Key Points
CXCR4 antagonist AMD3100 but not KRH3955 reverses the CXCL12 chemokine gradient across the bone marrow endothelium.
CXCR4 antagonists mobilize stem cells from the bone marrow by distinct mechanisms.
Abstract
Pharmacological mobilization of hematopoietic progenitor cells (HPCs) is used clinically to harvest HPCs for bone marrow transplants. It is now widely accepted that the CXCR4:CXCL12 chemokine axis plays a critical role in the retention of HPCs in the bone marrow, and CXCR4 antagonists have been developed for their mobilization. The first of this class of drugs to be US Food and Drug Administration-approved was the bicyclam AMD3100. In addition to mobilizing HPCs and leukocytes in naïve mice, AMD3100 has been shown to mobilize mesenchymal progenitor cells (MPCs) in vascular endothelial growth factor (VEGF-A) pretreated mice. AMD3100 binds to the transmembrane region of CXCR4 and is thought to mobilize HPCs by reversing the gradient of CXCL12 across the bone marrow endothelium. Consistent with this hypothesis, our data show that selective neutralization of CXCL12, with chalcone 4-phosphate (C4P), inhibited AMD3100-stimulated mobilization of HPCs and leukocytes in naïve mice and MPCs in VEGF-A pretreated mice. In contrast it is shown here that the CXCR4 antagonist KRH3955 that binds to the extracellular loop of CXCR4 does not reverse the CXCL12 chemokine gradient. However, this drug efficiently mobilizes HPCs, a response that is not inhibited by C4P. In contrast, KRH3955 does not mobilize MPCs in VEGF-A pretreated mice. These data suggest that CXCR4 antagonists that bind to distinct regions of the receptor mobilize progenitor cells by distinct molecular mechanisms.
Visual Abstract
Introduction
Chemokine receptors are critical for directed migration of leukocytes from the circulation to sites of inflammation.1 In addition, a number of chemokines play important roles in tissue homeostasis. One such chemokine is CXCL12 (C-X-C motif chemokine ligand 12), a chemokine produced constitutively in the bone marrow, which through binding to CXCR4 (C-X-C chemokine receptor type 4) expressed by leukocytes and hematopoietic stem/progenitor cells (HSPCs) plays a critical role in the retention and homing of these cells to the bone marrow.2-9 The small organic bicyclam molecule Plerixafor (AMD3100) is a CXCR4 antagonist that was originally developed as an anti-HIV agent and was shown to inhibit viral replication of the X4 HIV strain known to require CXCR4 for entry into T cells.10 However, during preclinical and phase I clinical studies, it was reported that administration of AMD3100 in mice and humans resulted in a rapid rise in circulating leukocytes and HSPCs within a matter of hours.11-13
Granulocyte colony-stimulating factor (G-CSF) is used clinically to mobilize HSPCs for bone marrow transplants (BMTs). Optimal mobilization requires daily administration of G-CSF over 4 to 6 days. In terms of its mechanism of action, G-CSF is thought to disrupt the CXCR4:CXCL12 chemokine retention axis by reducing local messenger RNA and protein levels of CXCL12.2,3,5,14 In approximately 20% of donors, the so-called poor mobilizers, G-CSF does not mobilize sufficient HSPCs to perform a BMT. AMD3100 was shown to act synergistically with G-CSF with respect to HSPC mobilization, and this led to AMD3100 being approved by the US Food and Drug Administration for clinical use in combination with G-CSF for poor mobilizers.13,15,16
Although it was originally thought that AMD3100 mobilized HSPCs as a result of direct disruption of the CXCR4:CXCL12 retention axis, through antagonism of CXCR4, a recent study reported that there was a rapid elevation of CXCL12 in the peripheral circulation of mice following AMD3100 administration, thereby reversing the gradient of CXCL12 across the bone marrow endothelium.13,17 The authors provided evidence that AMD3100 stimulated the translocation of CXCL12 across the bone marrow sinusoidal endothelium into the blood and that the chemokine gradient that was generated was critical in driving HSPC mobilization.17 These data have not as yet been corroborated by others.
In addition to hematopoietic progenitor cells (HPCs), AMD3100 has been reported to mobilize endothelial progenitor cells (EPCs) and mesenchymal progenitor cells (MPCs) from the bone marrow.18,19 Unlike EPCs and HPCs, in which AMD3100 alone was sufficient for mobilization, combination treatment with vascular endothelial growth factor (VEGF-A) was required for the mobilization of MPCs.18 It is currently not known how this combination treatment stimulates MPC mobilization.
In this article we have investigated the mobilization of HPCs and MPCs by AMD3100 and a chemically distinct CXCR4 antagonist, KRH3955.20,21 KRH3955 is reported to have a higher affinity for CXCR4 and a distinct target site to AMD3100.21 As with AMD3100, preliminary studies investigating the anti-HIV activity of KRH3955 showed that a single dose of this drug elevated leukocyte counts in peripheral blood.22 Here, we have made use of a specific CXCL12 neutraligand, chalcone 4-phosphate,23,24 to address the question of whether an elevation of CXCL12 in the plasma is required for mobilization of HPCs and MPCs by these distinct CXCR4 antagonists.
Our data corroborate the previous report that AMD3100 stimulates a reversal of the CXCL12 chemokine gradient across the bone marrow sinusoidal endothelium and moreover that an elevation in circulating CXCL12 is necessary for AMD3100-stimulated mobilization of leukocytes and HPCs into the blood. Furthermore, we demonstrate that this mechanism also underlies the mobilization of MPCs by AMD3100 in combination with VEGF-A. In contrast, KRH3955 does not elevate circulating concentrations of CXCL12, but it does mobilize HPCs and leukocytes, suggesting a distinct mechanism of action to AMD3100. Unexpectedly, KRH3955 in combination with VEGF-A failed to mobilize MPCs, suggesting that the reversed CXCL12 gradient is critical for their mobilization.
Methods
Mice
BALB/c female mice purchased from Harlan Laboratories were used throughout this study, which were between the ages of 8 and 12 weeks and weighing 18 to 22 g. Mice were age- and weight-matched for each experiment. All studies were carried out under the United Kingdom’s Animals (Scientific Procedures) Act of 1986 and local ethical approval from Imperial College London.
