Abstract
Serotonin transporter (SERT) inhibitors treat depression by elevating brain extracellular 5-hydroxytryptamine (5-HTExt). However, only one-third of patients respond adequately. Treatment-resistant depression (TRD) is a major unmet need. Interestingly, elevating 5-HTExt beyond what is achieved by a SERT inhibitor appears to treat TRD. Adjunctive administration of 5-hydroxytryptophan (5-HTP) safely elevates 5-HTExt beyond the SERT inhibitor effect in humans; but, 5-HTP cannot be a clinically viable drug because of its poor pharmacokinetics. A slow-release (SR) delivery mode would be predicted to overcome the pharmacokinetic limitations of 5-HTP, substantially enhance the pharmacological action, and transform 5-HTP into a clinically viable drug. Animal studies bear out this prediction. Thus, adjunct 5-HTP SR could be an important new treatment for TRD. Here we review the clinical and preclinical evidence.
Keywords: Antidepressant, depression, treatment-resistant depression, 5-hydroxytryptophan
Current therapies for treatment-resistant depression are inadequate
Depression is characterized by persistent depressed mood and/or anhedonia in conjunction with other mood and physical symptoms (Diagnostic and Statistical Manual of Mental Disorders, 5th Edition). According to statistics from the USA National Institute of Mental Health, 6.6 % of the population will suffer from depression each year. The mainstay of antidepressant therapy remains the serotonin transporter (SERT) inhibitors, predominantly selective serotonin reuptake-inhibitors (SSRIs) and dual serotonin and norepinephrine reuptake inhibitors (SNRIs). SERT inhibitors block reuptake of 5-HT from the extracellular space. This causes sustained elevation of brain extracellular serotonin (aka 5-hydroxytryptamine, 5-HTExt), which over time leads to an antidepressant response [1]. Unfortunately, SERT inhibitors achieve remission in only a third of patients [2]. As such, an estimated 2 % of the population suffers from treatment-resistant depression (TRD) [3]. Current treatments for TRD are of limited benefit [4], and new treatments are needed.
As reviewed below, multipronged clinical data suggest that elevating 5-HTExt beyond the effect achieved by SERT inhibitor monotherapy is therapeutic in TRD. Hence, a drug that, when administered adjunct to a SERT inhibitor, safely and in a sustained fashion, elevates 5-HTExt beyond the SSRI effect could be a new therapy for TRD. The aim of this article is two-fold: (i) To review the evidence that elevating 5-HTExt beyond the SERT inhibitor effect treat TRD. (ii) To present the hypothesis that adjunct treatment with a slow-release (SR) formulation of the 5-HT precursor 5-hydroxytryptophan (5-HTP; Figure 1) will be a safe and effective way to elevate 5-HTExt beyond the SERT inhibitor effect. Further, we highlight three critical points regarding 5-HTP pharmacology, not clearly recognized or articulated previously: (i) 5-HTP by itself only modestly elevates 5-HTExt, whereas adjunctive 5-HTP strongly and synergistically augments SERT inhibitor-induced 5-HTExt elevation. (ii) Combining 5-HTP with a SERT inhibitor appears quite safe in humans. (iii) Poor pharmacokinetics, i.e. rapid absorption and elimination, prohibit 5-HTP from being a clinically viable drug in its native, immediate release (IR), form. Importantly, convergent data suggest a SR delivery mode will remedy 5-HTP’s pharmacokinetic limitations and produce a drug with general therapeutic potential in TRD.
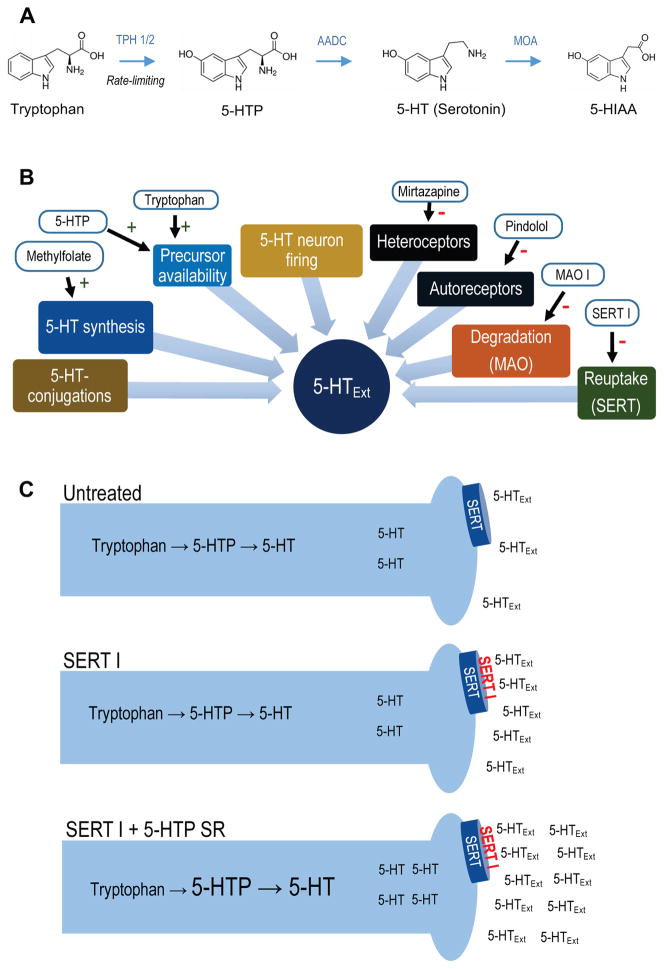
Figure 1.
(A) 5-HT metabolic pathway. Synthesis of 5-HTP from tryptophan via TPH 1 (periphery) or TPH 2 (CNS) is the rate-limiting step in 5-HT synthesis. 5-HTP is rapidly converted to 5-HT by the ubiquitous enzyme amino acid decarboxylase. 5-HT is metabolized to 5-HIAA, 5-HT’s main metabolite, by monoamine oxidase. (B) Simplified schematic of regulatory elements of CNS 5-HTExt. Drugs interacting with each element are indicated. (C) Schematic for adjunct 5-HTP SR mechanism-of-action. Adjunct exogenous 5-HTP increases endogenous 5-HT synthesis, increasing availability of 5-HT for net release by concomitant SERT inhibitor treatment. Abbreviations: 5-HT, 5-hydroxytryptamine (serotonin); 5-HTExt, extracellular 5-HT; 5-HTP, 5-hydroxytryptopan; 5-HIAA, 5-hydroxyindoleacetic acid; AADC, amino acid decarboxylase; MOA, monoamine oxidase; MOA I, monoamine oxidase inhibitor; SERT, serotonin transporter; SERT I, serotonin transporter inhibitor; SR, slow-release; TPH, tryptophan hydroxylase.
The need for sustained 5-HTExt elevation in depression therapy
5-HTExt elevation remains the best validated antidepressant mechanism [1]. Further, the evidence indicates that the 5-HTExt elevation must be sustained to achieve a clinically viable antidepressant effect.
Risk of relapse
A critical observation supporting the necessity of sustained elevation of 5-HTExt is that acutely lowering brain 5-HTExt by eliminating dietary tryptophan, a precursor of 5-HT, precipitates a return of depression symptoms in 50 % of patients otherwise remitted on a SERT inhibitor. The relapse occurs within hours [5–7]. In rat models of tryptophan depletion – where the procedure lowers plasma tryptophan as in humans (by 80 %) - brain 5-HTExt rapidly drops by 50 %, from the initial, SERT inhibitor-elevated level [8]. Thus, it appears an acute 50 % drop in brain 5-HTExt will trigger acute relapse in 50 % of depression patients otherwise treated to remission with a SERT inhibitor.
