Abstract
The objective of this study was to investigate the feasibility of rapid administration of iron via transdermal route as an alternative to parenteral route of administration. In vitro drug delivery studies were carried out using porcine epidermis mounted on Franz diffusion cells. The effect of chemical permeation enhancers and physical techniques (constant voltage iontophoresis, electroporation and combination of electroporation with iontophoresis) on the transport of ferric pyrophosphate (FPP) was studied. Transepidermal water loss (TEWL) and electrical resistance were measured in order to see the effect of these techniques on the skin barrier function. The amount of FPP permeated was not enhanced significantly with the use of any of the enhancers (P > 0.05). It was found that constant voltage iontophoresis (0.5, 2 or 4 V) for about 30 min across electroporated epidermis (120 V, 100 pulses, 10 ms at 5 Hz) enhanced the delivery of FPP over control in the range of 2- to 42-fold. Hence, a therapeutically required dose of iron could be delivered by transdermal route using electrically-mediated techniques.
Keywords: Ferric pyrophosphate, transdermal, constant voltage iontophoresis, electroporation, anemia
Introduction
Iron is a vital mineral required for carrying out various metabolic processes including oxygen transport, DNA synthesis and electron transport in our body. The decrease in the total body iron content leads to a condition known as iron deficiency anemia (IDA). This condition results due to inadequate dietary intake, poor absorption, or physiologic loss of iron from body. IDA is considered to be a major problem in developing countries, but recent epidemiological studies reported its prevalence even in developed countries.[1] IDA in children and pregnant women commonly results in major health disorders. In the case of children, if IDA is not diagnosed and treated during infancy it may result in decreased intellectual development and growth retardation.[2] Iron requirement is generally more in the case of pregnant women during second trimester and particularly high at the end of gestation.[3] Achieving such high levels of iron is extremely difficult with the regular diet and failure to do so increases the risk factor for both mother and fetus. Therefore, it is important to promptly treat this condition by iron supplementation.
The first-line therapy for treating IDA is by administration of iron supplements via oral route.[4] However, the response to this route of administration for long-term treatment is often poor due to less bioavailability, poor compliance, attention needed for daily self dosing, mild to severe GI and systemic side-effects.[5,6] Parenteral administration of iron is often preferred in some cases due to limitations and side-effects associated with oral delivery. Only colloidal formulations of iron (iron-gluconate, iron-dextran and iron-saccharate) are administered via parenteral route.[7] The orally administered iron salts cannot be administered parenterally as they are known to induce significant oxidative stress. On the other hand, although parenteral administration of colloidal iron has been a good alternative for replenishing the depleted body iron stores, it has been reported to have caused various side-effects like local pain, systemic ache, fever, arthralgia and rashes.[8,9] Moreover, the parenteral route does not allow the termination of therapy in the case of iron overload. Hence, we investigated the transdermal route as one of the delivery methods of iron.
Transdermal drug delivery is non-invasive and offers several advantages over conventional drug delivery systems such as oral and parenteral delivery. Hence, it is one of the most patient compliant non-oral methods of drug delivery. The controlled delivery of iron via transdermal route by constant current iontophoresis has been reported recently.[10] The objective of this project was to investigate the feasibility of rapid administration of iron via transdermal route as an alternative to parenteral route of administration. However, stratum corneum, the outer most layer of the skin, is known to limit the penetration of polar and hydrophilic molecules across the skin. Therefore, physical methods like electroporation and iontophoresis were used to enhance the transdermal delivery of FPP.
Ferric pyrophosphate (FPP: MW ~ 745 dalton) is a water soluble solid with a high stability constant (Log Kstab 22.3) and is capable of triggering direct transfer of transferrin to ferritin and promotes iron exchange between transferrin molecules.[11,12] FPP has been demonstrated to be safe and effective for transdermal, subcutaneous and i.v administration.[13] Therefore, FPP was chosen as the iron source for the transdermal delivery.
Materials and methods
Chemicals
Soluble ferric pyrophosphate (FPP), dimethyl sulfoxide, azone™, menthol, ethanol, isopropyl myristate, sodium lauryl sulfate and phosphate buffered saline (PBS, pH 7.1) were purchased from Sigma-Aldrich Inc, St Louis, MO, USA. FerroVer® Iron reagent was purchased from HACH, Loveland, CO, USA. All other chemicals and reagents used were of analytical grade. All solutions were prepared in deionized water.
