Abstract
Attention is critical for effective processing of incoming information and has long been identified as a potential area of dysfunction in people with schizophrenia (PSZ). In the realm of visual processing, both spatial attention and feature-based attention are involved in biasing selection toward task-relevant stimuli and avoiding distraction. Evidence from multiple paradigms has suggested that PSZ may hyperfocus and have a narrower “spotlight” of spatial attention. In contrast, feature-based attention seems largely preserved, with some suggestion of increased processing of stimuli sharing the target-defining feature. In the current study, we examined the spatial profile of feature-based distraction using a task in which participants searched for a particular color target and attempted to ignore distractors that varied in distance from the target location and either matched or mismatched the target color. PSZ differed from healthy controls in terms of interference from peripheral distractors that shared the target-color presented 200 ms before a central target. Specifically, PSZ showed an amplified gradient of spatial attention, with increased distraction to near distractors and less interference to far distractors. Moreover, consistent with hyperfocusing, individual differences in this spatial profile were correlated with positive symptoms, such that those with greater positive symptoms showed less distraction by target-colored distractors near the task-relevant location.
Keywords: Attention, schizophrenia, attentional capture, positive symptoms
Introduction
Everyday life demands dynamic adjustment of attention to balance the pursuit of current goals with the processing of other potentially important information from the environment. People with schizophrenia (PSZ) are typically impaired in measures of daily functioning (Green, Kern, & Heaton, 2004), and attentional dysfunction has long been implicated in this disease (Bleuler, 1911; Nuechterlein & Dawson, 1984). However, attention is a complex construct involving multiple interacting neural mechanisms that differentially impact performance, making it challenging to uncover the specific nature of attentional dysfunction in schizophrenia (Hemsley, 1975; Luck & Gold, 2008).
One fundamental attribute of attention is that a subset of information is given privileged processing, increasing its access to limited capacity memory stores and influence over behavior. This can occur in a variety of different domains, with attentional biasing of locations and features being of prime importance. These mechanisms are critical for interacting with the visual world, which involves optimizing information processing of spatially distributed input that varies in featural relevance to the observer. Spatial attention involves the facilitation of specific locations over others, and is associated with behavioral benefits (e.g.,Posner, Snyder, & Davidson, 1980), increases in early sensory activity (e.g., Martinez et al., 1999), and improved visual sensitivity for stimuli presented at attended locations (Bashinski & Bacharach, 1980). Spatial attention is often allocated to the point of fixation. That said, spatial attention will shift covertly to a peripheral location prior to making an eye movement to that location (Hoffman & Subramaniam, 1995; Kowler, Anderson, Dosher, & Blaser, 1995). For example, a person may fixate a friend’s face if there is uncertainty about whether a person is upset or not, using spatial attention to improve the processing of sensory detail. If the friend were to make a hand gesture, a person may then shift spatial attentional processing to this new potentially relevant information.
Attentional mechanisms also operate in nonspatial domains such as color, leading to a prioritization of sensory inputs that match attended feature values (Leonard & Egeth, 2008; Leonard, Lopez-Calderon, Kreither, & Luck, 2013; Saenz, Buracas, & Boynton, 2002; Treisman & Gelade, 1980; Wolfe, 1994). For example, if an observer knows a lost friend is wearing an orange shirt, feature-based attention would be used to facilitate the processing of people in the crowd wearing this color. This would help prioritize a location in the periphery, leading to a shift of spatial attention for further detailed examination. Together, feature-based attention and shifts of spatial attention help typical observers facilitate currently relevant information at fixation and also detect other potentially relevant information in the periphery for subsequent analysis.
Counterintuitively, recent work has suggested that attentional dysfunction in PSZ may be characterized by more intense processing of a smaller subset of information compared to healthy control subjects (HCS). This hyperfocusing hypothesis accounts for aberrant patterns of reaction time, accuracy, eye movements, and event-related potentials (ERPs), including experiments in which PSZ exhibit enhanced attentional benefits and enhanced working memory-related neural activity compared to HCS (e.g., Gray et al., 2014; Hahn et al., 2012; Leonard, Kaiser, et al., 2013; Luck et al., 2014).
With regard to hyperfocusing in the spatial domain, a large set of cuing experiments has suggested a narrowed but more intense scope of spatial attention in PSZ. PSZ show increased behavioral benefits when informed about the upcoming target location compared to when provided with a spatially-nonpredictive cue (Bustillo et al., 1997; Gold et al., 1992; Hahn et al., 2012; Liotti, Dazzi, & Umilta, 1993; Sapir, Henik, Dobrusin, & Hochman, 2001; Spencer et al., 2011). Similarly, other findings have suggested that PSZ are impaired in spreading attention broadly in space (Elahipanah, Christensen, & Reingold, 2010, 2011; Gray et al., 2014; Hahn et al., 2012). For example, Gray et al. (2014) found that PSZ were severely impaired at the Useful Field of View (UFOV) task—which requires simultaneous processing at fixation and in the periphery—suggesting a failure to distribute spatial attention broadly. Thus, PSZ may experience greater benefits when the task calls for narrow but intense focusing of spatial attention and greater costs when the task calls for a broad distribution of spatial attention.
In the feature domain, PSZ often show not only unimpaired feature-based attention (Clementz, Wang, & Keil, 2008; Mori et al., 1996), but even enhanced biasing consistent with hyperfocusing (Sawaki et al., in press). For example, PSZ can limit visual working memory encoding to objects of a relevant color or shape (Gold et al., 2006) and even showed greater filtering of objects possessing the irrelevant color or shape, suggesting an over-commitment to objects with the relevant feature. Similarly, attention is often captured by objects that match the current contents of working memory (Hollingworth, Matsukura, & Luck, 2013; Olivers, Meijer, & Theeuwes, 2006), and Luck et al. (2014) found that this effect was larger in PSZ compared to HCS, suggesting unusually intense working memory representations. Consistent with this, Leonard, Kaiser, et al. (2013) found that an electrophysiological signature of working memory maintenance was larger in PSZ relative to HCS when maintaining one object in working memory and ignoring a simultaneously presented distractor object.
