Abstract
Lymphomas are responsible for approximately 20% to 25% of annual cancer diagnoses in the adolescent and young adult (AYA) population. In 2006, the National Cancer Institute and the Lance Armstrong Foundation developed a joint Adolescent and Young Adult Oncology Progress Review Group (AYAO-PRG) to formally address the unique cancer burden of patients age 15 to 39 years. As part of their recommendations, the AYAO-PRG identified 5 imperatives for improving outcomes of AYAs with cancer. Broadly, the recommended areas of focus included research, awareness and education, investigational infrastructure, care delivery, and advocacy. In response to the challenges highlighted by the AYAO-PRG, the Lymphoma Research Foundation held the first AYA Lymphoma Research Foundation Symposium on 2 October 2015. At this symposium, clinicians and basic scientists from both pediatric and adult disciplines gave presentations describing the state of the science and proposed a collaborative research agenda built on the imperatives proposed by the AYAO-PRG. The following review presents an in-depth discussion of lymphoma management across pediatric and adult oncologic disciplines, focusing on Hodgkin lymphoma, mature B-cell lymphomas, and anaplastic large cell lymphoma.
Introduction
With ∼70 000 new diagnoses annually, cancer in the adolescent and young adult (AYA) population (age 15-39 years) has been a national public health challenge for decades.1-3 Despite therapeutic advances, improvements in the survival of AYAs trail behind those observed in children and older adults.4-6 Lymphomas are responsible for ∼20% to 25% of annual cancer diagnoses in AYAs, and studies suggest that lymphoma-related mortality is higher in AYAs than it is in younger children and older adults.6,7 These disparities are particularly striking in the setting of overall excellent outcomes and continued diagnostic and therapeutic advances in both Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL).
In 2006, the National Cancer Institute and the Lance Armstrong Foundation developed a joint AYA Oncology Progress Review Group (AYAO-PRG) to formally address the unique cancer burden of AYAs.8,9 As part of their recommendations, the AYAO-PRG identified 5 imperatives for improving outcomes of AYAs with cancer: research, awareness and education, investigational infrastructure, care delivery, and advocacy.
The imperatives highlighted by the AYAO-PRG provided the framework for the first Lymphoma Research Foundation AYA Symposium, held on 2 October 2015. As an initial step toward establishing an AYA-specific lymphoma research agenda, clinicians and basic scientists from pediatric and adult disciplines presented the state of the science in their respective fields. The following summary of the symposium provides an in-depth comparison of pediatric and adult approaches to lymphoma management focusing on Hodgkin lymphoma (HL), mature B-cell lymphomas, and anaplastic large cell lymphoma (ALCL).
HL
Epidemiology
Approximately 10 000 new cases of HL are diagnosed in the United States annually. Nodular sclerosis is the most common histologic subtype of classical HL in Europe and North America. The incidence of HL in AYAs is ∼37 per million in patients age 15 to 19 years and ∼50 per million in patients age 20 to 29 years. Overall, HL accounts for 16% of cancers in patients between the ages of 15 and 24 years.
Staging and risk stratification
Both adults and children with HL are staged according to the Ann Arbor Staging system with Cotswold modification.10 In a large retrospective analysis of children and adolescents with intermediate-risk HL, 4 conditions were predictive of reduced event-free survival (EFS): stage IV disease, large mediastinal adenopathy, albumin <3.5, and fever.11 These findings are currently undergoing prospective evaluation. In adults, the International Prognostic Score is used to identify patients with advanced-stage disease who might benefit from intensified therapy.12 The European Organisation for Research and Treatment of Cancer and the German Hodgkin Study Group both categorize limited-stage HL (stages I and II) into favorable or unfavorable based on the presence or absence of bulky mediastinal disease, elevated erythrocyte sedimentation rate, and/or B-symptoms. In general, survival in HL varies by risk group, with 5-year survival reaching 95% in low-risk patients, and ranging from 70% to 80% in high-risk patients.13
Biological features of HL
The tumor microenvironment in HL is primarily composed of reactive, nonneoplastic cells, with malignant Hodgkin Reed-Sternberg cells comprising a small percentage of classical HL tissue samples.14 Immunohistochemistry and gene-expression profiling of pretreatment whole-tissue biopsies have identified prognostic biomarkers that, although not well validated, have been used to stratify risk in some patients.15 By using NanoString technology (digital expression profiling on formalin-fixed paraffin-embedded tissue), a 23-gene outcome predictor was generated by using samples from adults enrolled on the Eastern Cooperative Oncology Group E2496 trial.16 These predictors were not associated with outcome in a cohort of children with intermediate-risk disease, raising questions about whether pediatric and adult HL have distinct biologic characteristics.17 Currently, there are not enough data to determine whether such differences are clinically meaningful. In a large retrospective study of pediatric patients, Cleary et al18 reported that the most common histologic subtypes in childhood HL were mixed cellularity and lymphocyte predominant. In a post hoc analysis of AYA patients treated on 2 German Hodgkin Study Group protocols, the predominant histologic subtype was nodular sclerosing.19 Taken together, these studies suggest the possibility that the distribution of histologic subtypes in HL varies by age.
Pediatric therapeutic approach
Risk-adapted combined modality therapy has resulted in outstanding survival outcomes in pediatric HL. Today, cooperative group trials focus primarily on maintaining survival while reducing long-term treatment sequelae.20-22 Because of gonadal and cardiac toxicity, pediatric regimens have shifted away from traditional MOPP (nitrogen mustard, vincristine, procarbazine, and prednisone) or ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) over the past 2 decades (Table 1).22 Traditionally, the standard approach of the Deutsche Arbeitsgemeinschaft für Leukämieforschung and German Society of Pediatric Oncology and Hematology-Hodgkin’s Disease (GPOH-HD) was to use OPPA-COPP (vincristine, procarbazine, prednisone, and doxorubicin-cyclophosphamide, vincristine, procarbazine, and prednisone). Because of its association with male infertility, procarbazine was replaced with etoposide and dacarbazine in the GPOH-HD-2002 trial.23 Outcomes after OEPA-COPDAC (vincristine, etoposide, prednisone, and doxorubicin-cyclophosphamide, vincristine, prednisone, and dacarbazine) in boys were comparable to outcomes after OPPA-COPP in girls. Thus OEPA-COPDAC is now widely used in Europe, Latin America, and in parts of North America. In North America, ABVE-PC (doxorubicin, bleomycin, vincristine, and etoposide-prednisone and cyclophosphamide) is the most commonly used therapeutic regimen for low- and intermediate-risk patients. BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) has proved to be an effective regimen in children and adolescents with high-risk HL (Table 1).24
Table 1.
Pediatric HL trials
| Reference | Trial acronym | Stage | Risk | Treatment | EFS | OS | |||
|---|---|---|---|---|---|---|---|---|---|
| Survival | % | Survival | % | ||||||
| 94 | CCG-P9426 | I, IIA, IIIA | ABVE × 4 + IFRT | 8-y | 86 | 8-y | 97 | ||
| 23 | GPOH-HD-2002 | I, IIA (treatment group 1) | OEPA × 2 (M); OPPA × 2 (F) | 3-y | 90 | 3-y | 97.9 | ||
| IIB, IIE, IIIA (treatment group 2) | OEPA × 2, COPDAC × 2 (M); OPPA × 2, COPP × 2 (F) | 3-y | 91.1 (M); 87.7 (F) | ||||||
| IIBE, IIIBE, IIIB, IV (treatment group 3) | OEPA × 2, COPDAC × 4 (M); OPPA × 2, COPP × 4 (F) | ||||||||
| 95 | CCG 5942 | IA, IB, IIA | Low | COPP/ABV × 4 ± IFRT | 10-y | 83.5 | 92.5 | ||
| 96 | IA, IB, IIA (with adverse features), IIB, III | Intermediate | COPP/ABV × 6 | 10-y (IFRT) | 91.2 | 97.1 | |||
| IV | High | COPP/ABV, CHOP, etoposide/cytarabine × 2 | 10-y (no IFRT) | 82.9 | 95.9 | ||||
| 97 | COG-AHOD0431 | IA, IIA (no bulk) | AVPC × 3 ± IFRT | 4-y | 79 | 4-y | 99.6 | ||
| 98 | Stanford, Dana-Farber, and St. Jude Consortium | IA, IIA (no bulk) | VAMP × 4 ± IFRT | 2-y | 90.8 | 2-y | 100 | ||
| 22 | P9425 | IB, IIA/IIIA1 (with large mediastinal adenopathy) or IIIA2 | Intermediate | ABVE-PC × 3 | 5-y | 84 | 5-y | 95 | |
| IIB, IIIB, IV | High | RER: IFRT SER: ABVE-PC × 2 + IFRT | |||||||
| 24 | COG-59704 | IIB/IIIB (with bulk), IV | High | BEACOPP × 4; RER (F): COPP/ABV × 4; RER (M): ABVD × 2 + IFRT; SER: BEACOPP × 4 + IFRT | 5-y | 93 | 5-y | 97 | |
| 25 | COG-AHOD0031 | IB, IA/IIA (with bulk), IIB, IIIA, IVA | ABVE-PC × 2 RER: ABVE × 2 ± IFRT; SER: ABVE-PC × 2 ± DECA × 2 + IFRT | 4-y | All 85 RER 86.9 SER 77.4 | 4-y | All 97.8 RER 98.5 SER 95.3 | ||
| 24 | AHOD0831 | IIIB, IVB | Very high | ABVE-PC × 2 RER: ABVE-PC × 2 + RT (bulk only) SER: ABVE-PC × 2 + ifosfamide/vinorelbine + ABVE-PC × 2 + RT (bulk and PET2-positive sites) | 4-y | 80.2 | 4-y | 95.9 | |
ABV, doxorubicin, bleomycin, and vinblastine; CCG, Children’s Oncology Group; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; COPDAC, cyclophosphamide, vincristine, prednisone, and dacarbazine; COPP, cyclophosphamide, vincristine, procarbazine, and prednisone; F, female; GPOH, German Society of Pediatric Oncology and Hematology; M, male; RER, rapid early responder; SER, slow early responder.