Risk of discontinuation syndrome
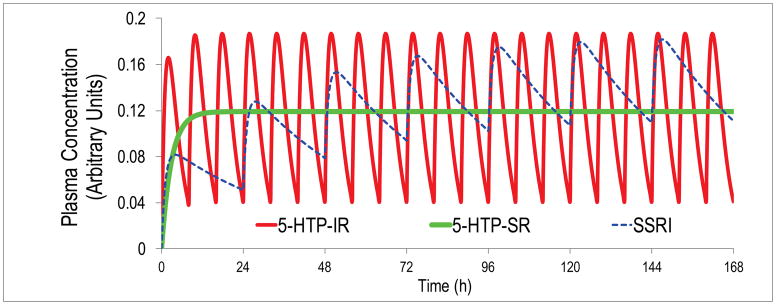
An additional consideration is that lapse of sustained elevation in 5-HTExt can precipitate specific adverse events. Specifically, missing even a single dose of a SERT inhibitor can occasionally precipitate discontinuation syndrome [9], characterized by dizziness, nausea, lethargy and headache. In animals, SSRI-induced 5-HTExt elevation rapidly reverts to baseline upon SSRI-withdrawal [10]. For the short-acting SNRI venlafaxine (T1/2 = 8h [average for parent compound and active metabolite]), the discontinuation syndrome is more frequent, and can occur within hours [11]. Because of the short T1/2, venlafaxine is used predominantly in its SR version. In a head-to-head antidepressant trial, venlafaxine SR was superior to venlafaxine IR [12]. All marketed SSRIs have T1/2 > 20h. This leads to < 0.3 fold steady-state drug level fluctuations, hence minimal fluctuations in SERT occupancy, and hence essentially stable 5-HTExt, so that discontinuation do not occur with once-daily dosing [13] (Figure 2).
Figure 2.
Pharmacokinetics (PK) simulation using one-compartment modeling and published human PK parameters for 5-HTP IR [60] and the canonical SSRI escitalopram [88]. Even at thrice-daily dosing at 8h intervals, an unrealistic level of adherence in outpatients, 5-HTP plasma levels will fluctuate 5-fold between doses. In contrast, during steady-state once-daily dosing of escitalopram, plasma escitalopram levels will fluctuate only about 0.3-fold. Also shown are 5-HTP plasma levels obtained during steady-state dosing with an ideal 5-HTP SR dosage form producing zero-order, constant, 5-HTP delivery.
Thus, for antidepressant therapy, 5-HTExt elevation must be sustained and cannot drop off, lest risk of relapse and discontinuation syndrome.
Elevating 5-HTExt beyond the SERT inhibitor effect has shown promise in treating TRD
Brain 5-HTExt levels are regulated on multiple levels
The SERT is one of several elements controlling 5-HTExt [14]. 5-HT synthesis [15], - degradation [16], -neuronal firing [17], -conjugations [18], and feed-back mechanisms [19] are all important determiners of 5-HTExt levels. For instance, in the rat brain, levels of glucuronide-conjugated 5-HTExt, which does not bind to 5-HT receptors, is twice that of free 5-HTExt [18]. In a naturalistic mouse model of brain 5-HT deficiency - due to a reduction-of-function mutation in tryptophan hydroxylase 2, the rate-limiting enzyme in brain 5-HT synthesis - chronic SSRI treatment only modestly elevated 5-HTExt [15, 20]. This implies that 5-HT reuptake is a less important determiner for 5-HTExt under 5-HT deficiency, a putative risk factor in depression [21]. From 5-HT neurobiology, it appears logical that selective SERT inhibition will not in all patients realize the full antidepressant potential of elevating 5-HTExt. Indeed, substantial preliminary clinical evidence suggests that elevating 5-HTExt beyond the SSRI inhibitor monotherapy effect has efficacy in TRD, or accelerates antidepressant onset [22–37]. Below we review key examples.
Adjunctive drugs elevating 5-HTExt beyond the effect of SERT inhibition augment the antidepressant effect
Adjunctive pindolol elevates 5-HTExt beyond the SSRI effect for a limited period early during SSRI treatment, by preferentially blocking inhibitory 5-HT1A auto-receptors [38, 39]. In double-blind trials, adjunctive pindolol accelerates the antidepressant onset [40, 41]. In contrast, pindolol augmentation has limited efficacy in TRD [24], consistent with that chronic SSRI treatment already inactivates 5-HT1A auto-receptors over time. Further, pindolol is disadvantaged by a short T1/2 of 4h [42] and a narrow therapeutic window [43]. Adjunctive treatment with the monoamine oxidase inhibitor (MAO I) moclobemide elevates 5-HTExt beyond the SSRI effect by inhibiting 5-HT degradation [44]. In open trials, adjunctive moclobemide is reported to treat TRD to SSRIs [26–28]. However, this strategy has the limitation that SSRI + moclobemide co-treatment occasionally triggers serious adverse events [29, 30]. In double-blind trials, adjunctive treatment with the 5-HT precursor tryptophan augments the efficacy of SERT inhibitors [31, 32]. However, this approach has the limitations that just a few percent of tryptophan is metabolized to 5-HT [45], and that tryptophan’s short T1/2 of 3h [46] necessitates frequent dosing. Likewise, as detailed below, adjunctive treatment with the immediate 5-HT precursor 5-HTP is reported to confer efficacy in TRD to SERT inhibitors. Recently, in double-blind trials, adjunctive treatment with methylfolate was reported to be effective in TRD patients who had failed SSRIs [33]. Methylfolate enhances the biosynthesis of tetrahydrobiopterin, a co-factor in 5-HT, DA, and NA synthesis [47]. It should be noted that adjunctive drugs that selectively elevate NAExt or DAExt fail to show efficacy in TRD [48, 49]. Therefore, methylfolate presumably acts by increasing brain 5-HT levels available for release by SSRI treatment, i.e. by elevating 5-HTExt beyond the SSRI effect. However, the extent to which methylfolate increases brain 5-HT levels is unknown. Altogether, clinical evidence, from use of five different adjunctive compounds, converges in suggesting that TRD can be treated by elevating 5-HTExt beyond the levels achieved by a SERT inhibitor.
Indirect pre-clinical evidence
Several presumably non-serotoninergic adjunctive drugs with varying degrees of evidence for efficacy in TRD in humans elevate 5-HTExt to varying degrees beyond the SERT inhibitor effect in rodents. These include adjunctive lithium [50], modafinil [51], and atypical antipsychotics [52, 53]. Thus, it is reasonable to hypothesize that 5-HTExt elevation plays a role in the therapeutic action in TRD of some adjunctive drugs that do not have direct 5-HTExt elevating effects.