Administration of drugs
Mice were administered AMD3100 (5 mg/kg intraperitonially [IP]; Sigma) or vehicle (phosphate-buffered saline [PBS]) 1 hour prior to the cull (unless stated otherwise in time-course experiments). Mice were administered KRH3955 (30 mg/kg by oral gavage; Kureha Corporation) or vehicle (water) 2 h prior to the cull (unless stated otherwise in time-course experiments). The CXCL12 neutraligand chalcone 4-phosphate (1.5 µmol/kg IV; Laboratoire d'Innovation Thérapeutique) or vehicle (PBS) was administered 60 min prior to the injection of AMD3100, KRH3955, or vehicle. Cytokine pretreatment of VEGF-A (100 µg/kg IP; PeproTech) or vehicle (PBS) was administered once daily for 4 days. Twenty-four hours after the last injection, mice were administered CXCR4 antagonist, as is stated above.
Cell isolation and colony assays
Peripheral blood was collected via cardiac puncture using a syringe precoated with and containing sodium citrate (100 μL; Sigma). Once the total volume was recorded, citrated blood was centrifuged to collect blood plasma when required. The remaining soft pellet containing cells was red blood cell-lysed, and to enumerate HPCs (colony-forming unit [CFU] HPCs), we added 1 × 105 cells to tissue culture-treated petri dishes containing 1 mL of Methocult (M3434; StemCell Technologies). Cultures were incubated at 37°C, 5% CO2, and quantified on day 12. To enumerate MPCs (CFU-Fs), we added 1 × 106 cells to tissue culture-treated 6-well plates containing 3 mL of Mesencult (05502; StemCell Technologies). Cultures were incubated at 37°C, 5% CO2, and medium was changed on day 7. CFU-Fs were quantified on day 21.
Harvest of bone marrow cells and supernatant
Femurs were harvested from mouse hind limbs. Bones were cleaned and kept on ice, before bone marrow cells were collected by bone marrow flush using a syringe containing D20 (Dulbecco’s modified Eagle medium supplemented with 20% fetal calf serum). For collection of bone marrow supernatant, bone marrow was flushed out with 0.5 mL of cold PBS per femur. Bone marrow cells were resuspended and centrifuged, and the cell-free supernatant was collected.
Flow cytometry analysis
Flow cytometry was used to determine the surface expression of CXCR4 on bone marrow-derived MPCs, HPCs, and endothelial cells. Cells were stained with fluorophore-conjugated monoclonal antibodies directed against CD45, CD31, platelet-derived growth factor receptor (PDGFR)-α, SCA-1, cKit, Lineage panel (cocktail), and CXCR4. Cells were acquired using a Fortessa flow cytometer (Becton Dickson). Data were analyzed using FlowJo software (version 7.6.5). Fluorescence minus one (FMO) controls were used to set gates appropriately for analysis. The mean fluorescence intensity was calculated using relevant control data (FMOs). Commercial antibodies (clones, fluorophores, and sources) are listed in supplemental Table 1.
CXCL12 enzyme-linked immunosorbent assay
CXCL12 content in bone marrow (BM) supernatant and peripheral blood (PB) plasma was quantified using paired antibodies (MAB350 and BAF351; RnD systems), and the assay was performed according to the manufacturer’s instructions. Recombinant mouse CXCL12 (RnD systems) was used to create a standard curve.
Data analysis and statistics
Data analysis was performed in GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Data are expressed as the mean and standard error of the mean (mean ± SEM), and statistical analysis was performed using one-way analysis of variance (ANOVA) with Dunnett or Bonferroni multiple comparison test, where appropriate. Results were considered statistically significant when P < .05 and are represented using asterisks (*P < .05; **P < .01; ***P < .001); n = number of mice per group. For further details on the number of mice per group, see supplemental Table 2.
Results
Bone marrow mesenchymal and hematopoietic progenitor cells and endothelial cells express high levels of CXCR4 on the cell surface
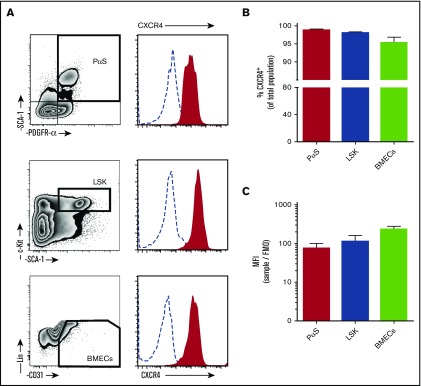
Previous research demonstrating expression of CXCR4 on MPCs was carried out on culture-expanded cells. Despite the fact that it is widely accepted that CXCL12 is important in directing injected MPCs to the site of injury/disease in vivo, results on surface expression of the receptor are inconsistent.25-29 In particular, several studies indicate that culture expansion of MPCs results to downregulated expression of CXCR4.25,29,30 Here, surface expression of CXCR4 was investigated on freshly isolated murine MPCs (PαS), HPCs (LSK), and BM endothelial cells (BMECs) (Figure 1A). MPCs expressed high levels of surface CXCR4, with levels comparable to HPCs and BMECs (Figure 1A-C).
Figure 1.
CXCR4 surface expression on bone marrow cell populations. Bone marrow cells were collected from mice by bone marrow flush for analysis of CXCR4 expression by flow cytometry. (A) Cells were stained with antibodies to detect mesenchymal progenitor cells (PαS; TER119−CD45−CD31−PDGFRα+SCA1+), hematopoietic progenitor cells (LSK; Lin−cKit+SCA1+), and BMECs (Lin−CD45−CD31+). Histograms of surface CXCR4 expression on cells (solid red areas) were determined by flow cytometry. Open dashed-line histograms represent FMO controls. CXCR4 positive percentage of cells within each population (B) and calculated mean fluorescence intensity for CXCR4 (C) (n = 4 mice per group). Data from 2 independent experiments are represented as mean ± SEM. MFI, mean fluorescence intensity.