The omission of involved-field radiation therapy (IFRT) in children with HL was originally restricted to early-stage disease. Recent studies have investigated AVPC (doxorubicin, vincristine, prednisone, and cyclophosphamide), VAMP (vinblastine, doxorubicin, methotrexate, and prednisone), and OEPA with and without IFRT (Table 1). Early-response evaluation with risk-based therapy was successfully tested in a trial with intermediate-risk patients on the Children’s Oncology Group (COG) AHOD0031 study. This protocol omitted radiation therapy (RT) in rapid early responders who achieved complete remission (CR) after 4 cycles of ABVE-PC (Table 1). Slow early responders received augmented chemotherapy with 2 cycles of DECA (dexamethasone, etoposide, cytarabine, and cisplatin) plus IFRT.25 Analyses revealed that IFRT (vs no IFRT) significantly improved 4-year EFS (IFRT, 89.3%; no IFRT, 77.9%; P = .019) in children with bulky stage I or II disease who also had anemia at the time of diagnosis.26
Adult therapeutic approach
Similar to the pediatric approach, risk-adapted therapy in adults with HL aims to minimize late effects and maximize cure. In adults, ABVD remains the backbone of therapy for most patients with HL. For early-stage nonbulky HL, the current approach is 2 to 4 cycles of ABVD plus 20 Gy IFRT.27 For bulky disease, standard protocols include 4 to 6 cycles of chemotherapy plus 30 Gy IFRT. In advanced-stage HL, dose-intensive BEACOPP is an effective regimen that, compared with ABVD, has better progression-free survival (PFS) but worse overall survival (OS) (PFS, 81%; OS, 68%) due to excessive toxicity (Table 2).28
Table 2.
Adult HL trials
| Reference | Trial acronym | Stage | Risk | Treatment | PFS | OS | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study arm | Survival | % | Study arm | Survival | % | |||||
| 99 | UK RAPID | IA, IIA (early) | ABVD × 3-4 ± IFRT | RT | 3-y | 94.6 | ||||
| No RT | 3-y | 90.8 | ||||||||
| 30 | CALGB-50604 | I and II (no bulk) | Low | ABVD × 4 (if PET2 negative) | 3-y | 92 | ||||
| ABVD × 2 plus BEACOPP × 2 + IFRT (if PET2 positive) | 3-y | 65 | ||||||||
| 27 | GHSG HD6 | IA, IIA (no bulk) | Low | ABVD × 4-6 cycles | ABVD | 12-y† | 87 | ABVD | 12-y | 94 |
| 20 Gy subtotal nodal RT ± 2 cycles of ABVD | RT ± ABVD | 12-y† | 92 | RT ± ABVD | 12-y | 87 | ||||
| 28, 100 | HD2000 | Early (nonbulky) | Unfavorable | ABVD × 4 + 30 Gy IFRT | 10-y | 69 | 85 | |||
| BEACOPP × 2, ABVD × 2, 30 Gy IFRT | 10-y | 75 | 84 | |||||||
| COPP-EBV-CAD | 10-y | 76 | 86 | |||||||
| 101 | GHSG HD15 | IIB (advanced, with bulky disease), III, IV | escBEACOPP × 8 | 5-y* | 84.4 | 91.9 | ||||
| escBEACOPP × 6 | 5-y* | 89.3 | 95.3 | |||||||
| BEACOPP × 8 | 5-y* | 85.4 | 94.5 | |||||||
| 31 | SWOG S0816 | Advanced | Interim PET-directed after ABVD × 2 | |||||||
| ABVD × 4 (PET negative) | 2-y | 64 | ||||||||
| escBEACOPP × 6 (PET positive) | 2-y | 82 | ||||||||
| 102 | RATHL | Advanced | Unfavorable | ABVD × 2 | 3-y | 85.7 | 97.2 | |||
| ABVD × 4 vs AVD × 4 (PET negative) | AVD | 3-y | 84.4 | 97.6 | ||||||
| BEACOPP vs BEACOPP-14 (PET positive) | BEACOPP | 3-y | 67.5 | 87.7 | ||||||
AVD, doxorubicin, vinblastine, and dacarbazine; COPP-EBV-CAD, cyclophosphamide, vincristine, procarbazine, and prednisone-epirubicin, bleomycin, and vinblastine-cyclophosphamide, doxorubicin, and dexamethasone; escBEACOPP, escalated BEACOPP; GHSG, German Hodgkin Study Group; RATHL, Response Adapted Therapy in Advanced Hodgkin Lymphoma; SWOG, Southwest Oncology Group.
Freedom from treatment failure.
Freedom from disease progression.
One of the most consistent predictors of survival in both pediatric and adult HL is interim response to chemotherapy, as measured by fluorodeoxyglucose positron emission tomography (FDG-PET).29 The Cancer and Leukemia Group B (CALGB) 50604 study (NCT01132807) demonstrated the prognostic significance of early response after 2 cycles of ABVD (PET2) in early-stage HL. Patients who were PET2-negative received 4 additional cycles of ABVD without RT and had a 3-year PFS of 92%. Those who were PET2-positive had a 2-year PFS of 65% despite receiving augmented therapy with 2 cycles of escalated BEACOPP in addition to RT.30 The prognostic importance of interim disease response also holds true for patients with advanced-stage disease.31
Traditional chemotherapeutic approaches consistently fall short in patients with primary refractory or relapsed HL. Brentuximab vedotin, an anti-CD30 antibody conjugate, 32 and programmed cell death protein 1 (PD-1) inhibitors (nivolumab and pembrolizumab) 33 have demonstrated remarkable activity in relapsed or refractory HL and are currently under investigation as part of first-line therapy (NCT02181738). Roemer et al34 recently reported that alterations in chromosome 9p24.1 and PD ligands (PD-L1 and PD-L2) are associated with reduced PFS, suggesting the potential benefit of checkpoint blockade in patients with these alterations.
Future perspectives
In both children and adults with HL, the goal of treatment is to maintain high cure rates while safely reducing overall treatment burden. Interim disease assessment with risk-adapted therapy has allowed for the omission of RT in a substantial proportion of both adults and children with chemotherapy-sensitive HL. Although both pediatric and medical oncologists seek to improve the therapeutic index in HL, consortium groups largely continue to work in parallel rather than in tandem toward this goal. Different approaches to risk stratification and response criteria render comparisons across consortia and across age groups challenging. Collaborative studies investigating age-related differences in prognostic biomarkers, biology of disease control, host immune response, and susceptibility to long-term toxicities are overdue.
