Abstract
An autoshaping procedure was used to test the notion that conditioned stimuli (CSs) gain greater incentive salience during adolescence than young adulthood under conditions of social isolation rearing and food restriction. Rats were single-housed and placed on food restriction during 10 daily training sessions in which a lever (CS+) was presented then followed immediately by a food unconditioned stimulus (US). A second lever (CS−) was presented on intermixed trials and was not reinforced. Despite the fact that food delivery was not contingent on the rats’ behavior, all rats exhibited behaviors directed towards the lever (i.e., sign-tracking). In the adolescent group, the rate of lever pressing and the percentage of trials with a lever press were higher than in young adults. Initially, group differences were observed when rats were retrained when the adolescents had reached young adulthood. These findings support the hypothesis that cues that come to predict reward become imbued with excessive motivational value in adolescents, perhaps contributing to the hyper-responsiveness to reward-related stimuli typically observed during this period of development.
INTRODUCTION
Adolescent humans and non-human animals alike exhibit increased novelty-seeking and exploratory behavior compared to either pre-adolescents or adults (Adriani, Chiarotti, & Laviola, 1998; Somerville, 2013; Meyer & Bucci, 2017; Stansfield & Kirstein, 2006). This behavioral phenotype is generally thought to be adaptive in that it promotes independence and facilitates gaining experience with the world, which are essential for an individual’s successful transition into adulthood (Crone & Dahl, 2012; Spear, 2010). However, heightened exploration and novelty-seeking can also lead to maladaptive risk-taking and impulsive behavior, increasing the risk of injury and substance abuse (Arnett, 1999; Casey & Jones, 2010; Chambers, Taylor, & Potenza, 2003; Spear, 2011). Accordingly, understanding the neural substrates and processes that contribute to behavior during adolescence is currently of great research interest.
One factor that is thought to have a particularly significant role in modulating behavior during adolescence is an increase in reward-sensitivity. Compared to adults, adolescents exhibit hyper-sensitivity to primary rewards (Somerville, Hare, & Casey, 2011; Steinberg, 2008; Adriani et al., 1998; Casey, Jones, & Hare, 2008; Fareri, Martin, & Delgado, 2008; Bjork et al., 2004; Geier, Terwilliger, Teslovich, Velanova & Luna, 2010; van Lejenhorst et al., 2010), as well as increased sensitivity to the rewarding qualities of drugs and alcohol (Vastola, Douglas, Varlinskaya, & Spear, 2002; Pautassi, Myers, Spear, Molina, & Spear, 2008; Vetter, Doremus-Fitzwater, & Spear, 2007). Importantly, adolescents also display increased responsiveness to otherwise neutral cues that come to predict reward (Galvan, 2013; Laviola, Marci, Morely-Fletcher & Adriani, 2003; Sturman & Moghaddam, 2011; Douglas, Varlinskaya, & Spear, 2003, 2004; Hare et al., 2008). In other words, in adolescents, conditioned stimuli (CSs) are more apt to elicit behaviors directed towards obtaining the reward. This is particularly significant in the context of drug use since an increased sensitivity to drug-related cues has been associated with addiction and substance abuse (Tomie, Grimes, & Pohorecky, 2008). One explanation for this increased sensitivity is that stimuli paired with the delivery of a drug become imbued with excessive incentive salience, or motivational value (Robinson & Berridge, 1993, 2001).
Incentive salience is often assessed through autoshaping procedures. In a standard autoshaping procedure in rats, like the one used in the present study, a lever serves as the CS+ and is presented for 10 sec. Upon lever retraction a food unconditioned stimulus (US) is immediately delivered into an adjacent food cup. A second lever is presented on intermixed trials but is not reinforced (CS−; Chang, Wheeler, & Holland, 2012a, 2012b; Chang, Todd, Bucci, & Smith, 2015; DeAngeli et al., 2015). Despite the fact that food delivery is not contingent on the rats’ behavior, they will typically approach the lever that is paired with food and exhibit a range of consummatory behaviors directed towards the lever (e.g., pressing, biting, etc.; Davey & Cleland, 1982). Collectively, these behaviors directed toward the CS+ lever are referred to as “sign-tracking” behaviors (Hearst & Jenkins, 1974). They are thought to reflect the acquisition of motivational value, or incentive salience of the CS per se, as a result of pairing the lever with food delivery (Berridge, 2004; Flagel et al., 2011). Sign-tracking behavior differs from “goal-tracking,” which consists of behavior that is directed toward the site of food delivery, such as approaching a food cup during presentation of the CS in anticipation of obtaining food reward (Boakes, 1977).
