Abstract
Reversible protein phosphorylation regulates virtually all aspects of life in the cell. As a result, dysregulation of protein kinases, the enzymes responsible for transferring phosphate groups from ATP to proteins, are often the cause or consequence of many human diseases including cancer. Almost three dozen protein kinase inhibitors (PKIs) have been approved for clinical applications since 1995, the vast majority of them for the treatment of cancer. According to the NCI, there are more than 100 types of cancer. However, FDA-approved PKIs only target 14 of them. Importantly, of the more than 500 protein kinases encoded by the human genome, only 22 are targets for currently approved PKIs, suggesting that the reservoir of PKIs still has room to grow significantly. In this short review we will discuss the most recent advances, challenges, and alternatives to currently adopted strategies in this burgeoning field.
The human genome, which may contain as few as 19,000 genes [1], encodes 538 protein kinases [2], representing almost 3 % of the total. Protein kinases are responsible for phosphorylating approximately one third of all proteins [3]. Unlike other post-translational modifications, phosphorylation is reversible and, in most cases transient, because phosphate groups can readily be removed by protein phosphatases. This mechanism greatly enhances the genome’s plasticity, and it can regulate protein function in virtually every imaginable way: To name a few examples, it can stimulate or inhibit enzymatic activity, protein degradation, or relocation within the cell. In many human diseases protein kinases are dysregulated, particularly in cancer [4]. Since most protein kinases stimulate cell growth and proliferation, cell survival and migration, they can, when overexpressed, amplified or constitutively active, assume oncogenic properties. It is thus not surprising that in the last few decades enormous efforts have been devoted to developing small molecules that specifically or selectively inhibit protein kinases. Initially, these efforts were hampered by two perceived difficulties: First, given that protein kinases share similar ATP-binding sites, it was considered impossible to develop compounds that inhibit just one kinase. Second, it was thought that these compounds would have to compete with millimolar ATP concentrations inside cells [5]. Eventually potent and specific kinase inhibitors could be developed, and the fact that many PKIs inhibit more than one kinase may actually be advantageous in cancer, since this would possibly allow the drug to be used in several types of cancer and prevent acquired drug resistance [5].
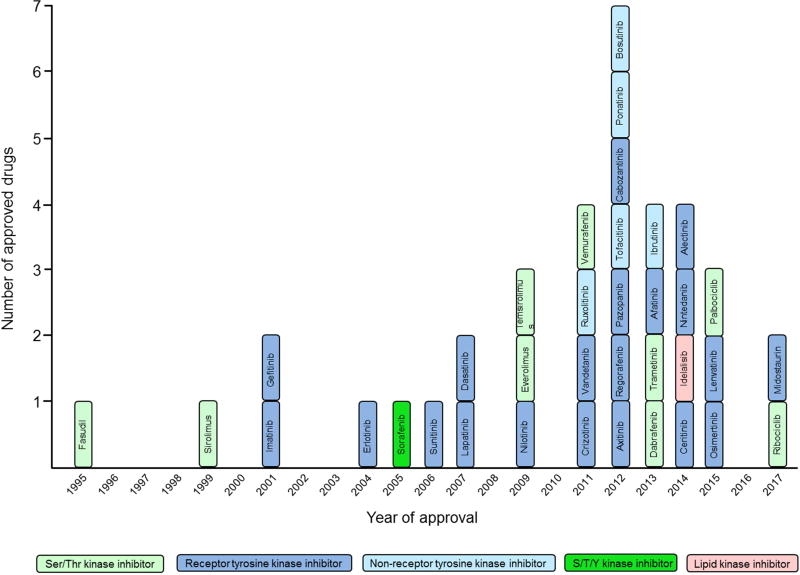
Kinase inhibitors are broadly classified as exclusively occupying the ATP-site (Type I), concurrently occupying the ATP site and an adjacent allosteric site (Type II) or solely occupying an allosteric site (Type III). The first kinase inhibitor that was approved for clinical use (1995 in Japan) is the ROCK inhibitor fasudil for treating cerebral vasospasms [2]. As of April 2017, 35 small molecule PKIs have been approved for clinical use, 31 of which are used in cancer therapy (Fig. 1). Sixteen of the originally intended 22 targets are tyrosine kinases. The subject of PKIs has been discussed, in great detail, in excellent recent reviews [5–11]. Here, we will briefly review the most recent advances in this field and its remaining challenges.
Figure 1.
FDA-approved small molecule kinase inhibitors.
