Abstract
Hypothesis
Mitoquinone (MitoQ) attenuates amikacin ototoxicity in guinea pigs.
Background
MitoQ, a mitochondria-targeted derivative of the antioxidant ubiquinone, has improved bioavailability and demonstrated safety in humans. Thus, MitoQ is a promising therapeutic approach for protecting against amikacin-induced ototoxicity.
Methods
Both oral and subcutaneous administration of MitoQ were tested. Amikacin-treated guinea pigs (n=12 to 18 per group) received water alone (control) or MitoQ 30mg/L-supplemented drinking water; or injected subcutaneously with 3 to 5 mg/kg MitoQ or saline (control). Auditory brainstem responses and distortion product otoacoustic emissions were measured before MitoQ or control solution administration and after amikacin injections. Cochlear hair cell damage was assessed using scanning electron microscopy and Western blotting.
Results
With oral administration, animals that received 30mg/L MitoQ had better hearing than controls at only 24 kHz at 3-weeks (p=0.017) and 6-weeks (p=0.027) post-amikacin. With subcutaneous administration, MitoQ-injected guinea pigs had better hearing than controls at only 24 kHz, 2-week post-amikacin (p=0.013). DPOAE amplitudes were decreased after amikacin injections, but were not different between treatments (p>0.05). Electron microscopy showed minor difference in outer hair cell loss between treatments. Western blotting demonstrated limited attenuation of oxidative stress in the cochlea of MitoQ-supplemented guinea pigs.
Conclusions
Oral or subcutaneous MitoQ provided limited protection against amikacin-induced hearing loss and cochlear damage in guinea pigs. Other strategies for attenuating aminoglycoside-induced ototoxicity should be explored.
1. INTRODUCTION
Aminoglycoside (AG) antibiotics such as gentamicin, neomycin, amikacin, and tobramycin are useful agents in the treatment of serious bacterial infections.1 These antimicrobials are commonly used worldwide because they are relatively inexpensive and in some countries they are available without a prescription. However, their use is complicated by adverse side effects, including ototoxicity, which may be manifested by debilitating dizziness and permanent hearing loss, dramatically compromising quality of life.
Several studies indicate that a primary mechanism in AG-ototoxicity is increased formation of reactive oxygen species (ROS). ROS are produced during normal cell respiration, when glucose and oxygen are converted to energy. However, entry of AG into outer hair cells (OHCs) leads to formation of an AG-iron complex, leading to increased ROS. Accumulation of ROS initiates a cascade of intracellular events that results in cell death.2 AG-generated ROS destroy the OHCs in the cochlea, beginning in the base, and extends to the apex and inner hair cells (IHCs) with increasing total dose.3 Aminoglycosides also causes atrophy of the stria vascularis, and damage spiral ligament fibrocytes, spiral ganglion neurons and cells supporting the organ of Corti.4–6
AG ototoxicity maybe prevented by administration of otoprotective agents, such as corticosteroids7,8 and antioxidants such as D-methionine, vitamin E and aspirin.9–12 Attempts to use large doses of antioxidants has been largely ineffective in humans, probably due to the limited cellular and mitochondrial uptake of these antioxidants.13 To overcome this limitation, a number of mitochondria-targeted antioxidants have been developed.14,15 The best-characterized is mitoquinone (MitoQ).
MitoQ consists of the antioxidant ubiquinone (CoQ10) linked to a triphenylphosphonium (TPP) cation. 16,20–22 Unlike CoQ10 which is extremely hydrophobic and accumulates in mitochondria to a limited extent, 16,17 the TPP moiety on MitoQ enables its accumulation at levels several hundred-fold within mitochondria.14,18–21 MitoQ has been extensively studied19,22,23 and has demonstrated safety in humans.24,25
In our earlier studies in guinea pigs, oral and subcutaneous MitoQ reduced gentamicin- and cisplatin-induced ototoxicity, respectively. 26, 27 To address clinical need, a therapeutic agent for AG-induced ototoxicity should work for a broad range of aminoglycosides, if not all. The goal of the present study was to evaluate if MitoQ attenuates ototoxicity of amikacin, another commonly used aminoglycoside, in a guinea pig model.