Mature B-cell NHL
Epidemiology
Together, diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL) comprise about 95% of mature B-cell lymphoma diagnoses annually. DLBCL makes up ∼30% to 35% of NHL in patients older than age 15 years and ∼18% in younger children.35 BL makes up ∼40% of NHL in children age 1 to 14 years and ∼20% of NHL in patients age 15 to 19 years. In patients older than 20 years, ∼5% of NHL is BL. The incidence of NHL increases with age, with 15 cases per million in AYAs age 15 to 19 years, 22 cases per million for those age 20 to 24 years, and 40 cases per million for those age 25 to 29 years.36
Staging and risk stratification
In adults, the Ann Arbor Staging System is used to describe disease stage at diagnosis and the International Prognostic Index (IPI) further characterizes risk. The IPI remains the strongest clinical predictor of outcome in adults. In children, the St. Jude/Murphy classification system has been updated to the International Pediatric NHL Staging System, which incorporates new histologic entities.37
Biological features of BL and DLBCL
Age-related biological differences in NHL subtypes have been well described in children and adults; however, they have not been fully characterized in AYAs. BL is divided into 3 clinico-epidemiologic subtypes: endemic, sporadic, and immunodeficiency-associated. The hallmark of BL in both adults and children is translocation of MYC (8q24) with an immunoglobulin locus on chromosome 14 (85% of cases), 2, or 8.38 In DLBCL, the frequency of MYC rearrangement depends on the method of detection. Frequency ranges from 5% to 10% using fluorescence in situ hybridization but is near 35% using immunohistochemistry.39 In a small study by Gualco et al,40 rearrangements were histologically identified in 37% of pediatric DLBCL patients (n = 6). Recently, mutations in ID3, TCF3, and cyclin D3 were shown to cooperate with MYC in BL. These mutations present possible therapeutic targets, particularly for patients who cannot tolerate conventional chemotherapy.41
DLBCL is biologically and clinicopathologically heterogeneous across age groups. The 2 main molecular subtypes of DLBCL are germinal center B-cell (GCB) and activated B-cell (ABC) DLBCL. GCB lymphoma is the predominant subtype in children and is associated with excellent outcomes.42 It is characterized by co-expression of CD10 and BCL6 with variable expression of BCL2. Approximately 30% of adults with GCB DLBCL have translocations involving BCL2, which is associated with favorable outcomes.43 The ABC subtype of DLBCL is more common in older adults and is associated with inferior survival.44 Chronic activation of B-cell receptor and MYD88 signalling in ABC DLBCL is characteristic, and leads to constitutive activation of the nuclear factor κB pathway.45 Primary mediastinal B-cell lymphoma (PMBCL) makes up ∼10% of DLBCL cases in children and older adults and up to 50% of cases in AYAs.46 It affects younger adults and has favorable survival. In contrast to other DLBCL subtypes, PMBCL lacks surface immunoglobulin expression, and BCL2, BCL6, and MYC rearrangements are rare.46,47
In children, DLBCL is a clinically aggressive disease characterized by MYC translocation (37%), low BCL2 expression, and high proliferation rates. Translocations involving BCL2 are infrequent, and up to 84% of cases overexpress MYC.48 A large proportion of adults with DLBCL have high protein expression of both MYC and BCL2, an entity termed “double-expresser” DLBCL. Double-expresser DLBCL is associated with poor outcomes after standard therapy.49 The presence of both MYC and BCL2 or BCL6 rearrangements constitutes another distinct subgroup of aggressive double-hit lymphomas.50 Recently recategorized in the updated World Health Organization Classification of Lymphoid Malignancies as “high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements,” this form of DLBCL is a highly proliferative disease with an aggressive course and poor outcome.46 Double-expresser and double-hit lymphomas seem to be uncommon in AYAs.
Pediatric therapeutic approach
In the United States, the current treatment approaches for pediatric BL and DLBCL are based on modifications of 2 successive French Society of Pediatric Oncology, French-American-British (FAB) Lymphomes Malins B (LMB), and Berlin-Frankfurt-Münster (BFM) cooperative group trials.51 The treatment backbones are largely similar in their emphasis on the importance of central nervous system (CNS)–directed therapy with high-dose methotrexate, high-dose cytarabine, and intrathecal methotrexate in patients with advanced disease or CNS involvement. The BFM therapy includes ifosfamide with lower doses of doxorubicin, cyclophosphamide, and etoposide. Despite different risk group stratification and varied therapeutic approaches, outcomes across protocols are comparable (long-term survival near 80%) (Table 3).51,52
Table 3.
Pediatric NHL trials
| Reference | Trial acronym | Stage | Treatment | EFS | OS | ||
|---|---|---|---|---|---|---|---|
| Survival | % | Survival | % | ||||
| B-cell NHL (BL, DLBCL, PMBCL) | |||||||
| 103 | FAB LMB96 | I and II (localized disease) | COPAD × 2 | 4-y | 98.3 | ||
| COP, COPADM 1-2, CYM, M1 | 4-y | 91.9 | |||||
| COP, COPADM 1-2, CYM, no M1 | 4-y | 92.5 | |||||
| 104 | FAB LMB96 | III and IV (advanced CNS negative) | COP, COPADM 1-2, CYVE, M1-M4 | 4-y | 90 | ||
| COP, COPADM 1-2, mini-CYVE, M1 | 4-y | 80 | |||||
| 105 | FAB LMB96 | All advanced, including CNS positive | COP, COPADM1+2, CYVE 1+2, M1, M2, M3, M4, CNS-positive patients received 13 IT injections plus an additional course of high-dose methotrexate between CYVE cycles | 4-y | 79 | 4-y | 82 |
| 106 | NHL-BFM 95 | I, II (R1, resected) | A, B | 3-y | 94 | ||
| I, II (R2, not resected), III (LDH <500 U/L) | VA, B, A, B | 3-y | 94 | ||||
| III (R3, LDH 500 to <1000 U/L) | V, AA, BB, CC, AA, BB | 3-y | 85 | ||||
| IV (LDH <1000 U/L, CNS negative) | |||||||
| III, IV (R4, LDH ≥1000 U/L and/or CNS positive) | V, AA, BB, CC, AA, BB, CC | 3-y | 81 | ||||
| 104 | FAB LMB96 | PMBCL | 5-y | 66 | 5-y | 73 | |
| 66 | NHL-BFM 04 | PMBCL | DA-EPOCH-R | 2-y | 92 | 2-y | 92 |
| T-cell NHL (ALCL) | |||||||
| 83,107 | NHL-BFM 90 | I, II-r I-, II-nr, III, IV | Prophase: dexamethasone, cyclophosphamide, ifosfamide, doxorubicin, high-dose methotrexate (5 g/m2) vs methotrexate (0.5 g/m2), cytarabine, etoposide ± vinblastine | 2-y | 75 | ||
All medications are part of systemic therapy unless otherwise indicated. Treatment course A: dexamethasone, vincristine, ifosfamide, cytarabine, etoposide, methotrexate (1 g/m2), and intrathecal methotrexate (12 mg) + intrathecal cytarabine (30 mg) + intrathecal prednisolone (10 mg); intrathecal doses adjusted for age in patients <3 years. Treatment course B: dexamethasone, vincristine, cyclophosphamide, doxorubicin, methotrexate (1 g/m2), and intrathecal methotrexate (12 mg) + intrathecal cytarabine (30 mg) + intrathecal prednisolone (10 mg); intrathecal doses adjusted for age in patients <3 years. Treatment courses AA and BB are the same as A and B, respectively, with the following dose adjustments: methotrexate (5 g/m2) and intrathecal methotrexate (6 mg) + intrathecal cytarabine (15 mg) + intrathecal prednisolone (5 mg); intrathecal doses adjusted for age in patients <3 years. Treatment course CC: dexamethasone, vindesine, cytarabine, etoposide, intrathecal methotrexate (12 mg) + intrathecal cytarabine (30 mg) + intrathecal prednisolone (10 mg).