Both spatial attention and feature-based attention are critical for functional visual perception, which involves processing a complex spatially-distributed input that varies in relevance to the observer. Leonard, Balestreri, and Luck (2015) recently investigated the interaction between spatial attention and feature-based biasing in typical college students using a behavioral measure of distractor interference. In that task, as well as the one used in the current experiment, participants viewed a rapid serial visual presentation (RSVP) stream of letters at the display center and searched for a target letter of a specific color within that stream. On some trials, irrelevant distractors were presented at locations that varied in distance from the task-relevant central stream (see Figure 1). These distractors could either share a feature with the target (target-colored distractors) or not (irrelevant-colored distractors). The results showed that target-colored distractors occurring 200 ms before target onset interfere with target processing, replicating previous research (Folk, Leber, & Egeth, 2002, 2008). Distractors presented at 500 ms typically show little interference, as participants would have time to reorient back to the central stream. This is called contingent capture of attention, because the ability of the distractor to capture attention is contingent on the match between the distractor and the currently attended color (as opposed to capture based on bottom-up physical salience). Critically, Leonard et al. (2015) found that this feature-based distraction depends on the distractor’s distance from the currently attended location, suggesting that spatial attention gates interference from target-like distractors in typical individuals.
Figure 1.
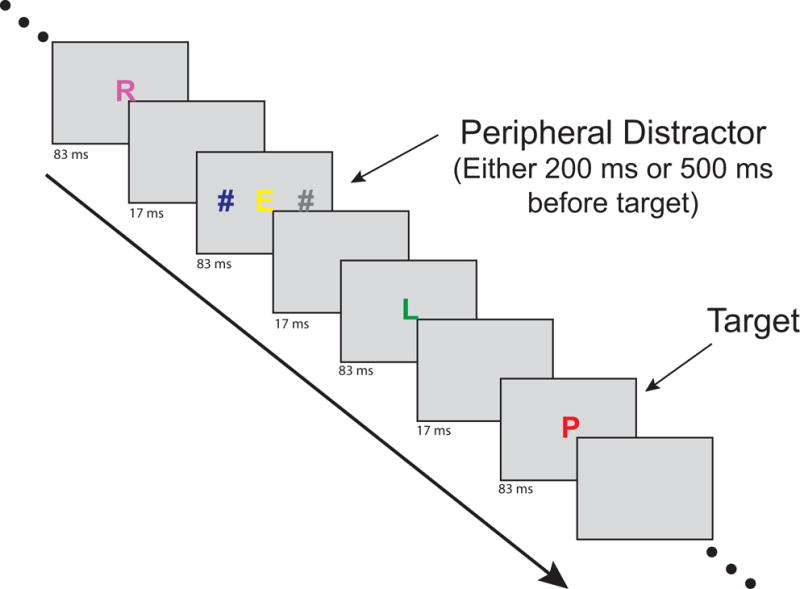
Example of timing and stimuli from the rapid serial visual presentation task in a distractor-present trial. Note that this is abbreviated as actual trials contained more letter frames.
In the current study, we used this same paradigm to examine how target similarity and spatial distance influence distraction in PSZ. The main question was whether PSZ would show a narrower focus of attention than HCS around the central, task-relevant location. This would be expected to lead to an amplified gradient of attention in PSZ, with greater distraction in PSZ than in HCS when the target-colored distractor was close to the task-relevant location and a sharper fall-off of distraction effects in PSZ than in HCS as the distractor was moved farther away. In addition, we predicted that PSZ and HCS would show equivalent levels of distraction by irrelevant-colored distractors. If PSZ are simply more distractible in general than HCS, then they should show enhanced interference from the distractor whether or not it possesses the target color. However, if PSZ are able to focus feature-based attention, as suggested by prior research, then only distractors sharing a target feature should result in task interference.
Methods
Participants
Forty-one people who met the criteria for schizophrenia (N=33) or schizoaffective disorder (N=8) and 45 HCS took part in this experiment. Four PSZ were excluded due to outlier performance, as defined by accuracy on no-distractor trials that was greater than 2 standard deviations below mean group performance (< 33% correct). The clinical description provided below and the demographics in Table 1 refer to the remaining 45 HCS and 37 PSZ (N=8 schizoaffective) included in the analyses.
Table 1.
Demographic information for sample.
| HCS N = 45 |
PSZ N = 37 |
Stats | |
|---|---|---|---|
| Age | 40.1 | 38.1 | t(80) = 0.85, p = 0.40 |
| Education (yrs) | 15.3 | 13.1 | t(80) = 4.7, p < 0.01 |
| Parental Education (yrs)1 | 14.3 | 13.9 | t(80) = 0.54, p = 0.59 |
| Male/Female (M:F) | 31:14 | 24:13 | χ2(1) = 0.15, p = 0.70 |
| Race (AA:W:O) | 14:28:3 | 11:21:5 | χ2(2) = 1.09, p = 0.58 |
Parental education is the average years of mother and father when both are available. Three participants in the HCS group and three participants in the PSZ group were only able to report education information about a single parent.
Diagnosis was based on the standard operational criteria in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM–IV–TR). A best estimate approach was used to establish diagnosis by combining material from past medical records, collateral informants (when available), and the results of the Structured Clinical Interview for DSM–IV–TR Axis I disorders (SCID-I). Final diagnosis was reached at a consensus conference. Symptom ratings were collected using the Brief Psychiatric Rating Scale (BPRS;Overall & Gorham, 1962), and composite factor scores were created for positive symptoms (BPRS-POS: grandiosity, suspiciousness, hallucinations, and unusual thought), negative symptoms (BPRS-NEG: blunted affect, uncooperativeness, motor retardation, emotional withdrawal), and disorganized symptoms (BPRS- DIS: concept disorganization, tension, mannerisms, excitement, disorientation). The PSZ were clinically stable outpatients who had been receiving the same antipsychotic medications, at the same dose, for at least 4 weeks before participation. PSZ were receiving the following types of medications: 3 were receiving first generation drugs as monotherapy, whereas 27 were taking second-generation antipsychotic monotherapy with clozapine being used most frequently (17 cases). In addition, 4 people were taking a combination of two second-generation antipsychotics, whereas 3 people were taking both first and second generation antipsychotics. Antidepressants were used by 19 people, 7 used anti-anxiety medications, 8 used mood stabilizers, and 5 used anticholinergics. Dosages were converted into chlorpromazine and haloperidol equivalents using the methods described by Andreasen, Pressler, Nopoulos, Miller, and Ho (2010).