Acute adjunctive 5-HTP elevates 5-HTExt beyond the SERT inhibitor effect
In rodents, at moderate parenteral doses (10–40 mg/kg), 5-HTP alone only modestly elevates 5-HTExt. In contrast, adjunctive 5-HTP strongly and synergistically elevates 5-HTExt beyond the SSRI effect [54, 55]. In one acute study in rats, 20 mg/kg 5-HTP or an SSRI elevated 5-HTExt by 100 % and 250 %, respectively, whereas 5-HTP plus the SSRI elevated 5-HTExt by 850 % [55]. The same synergism in rats was observed using an acute rise in plasma corticosteroids as a peripheral biomarker of an acute elevation in brain 5-HTExt [56]. Similarly, in human healthy volunteers, Lowe et al. found that oral 200 mg 5-HTP or an SSRI elevated cortisol by 35 % and 100 %, respectively. However, 5-HTP combined with the SSRI elevated cortisol by 500 % [57]. Notably, acute 5-HTP + SSRI, and to a lesser extent acute SSRI alone, caused rapid onset vomiting and nausea in some subjects [57], indicating that sudden surges in bodily 5-HT levels are not well tolerated. In depression or OCD patients, Meltzer et al. also administered acute oral 200 mg 5-HTP and measured the cortisol rise, either before or during chronic SSRI treatment. The cortisol rise after acute administration of 5-HTP was two-fold larger during SSRI treatment than prior to it [58]. No adverse events were observed in this study, conceivably because chronic SSRI administration adapts the gastrointestinal tract to increased 5-HT stimulation. Likewise, in depression patients Sargent et al. administered acute oral 100 mg 5-HTP and measured the cortisol rise, before and during chronic treatment with an SSRI. The cortisol rise after acute 100 mg 5-HTP was four-fold larger during SSRI treatment than prior it. Again, no adverse events were observed [59].
Combined, the published pre-clinical and clinical data suggest the following: (i) 5-HTP elevates 5-HTExt more potently when adjunct to a SERT inhibitor than when administered by its own. (ii) Adjunctive 5-HTP can elevate 5-HTExt beyond the SERT inhibitor effect safely.
5-HTP has shown promising antidepressant effects, but poor pharmacokinetics limits the therapeutic potential
The pharmacokinetics of 5-HTP
Native 5-HTP IR is a poor serotonergic antidepressant. As discussed above, effective antidepressant therapy requires sustained, minimally fluctuating 5-HTExt elevation [5, 9]. A T1/2 = 2h means that even at thrice-daily dosing 5-HTP plasma levels will fluctuate at least 5-fold at steady-state. This contrasts to the less than 0.3-fold steady-state plasma fluctuations of most SSRIs [13] (Figure 2). Further, 5-HTP’s fast-onset adverse events likely results from the rapid absorption and resultant 5-HT spikes upon administration. Co-administering a peripheral amino acid decarboxylase inhibitor (DCI) with 5-HTP will modestly extend the T1/2, several-fold enhance exposure, and not affect TMax [60]. Including a DCI in a 5-HTP SR drug could be beneficial, but could complicate formulation development, dosing, and safety.
5-HTP as an antidepressant
5-HTP has never been formally developed as a drug and optimized dosage forms and dosing regimens are unavailable. Further, all previous 5-HTP trials were small, including at most a few dozen subjects. In contrast, to ensure reasonable statistical power, a typical antidepressant proof-of-concept Phase II trial includes 50–100 subjects per arm [61]. Most trials used 5-HTP monotherapy; but, as noted above, 5-HTP may be more relevant as an adjunctive, augmentation therapy. For these reasons in aggregate, previous trials may inherently have underestimated the antidepressant potential of 5-HTP. Nevertheless, most 5-HTP antidepressant reports are positive [45]. Scholarly reviews conclude 5-HTP has shown promise as an antidepressant, and that more and better trials are warranted [45, 62]. Consistent with its pharmacology, 5-HTP antidepressant effect appears to be more consistent when adjunctive to another 5-HTExt-elevating antidepressant [45]. In the following we briefly review such adjunctive 5-HTP trials published in English (and see Table 1).
Table 1.
Clinical trials with adjunct 5-HTP immediate release in treatment-resistant depression
| Reference | Design | Arms & total daily dose | Dosing | DCI | Duration | Population | Finding | Safety | Comment |
|---|---|---|---|---|---|---|---|---|---|
| Alino et al 1976 [34] | Double-blind; HAMD | Nialamide (MAO I) 200 mg (N=15) vs. Nialamide + 5-HTP 200 mg (N=15) | BID, at breakfast and lunch | None noted | 15 days | Inpatients | Nialamide + 5-HTP superior (p<0.05) to Nialamide at day 15 | 2 patients in Nialamide + 5-HTP arm reported diarrhea | Doses of both drug titrated up over 5 days. |
| Hiele 1980 [35] | Open-label | Tricyclics (mostly) + 5-HTP (~200 mg/day) (N=99) | TID | Carbidopa, 150 mg/day | Variable | Outpatients; treatment-resistant on average for 18 months | 50% of patients full recovery | Transient hypomania in 1/3; “no significant side-effects” | Patients resistant to multiple drug treatments; dichotomous all-or-none response to adjunct 5-HTP |
| van Praag et al 1982 [36] | Double-blind; HAMD | Placebo vs. clomipramine 225 mg (tricyclic) vs. HTP 200 mg vs. clomipramine + 5-HTP (N=10, all groups) | TID | Carbidopa, 150 mg/day, 5-HTP groups only | 21 days | Inpatients | Both clomipramine and 5-HTP superior to placebo; clomipramine + 5-HTP superior to 3 other groups | Nausea | 5-HTP doses titrated up |
| Nardini et al 1983 [37] | Double-blind; HAMD | Clomipramine 50 mg (tricyclic) (N=13) vs. clomipramine + 5-HTP (300 mg/day) (N=13) | ? | None noted | 28 days | Inpatients | Clomipramine + 5-HTP superior (p<0.05) to Clomipramine at day 28 | Few adverse effects | No details on dosing regimen |
In a double-blind trial in depressed inpatients, Alino et al. [34] found that nialamide (MAO inhibitor) + 5-HTP (200 mg/day) was superior to nialamide alone. The worst reported adverse events were diarrhea. In an open-label case-series of 99 chronic TRD patients, most already on SERT inhibitor therapy, van Hiele [35] found that 5-HTP (average dose 200 mg/day) + DCI treatment induced a “remarkable recovery” in ~50 % of patients. Antidepressant responses tended to be all-or-none. Few adverse events were reported, mostly nausea. Hypomania occurred in 15 patients, which reversed upon lowering the 5-HTP dose. In a four-arm double-blind placebo-controlled trial in depressed inpatients, van Praag et al. [36] compared placebo with clomipramine (a SERT inhibitor), 5-HTP (200 mg/day) + DCI, and clomipramine + 5-HTP + DCI. Clomipramine + 5-HTP + DCI was superior to all other arms. Nausea was the most common adverse events. In a double-blind trial in depressed inpatients, Nardini et al. [37] found that clomipramine + 5-HTP (300 mg/day) was superior to clomipramine alone. Adverse events were reported to be few.
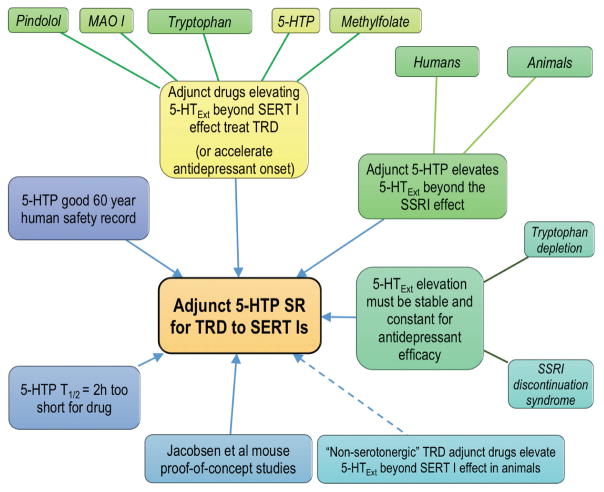
These pilot data on efficacy and safety of adjunct 5-HTP in TRD are encouraging. Similar pilot trials provided initial evidence of antidepressant efficacy of tricyclic antidepressants and ketamine [63, 64]. The data add to the larger rationale supporting adjunctive 5-HTP SR as a novel therapy for patients who respond inadequately to SERT inhibitors (Figure 3).
Figure 3.