AMD3100 induces a transient reversal of the bone marrow:blood CXCL12 chemokine gradient mediating rapid mobilization of leukocytes and hematopoietic progenitor cells
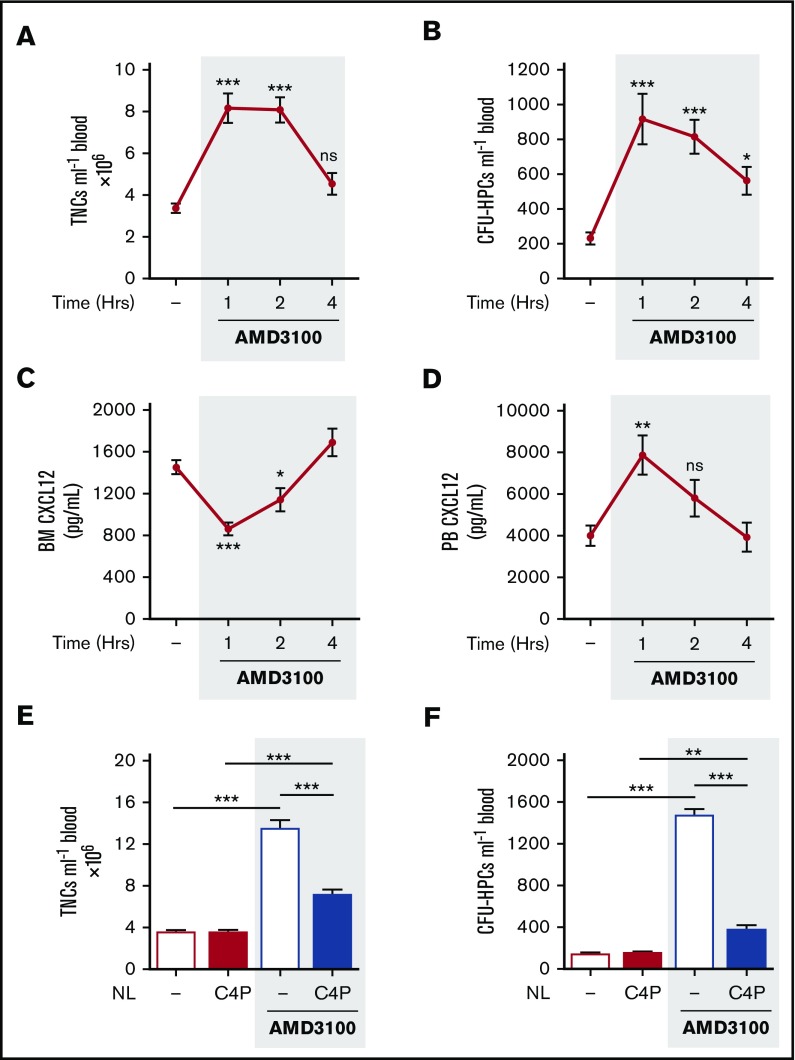
Single IP treatment of mice with AMD3100 (5 mg/kg) induced mobilization of leukocytes and HPCs, with peak numbers in the circulation after 1 hour. The number of circulating leukocytes returned to baseline after 4 hours (Figure 2A-B). Peak mobilization coincided with maximum changes in CXCL12 levels detected in the blood and bone marrow, with a significant decrease in the bone marrow and an increase in the blood, at 1h, returning to baseline by 4 hours (Figure 2C-D). These observations are consistent with the proposal that a rapid, but transient, reversal of the bone marrow:blood CXCL12 chemokine gradient mediates mobilization of leukocytes and HPCs into the blood following AMD3100 treatment.17
Figure 2.
AMD3100-induced changes in CXCL12 gradient coincided with and mediated mobilization of leukocytes and HPCs. (A-D) Mice were administered AMD3100 (5 mg/kg mouse IP) or vehicle (dash), and at varying time points (1, 2, and 4 hours), blood was collected for analysis of circulating total nucleated cells (TNCs) (A) and CFU-HPCs (B) (n = 11-14 mice per group). Femoral bone marrow and peripheral blood were collected for quantification of CXCL12 in bone marrow (BM) supernatant (C) and PB plasma (D), respectively (n = 7-14 mice per group). CXCL12 levels are shown as picograms per milliliter. Data of at least 3 independent experiments are represented as mean ± SEM. *P < .05; **P < .01; ***P < .001 (one-way ANOVA with Dunnett test). (E-F) Mice were administered AMD3100 or vehicle (dash) in the presence or absence of the CXCL12 neutraligand chalcone 4-phosphate (C4P; 1.5 µmol/kg mouse IV), and 1 hour later, blood was collected for analysis of circulating TNCs (E) and CFU-HPCs (F) (n = 5-10 mice per group). TNCs and CFU-HPCs are shown as cells and colonies per milliliter of blood, respectively. Data from at least 2 independent experiments are represented as mean ± SEM. **P < .01; ***P < .001 (one-way ANOVA with Bonferroni test). NL, neutraligand; ns, not significant.
Neutralization of CXCL12 suppresses mobilization of leukocytes and hematopoietic progenitor cells by AMD3100
Chalcone 4-phosphate (C4P) is a novel and highly soluble analog of chalcone 4. The compound is a prodrug; after in vivo cleavage of the phosphate group, it converts into the active chalcone 4 molecule that selectively binds CXCL12 and neutralizes its function.24 Chalcone 4 was shown to prevent induction of CXCR4 signaling and CXCL12-mediated CXCR4 internalization in vitro.23 To investigate whether elevated levels of circulating CXCL12 were required for mobilization of leukocytes and HPCs by AMD3100, we administered C4P IV (1.5 µmol/kg) to neutralize CXCL12 in the peripheral circulation. C4P treatment significantly reduced the number of circulating leukocytes and HPCs mobilized by AMD3100 (Figure 2E-F). These data suggest that the reversal of the bone marrow:blood CXCL12 gradient is required for the mobilization of leukocytes and HPCs by AMD3100.