2. MATERIALS AND METHODS
2.1. Animals
A total of 60 male albino guinea pigs initially weighing 235–250 grams (Charles River, Wilmington, MA) were used in two experiments (details below). All procedures were approved by the Institutional Animal Care and Use Committee (#201308097) and conforms to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Guinea pigs had free access to water and standard diet, and were observed daily for signs of vestibular dysfunction such as head tilt posturing, nystagmus, or abnormal gait. Subjects were also weighed daily during the amikacin injections and for the first 3 days following hearing tests.
In both experiments, animals were screened for normal auditory sensitivity using auditory brainstem responses (ABRs). Animals with normal ABR thresholds were randomly divided into treatment groups.
2.2. Efficacy of orally delivered MitoQ in preventing amikacin-induced cochlear damage and hearing loss in guinea pigs
2.2.1. Treatments
Subjects were divided into two treatments (n=18 per group). Power analysis, as described in details below under Statistical Analysis section, required 12 animals per group for auditory threshold testing, the primary outcome measure. The additional six guinea pigs in each group were used to run the additional Western blotting experiments, to address whether selective inhibition of mitochondrial ROS with MitoQ can attenuate the amikacin-induced oxidative stress in the cochlea. Group 1 received water alone (control) and group 2 received drinking water supplemented with 30mg/L MitoQ (as a β-cyclodextrin complex of the methane sulfonate salt; kindly donated by Drs. Michael Murphy and Robin Smith). This dose was used in our previous gentamicin study.26 Fresh MitoQ solutions were prepared twice a week. Water consumption was monitored and recorded.
After the 14-day MitoQ pre-treatment, all animals received 200mg/kg amikacin (Teva Pharmaceuticals, Sellersville, PA) daily for 14 days, injected subcutaneously. MitoQ was also supplemented during the amikacin injections and up to 3 weeks after the amikacin injections.
2.2.2. Evaluation of auditory function
ABR and distortion product otoacoustic emission (DPOAE) on both ears were obtained at baseline (prior to initiation of any treatment) and again at 3- and 6-week after amikacin injections. General anesthesia was achieved with subcutaneous injection of ketamine (30–50 mg/kg) and xylazine (2–5 mg/kg). Once anesthetized, the subject was placed in a sound-attenuated chamber on a heating pad to maintain body temperature at 37–38 °C. The ear canals and tympanic membranes were inspected to assure the ear canals were free of wax, no inflammation of tympanic membranes, and no effusion of the middle ears. ABR was tested first, followed by DPOAE.
ABRs
The ABR procedure was as previously described.26 Briefly, acoustic stimuli were 4, 8, and 16 and 24 kHz tone bursts (15-ms duration) generated using Tucker-Davis Technology (TDT, Alachua, FL) software (SigGenRP) and TDT System II/III hardware. Signals were presented to the external auditory meatus in a closed acoustic system through a tube connected to a transducer (Beyer DT-48, Beyer Dynamic, Farmingdale, NY). Sound levels for each frequency range from 0 to 100 dB SPL, using 5 dB steps. Evoked responses to 1024 tone presentations were amplified and filtered (RA4PA/RA4L1 Medusa, TDT) then digitized (RA16 Medusa, TDT) and averaged (BioSigRP, TDT). Threshold, tested separately for each ear, was defined as the lowest intensity of stimulation that yielded a repeatable waveform based on an identifiable ABR wave III or V.
DPOAEs
The DPOAEs were measured as described.28 Briefly, DPOAE measurements were completed using a TDT system, in combination with an ER10B+ microphone (Etymotic Research Inc., Elk Grove Village, IL) and two sound delivery tubes coupled to two loudspeakers (MF1 speakers, TDT). The tip of the probe assembly was gently pressed against the opening of the ear canal. Responses were elicited with two simultaneously presented ‘primary’ tones (frequencies f1 and f2) at an f2/f1 ratio of 1.2, with f2 frequencies of 4, 8, 16 and 24 kHz.