Schematic summarizing the multi-pronged clinical and pre-clinical data converging on adjunct 5-HTP SR as a new therapy for treatment-resistant depression (TRD). SERT I, serotonin transporter inhibitor.
5-HTP has a good oral human safety record
Experience from >100 published clinical trials and widespread nutraceutical use suggests that oral 5-HTP - in high milligram to low gram doses, alone or as an adjunct to other serotonergic drugs, with or without a DCI - has a low propensity to cause severe adverse events in humans [45, 65, 66].
5-HTP has not been reported to cause serotonin syndrome in humans
Serotonin syndrome is a toxic state caused by excessive 5-HTExt. Severe serotonin syndrome is rare, and almost exclusively caused by SERT inhibitor + MAO inhibitor co-treatment [67]. 5-HTP has never been associated with serotonin syndrome in humans. In published reports, > 250 humans have been dosed with 5-HTP + a SERT inhibitor, with no serious adverse events [35–37, 57–59, 68–70]. A MAO inhibitor blocks the metabolic flow through the 5-HT pathway, at the point of degradation, which might lead to extreme build-up of 5-HTExt. In contrast, 5-HTP increases 5-HT synthesis and the dynamic flow through the 5-HT pathway, which might not easily lead to 5-HTExt build-up.
In rodents, high parenteral acute bolus doses of 5-HTP, e.g. 100 mg/kg, in combination with an SSRI can cause transient serotonin syndrome [71]. However, this is an artefact of preclinical pharmacology methodology, i.e. extreme doses and non-oral routes of administration. Similar high parenteral doses of fluoxetine, methylphenidate, and caffeine often kill rodents [72], whereas in humans these compounds are extremely safe, in their appropriate oral doses and dosage forms.
Common 5-HTP adverse events are gastrointestinal
In humans, acute and long term treatment with 5-HTP, even at high doses, has minimal effects on cardiovascular, hepatic, renal, hematological, or urinalysis parameters (reviewed in [65, 73]). Similar, in rats, a 1-year toxicology study found no effects of oral high-dose 5-HTP (875 mg/kg/day, via the drinking water) on cardiovascular, hepatic, renal, hematological, body weight gain, organ histology, and organ weight parameters [74]. In humans, common adverse events seen with oral 5-HTP are mild to moderate, and gastrointestinal, e.g. nausea or stomach cramps, and less frequently diarrhea and vomiting (reviewed in [73]). Occasional adverse events include hypomania, headaches, lightheadedness, and palpitations. Often onset is rapid, which is likely due to rapid conversion of 5-HTP to 5-HT upon dosing with standard 5-HTP IR [35, 45, 57]. Interestingly, two studies report that using enteric coated 5-HTP capsules, which delays 5-HTP delivery until the intestine, substantially reduces gastrointestinal adverse events [35, 36]. This suggests a direct irritating effect of 5-HTP on the stomach. Most studies do not specify if they administered 5-HTP with enteric coating. On the other hand, vomiting and nausea could be centrally, rather than peripherally, mediated. Evidence for this is that upon acute bolus 5-HTP administration, DCI co-treatment (which reduces peripheral and increases central 5-HTP conversion to 5-HT) can induce nausea and vomiting at 5-HTP doses (100–200 mg) otherwise devoid of adverse events [60, 75, 76]. Further, acute co-treatment of 5-HTP IR 200 mg + SSRI causes vomiting and nausea [57], but acute 5-HTP IR 200 mg causes no adverse events when added after 4 weeks prior SSRI treatment [58]. In any case, 5-HTP gastrointestinal adverse events lessen or disappear over time [73], as occurs with SSRIs [77]. Overall, the evidence suggests that gastrointestinal adverse events after adjunctive 5-HTP can be greatly reduced if (i) the 5-HTP Cmax in plasma is minimized, (ii) appropriate 5-HTP formulations are used, and (iii) SSRI treatment has lasted several weeks prior to the start of 5-HTP dosing.
Slow-release delivery will transform the therapeutic potential of 5-HTP
A SR formulation delivers an active pharmaceutical ingredient (API) over many hours, thereby delaying the time (TMax) to peak plasma levels (CMax) and increasing the T1/2. An SR formulation is particularly beneficial when (i) T1/2 is very short, (ii) sustained API exposure is required, (iii) adverse events are linked to early high peak API levels, and (iv) less frequent dosing than necessary with the IR formulation is required. All points apply to 5-HTP. As parallels, some drugs with fast pharmacokinetics similar to 5-HTP’s are only safe and effective in their SR versions [78, 79]. The PK of 5-HTP IR - rapid absorption, short duration of action – is the opposite of that required by a serotonergic antidepressant, where constant and minimally fluctuating drug exposure is mandated [5–7, 9]. Unless taken very frequently, 5-HTP in its native IR form will not produce sustained exposure [60]. At the same time, adherence to more than once or twice daily dosing is unrealistic for antidepressants, which are taken for months or years, and mostly by outpatients [80–82]. A SR formulation would produce lower 5-HTP plasma Cmax, provide the necessary sustained 5-HTP exposure, allow for higher doses, and bring the dosing frequency to requisite once or twice daily. Thus, in theory a SR formulation would uniquely improve the therapeutic potential of 5-HTP as an antidepressant in an everyday clinical setting (Figure 4).
Figure 4.
Theoretical and experiential superiority of 5-HTP SR vs. 5-HTP IR.
In mouse models, 5-HTP SR transforms the therapeutic potential of 5-HTP
Directed by the clinical data reviewed above we carried out an adjunctive 5-HTP SR proof-of-concept study in mice [83]. We modeled 5-HTP SR using subcutaneous minipumps, which produces constant (zero-order) SR delivery. Adjunctive 5-HTP SR augmented the 5-HTExt-elevation induced by chronic SSRI, by 100 % in wildtype mice and by 800 % in mice with naturalistic 5-HT deficiency [15, 20], respectively. We observed no adverse events. Had minipump capacity not limited the 5-HTP SR dose to 100 mg/kg/day, even stronger 5-HTExt-augmentation could have been achieved. As expected, 5-HTP SR alone had only modest effects on 5-HTExt. When modeling adjunctive 5-HTP IR by administering 2 × 50 mg/kg (AM and PM) daily subcutaneous bolus 5-HTP injections, we observed large, transient spikes in 5-HTExt, accompanied by marked gastrointestinal adverse events and mild seizures. Low-dose adjunct 5-HTP IR, 2 × 3.125 mg/kg barely augmenting 5-HTExt, but still caused adverse events - even while the peak 5-HTP plasma levels were lower than the stable 5-HTP plasma levels resulting from 5-HTP SR 100 mg/kg/day. Recently we found that oral adjunct 5-HTP SR ~1000 mg/kg/day enhances brain 5-HT and plasma 5-HTP levels, and by extension 5-HTExt, several-fold stronger than 5-HTP SR via minipumps, and, again, with no adverse events (Jacobsen et al, unpublished). Thus, in our model systems, as compared to 5-HTP IR, 5-HTP SR potently and safely elevates 5-HTExt beyond the SSRI effect, and allows for higher safe 5-HTP exposure. While the contrast may be less stark in the clinic, our mouse data provide proof-of-principle of the therapeutic superiority of 5-HTP SR compared to 5-HTP IR.