Neutralization of CXCL12 abrogates pharmacological mobilization of mesenchymal progenitor cells
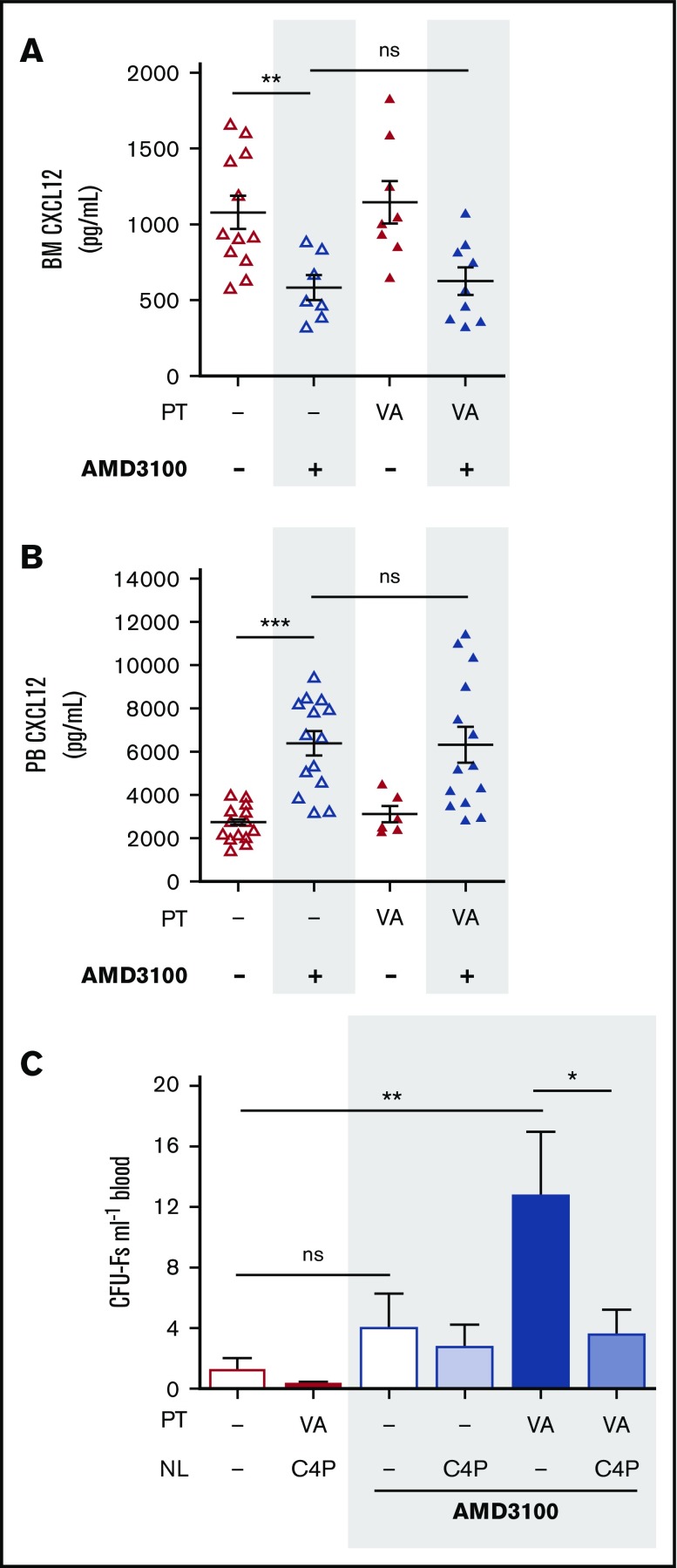
Pitchford et al demonstrated in 2009 that administration of AMD3100 was required to achieve MPC mobilization in mice pretreated with VEGF-A.18 It was therefore of interest to investigate whether mobilization of MPCs by AMD3100 was similarly dependent on changes in the CXCL12 chemokine gradient. Measurement of CXCL12 in blood and BM showed that in mice pretreated with VEGF-A over 4 days, AMD3100 stimulated the reversal of the CXCL12 chemokine gradient to the same extent as in mice treated with vehicle alone (Figure 3A-B). We show here that C4P treatment abolished mobilization of MPCs in mice receiving VEGF-A (100 µg/kg) and AMD3100 in combination (Figure 3C). This finding suggests that reversal of the CXCL12 chemokine gradient is an essential step for MPC mobilization by VEGF-A in combination with AMD3100.
Figure 3.
Neutralization of the CXCL12 chemokine gradient in VEGF-A pretreated mice. (A-B) Mice were pretreated with VEGF-A (VA; 100 µg/kg mouse IP) or vehicle (dash) once daily on 4 consecutive days. Twenty-four hours after the last injection, mice were administered AMD3100, and 1 hour later, mice were sacrificed. Femoral bone marrow and peripheral blood were collected for quantification of CXCL12 in BM supernatant (A) and PB plasma (B), respectively (n = 6-15 mice per group). CXCL12 levels are shown as picograms per milliliter. (C) Mice were pretreated with VEGF-A or vehicle (dash) once daily on 4 consecutive days. Twenty-four hours after the last injection, mice were administered AMD3100 or vehicle (dash) in the presence or absence of C4P, and 1 hour later, blood was collected for analysis of circulating CFU-Fs (n = 6-10 mice per group). CFU-Fs are shown as colonies per milliter of blood. Data from at least 2 independent experiments are represented as mean ± SEM. *P < .05; **P < .01; ***P < .001 (one-way ANOVA with Bonferroni test). VA, VEGF-A.