No significant differences were found between groups in age, race, sex, parental education, or handedness. As is typically found, the number of years of education was lower for PSZ, presumably because disease onset limits education attainment. Demographic information and statistical comparisons are provided in Table 1. For 43 HCS and 34 PSZ, the Wechsler Abbreviated Scale of Intelligence (WASI IQ; Wechsler, 1999) was administered, with PSZ performing significantly lower than HCS (103.8 vs. 116.8, respectively, t(75) = 4.7, p < 0.001).
All participants were free of other medical or neurologic comorbidity that might influence performance, including substance abuse or dependence within the last 12 months. This protocol was approved by the Institutional Review Board at the University of Maryland School of Medicine (#HP-00054557). All participants gave written informed consent before taking part in the study.
Stimuli
Stimuli were shown in a dimly lit room on a CRT monitor (60 Hz) with a gray background (46 cd/m2), with participants seated at a viewing distance of 70 cm. On each trial, a RSVP stream of 15 letters was presented at fixation (see Figure 1). Each letter appeared on the screen for 83 ms followed by a 17 ms blank frame, with the target letter occurring randomly between positions 8 and 12 in the stream. Letters were chosen without replacement (excluding QIOWM). Each letter subtended approximately 1.6 × 0.6° of visual angle. Depending on the instructions given for the block, the target was drawn in either red or blue. The colors of other letters in the stream were randomly chosen from the nontarget colors, which also included magenta, yellow, and green (all colors 11.9–12.7 cd/m2).
Distractor stimuli were hashtag characters (#) presented bilaterally for one 83 ms frame on 80% of trials. One of these lateralized distractors was always dark gray (12.1 cd/m2) and the other possessed the current target color (target-colored distractor trial) or the nontarget color (irrelevant-color distractor trial). Depending on the trial, these distractors (approximately 0.68 × 1.4° in size) were centered at 1, 1.5, 2.5, or 4.5° from the fixation point. The distractors always preceded the target stimulus and the stimulus onset asynchrony (lag) between the peripheral distractor frame and the target frame was either 200 or 500 ms.
Each participant performed two blocks of the task, with red as the target color in one block and blue as the target color in the other block (with order randomized across participants). Target-colored distractor trials, irrelevant-color distractor trials, and no-distractor trials were randomly intermixed. In total, 400 experimental trials were presented, including 80 no-distractor trials. There were 160 trials containing target-colored distractors and another 160 trials with irrelevant-colored distractors. For both of these distractor types, there were 20 trials for each pairing of lag (200 ms or 500 ms) and distractor distance (1, 1.5, 2.5, and 4.5°).
Task
Participants performed several short practice blocks until they were comfortable with the task. At first, the RSVP stream was presented at half speed without any peripheral distractors. In a second practice block, peripheral distractors were added on 80% of trials (as in the main experiment). A third practice block was completed at full speed with peripheral distractors on 80% of trials, followed by a final fourth practice block which was identical to the final experimental conditions. We examined the number of trials spent on each training stage in each group, and we found no significant differences in training time between groups for any of these stages (all p > 0.19). Participants were instructed to orally report the identity of the target letter at the end of the trial. They were also informed that the ‘#’ signs were irrelevant to the task and should be ignored. The task took ~45 minutes to complete.
Results
No-Distractor Trials
Letter report accuracy on the no-distractor trials provides a baseline against which to measure interference from distractor present trial types. Both groups performed this challenging task well above chance, with mean accuracy of 70.3% for HCS and 64.5% for PSZ (chance performance is 3.8%), indicating that both groups understood the instructions and were motivated. The difference in accuracy between HCS and PSZ was only a trend, t(80) = 1.88, p = 0.064, Cohen’s d = 0.41. However, interindividual differences in performance among both HCS and PSZ were large, and a normalized measure was therefore used to quantify distraction in subsequent analyses.
In PSZ, there was a significant negative correlation between no-distractor performance and the Brief Psychiatric Rating Scale composite positive symptom score (BPRS-POS) (Spearman’s rho = −0.32, p = 0.05), which is further considered in the Discussion. Although PSZ varied widely in their levels of negative symptoms, there was no correlation between no-distractor performance and a BPRS negative symptom factor score (Spearman’s rho = 0.02, p =0.98). Likewise, there was no significant relationship between no distractor performance and a factor score for disorganized symptoms (Spearman’s rho = 0.09, p = 0.60).
Capture Cost
In this task, the target-color distractor typically leads to impaired target detection performance. The degree of impairment is called the capture cost and is quantified as the reduction in accuracy on distractor-present trials relative to the no-distractor baseline trials. To minimize any confounding effects of the large interindividual and modest group differences in overall task performance, we created a normalized capture cost measure for each trial type by dividing the capture cost by the sum of the distractor present and distractor-absent accuracy. In other words, normalized capture cost = (distractor-absent accuracy − distractor-present accuracy) ÷ (distractor-absent accuracy + distractor-present accuracy).
Figure 2 shows normalized capture cost as a function of distractor distance, separately for the target-colored distractor trials (left panel) and the irrelevant-colored distractor trials (right panel). Consistent with previous work, both groups showed the expected effects of distractor timing, relevance and distance: capture costs mainly occurred for distractors that possessed the task-relevant color presented 200-ms before the target, and these costs were more pronounced with greater proximity to the target location (the upper-left panel in Figure 2). However, the spatial profile of distraction for the target-colored distractor trials appeared to differ between groups, with PSZ showing greater capture costs than HCS for distractors nearer to the central RSVP stream.
Figure 2.