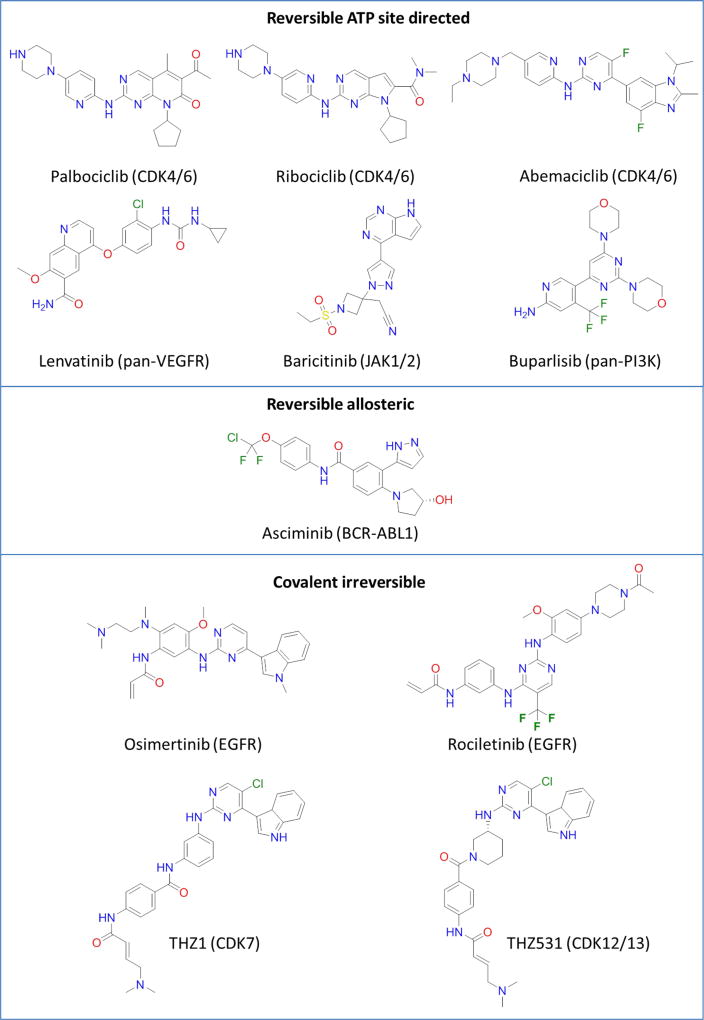
Reversible ATP site directed inhibitors
Among receptor tyrosine kinase inhibitors the pan-VEGFR inhibitor lenvatinib [12] was approved in 2015 for thyroid cancer and in 2016 for renal cell carcinoma in combination with the mTOR inhibitor everolimus (Fig. 2). Significant success has been reported in the development of serine-threonine specific kinase (STK) inhibitors, particularly of cyclin-dependent kinases (CDKs). Numerous studies during the early to mid-1990s have suggested a crucial role for CDK4 in cell cycle progression, particularly from G1 to S-phase. Key components of this event are: CDK4 with its partner Cyclin D and the tumor suppressors RB and p16Ink4 (for a review see [7]). The most important function of CDK4/cyclin D appears to be the initial step in sequential inactivating phosphorylations of the RB protein, which in turn leads to the expression of proteins necessary for DNA replication. At the same time, it was already realized that many cancers overexpress either D-type cyclins or CDK4, or they lack functional p16Ink4 or RB tumor suppressors. This and other observations led to the suggestion that ultimately cancer is a disease of the cell cycle. Using mouse models of mammary tumorigenesis, a crucial role of CDK4/cyclin D1 was demonstrated in a series of elegant studies: Thus, knock-in mice expressing a mutant cyclin D1 unable to activate CDK4/6 and CDK4-null mice are resistant to ErbB2-induced breast cancer [13,14]. More recently, it was shown that ablation of cyclin D3 or pharmacologic inhibition of CDK4 results in the selective killing of cancer cells without affecting normal tissues in mice carrying acute lymphoblastic leukemia [15]. It is nonetheless striking that it took until 2015 to gain approval for the first CDK inhibitor (palbociclib, targeting CDK4/6) in the treatment of metastatic breast cancer [10]. The latest addition to the portfolio of approved PKIs, ribociclib, is also a CDK4/6 inhibitor [7]. Like palboclib, ribociclib inhibits RB phosphorylation and causes G1 arrest in neuroblastoma cells [16]. Palbociclib and ribociclib are the only STK inhibitors approved since 2015. Another potent CDK4/6 inhibitor is abemaciclib (phase III) which has recently been shown to cause tumor regression in mouse models of several cancers including melanoma [17]. The success of CDK4/6 inhibitors also highlights the importance of outcome predictions to select patient populations that are likely to respond to the given drug. The inhibition of CDK4 is only efficacious in cells and patients that express functional RB [18]. Several inhibitors of other kinases involved in cell cycle progression are being evaluated in clinical trials, e.g. AZD1775 targeting Wee1 [19], alisertib targeting Aurora A [20] and LY2603618 targeting CHK1 [21]. Recent inhibitors of signaling kinases in clinical trials include the JAK1/JAK2 inhibitor baricitinib, an analogue of the FDA approved JAK2 inhibitor ruxolitinib, for refractory rheumatoid arthritis [22,23] and the pan-PI3K inhibitor buparlisib for advanced HR+/HER2 endocrine-resistant breast cancer [24,25] and other cancers [26–28]. In April 2017, the FDA approved the pan-kinase inhibitor midostaurin, a staurosporine analogue, for the treatment of newly diagnosed acute myeloid leukemia (AML) with FLT3 mutation [29].