Skin
Porcine skin is considered to be a good model for human skin in respect to the size and structure of the stratum corneum.[14,15] Porcine belly skin was obtained from a local abattoir. Pieces of skin wrapped in aluminum foil were heated to 60°C for 2 min and the epidermis was gently peeled off the skin. The fresh epidermis was placed on glass microscopic slides and kept dry at 4°C and was used within three days. Prior to use, the epidermis was thawed to room temperature by hydrating with water for 1 h.
Experimental set-up
A vertical Franz-type diffusion apparatus (Logan instruments, Somerset, NJ, USA) was used for all resistance and permeation measurements across the porcine epidermis. The temperature of the chamber was regulated at 37 ± 1°C by water circulation. A piece of porcine epidermis was placed between two compartments of the diffusion apparatus, one serving as the donor and other as the receiver compartment. The active diffusion area of the epidermis was 0.64 cm2. The receiver compartment (5 mL) was filled with PBS (adjusted to pH 5) to maintain sink conditions and 0.5 mL of the permeant solution was placed in the donor compartment. FPP solution was prepared by dissolving 50 mg in 1 mL deionized water and adjusting the pH to 5 using 1N HCl. The electrodes (Ag/AgCl) were placed 2 mm away from the skin in both the donor and the receiver compartments. Electrical pulsing was carried out using an ECM 830 Electro Square Porator (BTX Harvard apparatus, Holliston, MA, USA). Constant voltage iontophoresis was carried out using wave form generator (Agilent Technologies, Santa Clara, CA, USA).
Electrical measurements
The AC electrical resistance of the epidermis was measured by placing a load resistor of 100 kΩ in series with the epidermis. The voltage drop across the whole circuit and across the epidermis was measured using an electrical set-up consisting of a wave form generator and a digital multimeter (Agilent Technologies, Santa Clara, CA, USA). For measuring resistance, a low voltage of 100 mV was applied at 10 Hz and the skin resistance in kΩ was calculated. The piece of porcine epidermis which had a resistance greater than 20 kΩ.cm2 was only used for the permeation studies.
Permeation across porcine epidermis using chemical permeation enhancers
Chemical permeation enhancers such as 5% v/v dimethyl sulfoxide, 2% v/v azone™, 5% w/v menthol, 5% v/v ethanol, 4% v/v isopropyl myristate and 1% w/v sodium lauryl sulfate were used in order to see the effect of enhancers on permeation of FPP, in vitro. Samples were withdrawn at regular intervals of time for a period of 24 h from the receiver compartment and the iron content was measured at 510 nm by adding FerroVer® iron reagent using UV spectrophotometer.
Permeation across delipidized porcine epidermis
The porcine epidermis was mounted on Franz diffusion cell. Five hundred microliters of chloroform:methanol (2:1 v/v) was placed in the donor compartment and allowed to stand for 1 h in order to extract the lipids from epidermis, the receiver compartment was kept empty. After 1 h, the permeation studies were carried out by replacing the donor solution with 0.5 mL of FPP (50 mg/mL) solution. The receiver compartment was then filled with PBS adjusted to pH 5. Samples were withdrawn at regular intervals of time until 24 h and analyzed using UV spectrophotometer.
Constant voltage iontophoresis (IN)-mediated delivery of FPP
In the donor compartment of Franz diffusion apparatus, 0.5 mL of FPP (50 mg/mL) solution was placed and PBS adjusted to pH 5 was placed in the receiver compartment. Constant voltage cathodal iontophoresis was carried out by applying different voltages (0.5, 2 or 4 V) across the porcine epidermis mounted between the two compartments of the diffusion cell using the wave form generator source. Samples were withdrawn from the receiver compartment after 30 min and the iron content was measured by adding FerroVer® iron reagent at 510 nm using UV spectrophotometer. Passive permeation studies were carried out in similar set up at 0 V which served as control.
