Key Points
Patients with high-risk smoldering myeloma treated with 3-drug combinations have deep and durable responses with 63% MRD negativity.
Baseline mutations in high-risk smoldering myeloma and newly diagnosed myeloma are different, which suggests treatment-responsive biology.
Abstract
Early results of a prospective phase 2 clinical trial of carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide maintenance in high-risk smoldering myeloma showed promising results that were previously published. Here, we provide novel insights into the genetic landscape of high-risk smoldering myeloma and information on sustained minimal residual disease (MRD) negativity with an expanded cohort of patients. Eighteen patients with high-risk smoldering myeloma were enrolled between 29 May 2012, and 14 January 2014. We included patients with newly diagnosed multiple myeloma enrolled in a parallel trial who received the same therapy (reference group). The overall response rate was 100%. With median potential follow-up of 43.3 months, 10 (63%) remain in MRD negativity, and the estimated 4-year progression-free and overall survival rates are 71% and 100%, respectively. Importantly, we report differences in mutational patterns in patients with high-risk smoldering myeloma and newly diagnosed multiple myeloma, reflected in a lower frequency of mutations in significant myeloma genes (6.6% vs 45%) and NFKB pathway genes (6.6% vs 25%). Treatment with carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide maintenance was associated with a 100% response rate and 63% MRD negativity with a safety profile consistent with previous reports for this regimen. This study had a small numbers of participants, but there seemed to be important differences in the genetic landscape of patients with high-risk smoldering myeloma and those with newly diagnosed multiple myeloma, suggestive of a more treatment-responsive biology in early disease.
Visual Abstract
Introduction
Multiple myeloma (MM) is a plasma cell neoplasm and the second most common hematologic malignancy in adults.1 Large, independent studies have shown that the genetic and molecular landscape underlying MM pathogenesis is massively heterogeneous and includes several recurrent mutations in KRAS, NRAS, TP53, and other genes.2-4 However, no single mutation is seen in all or most patients with MM. Prospective studies have demonstrated that MM is consistently preceded by a precursor state, that is, monoclonal gammopathy of undetermined significance and smoldering myeloma.5,6 Smoldering myeloma is an earlier, asymptomatic stage of myeloma and is defined on the basis of clinical and laboratory characteristics. It carries an increased risk of progression to MM.7
Prior studies have suggested that a subset of patients with smoldering myeloma have a particularly high risk of developing MM, with a median time to progression of <2 years.8-10 The standard management of patients with high-risk smoldering myeloma is close clinical follow-up and initiation of treatment only when myeloma-defining end organ damage is observed.11 With the introduction of novel antimyeloma therapies with high efficacy and acceptable safety profiles, there is increasing interest in treatment trials that focus on high-risk smoldering myeloma.7 A recent randomized clinical trial by the Spanish Programa para el Tratamiento de Hemopatías Malignas (PETHEMA) myeloma group demonstrated improved time to progression to overt myeloma and overall survival (OS) with early initiation of lenalidomide and dexamethasone therapy compared with patients managed with standard observation.12,13 Despite the increasing interest in treatment studies that focus on high-risk smoldering myeloma, only limited information is available regarding the genetic profiles for this disease, but preliminary studies have suggested comparable mutational load and copy number profiles of myeloma cells in patients with either smoldering myeloma or MM.14,15
We previously reported results from 2 prospective studies that included patients with either high-risk smoldering myeloma or newly diagnosed MM.16 In both cohorts, all patients were treated uniformly with 8 cycles of combination therapy that included carfilzomib, lenalidomide, and dexamethasone (KRd) followed by 2 years of lenalidomide maintenance (KRd-R).
Here, we expand on our early results16 by enrolling additional patients with high-risk smoldering myeloma and capturing a longer median follow-up of almost 4 years. Our promising early results of high overall response rate and minimal residual disease (MRD) negativity among patients with high-risk smoldering myeloma who were treated with modern KRd-R combination therapy16 prompted us to prospectively investigate the baseline genetic landscape and patterns of mutations in our cohort of patients. As a reference group, we included 40 patients with newly diagnosed MM who received the same KRd-R therapy.16 Here we describe, to the best of our knowledge for the first time, differences in patterns of mutations in patients with high-risk smoldering myeloma and newly diagnosed MM by using prospectively enrolled patients treated on clinical trials.