To date, the relatively few studies that have directly compared sign-tracking behavior in adolescent and adult rats have yielded somewhat mixed results. Several earlier studies found that sign-tracking behavior was greater in adult rats compared to adolescents (Anderson & Spear, 2011; Doremus-Fitzwater & Spear, 2011). However, a subsequent report indicated that under certain conditions the opposite effect is observed (Anderson, Bush, & Spear, 2013). Specifically,Anderson et al. (2013) revealed that adolescents exhibit more sign-tracking than adults when housed individually and food restricted during training. In contrast, in the same study, rats that were housed in pairs and/or on free-feeding regimens exhibited either no age differences or more sign-tacking in adults than adolescents, replicating earlier studies using those conditions (Anderson & Spear, 2011; Doremus-Fitzwater & Spear, 2011).
The sensitivity of sign-tracking during adolescence to factors such as social versus individual housing may be particularly relevant for understanding contemporary human behavior. For example, direct interpersonal contact is in significant decline, especially among adolescents (Pea et al., 2012). Social impoverishment might enhance the sensitivity to reward-related cues during a developmental stage in which individuals are already vulnerable to risky behavior. Thus, the present study sought to replicate and extend the finding that sign-tracking behavior is greater in adolescents compared to young adults under conditions of social isolation and food-restriction (Anderson et al., 2013). Unlike prior studies, a CS− lever was included to determine if any observed age differences were specific to a stimulus that was paired with reward, rather than a general increase in activity. In addition, since the level of sign-tracking behavior can differ among rat strains (Fitzpatrick et al., 2013), we used a different strain of rats than prior studies to test the robustness and generalizability of the effect. Further, it is unclear whether increased sign-tracking behavior during adolescence persists into adulthood. Thus, after 10 daily training sessions, rats were given a break period and then training resumed for an additional seven sessions when the adolescent group had reached young adulthood. Finally, we assessed whether prior experience in the autoshaping procedure influences subsequent sign-tracking behavior.
MATERIALS AND METHODS
Subjects
Sixteen male Long Evans rats were obtained from Envigo Laboratories (Indianapolis, IN). Rats were weaned from their dam on post-natal day (PND) 21 and one group of 8 rats were shipped and received on the same day of weaning. A second group of 8 rats was received in the same shipment, but were 46 days old upon arrival. All rats were allowed a 9-day period to acclimate to the vivarium with food (Purina standard rat chow; Nestle Purina) and water available ad libitum, the final 3 days of which they were weighed and handled daily. Rats were then separated into individual cages and body weights were gradually reduced over a 3-day period to 85% of the daily weight of free-feeding age-matched control rats using growth charts generated from over 60 subjects (provided by the vendor), after which the behavioral procedures began. All rats remained food restricted until completion of behavioral training, with supplemental rat chow provided after each daily session to maintain the daily target weight. Throughout the experiment, rats were monitored and cared for in compliance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Behavioral Apparatus
Behavioral procedures were carried out in eight standard conditioning chambers (Med Associates, Georgia, VT) enclosed in sound-attenuating cubicles (62 × 56 × 56 cm). Exhaust fans provided airflow and background noise (~68 dB). The chambers had aluminum front and back walls and the ceiling and sidewalls were clear acrylic. The floors consisted of stainless steel rods (5 mm diameter) spaced 1.5 cm apart (center to center). Each chamber was outfitted with a food cup recessed in the center of the front wall. Retractable levers served as the conditioned stimuli (CSs) and were positioned to the left and right of the food cup. The levers were 4.8 cm in width, positioned 6.2 cm above the grid floor, and protruded 1.9 cm when extended. Background illumination was provided by a 2.8-W house light mounted 15 cm above the grid floor on the back wall. Four panel lights were present in the chamber but not used in this experiment. Three of the panel lights were located 10.8 cm above the floor; two of them were positioned 6.4 cm to the left or right of the food cup, one was located directly above the food cup, and one was centered 15 cm above the floor. The unconditioned stimulus (US) was two 45-mg grain-based food pellets (Bioserv, Flemington, NJ). Each chamber was equipped with a pair of infrared photocells located across the entrance of the food cup. A computer in an adjacent room controlled the behavioral apparatus and collected information about lever presses and food cup entries. The cubicles also contained surveillance cameras used to monitor the rats during behavioral training.
Behavioral Procedure
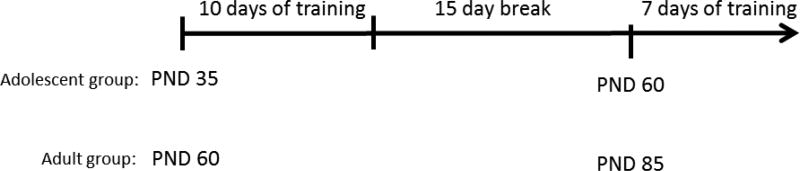
On the first day of the behavioral procedures, each rat was assigned to a chamber and received one 30-min magazine training session in which food pellets were delivered freely on a random time (RT) 30-s schedule, resulting in approximately 60 pellets being delivered. Conditioning in the autoshaping procedure began the following day (thus, on PND 35 for rats in the adolescent group and on PND 60 for the young adult group) and lasted for 10 days, as illustrated in Figure 1. Each daily conditioning session was 60-min in duration and consisted of 25 CS+ and 25 CS− trials with an average inter-trial interval (ITI) of 1 min. The CS+ trials consisted of a 10-sec extension of the lever and delivery of two food pellets upon retraction of the lever. The CS− trials consisted of a 10-sec extension with no delivery of food pellets. The trial order was random, although no more than two trials of the same type could occur consecutively. The levers were counterbalanced such that the CS+ lever was the right lever for half of the rats and the CS+ lever was the left lever for half of the rats.