Measures of DPOAE response growth (input-output) with increasing stimulus level were obtained at each of the f2 frequencies, with L1 ranging from 25 to 65 dB SPL, and L2 being 10 dB quieter than L1. Stimulus levels were decreased in 5-dB steps within each frequency. DPOAE amplitudes (2f1–f2) and adjacent noise floors were averaged, with all tests averaged over 10 seconds.
2.2.3. Evaluation of cochlear damage
After the final ABR and DPOAE measurements, animals were deeply anesthetized, and the otic bullae were immediately removed and processed for scanning electron microscopy (SEM) or subsequent biochemical assays.26
Scanning electron microscopy
Left ear bullae were opened and fixed by immersion in 4% paraformaldehyde for at least 24h. Specimens were processed for SEM as described.26 The numbers of missing OHCs at the basal, middle, and apical turns were qualitatively assessed by two observers blinded to the treatments.
Western blotting
The whole cochlea was dissected out, pulverized in liquid nitrogen and homogenized in RIPA buffer with protease and phosphatase inhibitors (ThermoFisher Scientific, Waltham, MA). Changes in protein expression of the mitochondrial respiratory chain subunits (NDUFB6 of complex I , COX IV of complex IV and alpha and beta subunit of complex V), apototic proteins (Bak, Bax, cyt c, AIF, PARP and caspase-8), and of the antioxidant enzymes manganese superoxide dismutase (MnSOD) and gluthathione reductase (GSR), were evaluated by Western blotting.26,29 All primary and secondary antibodies were purchased from commercial vendors (Cell Signaling Technology, Danvers, MA; Abcam Inc., Cambridge, MA; Life Technology/Thermo Fisher Scientific, Waltham, MA; GeneTex, Inc., Irvine, CA).
Protein carbonyls
Protein carbonyls, an index of oxidative damage to cellular proteins, were measured by Western blotting using an OxyBlot Protein Oxidation Detection kit (Chemicon, Temecula, CA) following the manufacturer's instructions.
2.2.4. Statistical analysis
The sample size was based on the outcomes from our previous studies using a 15 to 20 dB pre-specified difference in ABR between experimental and control groups. Power analysis was conducted using GPower 3.1 software comparing the difference in threshold shifts (hearing loss) between the treatment groups with α error probability set at 0.05 and the power to 0.90.
Hearing thresholds and Western blot data were the primary outcome measures. Western blot data and group differences in hearing outcomes at baseline and at 3- and 6-weeks post-amikacin were analyzed by two-tailed t-tests (JMP Pro™ 11.0, SAS Institute Inc., Cary, NC). Differences were considered significant for p ≤ 0.05.
2.3. Efficacy of subcutaneously injected MitoQ in preventing amikacin-induced cochlear damage and hearing loss in guinea pigs
In the second experiment, a subcutaneous administration of MitoQ was conducted. Guinea pigs were distributed to either control or MitoQ-supplemented group (n=12 per group). Animals were injected subcutaneously in the paraspinal region with either 5 mg/kg MitoQ, diluted in normal saline to 1 mg/ml, or equivalent volume of normal saline in the control group. The MitoQ dose was based from a study by Mukhopadhyay et al22 and our cisplatin study.27 MitoQ or saline was administered daily for 5 days before amikacin injections and 1 hour before receiving the daily dose of subcutaneous 200 mg/kg amikacin, for 14 days. The MitoQ dose was subsequently reduced to 3 mg/kg on Day 2 of the amikacin injections due to skin irritation at the site of MitoQ injections and weight loss in the MitoQ-injected guinea pigs. Due to time constraints, auditory function was re-assessed earlier (at 2- and 4-weeks post-amikacin) and only ABR was used to assess hearing outcomes. Differences in hearing outcomes between treatments were compared using a two-tailed t-test (p≤0.05; JMP Pro 11; SAS Institute, Inc., Cary, NC). All other procedures were as described for the oral MitoQ experiments.