KRH3955 rapidly mobilizes leukocytes and hematopoietic progenitor cells in the absence of a robust CXCL12 chemokine gradient
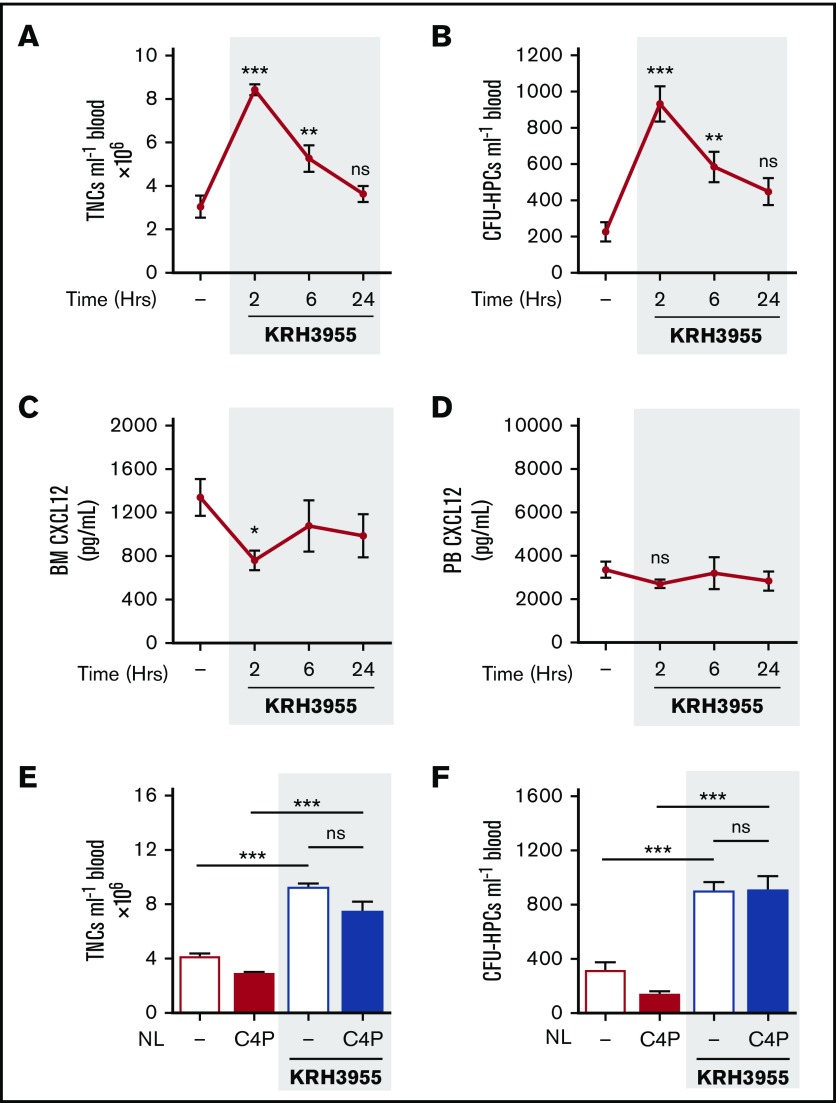
Previous work in monkeys demonstrated that a single dose of KRH3955 mobilizes leukocytes into peripheral blood.22 Our results show that a single administration of KRH3955 (30 mg/kg) induced mobilization of leukocytes and HPCs in mice, with largely elevated numbers in the circulation after 2 h. The number of circulating cells returned to baseline by 24 hours (Figure 4A-B). Peak mobilization coincided with a significant decrease in CXCL12 levels in the BM, but no changes were detected in the plasma levels of CXCL12 (Figure 4C-D). These findings indicate that KRH3955 does not stimulate the dramatic reversal of the bone marrow:blood CXCL12 chemokine gradient as is seen with AMD3100.
Figure 4.
Kinetics of KRH3955-induced mobilization of leukocytes and HPCs and studies on the bone marrow:blood CXCL12 chemokine gradient. (A-D) Mice were administered KRH3955 (30 mg/kg mouse by oral gavage) or vehicle (dash), and at varying time points (2, 6, and 24 hours), blood was collected for analysis of circulating TNCs (A) and CFU-HPCs (B) (n = 7-10 mice per group). Femoral bone marrow and peripheral blood were collected for quantification of CXCL12 in BM supernatant (C) and PB plasma (D), respectively (n = 7-17 mice per group). CXCL12 levels are shown as picograms per milliliter. Data from at least 2 independent experiments are represented as mean ± SEM. ***P < .001 (one-way ANOVA with Dunnett test). (E-F) Mice were administered KRH3955 or vehicle (dash) in the presence or absence of C4P, and 2 hours later, blood was collected for analysis of circulating TNCs (E) and CFU-HPCs (F) (n = 4-8 mice per group). TNCs and CFU-HPCs are shown as cells and colonies per milliliter of blood, respectively. Data from 2 independent experiments are represented as mean ± SEM. *P < .05; **P < .01; ***P < .001 (one-way ANOVA with Bonferroni test).
KRH3955-induced mobilization of leukocytes and hematopoietic progenitor cells is not mediated by an active CXCL12 chemokine gradient
No changes were detected in the number of circulating leukocytes and HPCs mobilized by KRH3955, when C4P was administered to mice (Figure 4E-F). These data indicate that KRH3955-stimulated mobilization of leukocytes and HPCs is not dependent on a CXCL12 chemokine gradient across the BM endothelium.
KRH3955 inhibits AMD3100-mediated elevation of blood CXCL12
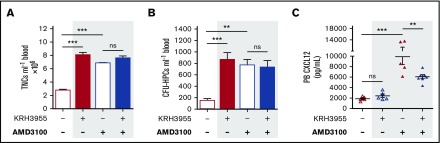
The 2 CXCR4 antagonists were administered in combination to see whether they demonstrated drug interaction in the context of mobilization. Treatment was delivered using optimal mobilization conditions that were previously determined for each drug. As is indicated in Figure 5A-B, no changes were detected in the number of mobilized leukocytes and HPCs following combination of KRH3955 and AMD3100, in comparison with either drug alone. The increase in circulating CXCL12 stimulated by AMD3100 alone was significantly reduced when AMD3100 was administered in combination with KRH3955 (Figure 5C).
Figure 5.
Combination treatment of KRH3955 and AMD3100; KRH3955 hinders CXCL12 changes stimulated by AMD3100. (A-C) Mice were administered KRH3955 or vehicle (dash), and 1 hour later, mice were further administered AMD3100 or vehicle (dash). One hour after the last injection, blood was collected for analysis of circulating TNCs (A) and CFU-HPCs (B) (n = 5-8 mice per group). TNCs and CFU-HPCs are shown as cells and colonies per milliliter of blood, respectively. (C) CXCL12 was quantified in PB plasma (n = 5-7 mice per group). CXCL12 levels are shown as picograms per milliliter. Data from 2 independent experiments were represented as mean ± SEM. **P < .01; ***P < .001 (one-way ANOVA with Bonferroni test).