Figure 1.
Schematic diagram of the experimental timeline. Rats in the Adolescent group began training in the autoshaping procedure on PND 35, while Adult rats began training on PND 60. After a 15 day break with no training, rats that were previously adolescents resumed training on PND 60. Rats in original Adult group resumed training on PND 85.
After the initial 10 training days, all rats remained food restricted and were weighed daily, but otherwise received no training or handling for the next 15 days in order to allow the rats in the adolescent group to reach young adulthood. After the 15-day break, daily conditioning sessions resumed for 7 days (referred to from here on out as ‘retraining’). Thus, rats that were previously trained as adolescents resumed training on PND 60 (i.e., the same age at which rats in the young-adult group began training) and rats that were in the young-adult group resumed training on PND 85.
Data Analysis
Sign-tracking
Sign-tracking behavior was assessed by recording the rate of lever pressing and the percentage of trials in which at least one lever press occurred. Prior to analysis, we tested for sphericity using Mauchly’s sphericity test. Not surprisingly, given the very low variance observed during CS− trials, there was a violation of sphericity for the rate of lever pressing and for percentage of trials with a lever press. As a result, the CS+ and CS− data were analyzed separately using a repeated measures ANOVA with Group (adolescent, young-adult) as the between-subjects variable and Session (days 1–10) as within-subjects variables.
Based on the prior finding of greater sign-tracking in adolescents compared to adults (Anderson et al., 2013), we predicted that the group difference would persist after the 15-day break. Thus, we conducted a planned comparison (corrected independent measures t-test) to assess group differences during the first session of re-training. Finally, we compared average lever responding in the young-adult group across sessions 1–7 of the original training phase to responding across retraining sessions 1–7 for the previously-adolescent group. Since these respective sessions occurred during PND 60–66 for both groups, this enabled us to assess whether prior experience in the autoshaping procedure influenced subsequent sign-tracking behavior.
Goal tracking
To assess goal-tracking behavior, the percentage of time during each lever presentation that the rat placed its snout into the food cup was calculated. Data from the initial phase of training (first 10 sessions) were analyzed using three-way mixed analyses of variance (ANOVAs) with Group (adolescent, young-adult) as the between-subjects variable and Cue (CS+, CS−) and Session (days 1–10) as within-subjects variables. Data from the retraining phase were similarly analyzed using a three-way ANOVA.
RESULTS
Lever pressing
Rate of responding
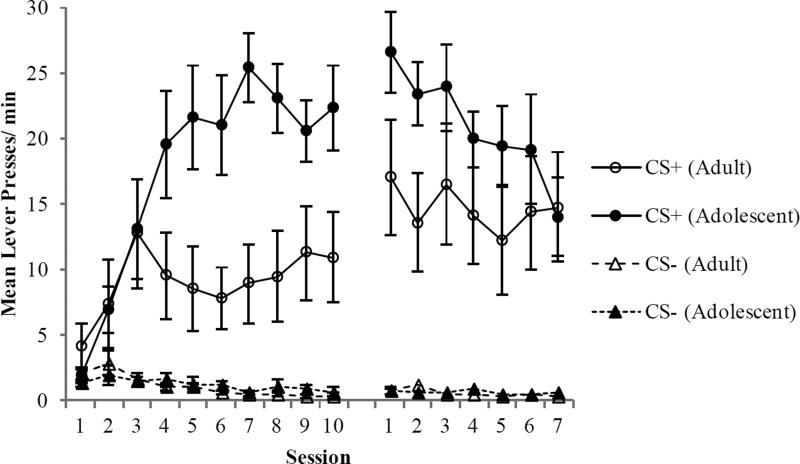
The rates of lever pressing by the two groups of rats are shown in Figure 2. For the initial 10 training sessions, a two-way ANOVA for the CS+ trials revealed that responding was higher in the adolescent rats compared to young adults. There were significant main effects of Group [F(1, 14) = 5.7, p < 0.03] and Session [F(9, 126) = 10.4, p < 0.001], and the a significant Group × Session interaction [F(9, 126) = 5.6, p < 0.001]. A two-way ANOVA for the CS− cue revealed a significant main effect of session [F(9, 126) = 4.6, p < 0.001], but no main effect of Group (p > 0.7) nor a Group × Session interaction (p > 0.5). Thus, adolescent rats exhibited higher rates of lever pressing during the CS+ than young adult rats, while no differences were observed in responding to the CS−.
Figure 2.
Lever pressing behavior (responses/min) during the autoshaping procedure. Compared to adults, adolescents exhibited an increase in sign-tracking as evidenced by significantly higher rates of responding when they were tested as adults. There were no group differences when training resumed after the adolescent group had reached adulthood. Data are means ± SEM.