Concluding Remarks
Antidepressant drug discovery is hampered by the poor predictability of animal “antidepressant-like” behavioral models [84]. Optimally, the rationale for a novel antidepressant should rest on strong clinical, as well as pre-clinical, data. The adjunctive 5-HTP SR therapeutic concept for TRD is founded in 5-HT biology, 5-HTP clinical and pre-clinical pharmacology, pharmacokinetics, and promising clinical pilot trials in TRD with 5-HTP and four other serotonergic adjuncts. In addition, we have shown in mice that chronic adjunctive 5-HTP SR safety and robustly elevates 5-HTExt beyond what is achieved by an SSRI, i.e. an antidepressant augmentation-like effect. As 5-HTP pharmacology appears similar between rodents and humans, we expect our mouse data will translate to humans. Based on previous clinical data, we project the therapeutic dose of adjunct 5-HTP SR will be 500–2000 mg per day [45]. Given the pharmacokinetics, physiochemical properties, and projected dose, realizing a 5-HTP SR formulation drug will be technologically feasible [85]. Many important drugs are specialized formulations of naturally occurring APIs [86, 87]. The ideal 5-HTP SR drug would produce essentially stable 5-HTP plasma levels at once or twice daily dosing. Defining the pharmacology of 5-HTP SR in clinical and preclinical paradigms opens a new line of inquiry (see Outstanding Questions Box). Indices of 5-HT deficiency segregate with suicidality, severe depression, and co-morbid borderline personality disorder, factors that predict poor SSRI treatment response (reviewed in [21]). Conceivably, adjunct 5-HTP SR will be particularly relevant for such patient populations. SSRIs are also approved for, but only partially effective in, OCD, PTSD, social anxiety, panic disorder, and generalized anxiety. Adjunctive 5-HTP SR could be therapeutically relevant also for these large indications. Further, a 5-HTP SR drug might also be effective as monotherapy, as an alternative to existing antidepressants.
In closing, strong data support that a high-performing adjunctive 5-HTP SR drug will be safe and effective in patients with depression, and potentially with other CNS indications, who fail to achieve adequate benefit from SERT inhibitor monotherapy.
Supplementary Material
Box 1. 5-HTP facts.
Stereochemistry: The natural occurring stereoisomer of 5-HTP is L-5-HTP.
Source: Exogenous 5-HTP is usually extracted from the African shrub Griffonia Simplicifolia.
Regulatory status: In the USA 5-HTP is regulated as a food supplement. There is no FDA-approved drug on the market containing 5-HTP as an active pharmaceutical ingredient. To our knowledge, no dosage form of 5-HTP has ever been formally developed as a drug and approved by a regulatory body for the treatment of a disease.
5-HTP absorption, distribution, metabolism, and excretion: No other metabolic fate for 5-HTP than decarboxylation to 5-HT is known. In humans, upon oral administration, 5-HTP is rapidly absorbed from the upper intestine, with a TMax of 1.5h [60]. Elimination is equally rapid, with a T1/2 of 2h [60, 89]. The human bioavailability of 5-HTP, when given alone, has not been determined. Whether 5-HTP is passively or actively absorbed is also not known, although, based on rat studies [90], it seems likely that luminal amino acid transporters, also involved in L-DOPA absorption, play a role. In contrast to 5-HT, which does not, 5-HTP crosses the blood-brain barrier, as assessed using radiolabeled 5-HTP tracers [91]. After oral administration of 5-HTP, e.g. of 300 mg/day [92], enough 5-HTP enters the brain to enhance 5-HT synthesis, as assessed by an increase in cerebrospinal fluid levels of 5-HIAA, the major 5-HT metabolite. When 5-HTP is co-administered with a peripheral amino acid decarboxylase inhibitor (DCI) - which reduces peripheral, but not brain, metabolism of 5-HTP - 5-HTP exposure is increased 5 to 15-fold, the T1/2 doubled to 4h, and bioavailability is 70% [93]. A few studies have examined the disposition of 5-HTP in animals. In rats, Shindo et al reported that 5-HTP is completely absorbed from the jejunum lumen via an active mechanism. 5-HTP transport into the brain also involves active transport. 5-HTP metabolism in the intestine and liver is 3 and 7 times slower, respectively, as compared to L-DOPA [90]. Such slower metabolism could account for the observed longer human T1/2 of 5-HTP (2h) vs. L-DOPA (1h) [94]. In mice, in a study discussed in the main text, we determined the T1/2 to be about 12 min [83], 10 times faster than in humans, in accordance with the typical mouse:human metabolism and dose-extrapolation scaling factor of about 10 [95].
Acknowledgments
This work was supported in part by grants from the National Institutes of Health MH79201 and MH60451 (MGC). Support from the Lennon Family Foundation to MGC for the initial part of this work is also greatly appreciated. JPRJ is the grateful recipient of an individual grant from The Lundbeck Foundation of Denmark. Professor Fan Yan’s assistance with producing the pharmacokinetic simulation (Figure 2) is greatly appreciated.
Glossary
MANDATORY ELEMENTS
- 5-hydroxytryptamine (5-HT, aka serotonin)
Signaling molecule in CNS and periphery
- Extracellular 5-HT (5-HTExt)
The “active” 5-HT pool signaling via 5-HT receptors
- 5-hydroxytryptophan immediate-release (5-HTP IR)
Standard, native 5-HTP
- 5-hydroxytryptophan slow-release (5-HTP SR)
Concept wherein 5-HTP is delivered as SR. In rodents 5-HTP SR can be modeled using minipumps or dietary administration
- Active pharmaceutical ingredient (API)
The compound in a dosage form/drug exerting the pharmacological action
- Adverse event
Undesirable experience associated with use of a medical product
- Blood-brain barrier (BBB)
Selective permeability barrier separating CNS extracellular fluid from the blood
- Cerebrospinal fluid (CSF)
Brain and spine extracellular fluid
- CMax
The peak concentration of the API following administration
- Depression
Mental disorder characterized by persistent feelings of sadness and loss of interest, together with additional symptoms, such as guilt, loss of energy, or suicidal ideation
- Discontinuation syndrome
Can occur when 5-HTExt-elevating antidepressants are stopped abruptly. Core symptoms include dizziness, nausea, lethargy, and headache
- Exposure
The API (concentration X time) area-under-the-curve after administration
- Hamilton depression (HAMD) scale
A multi-dimensional tool to create an aggregate score of depression severity
- Immediate-release
The dosage form delivers the entire API dose instantly
- Peripheral amino acid decarboxylase inhibitor (DCI)
Penetrates the BBB minimally, and therefore inhibits conversion of 5-HTP to 5-HT only peripherally. DCI co-administration increases 5-HTP brain exposure 5- to 15-fold and doubles the T1/2
- Pharmacokinetics
The study of how the body disposes of an API
- Pharmacodynamics
The study of what the API does to the body
- Selective serotonin-reuptake inhibitors (SSRIs)
Class of drugs that at therapeutic levels selectively inhibits the SERT (see below)
- Serotonin syndrome
Toxic syndrome caused by excessive 5-HTExt and characterized by neuromuscular excitation (e.g. clonus), autonomic excitation (e.g. hyperthermia), and altered mental state (e.g. agitation)
- Serotonin transporter (SERT)
Transports back into the neuron cytosol 5-HT released via vesicles to the extracellular space
- SERT inhibitor
Drug that inhibits the SERT. Includes the SSRIs, dual serotonin-noradrenaline reuptake inhibitors (SNRIs), and certain tricyclic antidepressants, e.g. clomipramine
- Slow-release (SR) formulation
The dosage form delivers the API dose gradually. The result is reduced CMax, delayed TMax, and increased T1/2. For APIs with T1/2 < 12h, a SR formulation often increase overall clinical effectiveness, by decreasing CMax-related onset adverse events, producing a sustained pharmacodynamics effect, and decreasing dosing frequency
- T1/2
The terminal elimination half-life of an API, measured after absorption is complete
- TMax
The time to CMax after dosing
- Treatment-resistant depression
Typically defined as failure to achieve remission with two or more adequate courses of antidepressants
- Tryptophan depletion
Acute administration of a tryptophan-devoid amino acid drink competes out uptake of tryptophan via brain amino-acid transporters. The result is an acute drop in brain 5-HT synthesis and in 5-HTExt (when elevated due to SERT inhibition)
Footnotes
Conflicts of Interest
JPRJ and MGC are inventors on US patents pertaining to the adjunct 5-HTP SR method-of-treatment, and hold stock in Evecxia Inc., a company founded to develop a 5-HTP SR drug. ADK and RRK serve on the Evecxia Scientific Advisory Board.