Methods
Study participants, treatment, and oversight
The details of the trial design and eligibility were published previously.16 Briefly, patients with high-risk smoldering myeloma or newly diagnosed MM were enrolled on 2 studies (NCT01402284 and NCT01572480). Smoldering myeloma was defined on the basis of International Myeloma Working Group (IMWG) 2003 guidelines17 and high risk of progression was determined on the basis of the Mayo Clinic risk models (serum monoclonal protein ≥3 g/dL, bone marrow plasma cell infiltration ≥10%, and abnormal serum free light chain ratio <0.125 or >8.0)8 and/or Spanish PETHEMA risk models (≥95% plasma cells with an abnormal immunophenotype by multicolor flow cytometry and evidence of serum immunoparesis).9 All patients were treated uniformly with eight 28-day cycles of combination therapy. Carfilzomib was administered as a 30-minute intravenous infusion on days 1, 2, 8, 9, 15, and 16 (starting dose of 20 mg/m2 on days 1 and 2 of cycle 1 and 36 mg/m2 thereafter), lenalidomide was administered orally on days 1 to 21 at a dose of 25 mg per day (not administered on day 1 of cycle 1), and dexamethasone was administered orally or intravenously on days 1, 2, 8, 9, 15, 16, 22, and 23 (20 mg for cycles 1-4 and 10 mg for cycles 5-8; dexamethasone was not administered on day 1 of cycle 1). All patients received lenalidomide maintenance for 2 years after completing 8 cycles of combination therapy. We obtained baseline bone marrow biopsy samples from patients enrolled in the 2 prospective clinical trials). The primary objective for the smoldering myeloma trial was response rate (very good partial response or better); secondary objectives were progression-free survival (PFS) and response duration,18 which were assessed after every cycle of induction and every 90 days during maintenance. Correlative studies including assessment of MRD status by multicolor flow cytometry (bone marrow aspirate; 10−5 sensitivity) were performed when patients achieved a complete response (CR) or after 8 cycles of induction and 1 or 2 years of maintenance lenalidomide.16 For patients who did not achieve a CR, MRD assessment was performed at the specified time points (end of induction and after 1 or 2 years of lenalidomide maintenance). The studies were approved by the Institutional Review Board at the National Cancer Institute, and all participants provided written informed consent.
Genetic studies and bioinformatic analysis
Genetic studies were performed on baseline bone marrow aspirates from which plasma cells were enriched by using Miltenyi anti-CD138 microbeads with MACS Manual Cell Separation. Enriched cells were stored at −80°C in Buffer RLT, and DNA and RNA were isolated by using the Qiagen AllPrep DNA/RNA Mini or Micro kits, depending on the number of cells. Genomic libraries were constructed on an Agilent Bravo Liquid Handling platform using a Translational Genomics Research Institute (TGen)–developed protocol for the KAPA Hyper Prep Kit optimized for 200-ng double-stranded DNA input with a 1:100 insert-to-adaptor ratio of a custom synthesized Illumina paired-end sequencing compatible adaptor. Exome enrichment was performed on 750 ng of each library using the Agilent SureSelect Human All Exon V6 kit with liquid handling protocols provided by Agilent; the only modification was the use of KAPA HiFi DNA polymerase for postcapture amplification. The enriched libraries were pooled and sequenced on Illumina HiSequation 2500 sequencers resulting in a median target coverage of 125× (range, 105-185×) after deduplication and quality control.