During the first session of retraining after the 15 day break period, responding continued to be nominally higher in the group that had originally trained as adolescents compared to the group that began training as young adults but the difference did not reach statistical significance [t(14) = 1.8, p = 0.08]. The comparison between the average level of responding in the young-adult group during the initial training phase and the average level of responding in the previously-adolescent group during retraining revealed that despite being exactly the same age during these periods, the rats with prior autoshaping experience as adolescents exhibited significantly more sign tracking than rats with no prior experience [means were 20.8 ± 2.2 and 8.4 ± 2.8 presses/min, respectively; t(14) = 3.5, p < 0.003].
Percentage of trials with a response
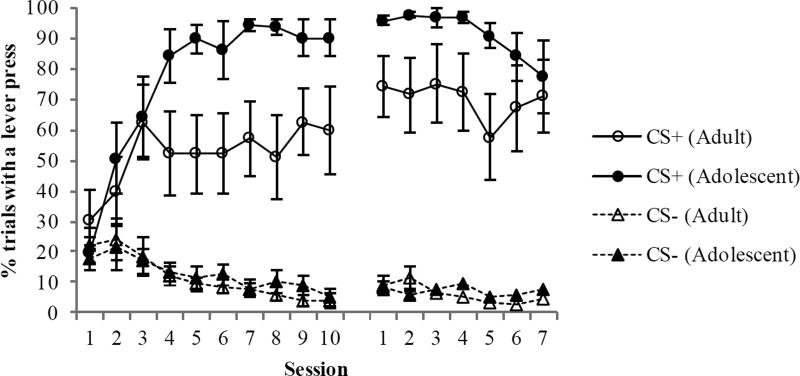
The percentage of trials that included a lever press is shown in Figure 3. Like the rate of lever pressing measure, we found that during CS+ trials, adolescent rats had a higher percentage of trials with a lever press than young adults. For the initial training phase, a repeated measures ANOVA revealed a significant Group × Session interaction [F(9, 126) = 3.4, p < 0.001], a significant main effect of Session [F(9, 126) = 11.6, p < 0.0001] and a marginally significant main effect of Group [F(1,14) = 4.4, p = 0.05]. Decomposition of the Group × Session interaction revealed that the percentage of trials with a response was higher in the adolescent group compared to the young-adult group during Sessions, 5, 7, 8, and 9 (ps < 0.05). A two-way ANOVA for the CS− cue revealed a significant main effect of Session [F(9, 126) = 6.0, p < 0.001], but no main effect of Group (p > 0.7) nor a Group × Session interaction (p > 0.9). Thus, the percentage of trials with a lever press was greater during several sessions in adolescent rats compared to young adult rats for the CS+, but comparable for the CS−.
Figure 3.
The percentage of trials in which a lever press occurred during the autoshaping procedure. Compared to adults, adolescents exhibited an increase in sign-tracking as evidenced by significantly higher percentage of trials with a lever press. There were no group differences when training resumed after the adolescent group had reached adulthood. Data are means ± SEM.
During the first session of retraining after the 15 day break period, the percentage of trials with a lever press continued to be higher in the group that had originally trained as adolescents compared to the group that began training as young adults [t(14) = 2.1, p < 0.05]. Finally, the comparison between the percentage of trials with a lever press in the young-adult group during the initial training phase and percentage of trials with a lever press in the previously-adolescent group during retraining revealed that the rats with prior autoshaping experience as adolescents exhibited significantly more sign tracking than rats with no prior experience [means were 91.1 ± 3.1% and 49.5± 9.9%, respectively; t(14) = 4.0, p < 0.002].
Food cup behavior
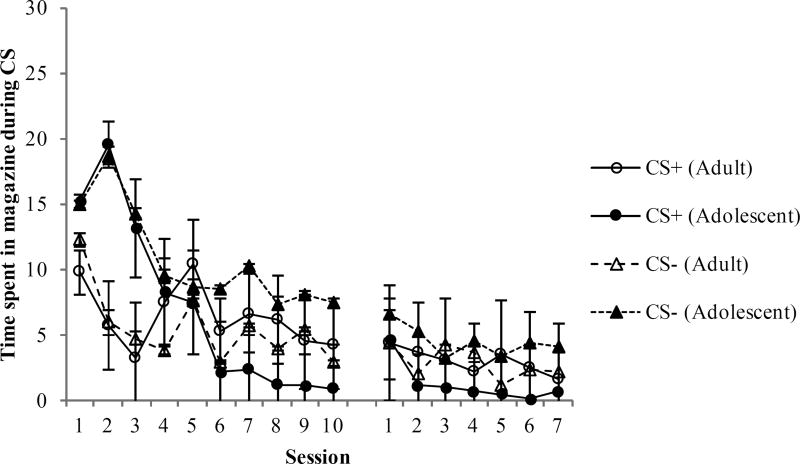
The percentage of time spent with the head in the food cup during lever presentation (i.e., goal-tracking) is shown in Figure 4. Adolescent rats exhibited more food cup behavior during presentation of either lever during the first few training sessions. Indeed, for the initial 10 training sessions, a three-way ANOVA revealed a main effect of Session [F(9, 126) = 7.1, p < 0.001] and a significant Group × Session interaction [F(9, 126) = 3.9, p < 0.001]. There were no other significant main effects or interactions (ps > 0.1). Follow-up analyses revealed that adolescents rats spent more time in the food cup during the first three sessions of training [F(1, 14) = 6.0, p < 0.03], but not for the last seven sessions (p > 0.8).