Electroporation facilitated permeation of FPP
The experimental set-up and resistance measurement was similar to that used for IN facilitated delivery. In electroporation set of studies, electrical pulses (120 V, 100 pulses) at different pulse lengths (1, 10 or 100 ms) and frequencies (1 or 5 Hz) were applied across porcine epidermis using an ECM 830 Electro Square Porator. Samples were withdrawn from the receiver compartment immediately after cessation of the electrical pulses and analyzed for iron content. Passive permeation studies were carried out for the same duration without application of electrical pulses which served as control.
In vitro delivery of FPP by IN across electroporated epidermis
FPP solution (0.5 mL of 50 mg/mL) and PBS adjusted to pH 5 was placed in the donor and receiver compartment, respectively of Franz diffusion apparatus. The porcine epidermis mounted between the two compartments of the diffusion cell was electroporated. Immediately, IN was initiated and run for 30 min at different voltages (0.5, 2 or 4 V). In control set, the epidermis was electroporated and the drug was allowed to permeate passively by application of 0 V through the circuit. Samples were collected immediately after electroporation and after 30 min of application of different voltages.
Measurement of Transepidermal Water Loss (TEWL)
In order to see the effect of chemical enhancers and physical techniques on the skin barrier function, TEWL was measured using a VapoMeter (Delfin Technologies, Kuopio, Finland). The porcine epidermis was sandwiched between two compartments of Franz diffusion cell. PBS adjusted to pH 5 was placed in the receiver compartment and the donor compartment was kept empty. In studies involving permeation enhancers the donor compartment was pretreated with enhancer solutions, donor was emptied, the moisture in the donor compartment and surface of the epidermis was removed by blotting with KimWipes. The epidermis was allowed to equilibrate. The whole set-up was placed in a temperature (37°C) and humidity (RH 55%) controlled chamber and the TEWL was measured by fixing the instrument directly on the donor compartment. Control set of experiments were carried using similar set up but without pulsing or treating the epidermis with enhancers.
Statistical analysis
Statistical analysis was carried out using Graph pad Prism 5 software. The t-test was selected as the test of significance and P value less than 0.05 was considered statistically significant. The data points provided in the graph are an average of three trials with error bars representing standard deviation.
Results and discussion
Passive permeation of FPP
Passive permeation of FPP across the porcine epidermis within 30 minutes was 0.78 ± 0.22 μg/cm2. Even after 24 h, the total amount of FPP permeated was only 31.13 ± 8.17 μg/cm2. This confirmed our earlier report regarding the poor skin permeability to FPP.[10] It is likely that the negative charge present on the FPP molecule, its hydrophilicity and large molecular weight (745 Da) are together responsible for the poor transdermal permeation. The amount of FPP permeated across porcine epidermis within 30 min was significantly high (P = 0.01) compared to the amount permeated across hairless rat skin which could be attributed to structural differences between the two membranes (the membrane thickness and availability of follicular pathway).[10] In this study, porcine epidermis model was used, as it is known to be one of the most appropriate models for investigation of electrically mediated drug delivery technologies.[14,15]
Effect of chemical permeation enhancers on passive delivery of FPP
Initially an attempt was made to enhance the permeation of FPP with the help of chemical permeation enhancers. Chemical permeation enhancers are known to act on the lipid domains of the stratum corneum and enhance overall permeability of skin to drugs. The initial average electrical resistance and TEWL of the porcine epidermis were 35.21 ± 2.45 kΩcm2 and 6.41 ± 0.32 g/m2/h, respectively. Upon treatment with the permeation enhancers for 24 h, the electrical resistance of the epidermis decreased significantly by ~ 40%. The TEWL values were not significantly different from control (P > 0.05). The drop in electrical resistance in case of delipidized epidermis was ~ 60% and the TEWL was increased by ~ 2-fold. Zhao and Singh have also reported an increase in TEWL across porcine epidermis due to lipid extraction which is in agreement with our observations.[16] However, the FPP permeation was not enhanced significantly with the use of any of the enhancers (Table 1, P > 0.05). The amount of FPP permeated across the delipidized porcine epidermis was 10-fold higher than untreated epidermis (control). It was interesting to note that alteration of lipid domains with chemical permeation enhancers did not lead to enhancement in the permeation of FPP significantly, whereas extraction of lipids from the epidermis did. The reason one could speculate is that the chemical permeation enhancers are likely to alter the lipid domain partially and superficially which would probably create less number of continuous pathways through the stratum corneum as compared to complete delipidation in which the pathways are more likely to be continuous. It appears that FPP requires continuous pathways to penetrate across the stratum corneum.