A tumor-only analysis was conducted by using the TGen Jetstream pipeline. Raw sequencing data were converted to FASTQ files by using bcl2fastq v1.8.4. The paired-end fastq files were aligned to the GRCh37 reference genome, hs37d5 version, from the 1000 Genomes Project by using the mem module of BWA v0.7.8 and SAMTOOLS v0.1.19 to produce BAM files. After alignment, the base quality scores were recalibrated and joint indel realignment was performed on the BAM files using GATK v3.1-1. Duplicate read pairs were marked using PICARD v1.111. These final BAM files were then used to identify potential somatic events using a comparative analysis against a common control sample, an identically processed NA12878 sample. DNA copy number abnormalities were identified by using tCoNuT (github.com/tgen/tCoNuT) followed by filtering of constitutional copy number variants found in NA12878 and the gold standard variants from the Database of Genomic Variants. Potential somatic single nucleotide variants and indels were identified by using 3 different somatic variant callers (SEURAT v2.6, STRELKA v1.0.13, and MUTECT v1.1.4) followed by filtering that required the sequencing depth at the position in question to be greater than 10 and the alternate allele ratio to be greater than 5%. Variants were removed and assumed to be constitutional variants if a matching variant with an allele count of 10 or more was reported in Single Nucleotide Polymorphism Database (dbSNP), 1000 Genomes Project, National Heart, Lung, and Blood Institute, or the Exome Aggregation Consortium (ExAC) databases. The remaining variants detected by at least two callers were further filtered to keep only those variants that were previously identified in the Catalogue of Somatic Mutations in Cancer (COSMIC) or that existed in a white list of cancer genes or were not observed in the aforementioned constitutional databases or an internal TGen database. These final variants were annotated by using snpEFF v4.2 with ensembl version 74 gene models.
Results
Between May 29, 2012, and January 14, 2014, 18 patients with high-risk smoldering myeloma were enrolled in the study (NCT01572480). Baseline demographics and disease characteristics are outlined in Table 1. Fifteen of the 18 patients had evaluable baseline bone marrow aspirate samples and were included in this analysis. As a reference group, we included bone marrow aspirate samples from 40 evaluable patients with newly diagnosed MM who were enrolled in a clinical trial with a similar treatment schedule (NCT01402284). Per the study protocols, bone marrow biopsies were obtained before treatment and after 1 single dose of carfilzomib. In this study, 29 patients had bone marrow aspirates collected before any treatment, and 27 had samples collected after 1 dose of carfilzomib because samples from day 1 were unavailable. To ensure the accuracy of the data, we performed whole-exome sequencing of paired samples collected before and after a single dose of carfilzomib for 4 patients, and the results were highly concordant (copy number estimate concordance range, 95.0%-99.6%; mutant allele frequency correlation range, 0.91-0.97).
Table 1.
Baseline demographic and disease characteristics
| Baseline characteristic | High-risk smoldering myeloma (n = 18) | |
|---|---|---|
| No. | % | |
| Median age, y (range) | 59 (40-73) | |
| Sex | ||
| Male | 9 | 50 |
| Female | 9 | 50 |
| Race | ||
| White | 14 | 89 |
| African American | 4 | 11 |
| Risk of progression | ||
| Mayo Clinic risk model* | ||
| High | 3 | 17 |
| Low/intermediate | 15 | 83 |
| Spanish PETHEMA risk model† | ||
| High | 16 | 89 |
| Low/intermediate | 2 | 11 |
| MM by the 2014 IMWG criteria11 | 6 | 33 |
| Heavy chain isotype | ||
| IgG | 16 | 89 |
| IgA | 1 | 5.5 |
| Light chain | 1 | 5.5 |
| Light chain isotype | ||
| κ | 13 | 72 |
| Λ | 5 | 28 |
| FISH/cytogenetics | ||
| 13q14 deletion | 4 | 22 |
| 17p13.1 deletion | 1 | 6 |
| IgH translocation | 4 | 22 |
| Median serum monoclonal protein, g/dL (range) | 1.9 (1.0-5.3) | |
| Bone marrow plasma cell infiltration | ||
| Median, % (range) | 30 (12.5-65) | |
| 10-60 | 12 | 67 |
| >60 | 6 | 33 |
FISH, fluorescent in situ hybridization; Ig, immunoglobulin.
High-risk features include bone marrow plasma cell infiltration >10%, serum monoclonal protein >3 g/dL, and free light chain ratio >8 or <0.125.
High-risk features include 95% or more plasma cells with abnormal immunophenotype by flow cytometry and serum immunoparesis.
Efficacy
The cutoff date for this analysis was October 20, 2016, and the median potential follow-up (median of intervals from on-study date until the analysis cutoff date) was 43.3 months. Per the clinical trial protocol, treatment response was assessed according to the International Myeloma Workshop consensus panel19 with the addition of near complete response20 (Table 2).