3. RESULTS
The mean guinea pig body mass was similar between treatment groups at the initiation of each experiment. Oral administration of MitoQ had no significant effect on the guinea pigs weight gain at the end of the treatments (54 and 66 grams for control and MitoQ, respectively; p=0.09). With subcutaneous administration, MitoQ-injected guinea pigs gained less weight compared to the controls (25g vs 38g; p=0.001).
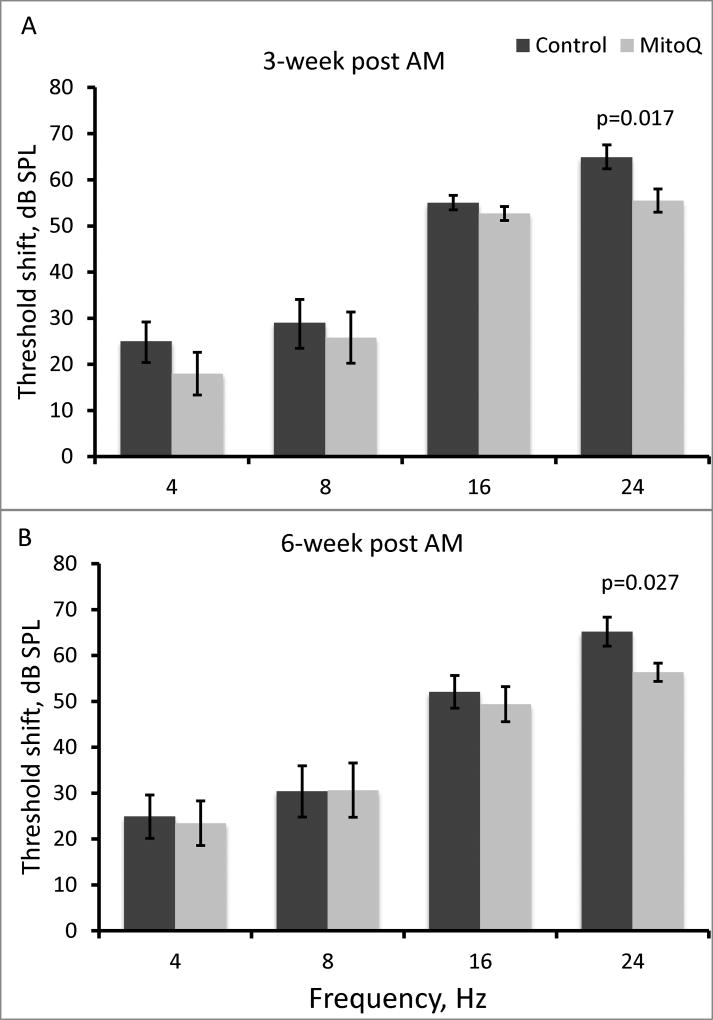
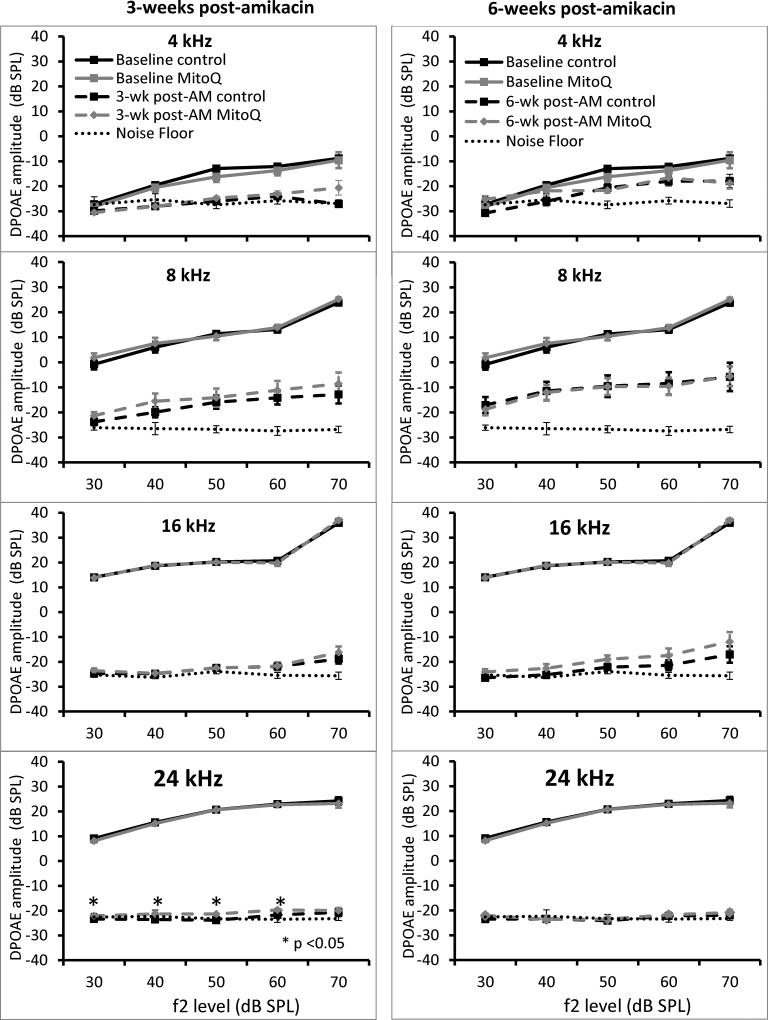
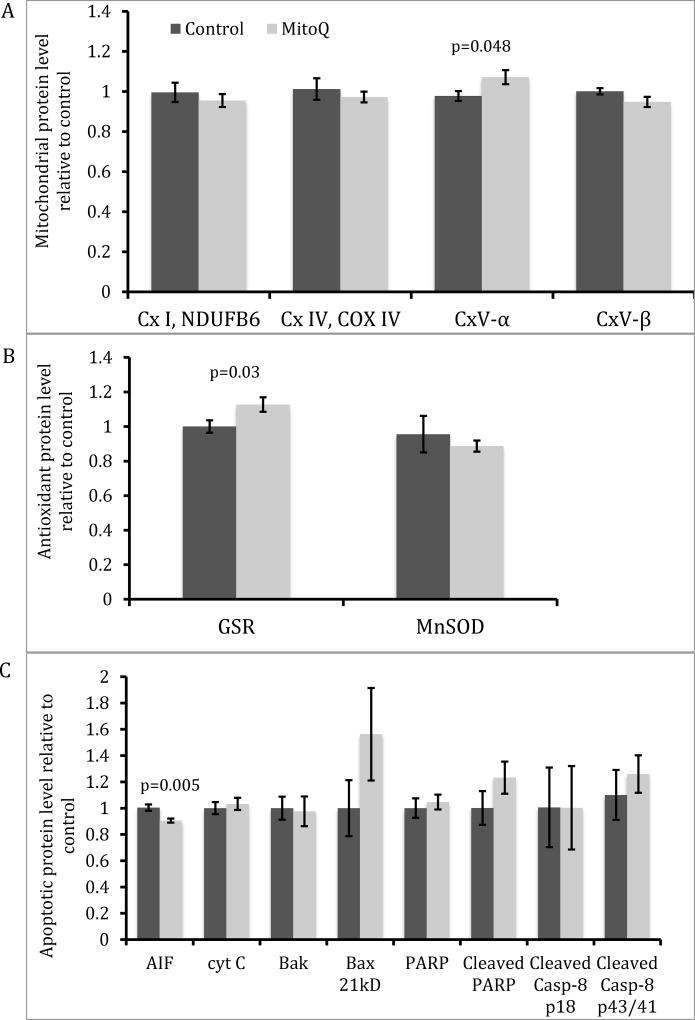
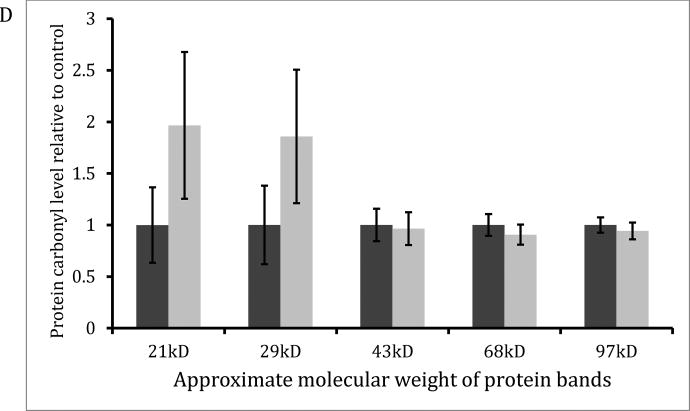
Pre-treatment hearing thresholds were not different between treatments for both oral and subcutaneous MitoQ. With oral administration, animals that received 30mg/L MitoQ had better hearing than controls at 24 kHz only at 3-weeks (Figure 1A; p=0.017) and 6-weeks (Figure 1B; p=0.027) post-amikacin. DPOAE amplitudes were decreased 