Table 1.
Amount of FPP permeated across porcine epidermis in 24 h using different permeation enhancers. Data expressed as mean ± SD (n = 3).
| Enhancer | Concentration(%) | Amt of FPP permeated in 24 h (μg/cm2) |
|---|---|---|
| Control(no enhancer) | – | 31.13 ± 8.17 |
| Dimethyl sulfoxide | 5 | 36.23 ± 12.56 |
| Azone™ | 2 | 56.81 ± 17.89 |
| Menthol | 5 | 27.27 ± 6.91 |
| Ethanol | 5 | 48.77 ± 15.42 |
| Isopropyl myristate | 4 | 25.71 ± 9.41 |
| Sodium lauryl sulfate | 1 | 34.23 ± 20.12 |
Constant voltage iontophoresis mediated delivery
Constant voltage iontophoresis for a duration of 30 min at 0.5 V was not effective whereas at 2 V and 4 V, the permeation enhancement was 3- and 10-fold higher than control (0.78 ± 0.22 μg/cm2, Figure 1). The electrical resistance of the epidermis during constant voltage iontophoresis dropped significantly leading to increase in current density across the epidermis. However, the current density remained well below 0.5 mA/cm2 which is the maximum acceptable current density for human application from the safety perspective.[17,18]
Figure 1.
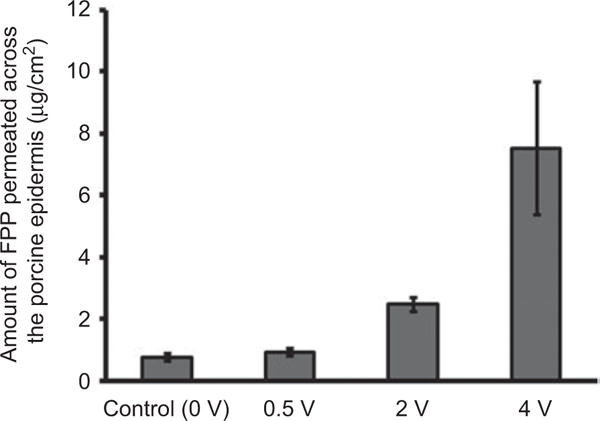
In vitro permeation of FPP across porcine epidermis on application of 0, 0.5, 2 and 4 V for 30 min. Data expressed as mean ± SD (n = 3).
Electroporation mediated delivery of FPP
Electroporation is a technique of application of short electrical pulses of moderate to high voltage to increase the permeability of skin.[19,20] The electrical pulses are known to create continuous transient aqueous pathways in the stratum corneum. In theory, the formation of continuous pathways (as in the case of delipidation of epidermis) was presumed to enhance the permeation of FPP. In these studies the pulse voltage (120 V) and the number of pulses (100 pulses) were fixed and only the pulse length and frequency was varied. The receiver compartment fluid was sampled immediately after cessation of pulses to assess the amount of FPP delivered due to electroporation of epidermis (or delivery during electroporation). The amount of FPP permeated by the application of different electrical protocols across porcine epidermis is represented in Figure 2.
Figure 2.
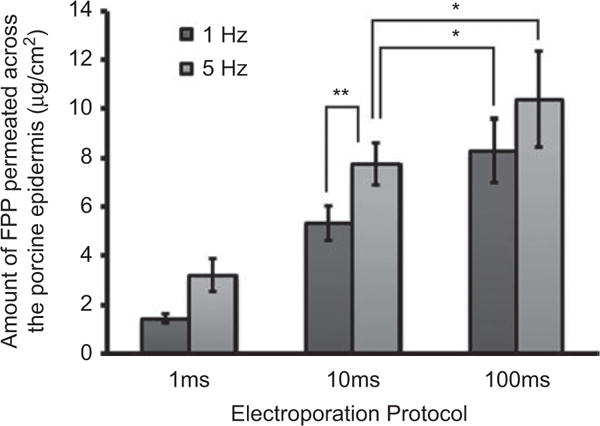
In vitro permeation of FPP across porcine epidermis on application of 120 V and 100 pulses at different pulse lengths (1, 10 or 100 ms) and frequencies (1 or 5 Hz). In case of control (passive permeation for 100 sec) experiments, detectable levels of FPP were not found when the studies were carried out for the same duration of time. Data expressed as mean ± SD (n = 3) (**P = 0.012, *P > 0.05).