Table 2.
Treatment responses after induction therapy and completion of 1 or 2 years of lenalidomide maintenance
| Response | Best response | End of induction therapy (8 cycles of KRd therapy) | After completion of 1 year of maintenance* | After completion of 2 years of maintenance* | ||||
|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | n/N | % | n/N | % | |
| ORR | 18/18 | 100 | 18/18 | 100 | 18/18 | 100 | 18/18 | 100 |
| ≥VGPR | 18/18 | 100 | 18/18 | 100 | 18/18 | 100 | 18/18 | 100 |
| nCR | 1/18 | 6 | 6/18 | 33 | 1/18 | 6 | 1/18 | 6 |
| sCR/CR | 16/18 | 89 | 11/18 | 61 | 16/18 | 89 | 16/18 | 89 |
| MRD-negative CR | 15/18 | 83 | 10/18 | 56 | 10/16 | 63 | 10/16 | 63† |
nCR, near complete response; n/N, number of patients achieving the response/number of patients assessed at the time point; ORR, overall response rate; sCR, stringent complete response; VGPR, very good partial response.
Lenalidomide maintenance: lenalidomide 10 mg once per day on days 1 to 21 on a 28-day cycle.
One patient refused to have a bone marrow biopsy procedure and 1 patient withdrew from therapy but was MRD negative after 6 months of maintenance therapy.
Table 2 provides the best overall responses (ie, the best response for an individual patient at any time point during follow-up) and landmark analysis responses (ie, at the end of combination therapy, after 1 or 2 years of lenalidomide maintenance). The best response rates were as follows: all 18 patients (100%) obtained a partial response or better, and 16 of 18 patients obtained a CR or a stringent CR. MRD assessment was performed by using multicolor flow cytometry (bone marrow aspirate; 10−5 sensitivity), and 15 of the 18 patients had attained MRD negativity or CR as best response.
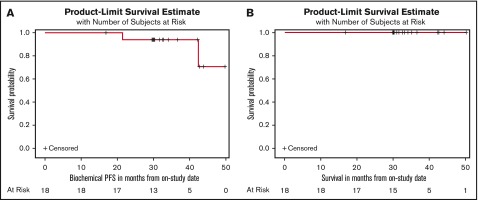
In the landmark analysis responses, we found that after completing the combination therapy and at the end of 2 years of lenalidomide maintenance, all 18 patients (100%) had at least a partial response, including 16 patients (89%) with a CR or stringent CR. Ten (63%) of 16 patients obtained an MRD-negative CR after 2 years of maintenance therapy (Table 2). By Kaplan-Meier analyses beginning at the on-study date, the estimated 36- and 48-month PFS rates for the intention-to-treat population (n = 18) were 94.1% (95% confidence interval, 65.0%-99.2%) and 70.6% (95% confidence interval, 16.0%-93.6%), respectively, and the estimated OS at 36 and 48 months was 100% (Figure 1A-B). If the revised IMWG 2014 criteria had been used at study enrollment, 1 of the 2 patients would have been classified as having MM.11
Figure 1.
Clinical outcomes in patients with high-risk smoldering myeloma after 8 cycles of KRd and 2 years of lenalidomide maintenance. Estimated (A) PFS and (B) OS.
Safety
All 18 patients were evaluated for adverse events, and there were no grade 5 adverse events. The adverse event profile was reported previously.16 Grade 3 to 4 adverse events occurring in >1 patient included lymphopenia (39%), neutropenia (28%), anemia (22%), diarrhea (17%), lung infection (17%), hypophosphatemia (11%), and thromboembolic event (11%). Serious adverse events included pulmonary infection in 2 patients (11%) and congestive heart failure in 1 patient (5.5%). Two patients discontinued therapy because of adverse events: 1 patient developed congestive heart failure after 6 cycles of combination therapy (assessed as being likely related to carfilzomib), and the other patient discontinued treatment after 9 months of lenalidomide maintenance because of grade 3 diarrhea and grade 2 rash (assessed as being likely related to lenalidomide).