3- and 6-week after amikacin injections at 4, 8, 16, and 24 kHz (Figure 2) and there were no differences between treatments except at 24 kHz, 3-weeks post-amikacin. The control guinea pigs showed a small (2–2.5 dB) but significant decrease in DPOAE amplitudes at L2 levels of 30, 40, 50 and 60 dB SPL compared to the MitoQ-supplemented guinea pigs (p=0.014, p=0.0004, and p=0.008, respectively). SEM showed OHC loss in both treatments but OHC loss was less in the MitoQ- supplemented guinea pigs (Figure 3). Oral supplementation of MitoQ had limited effect against amikacin-induced oxidative stress in the cochlea (Figure 4). Levels of the α-subunit of complex V (p=0.048; Figure 4A) and levels of the antioxidant GSR (p=0.03; Figure 4B) were higher, while levels of the apoptotic protein AIF were lower (p=0.005; Figure 4C) in cochleas of guinea pigs supplemented with MitoQ, compared to controls. MitoQ did not have an effect on other markers of oxidative stress that were measured in the study, as well as in the levels of protein carbonyls (Figure 4D).
Figure 1.
ABR threshold at 4, 8, 16, and 24 kHz at 3-weeks (A) and 6-weeks (B) after amikacin 200mg/kg and oral MitoQ treatment. Data represent mean ± SEM of control (n=18) and MitoQ (n=16) guinea pigs.
Figure 2.
DPOAE amplitudes at 4, 8, 16, and 24 kHz at 3-weeks (A) and 6-weeks (B) after amikacin 200mg/kg treatment and oral MitoQ treatment. Values are means ± SEM control (n=18) and MitoQ 30mg/L-supplemented guinea pigs (n = 16).
Figure 3.
Electron micrographs of the basal, middle and apical turn of cochleas from control and oral MitoQ-supplemented guinea pigs.
Figure 4.
Protein expression of mitochondrial proteins (A), antioxidants (B), and apoptotic proteins (C) in the cochlea of amikacin-treated guinea pigs with and without oral MitoQ. *p <0.05 compared to control.