COP, cyclophosphamide, vincristine, and prednisone; COPAD, cyclophosphamide, vincristine, prednisone, and doxorubicin; COPADM, cyclophosphamide, vincristine, prednisone, doxorubicin, and high-dose methotrexate (8 g/m2); CYM, cyclophosphamide, high-dose cytarabine, and high-dose methotrexate; CYVE, cytarabine, high-dose cytarabine (3 g/m2), etoposide (200 mg/m2), and dexamethasone; LDH, lactate dehydrogenase; LMB, Lymphoma Malins de Burkitt; M1, COPADM plus intrathecal methotrexate, cytarabine, and hydrocortisone; M2, cytarabine and etoposide; M3, cyclophosphamide, vincristine, prednisone, and doxorubicin; M4, cytarabine and etoposide; mini-CYVE, cytarabine, high-dose cytarabine (2 g/m2), etoposide (100 mg/m2), and dexamethasone; NR, not resected; R, resected; R1, stage I + II resected; R2, stage I + II not resected, stage III: LDH <500 U/L; R3, stage III: LDH 500 to <1000 U/L, stage IV: BM+ and LDH <1000 U/L; R4, stage III + IV: LDH ≥1000 U/L and/or CNS+; V, dexamethasone, and cyclophosphamide.
In 2010, Meinhardt and colleagues53 demonstrated the activity of first-line rituximab followed by standard chemotherapy for the treatment of mature B-cell NHL in children, and the COG piloted the addition of rituximab to an FAB/LMB96 chemotherapy backbone (ANHL01P1).54 This was further evaluated in an Intergroup trial for children and adolescents with B-cell NHL or B-cell acute lymphoblastic leukemia, which randomly assigned patients with high-risk NHL to receive either rituximab plus standard of care or standard of care only. Results suggested superiority of the rituximab arm.55
Although relapse in pediatric BL and DLBCL is infrequent, survival after disease recurrence is poor. There is no standard therapeutic approach for relapsed B-cell NHL in pediatrics although salvage chemotherapy with autologous hematopoietic cell transplantation is often used. Novel therapies, including chimeric antigen-receptor T-cell therapy, are currently being investigated.56
Adult therapeutic approach
Estimated 5-year survival in adults with DLBCL varies by IPI score. In adults with an IPI score of 0, OS is reported to be near 94% vs 50% in those with an IPI score ≥3.57 Early studies report survival ranging from 40% in patients over age 40 years up to 89% in all ages after the addition of rituximab.58 For the past 4 decades, CHOP-based therapy has been the de facto standard for treatment of adult DLBCL. The most notable improvement in outcomes came with the addition of rituximab to the CHOP backbone (R-CHOP).59 Except for the French regimen R-ACVBP (rituximab plus doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone), attempts to intensify R-CHOP have been unsuccessful. Récher et al60 reported superior efficacy of R-ACVBP vs R-CHOP in younger DLBCL patients who had low-intermediate IPI scores. Compared with R-CHOP, however, R-ACVBP was associated with more treatment-related toxicities; thus, at present, R-CHOP remains the standard approach for adults worldwide.61
Optimal therapy for PMBCL is a subject of debate. The MabThera International Trial (MInT) demonstrated comparable EFS in patients with low-risk DLBCL and PMBCL who received R-CHOP. In the study, the majority of patients with PMBCL required RT.62 In patients who had PMBCL with high-risk features, Soumerai et al63 reported an unacceptably high rate of primary refractory disease after R-CHOP. For DLBCL, early studies suggested increased efficacy with the infusional regimen DA-EPOCH (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin), which was subsequently trialed in PMBCL.63,64 A phase 2 trial of DA-EPOCH plus rituximab (DA-EPOCH-R) for PMBCL obviated the need for RT and demonstrated 5-year EFS of 93% and OS of 97%.65 Woessmann et al66 reported similar outcomes with this regimen in the pediatric NHL-BFM 04 trial. The ongoing Intergroup Trial ANHL1131 (NCT01595048) is investigating both the efficacy and long-term cardiac risks of DA-EPOCH-R in children and adolescents with PMBCL.
Several chemotherapy regimens are used to treat adult BL. The most common therapeutic approach in the United States is CODOX-M/IVAC (cyclophosphamide, doxorubicin, vincristine, and methotrexate/ifosfamide, cytarabine, and etoposide) plus rituximab.67,68 Though this regimen is associated with favorable outcomes, survival in pediatric BL remains superior. To reduce the incidence of treatment-related toxicities in patients with comorbidities, Corazzelli and colleagues69 modified the CODOX-M/IVAC regimen by adding rituximab and liposome-encapsulated cytarabine to increase antitumor activity while de-intensifying CNS prophylaxis. Despite high actual dose intensity, 93% CR rates, and fair tolerability, the modified regimen was associated with 49% PFS in patients older than age 60 years vs 93% PFS for patients younger than age 60 years) (Table 4). Reduced CNS-directed therapy did not increase CNS failure rate (3% to 4%). Hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) alternating with methotrexate and high-dose cytarabine is effective for BL, although it, too, is associated with significant treatment-related toxicities.70 Dunleavy et al71 reported excellent outcomes from a single-center study of low-risk BL patients treated with reduced-intensity DA-EPOCH-R (Table 4). For low-risk patients, the current standard is CODOX-M/IVAC.72 At present, a multicenter study of DA-EPOCH-R is ongoing in the United States (NCT00001337), and a randomized study of DA-EPOCH-R vs CODOX-M/IVAC is ongoing in Europe.
Table 4.
Adult NHL trials
| Reference | Trial acronym | Phase | Risk | Regimen | EFS | PFS | OS | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Survival | % | Survival | % | Survival | % | |||||
| BL | ||||||||||
| 108 | Low | Modified CODOX-M/IVAC × 3 + intrathecal chemotherapy | 2-y | 83 | 81 | |||||
| High | Modified CODOX-M/IVAC × 4 | 2-y | 65 | 73 | ||||||
| 67 | CODOX-M/IVAC ± rituximab | 3-y | 74*; 61† | |||||||
| 71 | Low | DA-EPOCH-R | 95 | 100 | ||||||
| 69 | II | Low | Hyper-CVAD with high-dose methotrexate, high-dose cytarabine + rituximab | 3-y | 80 | 88-89 | ||||
| 4-y | 75‡; 76§ | |||||||||
| DLBCL | ||||||||||
| 109 | III | R-CHOP-14 vs R-CHOP-21 | 2-y | 75 vs 75 | 2-y | 83 vs 81 | ||||
| 60 | III | R-ACVBP vs R-CHOP | 3-y | 87 vs 73 | 3-y | 92 vs 84 | ||||
| 110 | CALGB-50303 | R-CHOP vs DA-EPOCH-R | 3-y | 81 vs 79 | 3-y | 85 vs 85 | ||||
| PMBCL | ||||||||||
| 62, 111 | MInT | R-CHOP | 3-y | 78 | 3-y | 89 | ||||
| 64 | DA-EPOCH-R | 93 | 100 | |||||||
CODOX-M/IVAC, cyclophosphamide, vincristine, and doxorubicin-methotrexate/ifosfamide, etoposide, and cytarabine; MInT, MabThera International Trial.
Rituximab.
No rituximab.
Age >60 y.
Age <60 y.
Future perspectives
To date, no prospective studies have investigated whether AYAs would benefit from pediatric- or adult-like therapy for mature B-cell NHL. Future studies will likely focus on treatment de-intensification and on incorporation of targeted therapies whenever possible. The clinical significance of double-hit disease, MYC overexpression, and MYC translocation in AYAs remains unknown. Similarly, the molecular profile of mature B-cell NHL in this population has been understudied. Identification of genetic markers and establishment of age-associated prognostic factors are needed.
Mature T-cell NHL
Epidemiology
ALCL is a clinically aggressive T-cell NHL with bimodal age distribution. It accounts for ∼15% of NHL in children and ∼2% of NHL in adults.73 Systemic ALCL is subdivided into 2 groups on the basis of the presence or absence of an abnormal form of the anaplastic lymphoma kinase (ALK) protein74 on the lymphoma cell surface. In North America and Europe, ALK+ ALCL accounts for ∼7% of NHL and ALK– ALCL accounts for ∼5%.
Staging and risk stratification
Clinical features associated with poor outcomes in ALCL include elevated lactate dehydrogenase and skin, mediastinal, or visceral organ involvement. The ALK+ subtype predominates in children, and although young patients often present with advanced disease, 5-year OS in this population exceeds 70%.75,76
Biological features of ALCL
The first pathologic descriptions of ALCL were based on identification of large, multinucleated anaplastic (hallmark) cells. These cells are characterized by cytologic atypia and strong CD30 expression.77 The ALK translocation t(2;5)(p23;q25) results in the fusion of nucleophosmin and ALK genes leading to activation of a tyrosine kinase domain with unregulated cell proliferation.78 The lymphohistiocytic variant of ALK+ ALCL (∼10%) is characterized by large numbers of benign histiocytes with fewer large neoplastic cells. The small cell variant (∼5% to 10%) is characterized by predominance of small neoplastic cells and rare hallmark cells and is associated with worse outcomes in pediatrics.79,80 ALK positivity, an independent predictor of survival in ALCL, decreases in frequency with increasing age. In a study of 70 adults with ALCL, median age of ALK+ patients was 30 years, which was significantly younger than that of patients with ALK− ALCL. At present, relative incidence of ALK+ vs ALK– disease in the AYA population are not known.