Figure 2.
Chemical structures of representative kinase inhibitors.
Reversible allosteric inhibitors
For the longest time, PKIs have exclusively been functioning as ATP-competitive inhibitors. More recently, the field has also seen the advent of inhibitors targeting hydrophobic pockets outside the ATP binding site.[30] To date, there are just three FDA-approved allosteric inhibitors, the MEK1/2 inhibitor trametinib and the macrocyclic rapamycin analogs everolimus and temsirolimus targeting mTOR. While first generation catalytic-site ABL1 kinase inhibitors such as imatinib have led to a shift in the treatment of chronic myeloid leukemia (CML), second-generation inhibitors such as nilotinib were significantly active against imatinib-resistant mutants.and showed superior outcomes [31]. Recently a research group from Novartis reported on the development of a novel potent and selective allosteric inhibitor of BCR–ABL1 that binds to the myristoyl pocket [32]. ABL001 (asciminib) is a functional antagonist of BRC-ABL1 by inducing structural changes to the N terminus of ABL1 resulting in an enzymatically inactive kinase conformation. Acquired resistance was observed with single-agent therapy in mice, whereas the combination of asciminib and nilotinib led to complete disease control and eradicated CML xenograft tumors without recurrence after the cessation of treatment. These findings suggest that a combination treatment of catalytic and allosteric site inhibitors of BCR-ABL1 might lead to disease eradication and treatment-free remissions.
Covalent ATP site directed inhibitors
Many kinases have an exposed cysteine side chain in the ATP site that could be targeted for covalent reaction with compounds harboring an electrophilic Michael Acceptor in the right position [33–35]. Such covalent inhibitors are designed as affinity labels that interact with the target kinase in a two-step reaction, in which the formation of an irreversible enzyme-inhibitor complex is preceded by a rapidly reversible collision complex. In 2013, afatinib (targeting EGFR) and ibrutinib (targeting BTK) became the first FDA approved covalent kinase inhibitors. Another covalent EGFR inhibitor, osimertinib, was approved in 2015 for the treatment of non-small-cell lung cancer (NSCLC) characterized by the EGFR T790M mutation [36,37]. Novel covalent inhibitors of EGFR-T790M based on pyrazolopyrimidines[38] and indazoles[39] have been reported recently, and other inhibitors such as rociletinib are in late phase clinical trials for non-small cell lung cancer (NSLCL) [40]. Gray and colleagues developed novel covalent inhibitors to target specific kinase isoforms including JAK3 of the JAK family [41], as well as CDK7 [42,43] and CDK12 [44] which are critical for the transcriptional machinery to sustain the oncogenic state. Compounds THZ1 (targeting CDK7) and THZ531 (targeting CDK12) are unique in that they have been designed to target a Cys residue outside the ATP site, which provides new means of achieving covalent selectivity. To avoid potential off-target effects of irreversible acrylamide-based kinase inhibitors by forming permanent covalent adducts with cysteine residues of other proteins, the Taunton laboratory recently introduced a systemic approach towards the rational design of reversible covalent inhibitors [45]. This strategy was successfully employed to develop novel inhibitors of BTK and FGFR inhibitors with prolonged and tunable residence times in vivo [46].
Alternative approaches to PKI development
Next to inhibitors that directly decrease the enzymatic activity of PKs there are other, more indirect ways to interfere with kinase activity. Using CDKs as an example, to be fully active, these enzymes require i) synthesis of, and binding to, a regulatory cyclin subunit, ii) dissociation from inhibitor proteins, e.g. p16INK4A, and iii) phosphorylation by CDK7 and dephosphorylation by Cdc25. They can be inhibited by ubiquitin-dependent degradation of cyclins. Each one of these regulatory mechanisms is a potential drug target. To interfere with some of these processes would require the inhibition of protein-protein interactions, which is a difficult task, but as the example of the recently approved Bcl2 inhibitor venetoclax illustrates that it is not impossible [47]. Another promising avenue of drug discovery may be targeted protein degradation by so-called proteolysis-targeting chimeras (PROTACs) [48]. These bivalent heterobifunctional compounds recruit one of over 600 specific ubiquitin E3 ligases [49] to tag target proteins for subsequent degradation. The move from functional inhibition to destruction may also enable the targeting of proteins that have been deemed undruggable to the list of therapeutic targets. A different example of heterobivalent compounds is the mTOR inhibitor RapaLink-1, which consists of rapamycin linked to an ATP-competitive inhibitor [50]. This third-generation mTOR inhibitor effectively overcame acquired resistance to first- and second-generation inhibitors.