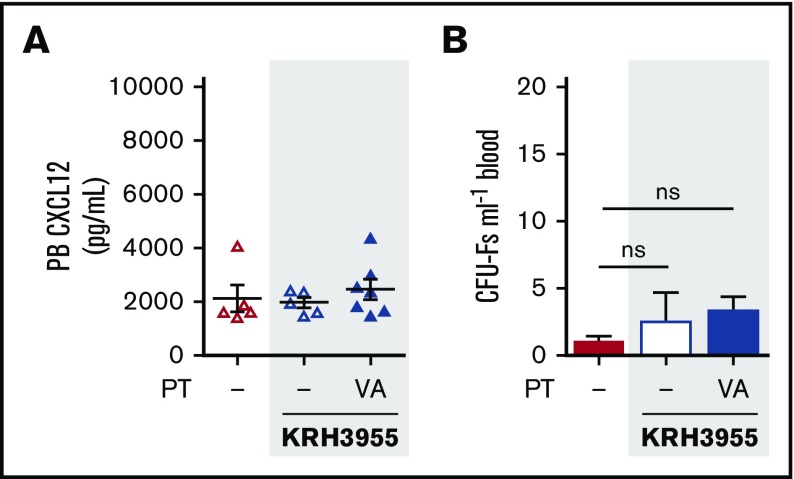
KRH3955 fails to mobilize mesenchymal progenitor cells in VEGF-A pretreated mice
We next assessed whether KRH3955, which fails to generate a robust reversal of the CXCL12 chemokine gradient (Figures 4C-D and 6A) was able to mobilize MPCs. Indeed, treatment with KRH3955 did not stimulate mobilization of MPCs in VEGF-A pretreated mice (Figure 6B). This indicates the importance of the CXCL12 gradient reversal in MPC mobilization.
Figure 6.
KRH3955 does not stimulate MPC mobilization in VEGF-A pretreated mice. (A-B) Mice were pretreated with VEGF-A or vehicle (dash) once daily on 4 consecutive days. Twenty-four hours after the last injection, mice were administered KRH3955 or vehicle (dash), and 2 h later, blood was collected for analysis of CXCL12 in PB plasma (A) and circulating CFU-Fs (B) (n = 5-7 mice per group). CXCL12 levels are shown as picograms per milliliter. CFU-Fs are shown as colonies per milliliter of blood. Data from 2 independent experiments are represented as mean ± SEM (one-way ANOVA with Bonferroni test). PT, pretreatment; VA, VEGF-A.
Discussion
HPCs may be mobilized in response to blood-borne chemokines that form a chemotactic gradient across the BM sinusoidal endothelium. Thus CCL11, CXCL8, and CXCL2 all induce a rapid mobilization of HPCs when injected into animals.31-33 The chemokine CXCL12 is produced constitutively in the BM, and there is now an extensive literature that is consistent with the notion that the CXCR4:CXCL12 chemokine axis is critical for HPC retention in the BM.34 Thus, for example, circadian variations in levels of CXCL12 in the BM have been shown to correlate with retention and egress of HPCs,35 and sustained overexpression of CXCL12 using an adenovirus and other drugs that are CXCR4 agonists have been shown to mobilize HPCs into the blood.36-38 CXCL12 is produced locally in damaged tissues and is reported to be critical for the mobilization and recruitment of BM stem/progenitor cells to the site of injury.39-45 Taken together, these studies indicate that the relative level of CXCL12 in the blood and BM is one factor that regulates progenitor cell retention/mobilization under homeostasis and in disease. Understanding this mechanism of retention has led to the development of CXCR4 antagonists as HPC-mobilizing drugs.
The BM endothelium expresses CXCR4, and complexes of CXCR4:CXCL12 have been reported to translocate CXCL12 across the BM endothelium in both directions.17,46 In one paper it has been reported that the CXCR4 antagonist AMD3100 enhances the translocation of CXCL12 across the BM endothelium into the blood, and indeed it has been suggested that this drug mobilizes HPCs by virtue of its ability to reverse the CXCL12 chemokine gradient.17 In this article, we have compared the ability of AMD3100 and a distinct CXCR4 antagonist, KRH3955, to mobilize leukocytes, HPCs, and MPCs into the blood and specifically investigated whether the reversal of the CXCL12 chemokine gradient plays a role in their ability to mobilize cells from the BM. Our results indicate that AMD3100, but not KRH3955, stimulates a rapid rise in plasma levels of CXCL12 coinciding with a decrease in the concentration of CXCL12 in the bone marrow and mobilization of leukocytes and HPCs into the blood. Moreover, use of the CXCL12 neutraligand, C4P, confirmed that CXCL12 activity is required for AMD3100-stimulated HPC and leukocyte mobilization. Our results are therefore consistent with those of Dar et al, who originally proposed (in 2011) that mobilization of HPCs by AMD3100 is an active process.17 Furthermore, we have shown that AMD3100 stimulated mobilization of MPCs, which requires pretreatment of mice with VEGF-A is also mediated by the same mechanism: C4P inhibiting this response.
In contrast we have shown that the CXCR4 antagonist KRH3955 stimulates leukocyte and HPC mobilization by a distinct mechanism, in that mobilization is not accompanied by any change in plasma levels of CXCL12 and is not inhibited by C4P. Previous studies in primates demonstrated that KRH3955 induces an increase in circulating leukocytes, but we are the first to demonstrate that KRH3955 mobilizes HPCs into the blood.22 Interestingly, KRH3955 does not mobilize MPCs in VEGF-A pretreated mice, suggesting that reversal of the CXCL12 chemokine gradient is critical for mobilization of MPCs.
Comparative in vitro studies of AMD3100 and KRH3955 revealed that KRH3955 exhibited a 100-fold lower IC50 for CXCL12 binding, a stronger binding affinity, and a slower dissociation rate than did AMD3100. Moreover, in functional assays, KRH3955 was a more potent anti-HIV drug than was AMD3100.21 Analysis of the mechanism whereby CXCL12 interacts with CXCR4 has shown that the chemokine interacts via a 2-step process. In the first step, CXCL12 binds to the extracellular region of CXCR4 via its β sheet and 50s loop, a step that does not lead to receptor conformational change or activation, whereas in the second step the interaction of the N terminus of CXCL12 with the transmembrane helices of CXCR4 leads to a conformational change of the receptor and activation of G-protein signaling.47 AMD3100 and KRH3955 have been shown to interact with CXCR4 at distinct sites. KRH3955 binds selectively to the extracellular loop of CXCR4, and AMD3100 binds to the transmembrane region.21,47 As such, it is predicted that KRH3955 inhibits binding of CXCL12 to both the extracellular loop and transmembrane region of CXCR4. In contrast, AMD3100 still permits CXCL12 binding to the extracellular loop; thus it is possible that CXCR4:CXCL12:AMD3100 complexes are formed. Given that higher plasma concentrations of CXCL12 are observed in vivo following AMD3100 treatment, we propose that the binding of AMD3100 to the transmembrane region of CXCR4 favors translocation of CXCL12 across the BM endothelium through formation of these complexes. In contrast, KRH3955 acts as a more traditional competitive antagonist, potentially mobilizing HPCs and leukocytes by directly disrupting the CXCR4:CXCL12 retention system. Therefore, it appears that KRH3955 stimulates a mechanistically distinct mobilization response in comparison with AMD3100.