Figure 4.
Time spent with the snout in the food cup during presentation of the lever during the autoshaping procedure. Adolescent rats spent more time in the food cup during both the CS+ and the CS− on the first few sessions, but otherwise there were no group differences in food cup behavior (goal-tracking).
During the additional training sessions after all rats reached adulthood there were no group differences in food cup behavior. A three-way ANOVA revealed only a significant main effect of Session [F(6, 84) = 7.6, p < 0.001]. There were no other significant main effects (ps > 0.3) or interactions (ps > 0.2).
DISCUSSION
The present study tested for age differences in sign-tracking behavior in male Long-Evans rats that were housed in isolation and food-restricted during training. During each conditioning session, rats were presented with a lever that was paired with food reward (CS+) and a second lever that was not reinforced (CS−). Although food delivery was not contingent upon pressing the lever, we found that during the initial 10-session training period, the rate of lever pressing and the percentage of trials with a lever press were significantly higher in adolescent rats than young adult rats during CS+ trials, a difference that persisted over the course of the 10 sessions. In comparison, responding during presentation of the CS− lever was uniformly low in both groups, indicating that age differences were specific to the CS+ lever. Goal-tracking behavior was also increased in the adolescent group, but only transiently at the outset of training and the increase was observed during both CS+ and CS− presentations. This likely reflects an initial general increase in approaching the food cup when a lever was presented. Thus, unlike the persistent increase in sign-tracking behavior, adolescents exhibited only a temporary, non-specific change in goal-tracking behavior compared to young adults.
It possible that the increased lever pressing we observed in adolescents was due to differences in the effects of stress experienced during shipping, since rats in the adolescent group were shipped at a younger age than rats in the young-adult group. Indeed, stress early in life often has more significant negative consequences than stress occurring in adulthood (McCormick & Green, 2013). However, our findings replicate those of Anderson et al. (2013), who similarly reported greater sign-tracking in adolescents compared to adults specifically when rats were isolation-housed and food restricted. Importantly, rats in the Anderson et al. (2013) study were bred in-house, and thus not prone to potential differences in stress induced by shipping. Moreover, rats in the present study were allowed nine days to acclimate to the colony before behavioral training began. Finally, it is important to note that group differences did not begin to emerge until after several training sessions. Thus, it seems unlikely that stress associated with shipping can explain the differences in responding that were observed.
The present data extend the findings of Anderson et al. (2013) by demonstrating that the increased sign-tracking behavior in adolescence is specific for a cue paired with reward, since responding to the CS− lever was comparably low in both adolescents and young adults. Thus, the observed age effects cannot be attributed to a general increase in activity or lever pressing behavior during adolescence. In addition, reproducing this effect in a strain of rats different from the ones used by Anderson et al. (2013) suggests that effects of social isolation and food restriction on sign-tracking in adolescents transcend variables such as strain, which is significant given that the level of sign-tracking behavior can differ among rat strains (Fitzpatrick et al., 2013).
Consistent with the present findings and those of Anderson et al. (2013), other studies have found that exposure to impoverished environments during development leads to increased sign-tracking in adulthood, and conversely, exposure to enriched environments reduces sign-tracking (Beckmann & Bardo, 2012; Lomanowska et al., 2011). Anderson and colleagues (2013) suggested that one explanation for these differences is that pair-housing may mitigate the stressful effects of food restriction on sign-tracking. However, they found that adolescents that were housed in isolation actually had lower levels of corticosterone than pair-housed adolescents, contrary to this explanation. Alternatively, sign-tracking might be increased under conditions of food-restriction and isolation housing because of imbalance in dopaminergic reward systems (Anderson et al., 2013). Specifically, both food restriction and isolation can alter dopamine levels in midbrain reward regions (Branch et al., 2013; Pothos, Hernandez, & Hoebel, 1995). As a result, animals may be more sensitive to external rewards and reward-related cues, leading to increased incentive salience and responding to CSs to relieve an existing reward system deficiency. A similar explanation has been proposed to explain the high co-occurrence of schizophrenia and substance abuse, namely that drug use alleviates an underlying deficit in reward systems (Green et al., 1999). Consistent with this idea, it has been shown that physical exercise can alleviate opiate withdrawal symptoms, purportedly by re-balancing dopaminergic systems (Saedi, Marghmaleki, & Alaei, 2016; Stoutenberg, Rethorst, Lawson, & Read, 2016; Zarrinkalam et al., 2016).