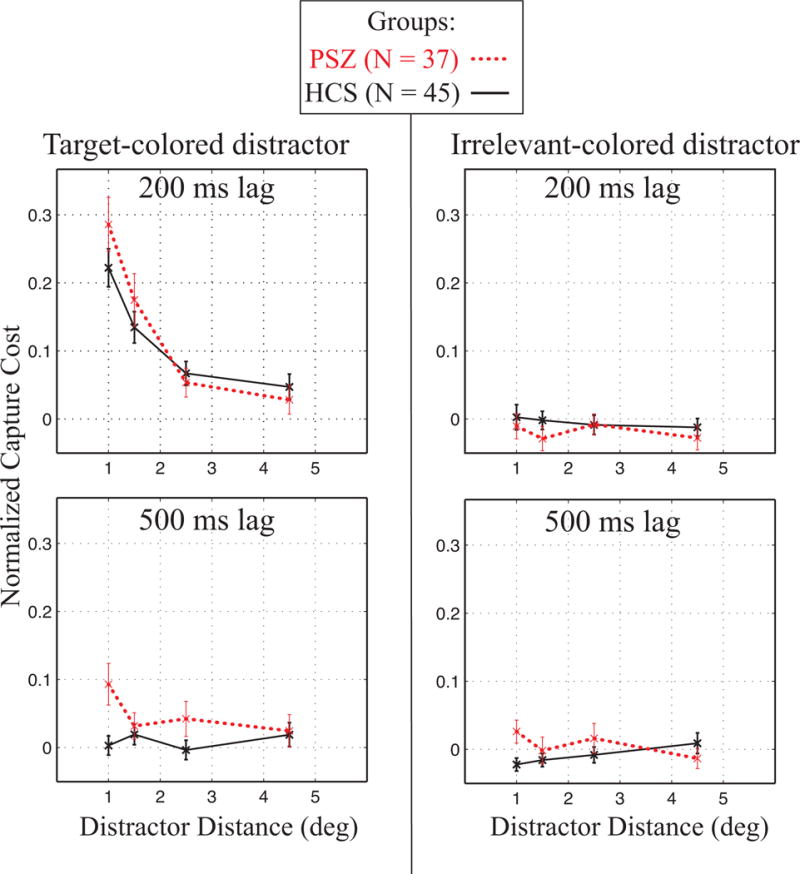
Normalized capture cost as a function of distractor distance. Left column shows data from the target-colored distractor trials and right column form the irrelevant-colored distractor trials. The top row shows data from the 200-ms lag trials and the bottom row shows data from the 500-ms lag trials (with lag referring to the SOA between the peripheral distractor and the central target). Error bars show standard error of the mean. PSZ showed an altered pattern of spatial distance, with enhanced distraction to nearby target-colored distractors compared to HCS.
To evaluate these effects, normalized capture cost was initially submitted to a 4-way ANOVA, with factors of group (HCS, PSZ), distractor relevance (target-colored, irrelevant-colored), distractor eccentricity (1, 1.5, 2.5, 4.5°), and distractor lag (200, 500 ms). Significant main effects were found for distractor relevance (F(1,80) = 66.83, p < 0.001), distractor distance (F(3,240) = 34.54, p < 0.001), and distractor lag (F(1,80) = 28.17, p < 0.001). In line with a spatial hyperfocusing account, there was an interaction of distractor distance and group (F(3,240) = 6.25, p < 0.001). As expected from previous research (Leonard et al., 2015), the basic capture effect—which was present mainly for target-color distractors at the 200-ms lag—led to a significant 3-way interaction of distractor relevance, lag, and distance (F(3,240) = 14.95, p < 0.001, ηp2 = 0.16).
To better understand the nature of these effects, follow-up 3-way ANOVAs were conducted separately for trials with target-colored distractors and trials with irrelevant-colored distractors. As evident in Figure 2, there was little capture cost for either HCS or PSZ on the irrelevant-colored distractor trials, regardless of distractor lag or distance. Accordingly, the ANOVA revealed no significant effects or interactions (smallest p = .10 for lag × group). However, the corresponding ANOVA for the target-colored distractor trials yielded significant effects of distance (F(3,240) = 45.13, p < 0.001) and lag (F(1,80) = 56.61, p < 0.001), as well as an interaction of distance and lag (F(3,240) = 22.90, p < 0.001). Critically, there was also an interaction of distance by group (F(3,240) = 4.54, p < 0.01, ηp2 = 0.05), suggesting a different spatial gradient of capture costs in PSZ than for HCS.
Matched group analysis
To verify that the above-described pattern was not an artifact of the normalization method used to minimize the impact of individual and group differences in baseline performance, we also performed a matched-group analysis on the original capture cost measure. Specifically, we extracted subgroups of PSZ and HCS who were matched on baseline performance and examined the simple capture cost measure in these subgroups. To create the subgroups, each PSZ was paired with a HCS of similar performance level in the no-distractor baseline condition; the mean difference between the paired individuals was 1.4% (range: 0–6.25%). This resulted in two groups of 33 participants, with mean no-distractor accuracy scores of 65.8% and 65.1% for the HCS and PSZ respectively (t(64) = 0.19, p = 0.85). As observed for the normalized capture cost measure in the whole sample, the overall ANOVA on non-normalized capture cost in these matched subgroups yielded a significant distance by group interaction (F(3,192) = 3.96, p < 0.01, ηp2 = 0.06). The distance by group interaction was also significant in a follow-up ANOVA that was limited to trials with target-colored distractors (F(3,192) = 2.63, p = 0.05, ηp2 = 0.04). There were no significant main effects or interactions involving group in a parallel ANOVA that was limited to trials with irrelevant-colored distractors. Thus, the sharper gradient of distraction observed in PSZ was not an artifact of the normalization procedure and cannot be explained by group performance differences.
Symptoms
Because the spatial profile of distractor interference might relate to hyperfocusing brought about by atypical dopamine functioning, we also examined individual differences in positive symptoms among people with schizophrenia. To assess this relationship, BPRS-POS scores were used to perform a median split of participants, resulting in a high positive symptom group (N = 19, mean BPRS-POS = 3.21) and a low positive symptom group (N = 18, mean BPRS-POS = 1.49). Figure 3 shows normalized capture cost for these symptom groups, plotted with HCS data for visual comparison. The largest differences between groups appear for the target-colored distractors presented 200 ms before the target (upper left panel of Figure 3). Specifically, PSZ in the high positive symptom group showed little capture by target-colored distractors unless they were immediately adjacent to the target location.