Pediatric therapeutic approach
Therapy for pediatric ALCL has evolved over the past 2 decades, with different consortia taking different approaches. The LSA2-L2 (cyclophosphamide, vincristine, methotrexate, daunorubicin, prednisone, cytarabine, thioguanine, asparaginase, hydroxyurea, and carmustine) regimen, developed at Memorial Sloan Kettering Cancer Center, is associated with 10-year survival of 65%.81 The majority of ALCL treatment regimens are based on the mature B-cell NHL literature. Seidemann et al82 reported results of the NHL-BFM 90 trial, which included a prephase of vincristine, cyclophosphamide, and dexamethasone followed by additional chemotherapy based on lymphoma stage (5-year EFS, 76%) (Table 3). In 2010, Le Deley and colleagues83 reported results of the ALCL99-vinblastine study, which investigated adding vinblastine maintenance therapy to the NHL-BFM 90 backbone in children with high-risk ALCL (Table 3). Per the BFM 90 protocol, patients received a prophase of low-dose cyclophosphamide and corticosteroid followed by 6 courses of moderate-dose or high-dose methotrexate, dexamethasone, ifosfamide, cyclophosphamide, etoposide, cytarabine, doxorubicin, and intrathecal chemotherapy over 4 months. High-risk patients were randomly assigned to receive additional vinblastine during and after therapy. Two-year EFS was 74.1%, and 2-year OS was 92.5% (Table 3).83
Brentuximab vedotin is associated with excellent outcomes in adult ALCL. Crizotinib, an orally available dual ALK/MET inhibitor, has also shown high response rates in phase 1 studies of adults with ALCL.84 The ongoing COG-ANHL12P1 study in children and adolescents with ALCL is investigating the efficacy of first-line crizotinib vs brentuximab vedotin, each in combination with multiagent chemotherapy (NCT01979536). Despite there being no standard therapeutic approach for relapsed pediatric ALCL, survival after recurrence is favorable. The French Society for Pediatric Oncology reported excellent outcomes after single-agent vinblastine in children with relapsed ALCL.85
Adult therapeutic approach
First-line therapy for adult ALCL is based largely on the treatment of DLBCL.86 The current recommendation for ALK+ ALCL in first remission is CHOP-like chemotherapy without transplantation. For relapsed disease, salvage chemotherapy with platinum-based regimens and stem cell transplantation is recommended. Several phase 2 studies have demonstrated efficacy of brentuximab vedotin in adults with ALCL. Pro et al87 reported an overall response rate of 86% in adults after receiving brentuximab vedotin for relapsed or refractory ALCL (58% CR), which resulted in approval by the US Food and Drug Administration for this indication. Currently, brentuximab vedotin is used primarily as a bridge to transplantation after relapse; however, its utility as part of up-front therapy in combination with CHP (cyclophosphamide, doxorubicin, and prednisone) is currently under investigation (NCT01777152).
Future directions
Despite excitement about the potential for targeted therapies in ALCL, it is still too soon to omit standard chemotherapy. Standard treatment of AYAs with ALCL generally includes CHOP-like therapy with or without early autologous stem cell transplantation. The benefit of more intensive pediatric-like therapy in AYAs has not been prospectively evaluated. As suggested in the above discussions, expanding our understanding of disease biology will drive the development of novel targeted therapies, which can then be brought to the clinic in the context of consortium-based trials.
Summary, challenges, and strategies for future research
The information gap in AYA lymphoma
This review highlights that even for similar lymphoma subtypes, treatment approaches by pediatric and medical oncologists reliably differ (Table 5).37,88 Largely due to low rates of clinical trial enrollment in AYAs, details about cancer biology, care delivery, and therapeutic efficacy in this population have been systematically understudied.3,89 As a result, for 15- to 39-year-olds, lymphoma treatment is not necessarily dictated by empiric evidence specific to the age group, but rather by community referral patterns, individual physician preference, and treatment location.90
Table 5.
Summary table of pediatric and adult therapeutic approaches in HL, BL, DLBCL, PMBCL, and ALCL
| Pediatric approach | Adult approach | |
|---|---|---|
| HL | ||
| Stage I/II (non-bulky) | ABVE × 2-4 ± RT; VAMP × 4 ± RT; OEPA × 2 ± RT | ABVD × 4-6 with risk-adapted use of RT |
| Stage I/II (bulky) | ABVE-PC × 4 ± RT; OEPA × 2, plus COPDAC × 2 ± RT | ABVD × 4-6 plus RT in most cases |
| Stage III/IV | ABVE-PC × 4-5 plus RT in most cases; OEPA × 2, plus COPDAC × 4 ± RT | ABVD × 6 |
| BL | ||
| Low risk | COPAD × 2 | R-CODOX-M × 3, DA-EPOCH-R, or rituximab, or R-hyper-CVAD |
| High risk | FAB LMB96 protocol + rituximab* | R-CODOX-M/IVAC or R-hyper-CVAD |
| DLBCL | Burkitt-like therapy | R-CHOP × 6 |
| PMBCL | DA-EPOCH-R × 6 | DA-EPOCH-R × 6 |
| ALCL | ALCL 99 protocol† | CHOEP × 6 |
CHOEP, cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone; R-CODOX-M, rituximab plus cyclophosphamide, vincristine, and doxorubicin-methotrexate; R-CODOX-M/IVAC,rituximab plus cyclophosphamide, vincristine, and doxorubicin-methotrexate/ifosfamide, etoposide, and cytarabine; R-hyper-CVAD, rituximab plus hyper-CVAD.
R-CHOP (rituximab plus cyclophosphamide, vincristine, and prednisone) prophase → 2 cycles of induction with R-COPADM × 2 → 2 cycles of maintenance with rituximab plus CYM plus intrathecal chemotherapy.
Cyclophosphamide-dexamethasone prophase followed by 6 alternating cycles of course A (dexamethasone, high-dose methotrexate, ifosfamide, cytarabine, and etoposide) and course B (dexamethasone, high-dose methotrexate, cyclophosphamide, and doxorubicin) chemotherapy.
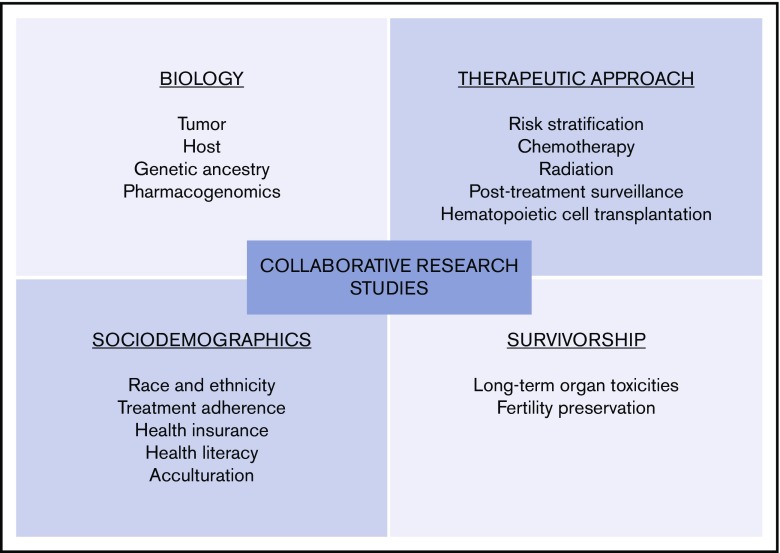
Clinical trials data and consensus recommendations establish standards of care in both pediatric and adult oncology. To develop consensus recommendations for AYAs with lymphoma, critical areas in need of further study include, but are not limited to, biology and molecular prognostic indicators, novel therapeutics, acute and long-term toxicities of therapy, and psychosocial outcomes (ie, financial burden of treatment, access-to-care, and therapy adherence) (Figure 1).91
Figure 1.
Key areas to advance research and improve outcomes in AYA lymphoma.