When an experiment was performed administering both AMD3100 and KRH3955 in vivo, the extent of HPC and leukocyte mobilization was no greater than it was with either drug alone. It is possible that maximal mobilization was achieved under these conditions. Notably, drug interaction was evident after assessment of plasma CXCL12 changes. KRH3955 administration significantly dampened the elevation of circulating CXCL12 mediated by AMD3100. It is possible that binding of KRH3955 with high affinity to the extracellular domain of CXCR4 displaces and further inhibits binding of CXCL12 (or binding of AMD3100) to the receptor. As a result, this hinders formation of CXCR4:CXCL12:AMD3100 complexes and thereby reduces the translocation of CXCL12 across the BM sinusoidal endothelium into the blood.
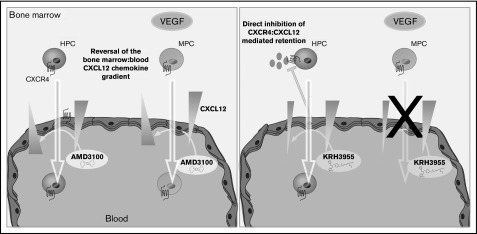
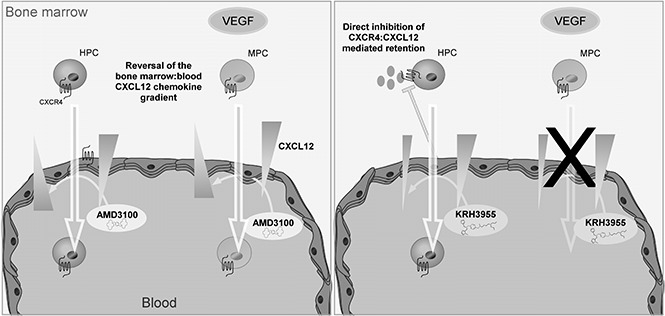
In conclusion, our data are consistent with the theory that AMD3100 mediates mobilization of leukocytes, HPCs, and MPCs via reversal of the CXCL12 chemokine gradient across the BM endothelium. KRH3955, on the other hand, which does not reverse the CXCL12 chemokine gradient, stimulates the mobilization of leukocytes and HPCs but not MPCs (Figure 7). These data suggest that generation of a CXCL12 chemokine gradient across the BM endothelium is vital for pharmacological mobilization of MPCs. Overall, this study indicates that CXCR4 antagonists mobilize cells from the BM by distinct mechanisms that are dependent on their CXCR4-binding sites.
Figure 7.
Chemically distinct CXCR4 antagonists mobilize progenitor cells by different mechanisms. AMD3100 treatment generates a CXCL12 gradient across the sinusoidal endothelium, which supports migration of HPCs and MPCs from the bone marrow into the circulation. On the other hand, KRH3955 directly disrupts CXCR4-mediated retention of HPCs, resulting in their mobilization, but does not generate the CXCL12 chemokine gradient critical for MPC mobilization.
Studying the efficacy of CXCR4 antagonists to mobilize HPCs and MPCs, and understanding the mechanism underlying this response, is important in selecting and applying these compounds used for stem cell mobilization. For example, designing a CXCR4 antagonist, such as AMD3100, that binds to the transmembrane region of CXCR4 and thereby stimulates the reversal of the CXCL12 gradient may be used to mobilize both HPCs and MPCs. This may be advantageous in the context of BMTs, in which MPCs are thought to improve engraftment of HPCs.48-50 In addition, MPC mobilization may enhance tissue repair in the context of tissue injury, such as cardiovascular disease or a musculoskeletal injury.45,49,51-53
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
KRH3955 was kindly donated by Kureha Corporation (Pharmaceuticals and Agrochemicals Division, Tokyo, Japan). Chalcone 4-phosphate was kindly donated by Dominique Bonnet (LabEx Medalis, Faculté de Pharmacie, Laboratoire d’Innovation Thérapeutique, Centre National de la Recherche Scientifique/Université de Strasbourg, Illkirch, France).
This work was supported by the Wellcome Trust (grant 095700) and by the National Heart and Lung Institute Foundation (A.N.R.).
Authorship
Contribution: A.N.R designed and performed experiments, analyzed data, made figures, and wrote and edited the manuscript; M.F. performed experiments; S.-P.W. performed experiments; D.B. provided compounds, advice on compound properties, and pharmacology; and S.M.R. led the project, designed the experiments, and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sara M. Rankin, Imperial College London, Sir Alexander Fleming Building, Office no. 351, Imperial College Rd, London SW7 2AZ, United Kingdom; e-mail: s.rankin@imperial.ac.uk.