Figure 3.
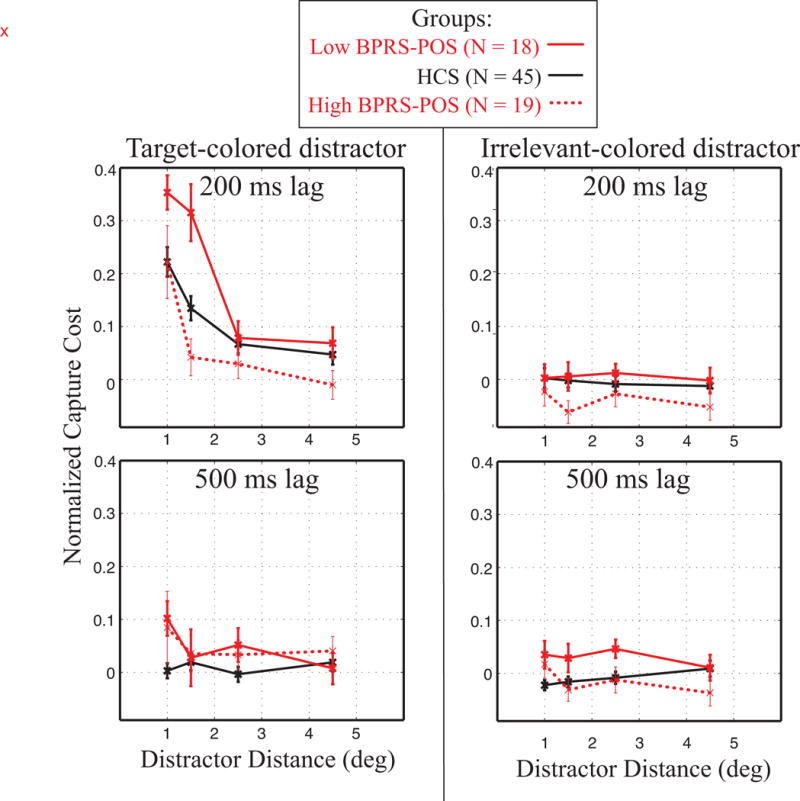
Normalized capture cost as shown in Figure 2 with groups divided based on severity of positive symptoms (BPRS-Pos score). For reference, control data are also shown. PSZ with high positive symptoms showed less capture to target-colored distractors than those with low positive symptoms, with a sharp fall-off in interference with increasing distance.
As in previous analyses, we first submitted normalized capture cost to a 4-way ANOVA, with distractor relevance (target-colored, irrelevant-colored), distractor eccentricity (1, 1.5, 2.5, 4.5°), and distractor lag (200, 500 ms), and group (low and high positive symptoms). Significant main effects were found for distractor relevance (F(1,35) = 24.31, p < 0.001), distance (F(3,105) = 24.78, p < 0.001), and lag (F(1,35) = 7.26, p = 0.01). The relationship between positive symptom level and atypical spatial processing was validated by a significant interaction of distractor distance and group (F(3,105) = 3.04, p = 0.03, ηp2 = 0.08). Once again the 3-way interaction of distractor relevance, lag, and distance was significant (F(3,105) = 9.20, p < 0.001, ηp2 = 0.21), justifying decomposing the analysis into two 3-way ANOVAs, examining symptom subgroup, distance, and lag for each distractor type.
For the target-colored distractors, there was a significant 3-way interaction of distance × lag × symptom subgroup (F(3,105) = 3.18, p = 0.03, ηp2 = 0.08). However, for the irrelevant-colored distractors, there was only a significant main effect of symptom subgroup (F(1,35) = 4.49, p = 0.04), and no significant interactions between symptom subgroup and other factors (all p’s > 0.5).
A median split was used for the preceding analyses because it is difficult to relate a continuous variable to the complex factorial interaction pattern observed in the present study. However, we conducted additional analyses using symptom severity as a continuous variable to examine correlations with performance in individual cells of the factorial design. Specifically, correlations were calculated between BPRS-POS symptom level and normalized capture cost after collapsing the data into near (1 and 1.5°) and far (2.5 and 4.5°) trials at the 200 ms lag. Of these 4 correlations that resulted from pairing distance (near, far) and task-relevance (target-colored, irrelevant-colored), the only significant correlation was between BPRS-POS and normalized capture cost for near target-colored distractors (Spearman’s rho = −0.37, p = 0.03). A scatterplot is shown in Figure 4, illustrating that individuals with high positive symptoms showed less interference from target-colored peripheral distractors that were near to the task-relevant location.
Figure 4.
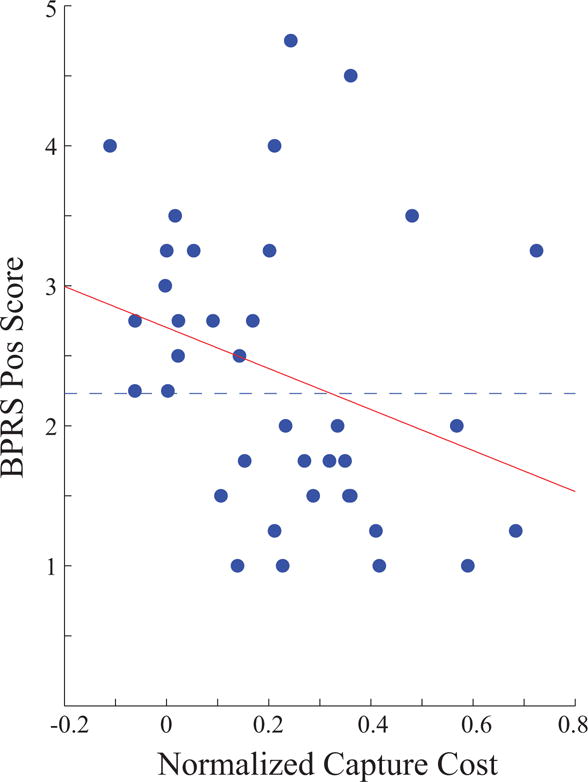
Scatter plot of BPRS-Pos scores and normalized capture cost for near target-colored distractors presented at 200-ms lag. Dotted line shows group division for median split analysis shown in Figure 3.