Genetic landscape and baseline mutational patterns in high-risk smoldering myeloma
The unprecedented clinical efficacy of early treatment of high-risk smoldering myeloma with combination therapy prompted us to investigate the baseline genetic landscape and patterns of mutations in patients with that disease. There were 18 patients in the study; 15 had sufficient DNA for the mutational analysis, and 13 samples passed the quality control for copy number analysis. We included 40 patients with newly diagnosed MM who received similar therapies as a control group.
Copy number analysis and nonsynonymous mutations in high-risk smoldering myeloma and newly diagnosed MM
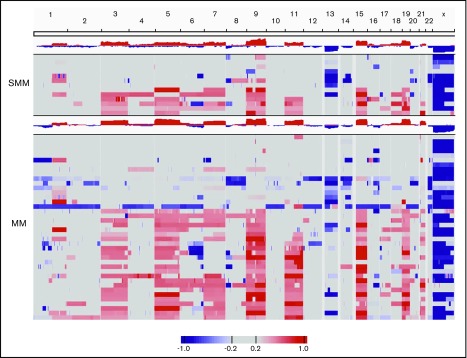
As a first step, we conducted copy number analysis of CD138-sorted bone marrow aspirate samples. Patients with either high-risk smoldering myeloma or newly diagnosed MM showed copy number deletions and gains typical of abnormal plasma cells, which confirmed the high purity of samples (Figure 2).
Figure 2.
Copy number analysis in patients with either high-risk smoldering myeloma (SMM) or newly diagnosed MM. The 13 patients with SMM and the 40 patients with MM with copy number estimates that passed quality control requirements are shown.
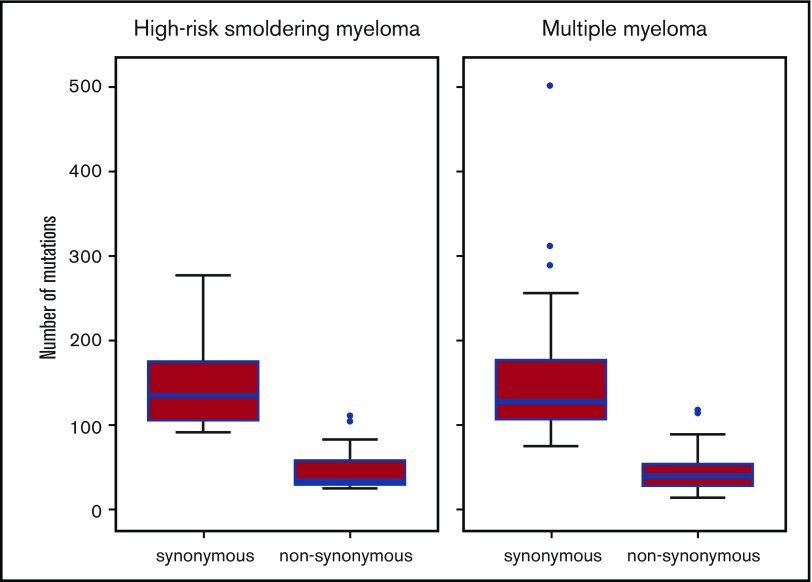
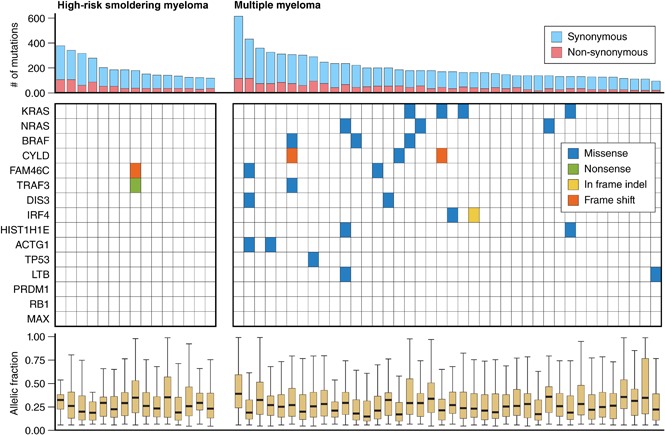
We then assessed the median number of nonsynonymous mutations in patients with either smoldering myeloma or newly diagnosed MM, and the results were comparable with 34 (interquartile range, 30-58) and 40 (interquartile range, 28-54) nonsynonymous single nucleotide variants per patient, respectively (Figure 3).