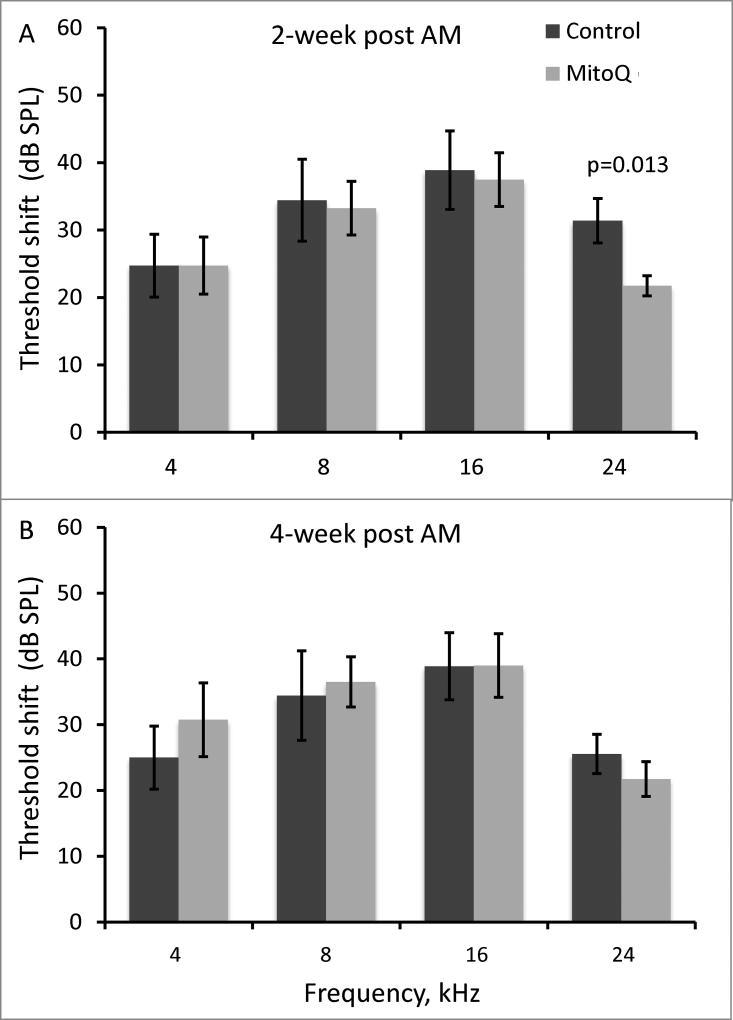
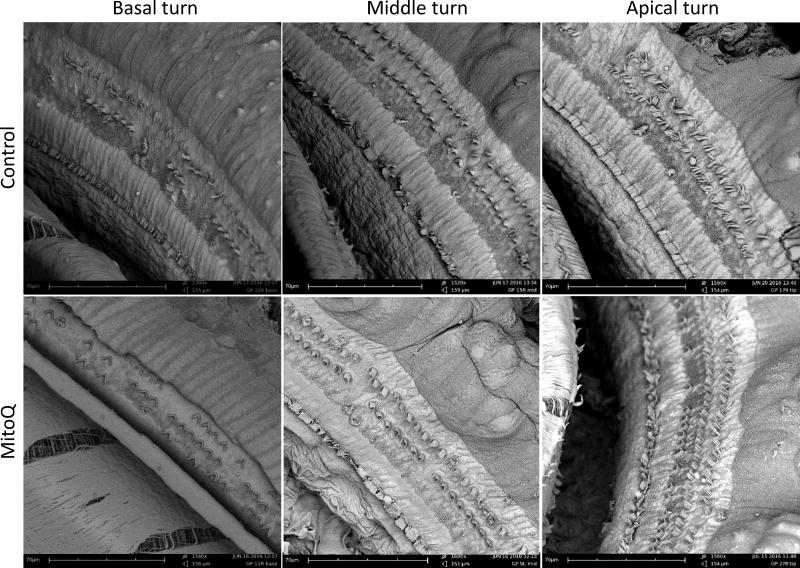
With subcutaneous administration, MitoQ-injected guinea pigs had better hearing than controls at only 24 kHz (p=0.013; Figure 5A) at 2-weeks after amikacin treatment. At 4-weeks post-amikacin, no differences in ABR threshold shifts were observed between the MitoQ- and saline-injected guinea pigs (p>0.05; Figure 5B). SEM showed no difference in OHC loss in the basal, middle, and apical turns in guinea pigs injected with MitoQ (Figure 6). Since subcutaneously injected MitoQ did not protect against amikacin-induced threshold shifts at 4-weeks post-amikacin and no difference in OHC loss was observed, no further biochemical or protein expression studies were conducted.