To test the specificity of this effect, the relationships between other symptom types and this distraction effect were also examined as exploratory analyses. There was no significant relationship between the BPRS negative symptom factor score and normalized capture cost (Spearman’s rho = 0.02, p = 0.98). Likewise, there was no significant relationship between the BPRS disorganization factor score and normalized capture cost (Spearman’s rho = 0.14, p = 0.42). There was also no relationship between WASI-IQ and this normalized capture cost measure (Spearman’s rho = 0.15, p = 0.38).
Medication Effects
No relationship between positive symptom scores and medication levels was found (chlorpromazine equivalent: Spearman’s rho = 0.18, p = 0.29; haloperidol equivalent: Spearman’s rho = 0.21, p = 0.21). Additionally, the high and low BPRS-POS groups did not differ on either haloperidol equivalents (t(35) = 1.12, p = 0.27) or chlorpromazine equivalents (t(35) = 0.5, p = 0.62). We also examined correlations between each of the two medication equivalents and normalized capture cost for the target-colored distractors at each combination of distance (near and far) and lag (200 and 500 ms). The only correlation that approached significance was between the near target-colored distractors at the 500-ms lag and the haloperidol equivalent measure (Spearman’s rho = −0.31, p = 0.07). No other correlations approached significance, suggesting that the symptom effects we observed for the target-colored distractors at the 200-ms lag were not a byproduct of medication.
Discussion
Previous research has shown that peripheral distractors containing a task-relevant color produce interference when presented 200 ms before a central target. Leonard et al. (2015) found a gradient of distraction in healthy young adults, with closer distractors causing more interference than those at more distant peripheral locations. The current results show that this pattern is exaggerated in PSZ, who show an amplified gradient of attention with a sharper fall-off of distraction effects as the target-colored distractors appear farther from the target location. These results are consistent with prior research showing that PSZ exhibit hyperfocusing on both objects containing task-relevant features and task-relevant locations.
There was no evidence of a general increase in distractibility among PSZ because the effects were limited to target-color distractors and were not found for irrelevant-color distractors. Even when highly salient distractors onset immediately adjacent to the target location 200 ms before the target, these distractors produced no interference in PSZ. This provides strong evidence of intact feature-based attention mechanisms in PSZ, such that only distractors sharing the target-feature were prioritized. Moreover, the sharper falloff in distraction over distance exhibited by PSZ is consistent with a tendency for hyperfocusing of spatial attention in this population (Elahipanah et al., 2010, 2011; Gray et al., 2014; Hahn et al., 2012). The present study indicates that these two effects interact, such that interference in PSZ is particularly high for distractors that both contain a task-relevant feature and are near the task-relevant location.
Because PSZ exhibited exaggerated distraction to objects that matched the target color and were near the task-relevant spatial location, the present results cannot be explained by impaired perception, poor task comprehension, failure to maintain a goal representation, decreased motivation, or poorer overall performance. These types of deficits would most likely have reduced the extent to which distractors that were similar to the target received “special treatment” and captured attention. Moreover, the same basic pattern of effects was obtained when we compared subgroups of PSZ and HCS who were equated for performance on the no-distractor baseline trials. Thus, the present results are well explained by the hypothesis that PSZ exhibit an exaggerated use of selective attention mechanisms that give privileged processing to objects that are similar to the target (both in color and location, in this case).
We also found a relationship between positive symptoms and interference from nearby target-colored distractors (see top left panel of Figure 3). Those PSZ with higher levels of positive symptoms showed less capture by target-colored distractors that were close to the target location. Although it is unusual to find relatively better cognitive performance with more severe symptoms of any type, this finding suggests that increased positive symptoms may be related to narrower spatial focusing (see Minas and Park (2007) for an alternative account). The connection between positive symptoms and increased spatial focus is suggested elsewhere in the literature as well. For example, Phillips and David (1997) compared PSZ groups that differed in delusion severity and found that compared to PSZ with fewer delusions, PSZ with more delusions made fewer fixations during a picture-viewing task, with significantly longer fixation durations. Even though this is a very different measure from that used in our current task, the observed effects are also consistent with a reduced flexibility for shifting spatial attention to a new location.
One caveat of the individual differences findings is that the task may be more difficult for PSZ with high positive symptoms, as suggested by the significant negative correlation between baseline performance and BPRS-POS score. That said, there was no significant difference in no-distractor performance between the high positive symptom subgroup (61.7%) and the low positive symptom subgroup (68.2%) (t(34) = 1.5, p = 0.14). However, participants with high positive symptoms may have needed to increase the focus of spatial attention to achieve a reasonable level of accuracy on the task. Nevertheless, our results still suggest a relationship between changes in the distribution of spatial attention and positive symptoms.
Hyperfocusing and dopaminergic processing
Hyperfocusing is generally consistent with neural network models that posit a relationship between dopamine and information representation in schizophrenia (Durstewitz & Seamans, 2008; Rolls, Loh, Deco, & Winterer, 2008). Prefrontal cortex (PFC) functioning is thought to operate on a spectrum between extreme states of D1-type and D2-type receptor activation. In a D1-dominated state, the network may settle into “deep basins of attraction” which allow stable representation to persist in the face of noise or other potentially distracting input. Alternatively, in a D2-dominated state, the network may represent broader (or more numerous) representations that are less stable and more susceptible to interference. Our finding of hyperfocusing in PSZ would be consistent with a bias toward the D1-dominated state. Indeed, Rolls et al. (2008, p. 697) discuss how maintaining spatial attention would be dependent on the dopaminergic state in PFC, which feeds back to bias processing of feedforward input to more posterior brain regions. However, this proposed mechanism of hyperfocusing is currently a speculation that awaits test.