Figure 3.
Number of synonymous and nonsynonymous mutations in patients with high-risk smoldering myeloma or newly diagnosed MM. The upper whisker extends from the hinge to the highest value that is within 1.5 × the interquartile range (IQR) of the hinge, in which the IQR is the distance between the first and third quartiles. The lower whisker extends from the hinge to the lowest value within 1.5 × IQR of the hinge. Data beyond the ends of the whiskers are outliers and are plotted as points (as specified by Tukey). The total number of mutations in high-risk smoldering myeloma (n = 15) was 3012, the median number per patient was 178 (IQR, 137-239), the median number of synonymous mutations per patient was 135 (IQR, 106-175), and median number of nonsynonymous mutations per patient was 34 (IQR, 30-58). Total number of mutations in newly diagnosed multiple myeloma (n = 40) was 8122, the median number of mutations per patient was 169 (IQR, 139-236), the median number of synonymous mutations per patient was 128 (IQR, 107-176), and the median number of nonsynonymous mutations per patient was 40 (IQR, 28-54). The blue horizontal bars represent the median number of mutations.
Mutations in significantly mutated genes in high-risk smoldering myeloma and newly diagnosed MM
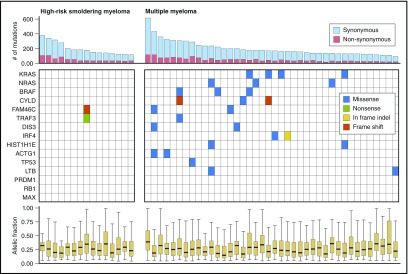
Next we assessed the frequency of nonsynonymous mutations in previously identified significantly mutated genes (Figure 4). Consistent with prior studies, 18 (45%) of 40 patients with newly diagnosed MM had a mutation in at least 1 of the 15 genes previously identified as significantly mutated genes.2,4 Strikingly, only 1 (6.6%) of 15 patients with high-risk smoldering myeloma had a mutation in these 15 genes. That individual patient had >60% plasma cells and would be considered to have MM on the basis of the revised IMWG 2014 criteria.11 As shown in Figure 4, that patient had mutations in both FAM46C and TRAF3.
Figure 4.
Frequency of mutations in significantly recurrent MM genes among patients with high-risk smoldering myeloma or newly diagnosed MM. Professional illustration by Patrick Lane, ScEYEnce Studios.
Mutations in genes associated with the NFKB pathway in high-risk smoldering myeloma and newly diagnosed MM
Prior studies have also demonstrated recurrent mutations in signaling pathways in MM.2,21 These include the NFKB pathway, coagulation cascade, and histone-modifying enzyme pathways among others. Although differences in mutations between individual genes in these pathways lack statistical significance, multiple genes associated with the pathway were mutated in MM patients, and they attained statistical significance in prior studies.2-4
Given that both the classical and the alternative NFKB pathways have been causally implicated in MM as potential targets for proteasome inhibitors, we were interested in mutations in genes associated with this pathway. Indeed, we identified these mutations in 1 (6.6%) of 15 patients with smoldering myeloma and 10 (25%) of 40 patients with newly diagnosed MM. We also looked for mutation in the coagulation cascade and the histone-modifying enzyme pathways; only 1 patient with newly diagnosed MM had a mutation in a gene in the histone-modifying enzyme pathway within this cohort.
Discussion
The introduction of highly effective antimyeloma therapies with acceptable safety profiles has led to clinical trials that focus on the treatment of patients with high-risk smoldering myeloma. Indeed, Mateos et al12 reported an improved time to progression and OS in patients with high-risk smoldering myeloma who received lenalidomide and dexamethasone. Population studies have estimated that ∼30% of all patients with smoldering myeloma would be considered high risk and would be considered as potential candidates for early initiation of treatment.22
On the basis of historical data and in the absence of active therapy, patients with high-risk smoldering myeloma have a median time to progression of <2 years.8-10 Here, we report unprecedented 63% sustained MRD negativity in patients with high-risk smoldering myeloma after completion of 8 cycles of KRd combination therapy followed by 2 years of lenalidomide maintenance. Furthermore, on the basis of small numbers of progression events (n = 2), extended clinical follow-up shows 3-year and 4-year PFS rates of 94% and 71%, respectively, and a 4-year OS rate of 100%.