There was significant enhancement (P < 0.05) in permeation of FPP with an increase in frequency at 1 ms and 10 ms pulse length. However, at 100 ms the permeation of FPP was not statistically significant between 1 Hz and 5 Hz. Moreover, the permeation at 100 ms pulses was not statistically different from 10 ms pulse protocol either. This was the reason why 10 ms and 5 Hz protocol was selected to assess the effect of combination of electroporation with iontophoresis. Nonetheless, it was found that, compared to the total transport when electroporation was combined with IN, the delivery by electroporation alone was less than 5%.
Constant voltage iontophoresis across electroporated epidermis
Iontophoresis was applied for 30 min across epidermis electroporated at 120 V, 100 pulses, 10 ms at 5 Hz. The current density across electroporated skin remained well below 0.5 mA/cm2. Again the transport of FPP was voltage-dependent and this time it was significantly higher than that observed across unelectroporated epidermis. Electroporation as a pretreatment could lead to significant enhancement in IN-mediated delivery of FPP. As evident from Figure 3, there was a remarkable enhancement in the delivery of FPP over control (7.75 ± 0.87 μg/cm2), significantly in the range of 2- to 42-fold.
Figure 3.
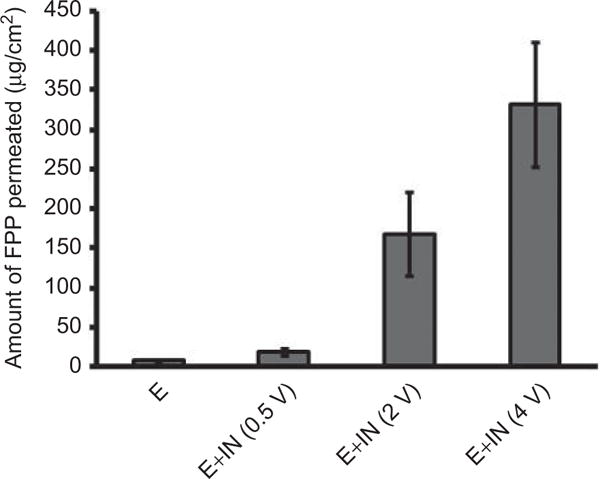
Amount of FPP permeated across electroporated porcine epidermis (120 V, 100 pulses of 10 ms pulse length at 5 Hz) followed by IN at different voltages for 30 min. ‘E’ represents amount of FPP permeated across electroporated epidermis without application of IN for a period of 30 min. Data expressed as mean ± SD (n = 3).
Conclusions
From the data obtained in this study, it is likely that a transdermal constant voltage iontophoretic patch of 20 cm2 can deliver ~ 6.5 mg of iron (near to therapeutic dose) within 30 min by the application of electroporation in combination with constant voltage iontophoresis for treating patients suffering from IDA.
Acknowledgments
This project was partially funded by Grant # HD061531A from Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD).