Although the present study was not designed to determine the neurobiological differences that underlie increased sign-tracking in adolescents, a substantial body of research suggests that it is likely related to age-differences in midbrain dopamine systems. Sign-tracking is known to be mediated by the nucleus accumbens and its dopaminergic afferents from the ventral tegmental areas (Flagel et al., 2011; Chang et al., 2012b; Saunders & Robinson, 2012). Functional neuroimaging studies have revealed that during adolescence, the maturation of the nucleus accumbens outpaces that of the prefrontal cortex, resulting in a functional imbalance in activity between subcortical reward-related regions and cortical control systems (Galván et al., 2006; Casey et al., 2008, 2010; Doremus-Fitzwater & Spear, 2016; Tottenham & Galván, 2016). The resulting hyperactivity of the nucleus accumbens is thought to potentiate reward processing and reward-related behavior. Consistent with this theory, we recently used a chemogenetic approach to simultaneously increase neural activity in nucleus accumbens and decrease activity in prefrontal cortex in adult rats. We found that the resulting recapitulation of the functional imbalance observed during adolescence impaired the ability of rats to successfully use environmental cues to inhibit reward-related behavior (Meyer & Bucci, 2016b). Moreover, the magnitude of this effect was highly similar to the difference we observed when we compared the same behavior in normal adolescents and adults (Meyer & Bucci, 2014; 2017).
An additional finding in the present study was that the high levels of sign-tracking observed during adolescence tended to persist into young adulthood. Specifically, during the first session of the retraining phase, which took place after rats in the adolescent group had reached young adulthood, responding continued to be nominally higher in the group that was originally trained as adolescents. This effect only reached marginal statistical significance, likely due to the relatively high variance observed once training resumed. However, we also found that prior experience in the autoshaping procedure during adolescence resulted in more sign-tracking behavior during young adulthood compared to rats that were trained as young adults but had no prior experience with the procedure. This is consistent with prior work demonstrating that training in an autoshaping procedure during adolescence resulted in increased sign-tracking when rats were tested as adults, compared to a non-pre-trained adult group (Anderson & Spear, 2011).
Together with prior reports, the present findings further identify the conditions in which reward-related cues may garner increased incentive salience during adolescence. This is critically important for understanding the vulnerability of this age group to maladaptive behaviors. Indeed, increased sensitivity to rewards and reward-related cues during adolescence may enhance the likelihood in engaging in drug seeking behavior and developing drug addiction (Casey & Jones, 2010; Chambers et al., 2003; Spear, 2011). Prior studies have demonstrated a peak in sensitivity to rewarding stimuli during adolescence in both humans and laboratory animals (cf Doremus-Fitzwater & Spear, 2016). For example, relative to adults, adolescents exhibit enhanced preference and consumption for palatable foods and tastes (Vaidya et al., 2004; Wilmouth & Spear, 2009; Friemel et al., 2010; Galván & McGlennen, 2013) as well as sensitivity to the rewarding properties of alcohol and other drugs of abuse (Brenhouse et al., 2008; Pautassi et al., 2008; Torres et al., 2008). Adolescents also exhibit increases in responses that facilitate reward attainment, indicating enhanced motivation to obtain reinforcers during this period. For example, adolescent rats will exert greater effort to obtain a food reward than adults, including climbing over barriers (Stolyarova & Izquierdo, 2015) and completing higher lever press requirements (Friemel et al., 2010) as well as perseverating on response patterns that previously triggered reward delivery (Andrzejewski et al., 2011; Sturman et al., 2010). Likewise, self-reports of reward- and sensation seeking also peak during adolescence in humans (Steinberg et al., 2009; Romer et al., 2010).
Consistent with the present data, adolescent rats are also hyper-responsive to environmental cues and contexts that come to predict reward. For example, our laboratory has previously shown that adolescents are resistant to the extinction of a reward-predictive cue (CS+) and will continue to nose-poke into the magazine where food was previously delivered during presentation of the CS+ for significantly longer than adult counterparts (Meyer & Bucci, 2016a). Similarly, in a conditioned place preference paradigm adolescent rats spend more time than adults in a chamber that was previously paired with a rewarding stimulus, such as a novel object or another rat, relative to an alternative chamber (Douglas et al., 2003, 2004). Moreover, adolescent humans have been shown to exhibit robust differences in reward-related brain activity coupled with faster reaction times and reduced inhibitory control when presented with appetitive stimuli (Galván et al., 2006; Somerville et al., 2011; Geier et al., 2010; Steinberg 2010).