Although we noted deep and durable responses in patients with either smoldering myeloma or newly diagnosed MM who were treated with KRd-R compared with patients with newly diagnosed MM, those with high-risk smoldering myeloma had a higher frequency of CR (94% vs 64%) and MRD negativity (63% vs 46%). These results suggest possible differences in the biology of earlier stages of the disease (ie, smoldering myeloma) compared with that of newly diagnosed MM, which motivated us to assess mutational patterns in patients with these diseases. Indeed, until now, only limited information based on small series was available regarding the genetic profiles of patients with high-risk smoldering myeloma. Although our study had a small number of patients, the strength of our data set is the genetic mutational analysis paired with prospective clinical treatment response data. Prior studies with small series of patients have reported comparable mutational burden (ie, median number of mutations) in patients with smoldering myeloma or MM.14,15 Here, we confirm these findings in the context of two parallel prospective treatment studies that used carfilzomib-based regimens to treat patients with high-risk smoldering myeloma or newly diagnosed MM. Furthermore, we show that even though the number of nonsynonymous mutations is comparable between the 2 groups, there are important differences in the patterns of mutations. Specifically, the frequency of mutations in significantly mutated MM genes (6.6% vs 45%) and genes associated with the NFKB pathway (6.6% vs 25%) are lower in patients with high-risk smoldering myeloma compared with those who have newly diagnosed MM. The biological underpinnings of these observations remain unknown. Although this is the largest study to date to assess genetic profiles and clinical outcomes in uniformly treated (using modern 3-drug combination therapy) patients with high-risk smoldering myeloma enrolled on a prospective clinical trial, the sample size precludes us from providing definitive conclusions. Therefore, these results need to be confirmed and expanded in future large data sets. Finally, another perspective on our observed MRD rate of 63% in patients with high-risk smoldering myeloma who were treated with KRd-R in this study, is the parallel ∼70% MRD negativity (10−5) in patients with newly diagnosed MM treated with bortezomib, lenalidomide, and dexamethasone (RVd) followed by high-dose melphalan and autologous stem cell transplantation (ASCT) and an additional 2 cycles of RVd followed by 1 year of lenalidomide maintenace.23
The clinical results from this study have prompted other investigators to confirm and expand on the use of KRd-R therapy in high-risk smoldering myeloma. For example, the Spanish PETHEMA myeloma group is currently enrolling 90 patients with high-risk smoldering myeloma on a single-arm phase 2 study that uses 6 cycles of KRd followed by high-dose melphalan and ASCT and an additional 2 cycles of KRd followed by 2 years of lenalidomide maintenance. Future studies will address the role of consolidative ASCT and other important questions (eg, the addition of monoclonal antibodies, the clinical value of improved MRD assays, and the impact of molecular profiling on clinical outcomes).
In this study, we used the exact same DNA whole-exome sequencing and statistical analysis as the Multiple Myeloma Research Foundation (MMRF) Compass study (n > 800).23 We have reviewed our data extensively, and we feel confident that they are accurate for the included 18 patients. Given the small sample size in our study and the observed results, we were unable to conduct further statistical analysis. Future larger studies are needed to improve our understanding of these topics. Avenues to consider in larger studies include stratifications (using predefined cutoffs) by plasma cell percentage, cytogenetic status, lactate dehydrogenase, and other factors.
In summary, our study shows that 63% of patients with high-risk smoldering myeloma treated with KRd-R therapy had sustained MRD negativity up to 4 years after starting therapy. Furthermore, for the first time, we show that patients with high-risk smoldering myeloma have a lower frequency of mutational burden in significantly mutated myeloma genes with less likelihood of NFKB pathway mutation involvement. When paired with clinical data, these observations support a more treatment-responsive biology compared with patients who have newly diagnosed MM.
Acknowledgments
The authors thank all of the patients who contributed to this study and the clinical and research staff at the Intramural Program of the National Cancer Institute (NCI), National Institutes of Health (NIH).