Recently, promising approaches have emerged by specifically targeting pseudokinases, which lack catalytic kinase activity but often function as scaffold proteins. Her3-dependent signaling in cancer cells was successfully inhibited with a heterobivalent compound, TX2-121-1, which contains a covalent inhibitor to selectively attack the ATP site of Her3 and a hydrophobic adamantane moiety to induce proteolytic degradation of thus modified protein [51]. A small molecule ligand of the JH2 pseudokinase domain of Tyk2, a member of the Janus tyrosine kinase family, was able to lock in a conformation that stabilizes an autoinhibitory interaction with the JH1 catalytic domain, thereby blocking Tyk2-mediated signaling important in autoimmunity [52]. A new class of pseudokinase antagonists was developed that stabilize a previously unrecognized inactive state of the scaffold protein KSR (kinase suppressor of Ras) [53]. In combination with MEK inhibitors, these compounds inhibited growth of Ras mutant cell lines, providing a new therapeutic strategy against Ras-driven cancers.
PKIs in diseases other than cancer
Adult cancers are typically characterized by aberrations in multiple signaling pathways, which has been and will continue to be a unique challenge for drug design and discovery. However, there are a number of other diseases that offer opportunities for PKI-based therapies. The Src family kinase inhibitor saracatinib undergoes clinical trials for Alzheimer’s disease, an example of efforts by the NIH to repurpose cancer drugs for the treatment of other diseases[54]. The rationale is that progression of the disease may be achievable by blocking the activity of Fyn kinase that has been shown to induce damage to brain synapses in mouse models of Alzheimer’s [55]. Recent examples of PKIs in clinical trials for diseases other than cancer are the JAK inhibitors filgotinib and peficitinib for moderate-to-severe Crohn's disease [56] and rheumatoid arthritis [57], respectively, the ASK1 inhibitor selonsertib for diabetic kidney disease [58], and the ROCK inhibitor netarsudil for glaucoma and ocular hypertension [59].
Challenges in PKI drug discovery
Overcoming resistance
Despite the initial efficacy of PKIs, cancer cells frequently develop resistance by engaging multiple mechanisms (reviewed in [2,11]). In the case of acquired mutations in key residues within their catalytic domain, this can be overcome by second and third generation inhibitors. Examples of such inhibitors are the aforementioned osimertinib and the ALK inhibitor alectinib (approved in 2014), which is used in the treatment of crizotinib-refractory NSCLC [60]. The efficacy of inhibitors developed against cancers driven by T790MEGFR can be jeopardized by yet another acquired EGFR mutation, C797S. Recent work of Eck and coworkers describes the rational design of an allosteric EGFR inhibitor EA1045 that is effective against EGFR mutants but spares wild-type receptors in biochemical assays. To effectively inhibit EGF-driven cell proliferation required the combination with cetuximab, an antibody that prevents receptor dimerization [61]. The well-documented success of RAF inhibitors, for instance in metastatic melanoma, depends on the MAPK pathway to be virtually silent. Unfortunately, these inhibitors paradoxically stimulate MAPK signaling in cells harboring oncogenic RAS, leading to the rapid development of other skin tumors. Recently a research team from Plexxikon Inc. developed second-generation RAF inhibitors (PLX7904 and PLX8394) that were effective against cells with mutant V600EBRAF without activating the MAPK pathway [62]. Other “paradox-breaking” compounds that were recently reported are CCT196969 and CCT242161, pan-RAF inhibitors that also inhibit SRC [63]. These compounds were effective against melanoma cells and patient-derived mouse xenografts resistant to BRAF and BRAF/MEK inhibitors.
Conclusions
Nowadays, tumors are increasingly being characterized by molecular diagnostics, resulting in the creation of new tumor subtypes that go far beyond the anatomic and histologic categories. For instance, NSCLC can be subdivided into more than 10 distinct genotypes, with activating KRAS and EGFR mutations being the most frequent driver aberrations (15–25 and 10–35%, respectively) [64]. Thus pairing a drug targeting a certain kinase with patients that have acquired mutations or amplifications in this kinase are more successful. On the other hand, the fact that PKIs, like all targeted therapies, are tailor-made to particular, possibly small, patient populations, increases the cost of therapy significantly, an aspect that is often neglected in academic drug discovery campaigns. Of the 35 approved PKIs, nine compounds selectively inhibit five STKs, whereas the other 26 PKIs target just 11 receptor tyrosine kinases (RTKs) and 5 non-receptor tyrosine kinase (NRTKs). Thus, only a very small subset of kinases are currently being exploited as a target for cancer therapy by FDA approved drugs, leaving a vast reservoir of yet poorly characterized kinases untapped. The second striking aspect is the absence, or near absence, of PKIs against several significant adult types of cancer, including pancreatic, prostate, colon and brain. The third is the fact that none of the approved drugs is purposefully aimed at pediatric tumors, which may be due to the expectation that such drugs would generate little revenue.
Acknowledgments
The authors acknowledge financial support from the National Institute of Child Health and Human Development (NIH/NICHD) grant HHSN275201300017C.