Combining resources and leveraging existing data
In children and older adults, analysis of prospectively collected clinical trial data enables frequent, evidence-based modifications of existing treatment paradigms. In patients treated in the community oncology setting, health outcomes researchers leverage tumor registries and insurance claims data to characterize care delivery and its impact on survival in large population-based cohorts. In AYA lymphoma, a combination of data from these 2 sources is a logical next step as we begin to address outstanding clinical questions. Synthesis of prospectively collected data from AYAs enrolled on pediatric and adult consortium trials will provide details about biology, toxicities, and survival in the cooperative group setting.92 Analysis of data from tumor registries and insurance files can provide insight into how community-level predictors (ie, access to care, socioeconomic status, treatment location) influence outcomes of AYAs treated for lymphoma in the community.93
In conclusion, lymphoma in the AYA population is a prime area for collaborative research between pediatric and medical oncologists, as well as between providers in the community and in the consortia. Community providers, often at the front line of diagnosis and management, are uniquely poised to establish a national tumor bank for AYA lymphoma. With broader understanding of prognostic molecular biomarkers in this patient population, we can plan innovative clinical trials whose inclusion criteria are based not only on age, but also on lymphoma subtype and disease biology. Developing well-designed therapeutic trials that span pediatric and adult groups, and that incorporate relevant biologic and health services correlatives, is critical to advancing research and improving outcomes in the AYA lymphoma population.
Acknowledgments
The authors thank Meghan Gutierrez (Chief Executive Officer, Lymphoma Research Foundation [LRF]) for her tireless leadership and for her unwavering commitment to improving the outcomes of AYAs with lymphoma; Molly Moody, Maxwell Mulcahy, and Whitney Steen (LRF) for coordinating and producing an outstanding symposium; the clinicians and scientists who shared their research and who contributed to the AYA Symposium in October 2015 (a full list of attendees is provided in the Appendix); and Ellen Walker and The Paul Foundation, the founding sponsor of the LRF AYA Initiative, for their steadfast dedication to this issue.
This work was supported in part by grant R25 CA094061 from the National Institutes of Health, National Cancer Institute (J.M.K.).
Appendix
Lymphoma Research Foundation AYA Symposium attendees: Sarah Alexander (The Hospital for Sick Children, University of Toronto); Burt Appel (Hackensack University Medical Center); Saro Armenian (City of Hope); Kristie Blum (The Ohio State University Comprehensive Cancer Center); Catherine Bollard (Children’s National Medical Center); Joshua Brody (Icahn School of Medicine at Mount Sinai); Ryan Cassaday (Fred Hutchinson Cancer Research Center); Jonathan Cohen (Winship Cancer Institute of Emory University); Peter D. Cole (Albert Einstein College of Medicine/The Children’s Hospital at Montefiore); Michael Douvas (University of Virginia School of Medicine); Kieron Dunleavy (George Washington University); Andy Evens (Tufts University School of Medicine); Adolfo Ferrando (Columbia University Medical Center); Chris Flowers (Winship Cancer Institute of Emory University); Debra Friedman (Vanderbilt University School of Medicine); Jill Ginsberg (The Children’s Hospital of Philadelphia); Leo Gordon (Feinberg School of Medicine at Northwestern University); Tom Gross (National Cancer Institute); Tara O. Henderson (The University of Chicago); David Hodgson (University of Toronto); Justine M. Kahn (Columbia University Medical Center); Frank Keller (Emory University School of Medicine); Kara Kelly (Roswell Park Cancer Institute); Anita Kumar (Memorial Sloan Kettering Cancer Center); Ann LaCasce (Dana-Farber Cancer Institute); Catherine Lai (National Institutes of Health); John Leonard (New York-Presbyterian Hospital Weill Cornell Medical Center); Megan Lim (University of Pennsylvania); Eric Lowe (Children’s Hospital of the King’s Daughters); Lindsay Morton (National Cancer Institute); Nmazuo Ozuah (Dana-Faber Cancer Institute); Barbara Pro (Northwestern University); Allison Rosenthal (Mayo Clinic, Scottsdale); Christian Steidl (BC Cancer Agency Research Centre); Deborah Stephens (Huntsman Cancer Institute at the University of Utah); Maria-Luisa Sulis (Columbia University Medical Center); and Michael Werner (Executive Vice President, Lymphoma Research Foundation Board of Directors).
Authorship
Contribution: J.M.K., N.W.O., K.D., T.O.H., K.K., and A.L. contributed to writing and reviewing this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Justine M. Kahn, Division of Pediatric Hematology/Oncology/Stem Cell Transplantation, Columbia University Medical Center, 161 Fort Washington Ave, IP7, New York, NY 10032; e-mail: jk2034@cumc.columbia.edu.
References
- 1.Pollock BH, Birch JM. Registration and classification of adolescent and young adult cancer cases. Pediatr Blood Cancer. 2008;50(5 Suppl):1090-1093. [DOI] [PubMed] [Google Scholar]
- 2.Bleyer A. Latest estimates of survival rates of the 24 most common cancers in adolescent and young adult Americans. J Adolesc Young Adult Oncol. 2011;1(1):37-42. [DOI] [PubMed] [Google Scholar]
- 3.Shaw PH, Reed DR, Yeager N, Zebrack B, Castellino SM, Bleyer A. Adolescent and young adult (AYA) oncology in the United States: A specialty in its late adolescence. J Pediatr Hematol Oncol. 2015;37(3):161-169. [DOI] [PubMed] [Google Scholar]
- 4.Kahn JM, Keegan TH, Tao L, Abrahão R, Bleyer A, Viny AD. Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer. 2016;122(17):2723-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keegan TH, Ries LA, Barr RD, et al. ; National Cancer Institute Next Steps for Adolescent and Young Adult Oncology Epidemiology Working Group. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009-1016. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg J, Waxman IM, Kelly KM, Morris E, Cairo MS. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009;144(1):24-40. [DOI] [PubMed] [Google Scholar]
- 7.Bleyer A, Viny A, Barr R. Cancer in 15- to 29-year-olds by primary site. Oncologist. 2006;11(6):590-601. [DOI] [PubMed] [Google Scholar]
- 8.Mathews-Bradshaw B, Johnson R, Kaplan S, Craddock K, Hayes-Lattin B. The history and accomplishments of the LIVESTRONG Young Adult Alliance. J Adolesc Young Adult Oncol. 2011;1(1):43-47. [DOI] [PubMed] [Google Scholar]
- 9.Smith AW, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer. 2016;122(7):988-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansell SM. Hodgkin lymphoma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(7):771-779. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz CL, Chen L, McCarten K, et al. Childhood Hodgkin International Prognostic Score (CHIPS) predicts event-free survival in Hodgkin lymphoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2017;64(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diefenbach CS, Li H, Hong F, et al. Evaluation of the International Prognostic Score (IPS-7) and a simpler prognostic score (IPS-3) for advanced Hodgkin lymphoma in the modern era. Br J Haematol. 2015;171(4):530-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diefenbach CS, Connors JM, Friedberg JW, et al. Hodgkin lymphoma: current status and clinical trial recommendations. J Natl Cancer Inst. 2016;109(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vardhana S, Younes A. The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica. 2016;101(7):794-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steidl C, Diepstra A, Lee T, et al. Gene expression profiling of microdissected Hodgkin Reed-Sternberg cells correlates with treatment outcome in classical Hodgkin lymphoma. Blood. 2012;120(17):3530-3540. [DOI] [PubMed] [Google Scholar]
- 16.Scott DW, Chan FC, Hong F, et al. Gene expression-based model using formalin-fixed paraffin-embedded biopsies predicts overall survival in advanced-stage classical Hodgkin lymphoma. J Clin Oncol. 2013;31(6):692-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mottok A, Johnston RL, Chan FC, et al. Prediction of primary treatment outcome using gene expression profiling of pre-treatment biopsies obtained from childhood and adolescent Hodgkin lymphoma patients [abstract]. Blood. 2015;126(230). Abstract 175. [Google Scholar]
- 18.Cleary SF, Link MP, Donaldson SS. Hodgkin’s disease in the very young. Int J Radiat Oncol Biol Phys. 1994;28(1):77-83. [DOI] [PubMed] [Google Scholar]