References
- 1.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17(4):413-423. [DOI] [PubMed] [Google Scholar]
- 3.Semerad CL, Christopher MJ, Liu F, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106(9):3020-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635-638. [DOI] [PubMed] [Google Scholar]
- 5.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687-694. [DOI] [PubMed] [Google Scholar]
- 6.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10(4):463-471. [DOI] [PubMed] [Google Scholar]
- 7.Martin C, Burdon PCE, Bridger G, Gutierrez-Ramos J-C, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19(4):583-593. [DOI] [PubMed] [Google Scholar]
- 8.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845-848. [DOI] [PubMed] [Google Scholar]
- 9.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901-1910. [DOI] [PubMed] [Google Scholar]
- 10.De Clercq E, Yamamoto N, Pauwels R, et al. Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob Agents Chemother. 1994;38(4):668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrix CW, Flexner C, MacFarland RT, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44(6):1667-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102(8):2728-2730. [DOI] [PubMed] [Google Scholar]
- 13.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lévesque J-P, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111(2):187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin C, Bridger GJ, Rankin SM. Structural analogues of AMD3100 mobilise haematopoietic progenitor cells from bone marrow in vivo according to their ability to inhibit CXCL12 binding to CXCR4 in vitro. Br J Haematol. 2006;134(3):326-329. [DOI] [PubMed] [Google Scholar]
- 16.Fricker SP. Physiology and pharmacology of plerixafor. Transfus Med Hemother. 2013;40(4):237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dar A, Schajnovitz A, Lapid K, et al. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25(8):1286-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4(1):62-72. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd RM, Capoccia BJ, Devine SM, et al. Angiogenic cells can be rapidly mobilized and efficiently harvested from the blood following treatment with AMD3100. Blood. 2006;108(12):3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joao HC, De Vreese K, Pauwels R, De Clercq E, Henson GW, Bridger GJ. Quantitative structural activity relationship study of bis-tetraazacyclic compounds. A novel series of HIV-1 and HIV-2 inhibitors. J Med Chem. 1995;38(19):3865-3873. [DOI] [PubMed] [Google Scholar]
- 21.Murakami T, Kumakura S, Yamazaki T, et al. The novel CXCR4 antagonist KRH-3955 is an orally bioavailable and extremely potent inhibitor of human immunodeficiency virus type 1 infection: comparative studies with AMD3100. Antimicrob Agents Chemother. 2009;53(7):2940-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakasone T, Kumakura S, Yamamoto M, Murakami T, Yamamoto N. Single oral administration of the novel CXCR4 antagonist, KRH-3955, induces an efficient and long-lasting increase of white blood cell count in normal macaques, and prevents CD4 depletion in SHIV-infected macaques: a preliminary study. Med Microbiol Immunol (Berl). 2013;202(2):175-182. [DOI] [PubMed] [Google Scholar]
- 23.Hachet-Haas M, Balabanian K, Rohmer F, et al. Small neutralizing molecules to inhibit actions of the chemokine CXCL12. J Biol Chem. 2008;283(34):23189-23199. [DOI] [PubMed] [Google Scholar]
- 24.Gasparik V, Daubeuf F, Hachet-Haas M, et al. Prodrugs of a CXC chemokine-12 (CXCL12) neutraligand prevent inflammatory reactions in an asthma model in vivo. ACS Med Chem Lett. 2011;3(1):10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104(9):2643-2645. [DOI] [PubMed] [Google Scholar]
- 26.Sordi V, Malosio ML, Marchesi F, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106(2):419-427. [DOI] [PubMed] [Google Scholar]
- 27.Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105(10):3793-3801. [DOI] [PubMed] [Google Scholar]
- 28.Rüster B, Göttig S, Ludwig RJ, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108(12):3938-3944. [DOI] [PubMed] [Google Scholar]
- 29.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24(4):1030-1041. [DOI] [PubMed] [Google Scholar]
- 30.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206-216. [DOI] [PubMed] [Google Scholar]
- 31.Palframan RT, Collins PD, Williams TJ, Rankin SM. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood. 1998;91(7):2240-2248. [PubMed] [Google Scholar]
- 32.Laterveer L, Lindley IJ, Hamilton MS, Willemze R, Fibbe WE. Interleukin-8 induces rapid mobilization of hematopoietic stem cells with radioprotective capacity and long-term myelolymphoid repopulating ability. Blood. 1995;85(8):2269-2275. [PubMed] [Google Scholar]
- 33.King AG, Horowitz D, Dillon SB, et al. Rapid mobilization of murine hematopoietic stem cells with enhanced engraftment properties and evaluation of hematopoietic progenitor cell mobilization in rhesus monkeys by a single injection of SB-251353, a specific truncated form of the human CXC chemokine GRObeta. Blood. 2001;97(6):1534-1542. [DOI] [PubMed] [Google Scholar]
- 34.Rankin SM. Chemokines and adult bone marrow stem cells. Immunol Lett. 2012;145(1-2):47-54. [DOI] [PubMed] [Google Scholar]
- 35.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442-447. [DOI] [PubMed] [Google Scholar]
- 36.Hattori K, Heissig B, Tashiro K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97(11):3354-3360. [DOI] [PubMed] [Google Scholar]
- 37.Perez LE, Alpdogan O, Shieh JH, et al. Increased plasma levels of stromal-derived factor-1 (SDF-1/CXCL12) enhance human thrombopoiesis and mobilize human colony-forming cells (CFC) in NOD/SCID mice. Exp Hematol. 2004;32(3):300-307. [DOI] [PubMed] [Google Scholar]
- 38.Tchernychev B, Ren Y, Sachdev P, et al. Discovery of a CXCR4 agonist pepducin that mobilizes bone marrow hematopoietic cells. Proc Natl Acad Sci USA. 2010;107(51):22255-22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362(9385):697-703. [DOI] [PubMed] [Google Scholar]
- 40.Kollet O, Shivtiel S, Chen YQ, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112(2):160-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858-864. [DOI] [PubMed] [Google Scholar]
- 42.Tögel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67(5):1772-1784. [DOI] [PubMed] [Google Scholar]
- 43.Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60(3):813-823. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Xiang LX, Shao JZ, et al. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J Cell Mol Med. 2010;14(6B):1494-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong SP, Rowley JE, Redpath AN, Tilman JD, Fellous TG, Johnson JR. Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol Ther. 2015;151:107-120. [DOI] [PubMed] [Google Scholar]
- 46.Dar A, Goichberg P, Shinder V, et al. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol. 2005;6(10):1038-1046. [DOI] [PubMed] [Google Scholar]
- 47.Kofuku Y, Yoshiura C, Ueda T, et al. Structural basis of the interaction between chemokine stromal cell-derived factor-1/CXCL12 and its G-protein-coupled receptor CXCR4. J Biol Chem. 2009;284(50):35240-35250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernardo ME, Fibbe WE. Mesenchymal stromal cells and hematopoietic stem cell transplantation. Immunol Lett. 2015;168(2):215-221. [DOI] [PubMed] [Google Scholar]
- 49.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11-22. [DOI] [PubMed] [Google Scholar]
- 50.Zhao K, Liu Q. The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. J Hematol Oncol. 2016;9(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Shapiro L, Flynn A. The clinical application of mesenchymal stem cells and cardiac stem cells as a therapy for cardiovascular disease. Pharmacol Ther. 2015;151:8-15. [DOI] [PubMed] [Google Scholar]
- 52.Grayson WL, Bunnell BA, Martin E, Frazier T, Hung BP, Gimble JM. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol. 2015;11(3):140-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith BD, Grande DA. The current state of scaffolds for musculoskeletal regenerative applications. Nat Rev Rheumatol. 2015;11(4):213-222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.