Abbreviations
- PKI
protein kinase inhibitor
- RTK
Receptor tyrosine kinase
- NRTK
Non-receptor tyrosine kinase
- STK
Serine/threonine-specific kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Annotated papers of special interest (*) or outstanding interest (**)
- 1.Ezkurdia I, Juan D, Rodriguez JM, Frankish A, Diekhans M, Harrow J, Vazquez J, Valencia A, Tress ML. Multiple evidence strands suggest that there may be as few as 19,000 human protein-coding genes. Hum Mol Genet. 2014;23:5866–5878. doi: 10.1093/hmg/ddu309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabbro D, Cowan-Jacob SW, Moebitz H. Ten things you should know about protein kinases: IUPHAR Review 14. Br J Pharmacol. 2015;172:2675–2700. doi: 10.1111/bph.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen P. The role of protein phosphorylation in human health and disease. Eur J Biochem. 2001;268:5001–5010. doi: 10.1046/j.0014-2956.2001.02473.x. [DOI] [PubMed] [Google Scholar]
- 4*.Fleuren ED, Zhang L, Wu J, Daly RJ. The kinome 'at large' in cancer. Nat Rev Cancer. 2016;16:83–98. doi: 10.1038/nrc.2015.18. Comprehensive analysis of the context-dependent functional role of specific kinases in cancer and how kinome remodelling modulates sensitivity to anticancer drugs. [DOI] [PubMed] [Google Scholar]
- 5**.Cohen P, Alessi DR. Kinase drug discovery--what's next in the field? ACS chemical biology. 2013;8:96–104. doi: 10.1021/cb300610s. Outstanding review by one of the leading experts in the field of protein phosphorylation summarizing the prospects and challenges of protein kinase inhibition in diseases other than cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015;36:422–439. doi: 10.1016/j.tips.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu P, Nielsen TE, Clausen MH. Small-molecule kinase inhibitors: an analysis of FDA-approved drugs. Drug Discovery Today. 2016;21:5–10. doi: 10.1016/j.drudis.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Wang P, Yang H, Zhou J, Li Y, Li X, Xue W, Yu C, Tian Y, Zhu F. Comparison of FDA Approved Kinase Targets to Clinical Trial Ones: Insights from Their System Profiles and Drug-Target Interaction Networks. Biomed Res Int. 2016;2016:2509385. doi: 10.1155/2016/2509385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17:93–115. doi: 10.1038/nrc.2016.138. Excellent and up-to-date review of therapeutic strategies focusing on proteins regulating the cell cycle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen NA, Kim TS, DeMatteo RP. Principles of Kinase Inhibitor Therapy for Solid Tumors. Ann Surg. 2017;265:311–319. doi: 10.1097/SLA.0000000000001740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14:5459–5465. doi: 10.1158/1078-0432.CCR-07-5270. [DOI] [PubMed] [Google Scholar]
- 13*.Landis MW, Pawlyk BS, Li T, Sicinski P, Hinds PW. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell. 2006;9:13–22. doi: 10.1016/j.ccr.2005.12.019. Original articles on the critical role of cyclin D and its partner CDK4 in mammalian tumorigenisis, demonstrating that functional inactivation of cyclin D1 or depletion of CDK4 wipes out breast cancer in vivo. [DOI] [PubMed] [Google Scholar]
- 14*.Yu Q, Sicinska E, Geng Y, Ahnstrom M, Zagozdzon A, Kong Y, Gardner H, Kiyokawa H, Harris LN, Stal O, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 2006;9:23–32. doi: 10.1016/j.ccr.2005.12.012. Original articles on the critical role of cyclin D and its partner CDK4 in mammalian tumorigenisis, demonstrating that functional inactivation of cyclin D1 or depletion of CDK4 wipes out breast cancer in vivo. [DOI] [PubMed] [Google Scholar]
- 15*.Choi YJ, Li X, Hydbring P, Sanda T, Stefano J, Christie AL, Signoretti S, Look AT, Kung AL, von Boehmer H, et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012;22:438–451. doi: 10.1016/j.ccr.2012.09.015. Original articles on the critical role of cyclin D and its partner CDK4 in mammalian tumorigenisis, demonstrating that functional inactivation of cyclin D1 or depletion of CDK4 wipes out breast cancer in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rader J, Russell MR, Hart LS, Nakazawa MS, Belcastro LT, Martinez D, Li Y, Carpenter EL, Attiyeh EF, Diskin SJ, et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res. 2013;19:6173–6182. doi: 10.1158/1078-0432.CCR-13-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Yadav V, Burke TF, Huber L, Van Horn RD, Zhang Y, Buchanan SG, Chan EM, Starling JJ, Beckmann RP, Peng SB. The CDK4/6 inhibitor LY2835219 overcomes vemurafenib resistance resulting from MAPK reactivation and cyclin D1 upregulation. Mol Cancer Ther. 2014;13:2253–2263. doi: 10.1158/1535-7163.MCT-14-0257. One of the first articles describing second generation PKIs, which are being developed in order to treat patients whose cancers have acquired resistance towards earlier targeted therapy. [DOI] [PubMed] [Google Scholar]