- 19.Eichenauer DA, Bredenfeld H, Haverkamp H, et al. Hodgkin’s lymphoma in adolescents treated with adult protocols: a report from the German Hodgkin study group. J Clin Oncol. 2009;27(36):6079-6085. [DOI] [PubMed] [Google Scholar]
- 20.Kelly KM. Hodgkin lymphoma in children and adolescents: improving the therapeutic index. Blood. 2015;126(22):2452-2458. [DOI] [PubMed] [Google Scholar]
- 21.Mauz-Körholz C, Metzger ML, Kelly KM, et al. Pediatric Hodgkin lymphoma. J Clin Oncol. 2015;33(27):2975-2985. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood. 2009;114(10):2051-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauz-Körholz C, Hasenclever D, Dörffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: the GPOH-HD-2002 study. J Clin Oncol. 2010;28(23):3680-3686. [DOI] [PubMed] [Google Scholar]
- 24.Kelly KM, Sposto R, Hutchinson R, et al. BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: a report from the Children’s Oncology Group. Blood. 2011;117(9):2596-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol. 2014;32(32):3651-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charpentier AM, Friedman DL, Wolden S, et al. Predictive factor analysis of response-adapted radiation therapy for chemotherapy-sensitive pediatric Hodgkin lymphoma: analysis of the Children’s Oncology Group AHOD 0031 Trial. Int J Radiat Oncol Biol Phys. 2016;96(5):943-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer RM, Gospodarowicz MK, Connors JM, et al. ; NCIC Clinical Trials Group; Eastern Cooperative Oncology Group. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366(5):399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Federico M, Luminari S, Iannitto E, et al. ; HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin’s lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol. 2009;27(5):805-811. [DOI] [PubMed] [Google Scholar]
- 29.Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25(24):3746-3752. [DOI] [PubMed] [Google Scholar]
- 30.Straus DJ, Pitcher B, Kostakoglu L, et al. Initial results of US Intergroup trial of response-adapted chemotherapy or chemotherapy/radiation therapy based on PET for non-bulky stage I and II Hodgkin lymphoma (HL) (CALGB/Alliance 50604) [abstract]. Blood. 2015;126(23). Abstract 578. [Google Scholar]
- 31.Press OW, Li H, Schöder H, et al. US Intergroup Trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: Southwest Oncology Group S0816. J Clin Oncol. 2016;34(17):2020-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinzani PL, Sasse S, Radford J, Shonukan O, Bonthapally V. Experience of brentuximab vedotin in relapsed/refractory Hodgkin lymphoma and relapsed/refractory systemic anaplastic large-cell lymphoma in the Named Patient Program: Review of the literature. Crit Rev Oncol Hematol. 2015;95(3):359-369. [DOI] [PubMed] [Google Scholar]
- 33.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34(23):2690-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hochberg J, El-Mallawany NK, Abla O. Adolescent and young adult non-Hodgkin lymphoma. Br J Haematol. 2016;173(4):637-650. [DOI] [PubMed] [Google Scholar]
- 36.Groves FD, Linet MS, Travis LB, Devesa SS. Cancer surveillance series: non-Hodgkin’s lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst. 2000;92(15):1240-1251. [DOI] [PubMed] [Google Scholar]
- 37.Jaglowski SM, Linden E, Termuhlen AM, Flynn JM. Lymphoma in adolescents and young adults. Semin Oncol. 2009;36(5):381-418. [DOI] [PubMed] [Google Scholar]
- 38.Love C, Sun Z, Jima D, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. 2012;44(12):1321-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrich AM, Nabhan C, Smith SM. MYC-associated and double-hit lymphomas: a review of pathobiology, prognosis, and therapeutic approaches. Cancer. 2014;120(24):3884-3895. [DOI] [PubMed] [Google Scholar]
- 40.Gualco G, Weiss LM, Harrington WJ Jr, Bacchi CE. Nodal diffuse large B-cell lymphomas in children and adolescents: immunohistochemical expression patterns and c-MYC translocation in relation to clinical outcome. Am J Surg Pathol. 2009;33(12):1815-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490(7418):116-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandlund JT, Downing JR, Crist WM. Non-Hodgkin’s lymphoma in childhood. N Engl J Med. 1996;334(19):1238-1248. [DOI] [PubMed] [Google Scholar]
- 43.Iqbal J, Sanger WG, Horsman DE, et al. BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol. 2004;165(1):159-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott DW, Mottok A, Ennishi D, et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J Clin Oncol. 2015;33(26):2848-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463(7277):88-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198(6):851-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miles RR, Raphael M, McCarthy K, et al. ; SFOP/LMB96/CCG5961/UKCCSG/NHL 9600 Study Group. Pediatric diffuse large B-cell lymphoma demonstrates a high proliferation index, frequent c-Myc protein expression, and a high incidence of germinal center subtype: Report of the French-American-British (FAB) international study group. Pediatr Blood Cancer. 2008;51(3):369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunleavy K. Double-hit lymphomas: current paradigms and novel treatment approaches. Hematology Am Soc Hematol Educ Program. 2014;2014:107-112. [DOI] [PubMed] [Google Scholar]
- 50.Dunleavy K. Optimal management of double-hit lymphoma. J Oncol Pract. 2016;12(3):241-242. [DOI] [PubMed] [Google Scholar]
- 51.Minard-Colin V, Brugières L, Reiter A, et al. Non-Hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol. 2015;33(27):2963-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cairo MS, Sposto R, Gerrard M, et al. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (≥ 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB LMB 96 study. J Clin Oncol. 2012;30(4):387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meinhardt A, Burkhardt B, Zimmermann M, et al. ; Berlin-Frankfurt-Münster group. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin Oncol. 2010;28(19):3115-3121. [DOI] [PubMed] [Google Scholar]
- 54.Goldman S, Smith L, Anderson JR, et al. Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children’s Oncology Group report. Leukemia. 2013;27(5):1174-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minard-Colin V, Auperin A, Pillon M, et al. Results of the randomized Intergroup trial Inter-B-NHL Ritux 2010 for children and adolescents with high-risk B-cell non-Hodgkin lymphoma (B-NHL) and mature acute leukemia (B-AL): Evaluation of rituximab (R) efficacy in addition to standard LMB chemotherapy (CT) regimen [abstract]. J Clin Oncol. 2016;34(15). Abstract 10507. [Google Scholar]
- 56.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22(5):941-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perkins AS, Friedberg JW. Burkitt lymphoma in adults. Hematology Am Soc Hematol Educ Program. 2008;341-348. [DOI] [PubMed] [Google Scholar]
- 59.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Récher C, Coiffier B, Haioun C, et al. ; Groupe d’Etude des Lymphomes de l’Adulte. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet. 2011;378(9806):1858-1867. [DOI] [PubMed] [Google Scholar]
- 61.Hoelzer D, Walewski J, Döhner H, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood. 2014;124(26):3870-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rieger M, Osterborg A, Pettengell R, et al. ; MabThera International Trial (MInT) Group. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol. 2011;22(3):664-670. [DOI] [PubMed] [Google Scholar]
- 63.Soumerai JD, Hellmann MD, Feng Y, et al. Treatment of primary mediastinal B-cell lymphoma with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone is associated with a high rate of primary refractory disease. Leuk Lymphoma. 2014;55(3):538-543. [DOI] [PubMed] [Google Scholar]
- 64.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoelzer D. Dose-adjusted EPOCH-R for Burkitt lymphoma. Clin Adv Hematol Oncol. 2014;12(11):777-779. [PubMed] [Google Scholar]
- 66.Woessmann W, Lisfeld J, Burkhardt B. NHL-BFM Study Group. Therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;369(3):282. [DOI] [PubMed] [Google Scholar]
- 67.Barnes JA, Lacasce AS, Feng Y, et al. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt’s lymphoma: a retrospective analysis. Ann Oncol. 2011;22(8):1859-1864. [DOI] [PubMed] [Google Scholar]
- 68.Evens AM, Carson KR, Kolesar J, et al. A multicenter phase II study incorporating high-dose rituximab and liposomal doxorubicin into the CODOX-M/IVAC regimen for untreated Burkitt’s lymphoma. Ann Oncol. 2013;24(12):3076-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corazzelli G, Frigeri F, Russo F, et al. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and ‘unclassifiable’ highly aggressive B-cell lymphoma. Br J Haematol. 2012;156(2):234-244. [DOI] [PubMed] [Google Scholar]