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75:671–678. [PubMed] [Google Scholar]
- 2.Tucker DM, Sandstead HH. Body iron stores and cortical arousal. In: Pollitt E, Leibel RL, editors. Iron deficiency: Brain biochemistry and behavior. New York: Raven Press Ltd; 1982. p. 161. [Google Scholar]
- 3.Milman N, Byg K, Bergholt T, Eriksen L, Hvas A. Body iron and individual iron prophylaxis in pregnancy-should the iron dose be adjusted according to serum ferritin? Ann Hematol. 2006;85:567–573. doi: 10.1007/s00277-006-0141-1. [DOI] [PubMed] [Google Scholar]
- 4.Crosby WH. The rationale for treating iron deficiency anemia. Arch Int Med. 1984;144:471–472. [PubMed] [Google Scholar]
- 5.Cook JD. Diagnosis and management of iron-deficiency anemia. Best Pract Res Clin Haematol. 2005;18:319–332. doi: 10.1016/j.beha.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Schultink W, van der Ree M, Matulessi P, Gross R. Low compliance with the iron-supplementation programw: A study among pregnant women in Jakarata, Indonesia. Am J Clin Nutr. 1993;57:135–139. doi: 10.1093/ajcn/57.2.135. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer RM, Schaefer L. Iron monitoring and supplementation: How do we achieve the best results? Nephrol Dial Transplant. 1998;13:9–12. doi: 10.1093/ndt/13.suppl_2.9. [DOI] [PubMed] [Google Scholar]
- 8.Hamstra RD, Block MH, Schocket AL. Intravenous iron dextran in clinical medicine. JAMA. 1980;243:1726–1731. [PubMed] [Google Scholar]
- 9.Zanen AL, Adriaansen HJ, van Bommel EFH, Posthuma R, Th de Jong GM. Oversaturation of transferrin after intravenous ferric gluconate (Ferrlecit B) in hemodialysis patients. Nephrol Dial Transplant. 1996;11:820–824. doi: 10.1093/oxfordjournals.ndt.a027405. [DOI] [PubMed] [Google Scholar]
- 10.Murthy SN, Vaka SRK. Irontophoresis™-Transdermal delivery of iron by iontophoresis. J Pharm Sci. 2009;98:2670–2676. doi: 10.1002/jps.21641. [DOI] [PubMed] [Google Scholar]
- 11.Morgan EH. Iron exchange between transferring molecules mediated by phosphate compounds and other cell metabolites. Biochim Biophys Acta. 1977;499:169–177. doi: 10.1016/0304-4165(77)90239-2. [DOI] [PubMed] [Google Scholar]
- 12.Ringbom A. In: Complexation in analytical chemistry: A guide for the critical selection of analytical methods based on complexation reactions in chemical analysis. Elving PJ, Kolthoff IM, editors. New York: Interscience Publishers; 1963. p. 16. [Google Scholar]
- 13.Gupta A, Amin NB, Besarab A. Dialysate iron therapy: Inclusion of soluble ferric pyrophosphate via the dialysate during hemodialysis. Kidney Int. 1999;55:1891–1898. doi: 10.1046/j.1523-1755.1999.00436.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferry LL, Argentieri G, Lochner DH. The comparative histology of porcine and guinea pig skin with respect to iontophoretic drug delivery. Pharma Acta Helv. 1995;70:43–56. doi: 10.1016/0031-6865(94)00050-6. [DOI] [PubMed] [Google Scholar]
- 15.Pliquett U, Gallo S, Hui SW, Gusbeth C, Neumann E. Local and transient structural changes in stratum corneum at high electric fields: Contribution of joule heating. Bioelectrochemistry. 2005;67:37–46. doi: 10.1016/j.bioelechem.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Zhao K, Singh J. In vitro percutaneous absorption enhancement of propranolol hydrochloride through porcine epidermis by terpenes/ethanol. J Control Release. 1999;62:359–366. doi: 10.1016/s0168-3659(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 17.Chang SL, Hofmann GA, Zhang L, Deftos LJ, Banga AK. Transdermal iontophoretic delivery of salmon calcitonin. Int J Pharm. 2000;200:107–113. doi: 10.1016/s0378-5173(00)00351-3. [DOI] [PubMed] [Google Scholar]
- 18.Singh P, Maibach HI. Iontophoresis: An alternative to the use of carriers in cutaneous drug delivery. Adv Drug Deliv Rev. 1996;18:379–394. [Google Scholar]
- 19.Denet AR, Vanbever R, Preat V. Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev. 2004;56:659–674. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Vanbever R, Preat V. In vivo efficacy and safety of skin electroporation. Adv Drug Deliv Rev. 1999;35:77–88. doi: 10.1016/s0169-409x(98)00064-7. [DOI] [PubMed] [Google Scholar]