- 18.Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. 2010;29:4018–4032. doi: 10.1038/onc.2010.154. [DOI] [PubMed] [Google Scholar]
- 19.Do K, Wilsker D, Ji J, Zlott J, Freshwater T, Kinders RJ, Collins J, Chen AP, Doroshow JH, Kummar S. Phase I Study of Single-Agent AZD1775 (MK-1775), a Wee1 Kinase Inhibitor, in Patients With Refractory Solid Tumors. J Clin Oncol. 2015;33:3409–3415. doi: 10.1200/JCO.2014.60.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Necchi A, Lo Vullo S, Mariani L, Raggi D, Giannatempo P, Calareso G, Togliardi E, Crippa F, Di Genova N, Perrone F, et al. An open-label, single-arm, phase 2 study of the Aurora kinase A inhibitor alisertib in patients with advanced urothelial cancer. Invest New Drugs. 2016;34:236–242. doi: 10.1007/s10637-016-0328-9. [DOI] [PubMed] [Google Scholar]
- 21.Laquente B, Lopez-Martin J, Richards D, Illerhaus G, Chang DZ, Kim G, Stella P, Richel D, Szcylik C, Cascinu S, et al. A phase II study to evaluate LY2603618 in combination with gemcitabine in pancreatic cancer patients. BMC Cancer. 2017;17:137. doi: 10.1186/s12885-017-3131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genovese MC, Kremer J, Zamani O, Ludivico C, Krogulec M, Xie L, Beattie SD, Koch AE, Cardillo TE, Rooney TP, et al. Baricitinib in Patients with Refractory Rheumatoid Arthritis. N Engl J Med. 2016;374:1243–1252. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- 23*.Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, Yakushin S, Ishii T, Emoto K, Beattie S, et al. Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. 2017;376:652–662. doi: 10.1056/NEJMoa1608345. Original article focusing on inhibitors targeting a kinase in a disease other than cancer. [DOI] [PubMed] [Google Scholar]
- 24.Martin M, Chan A, Dirix L, O'Shaughnessy J, Hegg R, Manikhas A, Shtivelband M, Krivorotko P, Batista Lopez N, Campone M, et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4) Ann Oncol. 2016 doi: 10.1093/annonc/mdw562. [DOI] [PubMed] [Google Scholar]
- 25.Geuna E, Milani A, Martinello R, Aversa C, Valabrega G, Scaltriti M, Montemurro F. Buparlisib, an oral pan-PI3K inhibitor for the treatment of breast cancer. Expert Opin Investig Drugs. 2015;24:421–431. doi: 10.1517/13543784.2015.1008132. [DOI] [PubMed] [Google Scholar]
- 26.Soulieres D, Faivre S, Mesia R, Remenar E, Li SH, Karpenko A, Dechaphunkul A, Ochsenreither S, Kiss LA, Lin JC, et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017;18:323–335. doi: 10.1016/S1470-2045(17)30064-5. [DOI] [PubMed] [Google Scholar]
- 27.Vansteenkiste JF, Canon JL, Braud FD, Grossi F, De Pas T, Gray JE, Su WC, Felip E, Yoshioka H, Gridelli C, et al. Safety and Efficacy of Buparlisib (BKM120) in Patients with PI3K Pathway-Activated Non-Small Cell Lung Cancer: Results from the Phase II BASALT-1 Study. J Thorac Oncol. 2015;10:1319–1327. doi: 10.1097/JTO.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ragon BK, Kantarjian H, Jabbour E, Ravandi F, Cortes J, Borthakur G, DeBose L, Zeng Z, Schneider H, Pemmaraju N, et al. Buparlisib, a PI3K inhibitor, demonstrates acceptable tolerability and preliminary activity in a phase I trial of patients with advanced leukemias. Am J Hematol. 2017;92:7–11. doi: 10.1002/ajh.24568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levis M. Midostaurin approved for FLT3-mutated AML. Blood. 2017 doi: 10.1182/blood-2017-05-782292. [DOI] [PubMed] [Google Scholar]
- 30.Wu P, Clausen MH, Nielsen TE. Allosteric small-molecule kinase inhibitors. Pharmacol Ther. 2015;156:59–68. doi: 10.1016/j.pharmthera.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 31**.Branford S, Yeung DT, Parker WT, Roberts ND, Purins L, Braley JA, Altamura HK, Yeoman AL, Georgievski J, Jamison BA, et al. Prognosis for patients with CML and >10% BCR-ABL1 after 3 months of imatinib depends on the rate of BCR-ABL1 decline. Blood. 2014;124:511–518. doi: 10.1182/blood-2014-03-566323. Original article on the rational design of a highly potent allosteric inhibitor of BCR-ABL1 and its potential for disease eradication and treatment-free remissions of CML. [DOI] [PubMed] [Google Scholar]
- 32.Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, Marzinzik AL, Pelle X, Donovan J, Zhu W, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017;543:733–737. doi: 10.1038/nature21702. [DOI] [PubMed] [Google Scholar]