The present results also show variation in distractor interference as a function of positive symptoms, providing further, albeit indirect, evidence of a relationship between dopaminergic dysfunction and hyperfocusing. There has long been evidence suggesting a relationship between schizophrenia and dopaminergic functioning (e.g., Braver, Barch, & Cohen, 1999; Joseph, Frith, & Waddington, 1979; Owen et al., 1978), although the precise nature of how these abnormalities relate to positive symptoms and their development is still debated (Crow, 1985; Davis, Kahn, Ko, & Davidson, 1991; Mackay, 1980). It should be noted that the relationship we found was in medicated participants and does not necessarily provide direct insight into the potential underlying variation caused by disease (Mathalon & Ford, 2012). In any case, our results indicate that positive symptoms in PSZ relate to variation in attentional states that alters processing of potentially relevant peripheral information.
Clinical and practical relevance
In the context of the current experimental task, focused spatial attention is beneficial because peripheral distractors are never relevant and only serve to interfere with performance. However, optimal information processing in the everyday environment requires the ability to adjust between broad and narrow scopes of spatial attention on demand. For example, although maintaining eye contact while speaking with a friend is important, failing to notice that a new person has entered the room entirely would also be socially inappropriate. At the other extreme, continually switching gaze to other events during a conversation would also be problematic. The inability to flexibly adjust the degree of spatial focus could be the source of some functional difficulties in PSZ. Failures to update context has been noted as a critical deficit in schizophrenia (Cohen, Barch, Carter, & Servan-Schreiber, 1999). In everyday life, taking note of changes in context requires that processing resources be shifted from foveal information to potentially relevant peripheral information. Indeed, we have recently found event-related potential evidence that PSZ show reduced sensory processing of peripheral information when the task involves selectively attending to fixation (Kreither et al., 2017). Consequently, spatial hyperfocusing could be responsible for failures of PSZ to integrate global information needed for optimal functioning.
Given the possible effects that hyperfocusing might have on everyday functioning, it is interesting that our results suggest a specific relationship between hyperfocusing and positive symptoms. Previous literature has also suggested differential relationships between symptom type and specific domains of functioning (Dominguez Mde, Viechtbauer, Simons, van Os, & Krabbendam, 2009; Zakzanis, 1998). For example, Addington and Addington (1993) found that outcome was related to positive symptom level. Further research on individual differences in hyperfocusing and its underlying neural mechanisms may provide insight into understanding the heterogeneity of schizophrenia and other disorders that potentially share an overlapping etiology.
Supplementary Material
Scientific Summary.
This study suggests that people with schizophrenia may have an atypical tendency to hyperfocus on a narrow region of visual space, and that this may be increased in those with high positive symptoms. This knowledge may be useful in designing new treatments for cognitive dysfunction in people with schizophrenia.
Acknowledgments
This work was made possible by grant R01MH065034 to JG and SL from the National Institute of Mental Health. These results were previously presented at the annual meeting of the Psychonomic Society in 2015.
References
- Addington J, Addington D. Premorbid functioning, cognitive functioning, symptoms and outcome in schizophrenia. J Psychiatry Neurosci. 1993;18(1):18–23. [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashinski HS, Bacharach VR. Enhancement of perceptual sensitivity as a result of selectively attending to spatial locations. Perception & Psychophysics. 1980;28(3):241–248. doi: 10.3758/bf03204380. [DOI] [PubMed] [Google Scholar]
- Bleuler E. In: Dementia Praecox or the Group of Schizophrenias. Zinkin L, translator. New York: International Universities Press; 1911. [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biol Psychiatry. 1999;46(3):312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Thaker G, Buchanan RW, Moran M, Kirkpatrick B, Carpenter WT., Jr Visual information-processing impairments in deficit and nondeficit schizophrenia. Am J Psychiatry. 1997;154(5):647–654. doi: 10.1176/ajp.154.5.647. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Wang J, Keil A. Normal electrocortical facilitation but abnormal target identification during visual sustained attention in schizophrenia. J Neurosci. 2008;28(50):13411–13418. doi: 10.1523/JNEUROSCI.4095-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108(1):120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Crow TJ. The two-syndrome concept: origins and current status. Schizophr Bull. 1985;11(3):471–486. doi: 10.1093/schbul/11.3.471. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148(11):1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Dominguez Mde G, Viechtbauer W, Simons CJ, van Os J, Krabbendam L. Are psychotic psychopathology and neurocognition orthogonal? A systematic review of their associations. Psychol Bull. 2009;135(1):157–171. doi: 10.1037/a0014415. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry. 2008;64(9):739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Elahipanah A, Christensen BK, Reingold EM. Visual search performance among persons with schizophrenia as a function of target eccentricity. Neuropsychology. 2010;24(2):192–198. doi: 10.1037/a0017523. [DOI] [PubMed] [Google Scholar]
- Elahipanah A, Christensen BK, Reingold EM. Controlling the spotlight of attention: Visual span size and flexibility in schizophrenia. Neuropsychologia. 2011;49(12):3370–3376. doi: 10.1016/j.neuropsychologia.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Folk CL, Leber AB, Egeth HE. Made you blink! Contingent attentional capture produces a spatial blink. Perception & Psychophysics. 2002;64(5):741–753. doi: 10.3758/bf03194741. [DOI] [PubMed] [Google Scholar]
- Folk CL, Leber AB, Egeth HE. Top-down control settings and the attentional blink: Evidence for nonspatial contingent capture. Visual Cognition. 2008;16(5):616–642. [Google Scholar]
- Gold JM, Fuller RL, Robinson B, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. Journal of Abnormal Psychology. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- Gold JM, Randolph C, Coppola R, Carpenter CJ, Goldberg TE, Weinberger DR. Visual orienting in schizophrenia. Schizophr Res. 1992;7(3):203–209. doi: 10.1016/0920-9964(92)90013-u. [DOI] [PubMed] [Google Scholar]
