M., Eicke S., Mettler T., Messerli G., Hörtensteiner S., Zeeman S.C. (2009). Blocking the metabolism of starch breakdown products in Arabidopsis leaves triggers chloroplast degradation. Mol. Plant 2: 1233–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueldo D.J., van der Hoorn R.A.L. (2017). Plant life needs cell death, but does plant cell death need Cys proteases? FEBS J. 284: 1577–1585. [DOI] [PubMed] [Google Scholar]
- Sun Q., Emanuelsson O., van Wijk K.J. (2004). Analysis of curated and predicted plastid subproteomes of Arabidopsis. Subcellular compartmentalization leads to distinctive proteome properties. Plant Physiol. 135: 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Fu T., Chen N., Guo J., Ma J., Zou M., Lu C., Zhang L. (2010). The stromal chloroplast Deg7 protease participates in the repair of photosystem II after photoinhibition in Arabidopsis. Plant Physiol. 152: 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Niwa H., Yokota N., Kubota K., Inoue H. (2008). Widespread tissue expression of nepenthesin-like aspartic protease genes in Arabidopsis thaliana Plant Physiol. Biochem. 46: 724–729. [DOI] [PubMed] [Google Scholar]
- Tanz S.K., Castleden I., Hooper C.M., Small I., Millar A.H. (2014). Using the SUBcellular database for Arabidopsis proteins to localize the DEG protease family. Front. Plant Sci. 5: 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrago L., Laugier E., Rey P. (2009). Protein-repairing methionine sulfoxide reductases in photosynthetic organisms: gene organization, reduction mechanisms, and physiological roles. Mol. Plant 2: 202–217. [DOI] [PubMed] [Google Scholar]
- Teixeira P.F., Glaser E. (2013). Processing peptidases in mitochondria and chloroplasts. Biochim. Biophys. Acta 1833: 360–370. [DOI] [PubMed] [Google Scholar]
- Tohge T., Fernie A.R. (2012). Co-expression and co-responses: within and beyond transcription. Front. Plant Sci. 3: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano-Morales R., Xoconostle-Cázares B., Cabrera-Ponce J.L., Hinojosa-Moya J., Ruiz-Salas J.L., Galván-Gordillo S.V., Guevara-González R.G., Ruiz-Medrano R. (2015). AtTCTP2, an Arabidopsis thaliana homolog of translationally controlled tumor protein, enhances in vitro plant regeneration. Front. Plant Sci. 6: 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trösch R., Mühlhaus T., Schroda M., Willmund F. (2015). ATP-dependent molecular chaperones in plastids--More complex than expected. Biochim. Biophys. Acta 1847: 872–888. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Fujisawa Y., Mino T., Yuasa K. (2011). Identification of a plant aminopeptidase with preference for aromatic amino acid residues as a novel member of the prolyl oligopeptidase family of serine proteases. J. Biochem. 150: 525–534. [DOI] [PubMed] [Google Scholar]
- Van Aken O., Van Breusegem F. (2015). Licensed to kill: Mitochondria, chloroplasts, and cell death. Trends Plant Sci. 20: 754–766. [DOI] [PubMed] [Google Scholar]
- van der Hoorn R.A. (2008). Plant proteases: from phenotypes to molecular mechanisms. Annu. Rev. Plant Biol. 59: 191–223. [DOI] [PubMed] [Google Scholar]
- van Wijk K.J. (2015). Protein maturation and proteolysis in plant plastids, mitochondria, and peroxisomes. Annu. Rev. Plant Biol. 66: 75–111. [DOI] [PubMed] [Google Scholar]
- Vierstra R.D. (2009). The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 10: 385–397. [DOI] [PubMed] [Google Scholar]
- Vlot A.C., Liu P.P., Cameron R.K., Park S.W., Yang Y., Kumar D., Zhou F., Padukkavidana T., Gustafsson C., Pichersky E., Klessig D.F. (2008). Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance in Arabidopsis thaliana Plant J. 56: 445–456. [DOI] [PubMed] [Google Scholar]
- Vögtle F.N., Wortelkamp S., Zahedi R.P., Becker D., Leidhold C., Gevaert K., Kellermann J., Voos W., Sickmann A., Pfanner N., Meisinger C. (2009). Global analysis of the mitochondrial N-proteome identifies a processing peptidase critical for protein stability. Cell 139: 428–439. [DOI] [PubMed] [Google Scholar]
- Wagner R., Aigner H., Funk C. (2012). FtsH proteases located in the plant chloroplast. Physiol. Plant. 145: 203–214. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zheng X., Liu Y. (2016). Functional links between microtubules, autophagy and leaf starch degradation in plants. Plant Signal. Behav. 11: e1201626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yu B., Zhao J., Guo J., Li Y., Han S., Huang L., Du Y., Hong Y., Tang D., Liu Y. (2013). Autophagy contributes to leaf starch degradation. Plant Cell 25: 1383–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welchen E., García L., Mansilla N., Gonzalez D.H. (2014). Coordination of plant mitochondrial biogenesis: keeping pace with cellular requirements. Front. Plant Sci. 4: 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J., Park E., Dinesh-Kumar S.P. (2014). Differential processing of Arabidopsis ubiquitin-like Atg8 autophagy proteins by Atg4 cysteine proteases. Proc. Natl. Acad. Sci. USA 111: 863–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson J.D., Joens M.S., Sinson A.B., Gilkerson J., Salomé P.A., Weigel D., Fitzpatrick J.A., Chory J. (2015). Ubiquitin facilitates a quality-control pathway that removes damaged chloroplasts. Science 350: 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N., Doelling J.H., Falbel T.G., Durski A.M., Vierstra R.D. (2000). The ubiquitin-specific protease family from Arabidopsis. AtUBP1 and 2 are required for the resistance to the amino acid analog canavanine. Plant Physiol. 124: 1828–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xu R., Ma C.J., Vlot A.C., Klessig D.F., Pichersky E. (2008). Inactive methyl indole-3-acetic acid ester can be hydrolyzed and activated by several esterases belonging to the AtMES esterase family of Arabidopsis. Plant Physiol. 147: 1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Sun X., Zhang L. (2008). An Arabidopsis ctpA homologue is involved in the repair of photosystem II under high light. Chin. Sci. Bull. 53: 1021–1026. [Google Scholar]
- Yoshida K., Hisabori T. (2016). Adenine nucleotide-dependent and redox-independent control of mitochondrial malate dehydrogenase activity in Arabidopsis thaliana. Biochim. Biophys. Acta 1857: 810–818. [DOI] [PubMed] [Google Scholar]
- Yoshioka-Nishimura M., Yamamoto Y. (2014). Quality control of photosystem II: the molecular basis for the action of FtsH protease and the dynamics of the thylakoid membranes. J. Photochem. Photobiol. B 137: 100–106. [DOI] [PubMed] [Google Scholar]
- Zhang Z.J., Peck S.C. (2011). Simplified enrichment of plasma membrane proteins for proteomic analyses in Arabidopsis thaliana. Proteomics 11: 1780–1788. [DOI] [PubMed] [Google Scholar]
- Zientara-Rytter K., Sirko A. (2016). To deliver or to degrade - an interplay of the ubiquitin-proteasome system, autophagy and vesicular transport in plants. FEBS J. 283: 3534–3555. [DOI] [PubMed] [Google Scholar]
- Zybailov B., Rutschow H., Friso G., Rudella A., Emanuelsson O., Sun Q., van Wijk K.J. (2008). Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One 3: e1994. [DOI] [PMC free article] [PubMed] [Google Scholar]