In summary, the results of the present study indicate that appetitive CSs can gain greater motivational value in adolescents compared to young adults under conditions of environmental stressors such as social impoverishment and food restriction. Increased reward seeking may have an adaptive function for adolescent development; in particular, the enhanced salience of feedback from interactions with the environment may serve to facilitate cognitive processes and contribute to the development of emotional regulation (Spear, 2000; Casey, 2015; Telzer, 2016). At the same time, heightened incentive salience during adolescence may contribute to an increased vulnerability to engage in maladaptive behavior, such as drug use. Indeed, addiction and substance abuse are associated with increased sensitivity to drug-related cues, resulting from the sensitization of midbrain dopamine neurons in response to drugs that imbues the drug-related stimuli with excessive incentive salience (Robinson & Berridge, 1993, 2001). Moreover, it is during adolescence that individuals are particularly vulnerable to developing drug abuse and addiction (U.S. DHHS, 2014).
Acknowledgments
Research supported by NIH grants R01DA027688 to D.J.B. and F31MH107138 to H.C.M, and a First-Year Summer Research Award from the Dartmouth College Undergraduate Deans Office and the Office of Undergraduate Advising and Research to S.B.M. The authors thank Dr. Stephen Chang for comments on prior versions of the manuscript.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behavioral Neuroscience. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Spear LP. Autoshaping in adolescence enhances sign-tracking behavior in adulthood: impact on ethanol consumption. Pharmacology, Biochemistry, and Behavior. 2011;98(2):250–60. doi: 10.1016/j.pbb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Bush PC, Spear LP. Environmental manipulations alter age differences in attribution of incentive salience to reward-paired cues. Behavioural Brain Research. 2013;257:83–9. doi: 10.1016/j.bbr.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski ME, Schochet TL, Feit EC, Harris R, Mc Kee BL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behavioral Neuroscience. 2011;125:93–105. doi: 10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Adolescent storm and stress, reconsidered. The American Psychologist. 1999;54:317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Bardo MT. Environmental enrichment reduces attribution of incentive salience to a food-associated stimulus. Behavioral Brain Research. 2012;226:331–4. doi: 10.1016/j.bbr.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiology and Behavior. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–80. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes R. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant–Pavlovian interactions. Lawrence Erlbaum Associates; Hillsdale, NJ: 1977. pp. 67–97. [Google Scholar]
- Branch SY, Goertz RB, Sharpe AL, Pierce J, Roy S, Ko D, Paladini CA, Beckstead MJ. Food restriction increases glutamate receptor-mediated burst firing of dopamine neurons. Journal of Neuroscience. 2013;33:13861–7. doi: 10.1523/JNEUROSCI.5099-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen S. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behavioral Neuroscience. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galván A. The adolescent brain and risky decisions. Developmental Reviews. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, Somerville LH. The storm and stress of adolescence: insights from human imaging and mouse genetics. Developmental Psychobiology. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. The American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Wheeler DS, Holland PC. Effects of lesions of the amygdala central nucleus on autoshaped lever pressing. Brain Research. 2012a;1450:49–56. doi: 10.1016/j.brainres.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Wheeler DS, Holland PC. Roles of nucleus accumbens and basolateral amygdala in autoshaped lever pressing. Neurobiology of Learning and Memory. 2012b;97:441–451. doi: 10.1016/j.nlm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Todd TP, Bucci DJ, Smith KS. Chemogenetic manipulation of ventral pallidal neurons impairs acquisition of sign-tracking in rats. European Journal of Neuroscience. 2015;42:3105–3116. doi: 10.1111/ejn.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- DeAngeli NE, Todd TP, Chang S, Yeh HH, Yeh P, Bucci DJ. Exposure to kynurenic acid during adolescence impacts autoshaping and long-term potentiation in adulthood. Frontiers in Behavioral Neuroscience. 2015;8 doi: 10.3389/fnbeh.2014.00451. Article 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Reward-centricity and attenuated aversions: An adolescent phenotype emerging from studies in laboratory animals. Neuroscience and Biobehavioral Reviews. 2016;70:121–134. doi: 10.1016/j.neubiorev.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behavioral Neuroscience. 2011;125:661–7. doi: 10.1037/a0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiology and Behavior. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Developmental Psychobiology. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Fareri D, Martin L, Delgado M. Reward-related processing in the human brain: Developmental considerations. Development and Psychopathology. 2008;20:1191–1211. doi: 10.1017/S0954579408000576. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, Yager LM, Meyer PJ, Lovic V, Saunders BT, Parker CC, Gonzales NM, Aryee E, Flagel SB, Palmer AA, Robinson TE, Morrow JD. Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague–Dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PLoS One. 2013;8:e75042. doi: 10.1371/journal.pone.0075042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friemel CM, Spanagel R, Schneider M. Reward sensitivity for a palatable food reward peaks during pubertal developmental in rats. Frontiers in Behavioral Neuroscience. 2010;4:39. doi: 10.3389/fnbeh.2010.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. The Teenage Brain Sensitivity to Rewards. Current Directions in Psychological Science. 2013;22:88–93. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A, McGlennen K. Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. Journal of Cognitive Neuroscience. 2013;25:284–296. doi: 10.1162/jocn_a_00326. [DOI] [PubMed] [Google Scholar]