- Gray BE, Hahn B, Robinson B, Harvey A, Leonard CJ, Luck SJ, Gold JM. Relationships between divided attention and working memory impairment in people with schizophrenia. Schizophr Bull. 2014;40(6):1462–1471. doi: 10.1093/schbul/sbu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern MS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophrenia Research. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Hahn B, Robinson B, Harvey AN, Kaiser ST, Leonard CJ, Luck SJ, Gold JM. Visuospatial attention in schizophrenia: Deficits in broad monitoring. Journal of Abnormal Psychology. 2012;121(1):119–128. doi: 10.1037/a0023938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley DR. A two-stage model of attention in schizophrenia research. Br J Soc Clin Psychol. 1975;14(1):81–89. doi: 10.1111/j.2044-8260.1975.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Perception & Psychophysics. 1995;57(6):787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Hollingworth A, Matsukura M, Luck SJ. Visual working memory modulates rapid eye movements to simple onset targets. Psychol Sci. 2013;24(5):790–796. doi: 10.1177/0956797612459767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph MH, Frith CD, Waddington JL. Dopaminergic mechanisms and cognitive deficit in schizophrenia. A neurobiological model. Psychopharmacology (Berl) 1979;63(3):273–280. doi: 10.1007/BF00433561. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Research. 1995;35(13):1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Kreither J, Lopez-Calderon J, Leonard CJ, Robinson BM, Ruffle A, Hahn B, Luck SJ. Electrophysiological Evidence for Hyperfocusing of Spatial Attention in Schizophrenia. J Neurosci. 2017;37(14):3813–3823. doi: 10.1523/JNEUROSCI.3221-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Balestreri A, Luck SJ. Interactions between space-based and feature-based attention. J Exp Psychol Hum Percept Perform. 2015;41(1):11–16. doi: 10.1037/xhp0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Egeth HE. Attentional guidance in singleton search: An examination of top-down, bottom-up, and intertrial factors. Visual Cognition. 2008;16(8):1078–1091. [Google Scholar]
- Leonard CJ, Kaiser ST, Robinson BM, Kappenman ES, Hahn B, Gold JM, Luck SJ. Toward the neural mechanisms of reduced working memory capacity in schizophrenia. Cerebral Cortex. 2013;23(7):1582–1892. doi: 10.1093/cercor/bhs148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Lopez-Calderon J, Kreither J, Luck SJ. Rapid feature-driven changes in the attentional window. J Cogn Neurosci. 2013;25(7):1100–1110. doi: 10.1162/jocn_a_00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Dazzi S, Umilta C. Deficits of the automatic orienting of attention in schizophrenic patients. J Psychiatr Res. 1993;27(1):119–130. doi: 10.1016/0022-3956(93)90056-8. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64(1):34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, McClenon C, Beck VM, Hollingworth A, Leonard CJ, Hahn B, Gold JM. Hyperfocusing in schizophrenia: Evidence from interactions between working memory and eye movements. J Abnorm Psychol. 2014;123(4):783–795. doi: 10.1037/abn0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay AV. Positive and negative schizophrenic symptoms and the role of dopamine. Br J Psychiatry. 1980;137:379–383. doi: 10.1192/bjp.137.4.379. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Hillyard SA. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nature Neuroscience. 1999;2:364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM. Neurobiology of schizophrenia: search for the elusive correlation with symptoms. Front Hum Neurosci. 2012;6:136. doi: 10.3389/fnhum.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minas RK, Park S. Attentional window in schizophrenia and schizotypal personality: Insight from negative priming studies. Appl Prev Psychol. 2007;12(3):140–148. doi: 10.1016/j.appsy.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Tanaka G, Ayaka Y, Michitsuji S, Niwa H, Uemura M, Ohta Y. Preattentive and focal attentional processes in schizophrenia: a visual search study. Schizophr Res. 1996;22(1):69–76. doi: 10.1016/0920-9964(96)00049-7. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophrenia Bulletin. 1984;10(2):160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- Olivers CN, Meijer F, Theeuwes J. Feature-based memory-driven attentional capture: visual working memory content affects visual attention. J Exp Psychol Hum Percept Perform. 2006;32(5):1243–1265. doi: 10.1037/0096-1523.32.5.1243. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Owen F, Cross AJ, Crow TJ, Longden A, Poulter M, Riley GJ. Increased dopamine-receptor sensitivity in schizophrenia. Lancet. 1978;2(8083):223–226. doi: 10.1016/s0140-6736(78)91740-3. [DOI] [PubMed] [Google Scholar]
- Phillips ML, David AS. Visual scan paths are abnormal in deluded schizophrenics. Neuropsychologia. 1997;35(1):99–105. doi: 10.1016/s0028-3932(96)00061-9. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR, Davidson BJ. Attention and the detection of signals. Journal of Experimental Psychology: General. 1980;109:160–174. [PubMed] [Google Scholar]
- Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9(9):696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- Saenz M, Buracas GT, Boynton GM. Global effects of feature-based attention in human visual cortex. Nat Neurosci. 2002;5(7):631–632. doi: 10.1038/nn876. [DOI] [PubMed] [Google Scholar]
- Sapir A, Henik A, Dobrusin M, Hochman EY. Attentional asymmetry in schizophrenia: disengagement and inhibition of return deficits. Neuropsychology. 2001;15(3):361–370. doi: 10.1037//0894-4105.15.3.361. [DOI] [PubMed] [Google Scholar]
- Sawaki R, Kreither J, Leonard CJ, Kaiser ST, Hahn B, Gold JM, Luck SJ. Hyperfocusing of attention on goal-related information in schizophrenia: Evidence from electrophysiology. Journal of Cognitive Neuroscience. doi: 10.1037/abn0000209. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Valdman O, Niznikiewicz MA, Shenton ME, McCarley RW. Enhanced facilitation of spatial attention in schizophrenia. Neuropsychology. 2011;25(1):76–85. doi: 10.1037/a0020779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cogn Psychol. 1980;12(1):97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Asessment; 1999. [Google Scholar]
- Wolfe JM. Guided search 2.0: A revised model of visual search. Psychonomic Bulletin & Review. 1994;1:202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK. Neuropsychological correlates of positive vs. negative schizophrenic symptomatology. Schizophr Res. 1998;29(3):227–233. doi: 10.1016/s0920-9964(97)00102-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