- 33.Leproult E, Barluenga S, Moras D, Wurtz JM, Winssinger N. Cysteine mapping in conformationally distinct kinase nucleotide binding sites: application to the design of selective covalent inhibitors. J Med Chem. 2011;54:1347–1355. doi: 10.1021/jm101396q. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Sabnis Y, Zhao Z, Zhang T, Buhrlage SJ, Jones LH, Gray NS. Developing irreversible inhibitors of the protein kinase cysteinome. Chem Biol. 2013;20:146–159. doi: 10.1016/j.chembiol.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Z, Liu Q, Bliven S, Xie L, Bourne PE. Determining Cysteines Available for Covalent Inhibition Across the Human Kinome. J Med Chem. 2017;60:2879–2889. doi: 10.1021/acs.jmedchem.6b01815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yver A. Osimertinib (AZD9291)-a science-driven, collaborative approach to rapid drug design and development. Ann Oncol. 2016;27:1165–1170. doi: 10.1093/annonc/mdw129. [DOI] [PubMed] [Google Scholar]
- 37.Zugazagoitia J, Ferrer I, Paz-Ares L. Osimertinib in EGFR-mutant NSCLC: how to select patients and when to treat. Lancet Oncol. 2016;17:1622–1623. doi: 10.1016/S1470-2045(16)30506-X. [DOI] [PubMed] [Google Scholar]
- 38.Engel J, Becker C, Lategahn J, Keul M, Ketzer J, Muhlenberg T, Kollipara L, Schultz-Fademrecht C, Zahedi RP, Bauer S, et al. Insight into the Inhibition of Drug- Resistant Mutants of the Receptor Tyrosine Kinase EGFR. Angew Chem Int Ed Engl. 2016;55:10909–10912. doi: 10.1002/anie.201605011. [DOI] [PubMed] [Google Scholar]
- 39.Tomassi S, Lategahn J, Engel J, Keul M, Tumbrink HL, Ketzer J, Muhlenberg T, Baumann M, Schultz-Fademrecht C, Bauer S, et al. Indazole-Based Covalent Inhibitors To Target Drug-Resistant Epidermal Growth Factor Receptor. J Med Chem. 2017;60:2361–2372. doi: 10.1021/acs.jmedchem.6b01626. [DOI] [PubMed] [Google Scholar]
- 40.Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, Papadimitrakopoulou V, Solomon BJ, Oxnard GR, Dziadziuszko R, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372:1700–1709. doi: 10.1056/NEJMoa1413654. [DOI] [PubMed] [Google Scholar]
- 41.Tan L, Akahane K, McNally R, Reyskens KM, Ficarro SB, Liu S, Herter-Sprie GS, Koyama S, Pattison MJ, Labella K, et al. Development of Selective Covalent Janus Kinase 3 Inhibitors. J Med Chem. 2015;58:6589–6606. doi: 10.1021/acs.jmedchem.5b00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwiatkowski N, Zhang T, Rahl PB, Abraham BJ, Reddy J, Ficarro SB, Dastur A, Amzallag A, Ramaswamy S, Tesar B, et al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature. 2014;511:616–620. doi: 10.1038/nature13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cayrol F, Praditsuktavorn P, Fernando TM, Kwiatkowski N, Marullo R, Calvo-Vidal MN, Phillip J, Pera B, Yang SN, Takpradit K, et al. THZ1 targeting CDK7 suppresses STAT transcriptional activity and sensitizes T-cell lymphomas to BCL2 inhibitors. Nat Commun. 2017;8:14290. doi: 10.1038/ncomms14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Zhang T, Kwiatkowski N, Olson CM, Dixon-Clarke SE, Abraham BJ, Greifenberg AK, Ficarro SB, Elkins JM, Liang Y, Hannett NM, et al. Covalent targeting of remote cysteine residues to develop CDK12 and CDK13 inhibitors. Nat Chem Biol. 2016;12:876–884. doi: 10.1038/nchembio.2166. Original article on the rational design of covalent irreversible, selective CDK inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.London N, Miller RM, Krishnan S, Uchida K, Irwin JJ, Eidam O, Gibold L, Cimermancic P, Bonnet R, Shoichet BK, et al. Covalent docking of large libraries for the discovery of chemical probes. Nat Chem Biol. 2014;10:1066–1072. doi: 10.1038/nchembio.1666. Original articles on the rational design of covalent reversible kinase inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Bradshaw JM, McFarland JM, Paavilainen VO, Bisconte A, Tam D, Phan VT, Romanov S, Finkle D, Shu J, Patel V, et al. Prolonged and tunable residence time using reversible covalent kinase inhibitors. Nat Chem Biol. 2015;11:525–531. doi: 10.1038/nchembio.1817. Original articles on the rational design of covalent reversible kinase inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mato AR, Hill BT, Lamanna N, Barr PM, Ujjani CS, Brander DM, Howlett C, Skarbnik AP, Cheson BD, Zent CS, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol. 2017;28:1050–1056. doi: 10.1093/annonc/mdx031. [DOI] [PubMed] [Google Scholar]