References
- 1.Blier P, El Mansari M. Serotonin and beyond: therapeutics for major depression. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120536. doi: 10.1098/rstb.2012.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trivedi MH, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American journal of psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Nemeroff CB. Prevalence and management of treatment-resistant depression. The Journal of clinical psychiatry. 2007;68(Suppl 8):17–25. [PubMed] [Google Scholar]
- 4.Thase ME. Using adjunctive treatments when first-line antidepressants fail. J Clin Psychiatry. 2012;73:e01. doi: 10.4088/JCP.10126tx4c. [DOI] [PubMed] [Google Scholar]
- 5.Delgado PL, et al. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47:411–418. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- 6.Delgado PL. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(Suppl 4):22–26. [PubMed] [Google Scholar]
- 7.Moreno FA, et al. Tryptophan depletion and risk of depression relapse: a prospective study of tryptophan depletion as a potential predictor of depressive episodes. Biological psychiatry. 2000;48:327–329. doi: 10.1016/s0006-3223(00)00893-3. [DOI] [PubMed] [Google Scholar]
- 8.Bel N, Artigas F. Reduction of serotonergic function in rat brain by tryptophan depletion: effects in control and fluvoxamine-treated rats. Journal of neurochemistry. 1996;67:669–676. doi: 10.1046/j.1471-4159.1996.67020669.x. [DOI] [PubMed] [Google Scholar]
- 9.Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24:183–197. doi: 10.2165/00002018-200124030-00003. [DOI] [PubMed] [Google Scholar]
- 10.Ceglia I, et al. Effects of chronic treatment with escitalopram or citalopram on extracellular 5-HT in the prefrontal cortex of rats: role of 5-HT1A receptors. British journal of pharmacology. 2004;142:469–478. doi: 10.1038/sj.bjp.0705800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dallal A, Chouinard G. Withdrawal and rebound symptoms associated with abrupt discontinuation of venlafaxine. J Clin Psychopharmacol. 1998;18:343–344. doi: 10.1097/00004714-199808000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham LA. Once-daily venlafaxine extended release (XR) and venlafaxine immediate release (IR) in outpatients with major depression. Venlafaxine XR 208 Study Group. Annals of clinical psychiatry: official journal of the American Academy of Clinical Psychiatrists. 1997;9:157–164. doi: 10.1023/a:1026277907818. [DOI] [PubMed] [Google Scholar]
- 13.van Harten J. Clinical pharmacokinetics of selective serotonin reuptake inhibitors. Clin Pharmacokinet. 1993;24:203–220. doi: 10.2165/00003088-199324030-00003. [DOI] [PubMed] [Google Scholar]
- 14.Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nature reviews. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen JP, et al. Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice. Molecular psychiatry. 2012;17:694–704. doi: 10.1038/mp.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evrard A, et al. Altered regulation of the 5-HT system in the brain of MAO-A knock-out mice. Eur J Neurosci. 2002;15:841–851. doi: 10.1046/j.1460-9568.2002.01917.x. [DOI] [PubMed] [Google Scholar]
- 17.Gartside SE, et al. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. British journal of pharmacology. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uutela P, et al. Analysis of intact glucuronides and sulfates of serotonin, dopamine, and their phase I metabolites in rat brain microdialysates by liquid chromatography-tandem mass spectrometry. Analytical chemistry. 2009;81:8417–8425. doi: 10.1021/ac901320z. [DOI] [PubMed] [Google Scholar]
- 19.Sharp T, et al. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Beaulieu JM, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobsen JP, et al. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. 2012;367:2444–2459. doi: 10.1098/rstb.2012.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Artigas F, et al. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends in neurosciences. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- 23.Whale R, et al. Pindolol augmentation of serotonin reuptake inhibitors for the treatment of depressive disorder: a systematic review. Journal of psychopharmacology. 2010;24:513–520. doi: 10.1177/0269881108097714. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin GM, et al. Presynaptic serotonin receptor-mediated response in mice attenuated by antidepressant drugs and electroconvulsive shock. Nature. 1985;317:531–533. doi: 10.1038/317531a0. [DOI] [PubMed] [Google Scholar]
- 25.Sargent P, et al. Effect of paroxetine and nefazodone on 5-HT1A receptor sensitivity. Psychopharmacology. 1997;132:296–302. doi: 10.1007/s002130050348. [DOI] [PubMed] [Google Scholar]
- 26.Hawley CJ, et al. Safety and tolerability of combined treatment with moclobemide and SSRIs: a systematic study of 50 patients. International clinical psychopharmacology. 1996;11:187–191. doi: 10.1097/00004850-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Ebert D, et al. Combined SSRI-RIMA treatment in refractory depression. Safety data and efficacy. Psychopharmacology. 1995;119:342–344. doi: 10.1007/BF02246301. [DOI] [PubMed] [Google Scholar]
- 28.Joffe RT, Bakish D. Combined SSRI-moclobemide treatment of psychiatric illness. J Clin Psychiatry. 1994;55:24–25. [PubMed] [Google Scholar]
- 29.FitzSimmons CR, Metha S. Serotonin syndrome caused by overdose with paroxetine and moclobemide. J Accid Emerg Med. 1999;16:293–295. doi: 10.1136/emj.16.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillman K. Moclobemide and the risk of serotonin toxicity (or serotonin syndrome) CNS drug reviews. 2004;10:83–85. doi: 10.1111/j.1527-3458.2004.tb00005.x. author reply 86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walinder J, et al. Potentiation of the antidepressant action of clomipramine by tryptophan. Arch Gen Psychiatry. 1976;33:1384–1389. doi: 10.1001/archpsyc.1976.01770110112012. [DOI] [PubMed] [Google Scholar]
- 32.Thomson J, et al. The treatment of depression in general practice: a comparison of L-tryptophan, amitriptyline, and a combination of L-tryptophan and amitriptyline with placebo. Psychological medicine. 1982;12:741–751. doi: 10.1017/s0033291700049047. [DOI] [PubMed] [Google Scholar]
- 33.Papakostas GI, et al. l-Methylfolate as Adjunctive Therapy for SSRI-Resistant Major Depression: Results of Two Randomized, Double-Blind, Parallel-Sequential Trials. The American journal of psychiatry. 2012;169:1267–1274. doi: 10.1176/appi.ajp.2012.11071114. [DOI] [PubMed] [Google Scholar]
- 34.Alino JJ, et al. 5-Hydroxytryptophan (5-HTP) and a MAOI (nialamide) in the treatment of depressions. A double-blind controlled study. Int Pharmacopsychiatry. 1976;11:8–15. doi: 10.1159/000468207. [DOI] [PubMed] [Google Scholar]
- 35.van Hiele LJ. l-5-Hydroxytryptophan in depression: the first substitution therapy in psychiatry? The treatment of 99 out-patients with ‘therapy-resistant’ depressions. Neuropsychobiology. 1980;6:230–240. doi: 10.1159/000117757. [DOI] [PubMed] [Google Scholar]
- 36.van Praag HM. Serotonin precursors in the treatment of depression. Advances in biochemical psychopharmacology. 1982;34:259–286. [PubMed] [Google Scholar]