This work was supported by the Intramural Program at the NCI, NIH; by Memorial Sloan Kettering Cancer Center Core Grant P30 CA008748 from the NCI, NIH (S.M., N.K., M.H., E.P., and O.L.); and by grant 2015-00564 from the Swedish Research Council (M.H.). Carfilzomib and lenalidomide and funding for parts of the correlative assays were provided by Onyx and Celgene (to NCI, NIH) as part of a cooperative research and development agreement.
Onyx and Celgene had no role in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Presented in part at the 52nd annual meeting of the American Society of Clinical Oncology, Chicago, IL, 3-7 June 2016, and the 58th annual meeting of the American Society of Hematology, San Diego, CA, 3-6 December 2016.
Authorship
Contribution: S.M., D.K., N.K., and O.L. designed the study; S.M., D.K., N.K., M.R., E.M., M.B., N.T., M.K., and C.M. managed patients; Y.Z., A.Z., L.L., and R.C. performed correlative studies; A.C., M.W., M.B., and J.J.K. performed whole-exome sequencing and bioinformatic analysis; S.M.S. performed statistical analysis; M.H., E.P., W.D.F., and W.H.W. helped with data analysis; and all authors provided critical review of the manuscript and approved the last version of the manuscript.
Conflict-of-interest disclosure: O.L. has given scientific talks at meetings funded by unrestricted grants from Celgene, Amgen, Bristol-Myers Squibb, Novartis, Takeda, and Janssen; has served on independent data monitoring committees for Takeda, Merck, and Janssen; serves as chairman for the Medscape Continuing Medical Education Program for Myeloma (2015-2017); and has received grant support from Takeda, Celgene, Amgen, and Janssen. S.M. has served as principal investigator in clinical trials supported by Juno Therapeutics and Takeda Oncology. The remaining authors declare no competing financial interests.
The current affiliation for L.L. is Paradigm Shift Therapeutics, Rockville, MD.
Correspondence: Ola Landgren, Myeloma Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: landgrec@mskcc.org.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2.Lohr JG, Stojanov P, Carter SL, et al. ; Multiple Myeloma Research Consortium. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell. 2014;25(1):91-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolli N, Avet-Loiseau H, Wedge DC, et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat Commun. 2014;5:2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker BA, Boyle EM, Wardell CP, et al. Mutational spectrum, copy number changes, and outcome: results of a sequencing study of patients with newly diagnosed myeloma. J Clin Oncol. 2015;33(33):3911-3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412-5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113(22):5418-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghobrial IM, Landgren O. How I treat smoldering multiple myeloma. Blood. 2014;124(23):3380-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111(2):785-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Persona E, Vidriales MB, Mateo G, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110(7):2586-2592. [DOI] [PubMed] [Google Scholar]
- 10.Cherry BM, Korde N, Kwok M, et al. Modeling progression risk for smoldering multiple myeloma: results from a prospective clinical study. Leuk Lymphoma. 2013;54(10):2215-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. [DOI] [PubMed] [Google Scholar]
- 12.Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447. [DOI] [PubMed] [Google Scholar]
- 13.Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(8):1127-1136. [DOI] [PubMed] [Google Scholar]
- 14.Walker BA, Wardell CP, Melchor L, et al. Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia. 2014;28(2):384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao S, Choi M, Heuck C, et al. Serial exome analysis of disease progression in premalignant gammopathies. Leukemia. 2014;28(7):1548-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1(6):746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749-757. [PubMed] [Google Scholar]
- 18.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328-e346. [DOI] [PubMed] [Google Scholar]
- 19.Rajkumar SV, Harousseau JL, Durie B, et al. ; International Myeloma Workshop Consensus Panel 1. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609-2617. [DOI] [PubMed] [Google Scholar]
- 21.Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471(7339):467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristinsson SY, Holmberg E, Blimark C. Treatment for high-risk smoldering myeloma. N Engl J Med. 2013;369(18):1762-1763. [DOI] [PubMed] [Google Scholar]
- 23.Attal M, Lauwers-Cances V, Hulin C, et al. ; IFM 2009 Study. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]