- Galván A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AI, Zimmet SV, Strous RD, Schildkraut JJ. Clozapine for comorbid substance use disorder and schizophrenia: do patients with schizophrenia have a reward deficiency syndrome that can be ameliorated by clozapine? Harvard Review of Psychiatry. 1999;6:287–296. doi: 10.3109/10673229909017206. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst E, Jenkins H. Sign-Tracking: The Stimulus-Reinforcer Relation and Directed Action (Monograph of the Psychonomic Society 1974) 1974. [Google Scholar]
- Lomanowska AM, Lovic V, Rankine MJ, Mooney SJ, Robinson TE, Kraemer GW. Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behavioral Brain Research. 2011;220:91–9. doi: 10.1016/j.bbr.2011.01.033. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Green MR. From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience. 2013;249:242–57. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- Meyer HC, Bucci DJ. Non-linear development of negative occasion setting. Behavioural Processes. 2017;137:33–39. doi: 10.1016/j.beproc.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Bucci DJ. Age differences in appetitive Pavlovian conditioning and extinction in rats. Physiology & Behavior. 2016a;167:354–362. doi: 10.1016/j.physbeh.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Bucci DJ. Imbalanced activity in the orbitofrontal cortex and nucleus accumbens impairs behavioral inhibition. Current Biology. 2016;26:2834–2839. doi: 10.1016/j.cub.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Bucci DJ. The ontogeny of learned inhibition. Learning & Memory. 2014;21:143–152. doi: 10.1101/lm.033787.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pea R, Nass C, Meheula L, Rance M, Kumar A, Bamford H, Nass M, Simha A, Stillerman B, Yang S, Zhou M. Media use, face-to-face communication, media multitasking, and social well-being among 8- to 12-year-old girls. Developmental Psychology. 2012;48:327–36. doi: 10.1037/a0027030. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Hernandez L, Hoebel BG. Chronic food deprivation decreases extracellular dopamine in the nucleus accumbens: implications for a possible neurochemical link between weight loss and drug abuse. Obesity Research Supplement. 1995;4:525S–529S. doi: 10.1002/j.1550-8528.1995.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcoholism: Clinical and Experimental Research. 2008;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–14. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Romer D, Duckworth AL, Sznitman S, Park S. Can adolescents learn self-control? Delay of gratification in the development of control over risk taking. Prevention Science. 2010;11:319–330. doi: 10.1007/s11121-010-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedi Marghmaleki V, Alaei H. Effect of Treadmill Running on Morphine Dependence Before and After Medial Prefrontal Cortex Lesion in Rats. Asian Journal of Sports Medicine. 2016;7:e35181. doi: 10.5812/asjsm.35181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. European Journal of Neuroscience. 2012;36:2521–32. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH. The teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science. 2013;22:121–127. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The behavioral neuroscience of adolescence. New York: Norton; 2010. [Google Scholar]
- Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: Setting the stage for alcohol use disorders? Child Development Perspectives. 2011;5:231–238. doi: 10.1111/j.1750-8606.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Developmental Psychobiology. 2006;48:10–5. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Reviews. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Development. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Stolyarova A, Izquierdo A. Distinct patterns of outcome valuation and amygdala-prefrontal cortex synaptic remodeling in adolescence and adulthood. Frontiers in Behavioral Neuroscience. 2015;9:115. doi: 10.3389/fnbeh.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoutenberg M, Rethorst CD, Lawson O, Read JP. Exercise training - A beneficial intervention in the treatment of alcohol use disorders? Drug and Alcohol Dependence. 2016;160:2–11. doi: 10.1016/j.drugalcdep.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B. Reduced neuronal inhibition and coordination of adolescent prefrontal cortex during motivated behavior. Journal of Neuroscience. 2011;31:1471–1478. doi: 10.1523/JNEUROSCI.4210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman DA, Mandell DR, Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behavioral Neuroscience. 2010;124:16–25. doi: 10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH. Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience. 2016;17:57–67. doi: 10.1016/j.dcn.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Research Reviews. 2008;58:121–35. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O'Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacology, Biochemistry and Behavior. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Galván A. Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neuroscience and Biobehavioral Reviews. 2016;70:217–227. doi: 10.1016/j.neubiorev.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- Vaidya JG, Grippo AJ, Johnson AK, Watson D. A comparative developmental study of impulsivity in rats and humans: the role of reward sensitivity. Annals of the New York Academy of Sciences. 2004;1021:395–398. doi: 10.1196/annals.1308.051. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiology and Behavior. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary access conditions. Alcoholism Clinical and Experimental Research. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: taste reactivity and voluntary sucrose consumption. Pharmacology, Biochemistry and Behavior. 2009;92:566–573. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinkalam E, Heidarianpour A, Salehi I, Ranjbar K, Komaki A. Effects of endurance, resistance, and concurrent exercise on learning and memory after morphine withdrawal in rats. Life Science. 2016;157:19–24. doi: 10.1016/j.lfs.2016.05.034. [DOI] [PubMed] [Google Scholar]