- 37.Nardini M, et al. Treatment of depression with L-5-hydroxytryptophan combined with chlorimipramine, a double-blind study. Int J Clin Pharmacol Res. 1983;3:239–250. [PubMed] [Google Scholar]
- 38.Gobert A, Millan MJ. Modulation of dialysate levels of dopamine, noradrenaline, and serotonin (5-HT) in the frontal cortex of freely-moving rats by (−)-pindolol alone and in association with 5-HT reuptake inhibitors: comparative roles of beta-adrenergic, 5-HT1A, and 5-HT1B receptors. Neuropsychopharmacology. 1999;21:268–284. doi: 10.1016/S0893-133X(99)00035-4. [DOI] [PubMed] [Google Scholar]
- 39.Romero L, et al. Effect of pindolol on the function of pre- and postsynaptic 5-HT1A receptors: in vivo microdialysis and electrophysiological studies in the rat brain. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 1996;15:349–360. doi: 10.1016/0893-133X(95)00240-E. [DOI] [PubMed] [Google Scholar]
- 40.Bordet R, et al. Effect of pindolol on onset of action of paroxetine in the treatment of major depression: intermediate analysis of a double-blind, placebo-controlled trial. Reseau de Recherche et d’Experimentation Psychopharmacologique. Am J Psychiatry. 1998;155:1346–1351. doi: 10.1176/ajp.155.10.1346. [DOI] [PubMed] [Google Scholar]
- 41.Zanardi R, et al. Faster onset of action of fluvoxamine in combination with pindolol in the treatment of delusional depression: a controlled study. J Clin Psychopharmacol. 1998;18:441–446. doi: 10.1097/00004714-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Borchard U. Pharmacokinetics of beta-adrenoceptor blocking agents: clinical significance of hepatic and/or renal clearance. Clinical physiology and biochemistry. 1990;8(Suppl 2):28–34. [PubMed] [Google Scholar]
- 43.Rabiner EA, et al. beta-blocker binding to human 5-HT(1A) receptors in vivo and in vitro: implications for antidepressant therapy. Neuropsychopharmacology. 2000;23:285–293. doi: 10.1016/S0893-133X(00)00109-3. [DOI] [PubMed] [Google Scholar]
- 44.Bonnet U. Moclobemide: evolution, pharmacodynamic, and pharmacokinetic properties. CNS drug reviews. 2002;8:283–308. doi: 10.1111/j.1527-3458.2002.tb00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner EH, et al. Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther. 2006;109:325–338. doi: 10.1016/j.pharmthera.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Green AR, et al. Metabolism of an oral tryptophan load. I: Effects of dose and pretreatment with tryptophan. British journal of clinical pharmacology. 1980;10:603–610. doi: 10.1111/j.1365-2125.1980.tb00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69:1352–1353. doi: 10.4088/jcp.v69n0901. [DOI] [PubMed] [Google Scholar]
- 48.Patkar AA, et al. A randomized, double-blind, placebo-controlled trial of augmentation with an extended release formulation of methylphenidate in outpatients with treatment-resistant depression. Journal of clinical psychopharmacology. 2006;26:653–656. doi: 10.1097/01.jcp.0000246212.03530.fd. [DOI] [PubMed] [Google Scholar]
- 49.Oakes TM, et al. Edivoxetine compared to placebo as adjunctive therapy to selective serotonin reuptake inhibitors in the prevention of symptom re-emergence in major depressive disorder. Curr Med Res Opin. 2015:1–11. doi: 10.1185/03007995.2015.1037732. [DOI] [PubMed] [Google Scholar]
- 50.Wegener G, et al. Increased extracellular serotonin level in rat hippocampus induced by chronic citalopram is augmented by subchronic lithium: neurochemical and behavioural studies in the rat. Psychopharmacology. 2003;166:188–194. doi: 10.1007/s00213-002-1341-6. [DOI] [PubMed] [Google Scholar]
- 51.Ferraro L, et al. Modafinil enhances the increase of extracellular serotonin levels induced by the antidepressant drugs fluoxetine and imipramine: a dual probe microdialysis study in awake rat. Synapse. 2005;55:230–241. doi: 10.1002/syn.20111. [DOI] [PubMed] [Google Scholar]
- 52.Bjorkholm C, et al. Adjunctive treatment with asenapine augments the escitalopram-induced effects on monoaminergic outflow and glutamatergic neurotransmission in the medial prefrontal cortex of the rat. Int J Neuropsychopharmacol. 2015:18. doi: 10.1093/ijnp/pyu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chernoloz O, et al. Electrophysiological studies in the rat brain on the basis for aripiprazole augmentation of antidepressants in major depressive disorder. Psychopharmacology. 2009;206:335–344. doi: 10.1007/s00213-009-1611-7. [DOI] [PubMed] [Google Scholar]
- 54.Jacobsen JP, et al. Insensitivity of NMRI mice to selective serotonin reuptake inhibitors in the tail suspension test can be reversed by co-treatment with 5-hydroxytryptophan. Psychopharmacology. 2008;199:137–150. doi: 10.1007/s00213-008-1142-7. [DOI] [PubMed] [Google Scholar]
- 55.Perry KW, Fuller RW. Extracellular 5-hydroxytryptamine concentration in rat hypothalamus after administration of fluoxetine plus L-5-hydroxytryptophan. J Pharm Pharmacol. 1993;45:759–761. doi: 10.1111/j.2042-7158.1993.tb07105.x. [DOI] [PubMed] [Google Scholar]
- 56.Fuller RW, et al. Potentiation of the L-5-hydroxytryptophan-induced elevation of plasma corticosterone levels in rats by a specific inhibitor of serotonin uptake. Res Commun Chem Pathol Pharmacol. 1975;10:193–196. [PubMed] [Google Scholar]
- 57.Lowe SL, et al. L-5-Hydroxytryptophan augments the neuroendocrine response to a SSRI. Psychoneuroendocrinology. 2006;31:473–484. doi: 10.1016/j.psyneuen.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Meltzer H, et al. Fluoxetine, but not tricyclic antidepressants, potentiates the 5-hydroxytryptophan-mediated increase in plasma cortisol and prolactin secretion in subjects with major depression or with obsessive compulsive disorder. Neuropsychopharmacology. 1997;17:1–11. doi: 10.1016/S0893-133X(96)00280-1. [DOI] [PubMed] [Google Scholar]
- 59.Sargent PA, et al. Brain 5-HT neurotransmission during paroxetine treatment. The British journal of psychiatry: the journal of mental science. 1998;172:49–52. doi: 10.1192/bjp.172.1.49. [DOI] [PubMed] [Google Scholar]
- 60.Gijsman HJ, et al. Placebo-controlled comparison of three dose-regimens of 5-hydroxytryptophan challenge test in healthy volunteers. J Clin Psychopharmacol. 2002;22:183–189. doi: 10.1097/00004714-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 61.Post A, et al. A Selective Nociceptin Receptor Antagonist to Treat Depression: Evidence from Preclinical and Clinical Studies. Neuropsychopharmacology. 2016;41:1803–1812. doi: 10.1038/npp.2015.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iovieno N, et al. Second-tier natural antidepressants: review and critique. Journal of affective disorders. 2011;130:343–357. doi: 10.1016/j.jad.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 63.Lopez-Munoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15:1563–1586. doi: 10.2174/138161209788168001. [DOI] [PubMed] [Google Scholar]
- 64.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biological psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 65.Das YT, et al. Safety of 5-hydroxy-L-tryptophan. Toxicol Lett. 2004;150:111–122. doi: 10.1016/j.toxlet.2003.12.070. [DOI] [PubMed] [Google Scholar]
- 66.NIH TOXNET 5-HTP. 2007 http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+4295.