- 48**.Lai AC, Crews CM. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16:101–114. doi: 10.1038/nrd.2016.211. Contemporary review on the rational design of proteolysis-targeting chimera (PROTAC) drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipkowitz S, Weissman AM. RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat Rev Cancer. 2011;11:629–643. doi: 10.1038/nrc3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Rodrik-Outmezguine VS, Okaniwa M, Yao Z, Novotny CJ, McWhirter C, Banaji A, Won H, Wong W, Berger M, de Stanchina E, et al. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature. 2016;534:272–276. doi: 10.1038/nature17963. Original article on the rational design of bivalent mTOR inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie T, Lim SM, Westover KD, Dodge ME, Ercan D, Ficarro SB, Udayakumar D, Gurbani D, Tae HS, Riddle SM, et al. Pharmacological targeting of the pseudokinase Her3. Nat Chem Biol. 2014;10:1006–1012. doi: 10.1038/nchembio.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tokarski JS, Zupa-Fernandez A, Tredup JA, Pike K, Chang C, Xie D, Cheng L, Pedicord D, Muckelbauer J, Johnson SR, et al. Tyrosine Kinase 2-mediated Signal Transduction in T Lymphocytes Is Blocked by Pharmacological Stabilization of Its Pseudokinase Domain. J Biol Chem. 2015;290:11061–11074. doi: 10.1074/jbc.M114.619502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53**.Dhawan NS, Scopton AP, Dar AC. Small molecule stabilization of the KSR inactive state antagonizes oncogenic Ras signalling. Nature. 2016;537:112–116. doi: 10.1038/nature19327. Original article on the rational design of a pseudokinase antagonist to inhibit oncogenic Ras signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wadman M. NIH gambles on recycled drugs. Nature. 2013;499:263–264. doi: 10.1038/499263a. [DOI] [PubMed] [Google Scholar]
- 55.Folch J, Petrov D, Ettcheto M, Abad S, Sanchez-Lopez E, Garcia ML, Olloquequi J, Beas-Zarate C, Auladell C, Camins A. Current Research Therapeutic Strategies for Alzheimer's Disease Treatment. Neural Plast. 2016;2016:8501693. doi: 10.1155/2016/8501693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, Klopocka M, Goldis A, Wisniewska-Jarosinska M, Baranovsky A, et al. Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–275. doi: 10.1016/S0140-6736(16)32537-5. [DOI] [PubMed] [Google Scholar]
- 57.Papp K, Pariser D, Catlin M, Wierz G, Ball G, Akinlade B, Zeiher B, Krueger JG. A phase 2a randomized, double-blind, placebo-controlled, sequential dose-escalation study to evaluate the efficacy and safety of ASP015K, a novel Janus kinase inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol. 2015;173:767–776. doi: 10.1111/bjd.13745. [DOI] [PubMed] [Google Scholar]
- 58.Lin JH, Zhang JJ, Lin SL, Chertow GM. Design of a phase 2 clinical trial of an ASK1 inhibitor, GS-4997, in patients with diabetic kidney disease. Nephron. 2015;129:29–33. doi: 10.1159/000369152. [DOI] [PubMed] [Google Scholar]
- 59.Lewis RA, Levy B, Ramirez N, Kopczynski CC, Usner DW, Novack GD Group PCS. Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol. 2016;100:339–344. doi: 10.1136/bjophthalmol-2015-306778. [DOI] [PubMed] [Google Scholar]
- 60.McKeage K. Alectinib: a review of its use in advanced ALK-rearranged non-small cell lung cancer. Drugs. 2015;75:75–82. doi: 10.1007/s40265-014-0329-y. [DOI] [PubMed] [Google Scholar]
- 61**.Jia Y, Yun CH, Park E, Ercan D, Manuia M, Juarez J, Xu C, Rhee K, Chen T, Zhang H, et al. Overcoming EGFRT790M) and EGFRC797S) resistance with mutant-selective allosteric inhibitors. Nature. 2016;534:129–132. doi: 10.1038/nature17960. Original article on the rational design of mutant-selective allosteric EGFR inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62**.Zhang C, Spevak W, Zhang Y, Burton EA, Ma Y, Habets G, Zhang J, Lin J, Ewing T, Matusow B, et al. RAF inhibitors that evade paradoxical MAPK pathway activation. Nature. 2015;526:583–586. doi: 10.1038/nature14982. Original articles on novel paradox-breaking RAF inhibitors. [DOI] [PubMed] [Google Scholar]
- 63**.Girotti MR, Lopes F, Preece N, Niculescu-Duvaz D, Zambon A, Davies L, Whittaker S, Saturno G, Viros A, Pedersen M, et al. Paradox-breaking RAF inhibitors that also target SRC are effective in drug-resistant BRAF mutant melanoma. Cancer Cell. 2015;27:85–96. doi: 10.1016/j.ccell.2014.11.006. Original articles on novel paradox-breaking RAF inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meador CB, Micheel CM, Levy MA, Lovly CM, Horn L, Warner JL, Johnson DB, Zhao Z, Anderson IA, Sosman JA, et al. Beyond histology: translating tumor genotypes into clinically effective targeted therapies. Clin Cancer Res. 2014;20:2264–2275. doi: 10.1158/1078-0432.CCR-13-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]