- 70.Thomas DA, Faderl S, O’Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106(7):1569-1580. [DOI] [PubMed] [Google Scholar]
- 71.Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013;369(20):1915-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lacasce A, Howard O, Lib S, et al. Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased toxicity. Leuk Lymphoma. 2004;45(4):761-767. [DOI] [PubMed] [Google Scholar]
- 73.Drexler HG, Gignac SM, von Wasielewski R, Werner M, Dirks WG. Pathobiology of NPM-ALK and variant fusion genes in anaplastic large cell lymphoma and other lymphomas. Leukemia. 2000;14(9):1533-1559. [DOI] [PubMed] [Google Scholar]
- 74.Pileri SA, Pulford K, Mori S, et al. Frequent expression of the NPM-ALK chimeric fusion protein in anaplastic large-cell lymphoma, lympho-histiocytic type. Am J Pathol. 1997;150(4):1207-1211. [PMC free article] [PubMed] [Google Scholar]
- 75.Stein H, Foss HD, Dürkop H, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96(12):3681-3695. [PubMed] [Google Scholar]
- 76.Savage KJ, Harris NL, Vose JM, et al. ; International Peripheral T-Cell Lymphoma Project. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496-5504. [DOI] [PubMed] [Google Scholar]
- 77.Jaffe ES. Anaplastic large cell lymphoma: the shifting sands of diagnostic hematopathology. Mod Pathol. 2001;14(3):219-228. [DOI] [PubMed] [Google Scholar]
- 78.Falini B. Anaplastic large cell lymphoma: pathological, molecular and clinical features. Br J Haematol. 2001;114(4):741-760. [DOI] [PubMed] [Google Scholar]
- 79.Alexander S, Kraveka JM, Weitzman S, et al. Advanced stage anaplastic large cell lymphoma in children and adolescents: results of ANHL0131, a randomized phase III trial of APO versus a modified regimen with vinblastine: a report from the children’s oncology group. Pediatr Blood Cancer. 2014;61(12):2236-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lamant L, McCarthy K, d’Amore E, et al. Prognostic impact of morphologic and phenotypic features of childhood ALK-positive anaplastic large-cell lymphoma: results of the ALCL99 study. J Clin Oncol. 2011;29(35):4669-4676. [DOI] [PubMed] [Google Scholar]
- 81.Mora J, Filippa DA, Qin J, Wollner N. Lymphoblastic lymphoma of childhood and the LSA2-L2 protocol: the 30-year experience at Memorial-Sloan-Kettering Cancer Center. Cancer. 2003;98(6):1283-1291. [DOI] [PubMed] [Google Scholar]
- 82.Seidemann K, Tiemann M, Schrappe M, et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Münster Group Trial NHL-BFM 90. Blood. 2001;97(12):3699-3706. [DOI] [PubMed] [Google Scholar]
- 83.Le Deley MC, Rosolen A, Williams DM, et al. Vinblastine in children and adolescents with high-risk anaplastic large-cell lymphoma: results of the randomized ALCL99-vinblastine trial. J Clin Oncol. 2010;28(25):3987-3993. [DOI] [PubMed] [Google Scholar]
- 84.Mossé YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14(6):472-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brugières L, Pacquement H, Le Deley MC, et al. Single-drug vinblastine as salvage treatment for refractory or relapsed anaplastic large-cell lymphoma: a report from the French Society of Pediatric Oncology. J Clin Oncol. 2009;27(30):5056-5061. [DOI] [PubMed] [Google Scholar]
- 86.Eyre TA, Khan D, Hall GW, Collins GP. Anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: current and future perspectives in adult and paediatric disease. Eur J Haematol. 2014;93(6):455-468. [DOI] [PubMed] [Google Scholar]
- 87.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190-2196. [DOI] [PubMed] [Google Scholar]
- 88.Parsons HM, Harlan LC, Schmidt S, et al. ; AYA HOPE Collaborative Group. Who Treats Adolescents and Young Adults with Cancer? A Report from the AYA HOPE Study. J Adolesc Young Adult Oncol. 2015;4(3):141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferrari A, Montello M, Budd T, Bleyer A. The challenges of clinical trials for adolescents and young adults with cancer. Pediatr Blood Cancer. 2008;50(5 Suppl):1101-1104. [DOI] [PubMed] [Google Scholar]
- 90.Bleyer A. The adolescent and young adult gap in cancer care and outcome. Curr Probl Pediatr Adolesc Health Care. 2005;35(5):182-217. [DOI] [PubMed] [Google Scholar]
- 91.Zebrack B, Bleyer A, Albritton K, Medearis S, Tang J. Assessing the health care needs of adolescent and young adult cancer patients and survivors. Cancer. 2006;107(12):2915-2923. [DOI] [PubMed] [Google Scholar]
- 92.Fernández KS, Schwartz CL, Chen L, Constine LS, Chauvenet A, de Alarcón PA. Outcome of adolescents and young adults compared to children with Hodgkin lymphoma treated with response-based chemotherapy on pediatric protocols: a Children’s Oncology Group report [published online ahead of print 14 June 2017]. Pediatr Blood Cancer. doi:10.1002/pbc.26681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boscoe FP, Schrag D, Chen K, Roohan PJ, Schymura MJ. Building capacity to assess cancer care in the Medicaid population in New York State. Health Serv Res. 2011;46(3):805-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tebbi CK, Mendenhall NP, London WB, et al. Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2012;59(7):1259-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nachman JB, Sposto R, Herzog P, et al. ; Children’s Cancer Group. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002;20(18):3765-3771. [DOI] [PubMed] [Google Scholar]
- 96.Wolden SL, Chen L, Kelly KM, et al. Long-term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin’s lymphoma--a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(26):3174-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117(6):1806-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Metzger ML, Weinstein HJ, Hudson MM, et al. Association between radiotherapy vs no radiotherapy based on early response to VAMP chemotherapy and survival among children with favorable-risk Hodgkin lymphoma. JAMA. 2012;307(24):2609-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372(17):1598-1607. [DOI] [PubMed] [Google Scholar]
- 100.Merli F, Luminari S, Gobbi PG, et al. Long-term results of the HD2000 trial comparing ABVD versus BEACOPP versus COPP-EBV-CAD in untreated patients with advanced Hodgkin lymphoma: a study by Fondazione Italiana Linfomi. J Clin Oncol. 2016;34(11):1175-1181. [DOI] [PubMed] [Google Scholar]
- 101.Engert A, Haverkamp H, Kobe C, et al. ; German Hodgkin Study Group; Swiss Group for Clinical Cancer Research; Arbeitsgemeinschaft Medikamentöse Tumortherapie. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379(9828):1791-1799. [DOI] [PubMed] [Google Scholar]
- 102.Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374(25):2419-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patte C, Auperin A, Gerrard M, et al. ; FAB/LMB96 International Study Committee. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109(7):2773-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gerrard M, Cairo MS, Weston C, et al. ; FAB LMB96 International Study Committee. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Br J Haematol. 2008;141(6):840-847. [DOI] [PubMed] [Google Scholar]
- 105.Cairo MS, Gerrard M, Sposto R, et al. ; FAB LMB96 International Study Committee. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109(7):2736-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Woessmann W, Seidemann K, Mann G, et al. ; BFM Group. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood. 2005;105(3):948-958. [DOI] [PubMed] [Google Scholar]
- 107.Wrobel G, Mauguen A, Rosolen A, et al. ; European Inter-Group for Childhood, Non-Hodgkin Lymphoma (EICNHL). Safety assessment of intensive induction therapy in childhood anaplastic large cell lymphoma: report of the ALCL99 randomised trial. Pediatr Blood Cancer. 2011;56(7):1071-1077. [DOI] [PubMed] [Google Scholar]
- 108.Mead GM, Barrans SL, Qian W, et al. ; UK National Cancer Research Institute Lymphoma Clinical Studies Group; Australasian Leukaemia and Lymphoma Group. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial). Blood. 2008;112(6):2248-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880):1817-1826. [DOI] [PubMed] [Google Scholar]
- 110.Wilson WH, sin-Ho J, Pitcher BN, et al. Phase III randomized study of R-CHOP versus DA-EPOCH-R and molecular analysis of untreated diffuse large B-cell lymphoma: CALGB/Alliance 50303 [abstract]. Blood. 2016;128(22). Abstract 469. [Google Scholar]
- 111.Pfreundschuh M, Kuhnt E, Trümper L, et al. ; MabThera International Trial (MInT) Group. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013-1022. [DOI] [PubMed] [Google Scholar]