- 67.Buckley NA, et al. Serotonin syndrome. British Medical Journal. 2014;348:g1626. doi: 10.1136/bmj.g1626. [DOI] [PubMed] [Google Scholar]
- 68.Sargent PA, et al. Clomipramine enhances the cortisol response to 5-HTP: implications for the therapeutic role of 5-HT2 receptors. Psychopharmacology (Berl) 1998;140:120–122. doi: 10.1007/s002130050747. [DOI] [PubMed] [Google Scholar]
- 69.Zmilacher K, et al. L-5-hydroxytryptophan alone and in combination with a peripheral decarboxylase inhibitor in the treatment of depression. Neuropsychobiology. 1988;20:28–35. doi: 10.1159/000118469. [DOI] [PubMed] [Google Scholar]
- 70.van Praag HM, et al. 5-hydroxytryptophan in combination with clomipramine in “therapy-resistant” depressions. Psychopharmacologia. 1974;38:267–269. doi: 10.1007/BF00421379. [DOI] [PubMed] [Google Scholar]
- 71.Haberzettl R, et al. The murine serotonin syndrome - evaluation of responses to 5-HT-enhancing drugs in NMRI mice. Behav Brain Res. 2015;277:204–210. doi: 10.1016/j.bbr.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 72.Register of Toxic Effects of Chemical Substances - Center For Disease Control And Prevention.
- 73.Byerley WF, et al. 5-Hydroxytryptophan: a review of its antidepressant efficacy and adverse effects. J Clin Psychopharmacol. 1987;7:127–137. [PubMed] [Google Scholar]
- 74.Preuss HG, et al. Does 5-hydroxytryptophan cause acute and chronic toxic perturbations in rats? Toxicol Mech Methods. 2006;16:281–286. doi: 10.1080/15376520500195616. [DOI] [PubMed] [Google Scholar]
- 75.Smarius LJ, et al. Pharmacology of rising oral doses of 5-hydroxytryptophan with carbidopa. J Psychopharmacol. 2008;22:426–433. doi: 10.1177/0269881107082025. [DOI] [PubMed] [Google Scholar]
- 76.Schruers K, et al. L-5-hydroxytryptophan induced increase in salivary cortisol in panic disorder patients and healthy volunteers. Psychopharmacology (Berl) 2002;161:365–369. doi: 10.1007/s00213-002-1072-8. [DOI] [PubMed] [Google Scholar]
- 77.Peretti S, et al. Safety and tolerability considerations: tricyclic antidepressants vs. selective serotonin reuptake inhibitors. Acta psychiatrica Scandinavica Supplementum. 2000;403:17–25. doi: 10.1111/j.1600-0447.2000.tb10944.x. [DOI] [PubMed] [Google Scholar]
- 78.Croom KF, Wellington K. Modified-release nifedipine: a review of the use of modified-release formulations in the treatment of hypertension and angina pectoris. Drugs. 2006;66:497–528. doi: 10.2165/00003495-200666040-00007. [DOI] [PubMed] [Google Scholar]
- 79.Brooks BR, et al. Treatment of pseudobulbar affect in ALS with dextromethorphan/quinidine: a randomized trial. Neurology. 2004;63:1364–1370. doi: 10.1212/01.wnl.0000142042.50528.2f. [DOI] [PubMed] [Google Scholar]
- 80.Granger AL, et al. An assessment of patient preference and adherence to treatment with Wellbutrin SR: a web-based survey. J Affect Disord. 2006;90:217–221. doi: 10.1016/j.jad.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 81.Claxton AJ, et al. A systematic review of the associations between dose regimens and medication compliance. Clinical therapeutics. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 82.Llor C, et al. The higher the number of daily doses of antibiotic treatment in lower respiratory tract infection the worse the compliance. The Journal of antimicrobial chemotherapy. 2009;63:396–399. doi: 10.1093/jac/dkn472. [DOI] [PubMed] [Google Scholar]
- 83.Jacobsen JP, et al. SSRI Augmentation by 5-Hydroxytryptophan Slow Release: Mouse Pharmacodynamic Proof of Concept. Neuropsychopharmacology. 2016;41:2324–2334. doi: 10.1038/npp.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Insel TR, et al. Innovative solutions to novel drug development in mental health. Neuroscience and biobehavioral reviews. 2013 doi: 10.1016/j.neubiorev.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thombre AG. Assessment of the feasibility of oral controlled release in an exploratory development setting. Drug Discov Today. 2005;10:1159–1166. doi: 10.1016/S1359-6446(05)03551-8. [DOI] [PubMed] [Google Scholar]
- 86.McCormack PL, Keating GM. Prolonged-release nicotinic acid: a review of its use in the treatment of dyslipidaemia. Drugs. 2005;65:2719–2740. doi: 10.2165/00003495-200565180-00014. [DOI] [PubMed] [Google Scholar]
- 87.Capildeo R. Implications of the 5-year CR FIRST trial. Sinemet CR Five-Year International Response Fluctuation Study. Neurology. 1998;50:S15–17. doi: 10.1212/wnl.50.6_suppl_6.s15. discussion S44–18. [DOI] [PubMed] [Google Scholar]
- 88.Nilausen DO, et al. The perception and pharmacokinetics of a 20-mg dose of escitalopram orodispersible tablets in a relative bioavailability study in healthy men. Clin Ther. 2011;33:1492–1502. doi: 10.1016/j.clinthera.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 89.Magnussen I, Engbaek F. The effects of aromatic amino acid decarboxylase inhibitors on plasma concentrations of 5-hydroxytryptophan in man. Acta Pharmacol Toxicol (Copenh) 1978;43:36–42. doi: 10.1111/j.1600-0773.1978.tb02229.x. [DOI] [PubMed] [Google Scholar]
- 90.Shindo H, et al. Mechanism of intestinal absorption and brain uptake of L-5-hydroxytryptophan in rats, as compared to those of L-3,4-dihydroxyphenylalanine. Chem Pharm Bull (Tokyo) 1977;25:1417–1425. doi: 10.1248/cpb.25.1417. [DOI] [PubMed] [Google Scholar]
- 91.Agren H, et al. Low brain uptake of L-[11C]5-hydroxytryptophan in major depression: a positron emission tomography study on patients and healthy volunteers. Acta Psychiatr Scand. 1991;83:449–455. doi: 10.1111/j.1600-0447.1991.tb05574.x. [DOI] [PubMed] [Google Scholar]
- 92.Takahashi S, et al. Effect of l-5-hydroxytryptophan on brain monoamine metabolism and evaluation of its clinical effect in depressed patients. J Psychiatr Res. 1975;12:177–187. doi: 10.1016/0022-3956(75)90025-4. [DOI] [PubMed] [Google Scholar]
- 93.Magnussen I. Effects of carbidopa on the cerebral accumulation of exogenous L-5-hydroxytryptophan in mice. Acta Pharmacol Toxicol (Copenh) 1984;55:199–202. doi: 10.1111/j.1600-0773.1984.tb02037.x. [DOI] [PubMed] [Google Scholar]
- 94.Khor SP, Hsu A. The pharmacokinetics and pharmacodynamics of levodopa in the treatment of Parkinson’s disease. Curr Clin Pharmacol. 2007;2:234–243. doi: 10.2174/157488407781668802. [DOI] [PubMed] [Google Scholar]
- 95.Sharma V, McNeill JH. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol. 2009;157:907–921. doi: 10.1111/j.1476-5381.2009.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.