Figure 5.
ABR threshold at 4, 8, 16, and 24 kHz at 2-weeks (A) and 4-weeks (B) after amikacin 200mg/kg and subcutaneous MitoQ treatment. Data represent mean ± SEM of control (n=11) and MitoQ (n=12) guinea pigs.
Figure 6.
Electron micrographs of the basal, middle and apical turn of cochleas from control and MitoQ-injected guinea pigs.
In both experiments, no animals showed evidence of vestibulopathy (eg, head tilt posturing, nystagmus, or abnormal gait).
4. DISCUSSION
MitoQ has been shown to protect against a variety of oxidative insults when given intraperitoneally, intravenously or orally.18,19,23,30–32 Oral and subcutaneous MitoQ also reduced gentamicin- and cisplatin-induced ototoxicity, respectively, in guinea pigs.26,27 However, the present study demonstrates that MitoQ delivered orally or subcutaneously has minimal effect in reducing amikacin-induced hearing loss, OHC loss, and oxidative stress in the cochlea.
Oral MitoQ administered at 30mg/L, the same dose that showed efficacy against gentamicin,26 showed only modest reduction of amikacin-induced hearing loss. Lower hearing loss at 3- and 6-weeks post-amikacin was observed in MitoQ-supplemented guinea pigs, but only at the highest frequency tested. The higher frequencies are known to be the most vulnerable to ototoxicity.33,34 The limited benefit of MitoQ on hearing thresholds was supported by nominal impact of oral MitoQ on biochemical indices of oxidative damage, antioxidant status, and mitochondrial proteins in the cochlea, as well as SEM findings of massive OHC loss across all the treatments. Subcutaneously injected MitoQ showed similar results.
In contrast to the prior study on protection from gentamicin ototoxicity,26 we administered MitoQ for a few weeks after completion of amikacin exposure. This raises the possibility that the timing, dosing, and duration of MitoQ may be important in order to protect against amikacin-induced ototoxicity. However, we have also observed MitoQ protection against cisplatin ototoxicity with treatment limited to before and during ototoxic exposure.
MitoQ is not without potential side effects. We previously observed a potential toxicity when gentamicin and MitoQ were injected simultaneously in guinea pigs, and the combination of gentamicin and MitoQ disrupts mitochondrial membrane potential in vitro.35 We have also demonstrated that gentamicin MICs can be enhanced by MitoQ.36 These observations indicate a possibility that gentamicin action or toxicity could be potentiated by MitoQ administration. We also observed that subcutaneously injected MitoQ, where uptake of MitoQ is presumably higher than oral MitoQ, was associated with lower weight gain in the present study and in the cisplatin study.27 Other studies also suggest that MitoQ has a narrow therapeutic window, showing dose-dependent benefits or toxicity in stem cells.37
In conclusion, oral or subcutaneous MitoQ administration showed limited protection against amikacin-induced oxidative damage in the cochlea, OHC loss, and hearing loss in guinea pigs. There remain many questions that need to be addressed if MitoQ is to be developed as a therapeutic against aminoglycoside-induced ototoxicity. Other strategies for attenuating aminoglycoside-induced ototoxicity should be explored.
Acknowledgments
The authors thank Drs. Michael Murphy and Robin Smith for generously providing the MitoQ. This research was funded by the National Institute on Deafness and Other Communicative Disorder (NIDCD) of the National Institutes of Health (NIH) under award number R03DC013659 to C. Dirain. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no financial conflicts to disclose.
References
- 1.Mascaretti O. Bacteria versus antibacterial agents: an integrated approach. ASM Press; Washington, DC: 2003. pp. 229–333. [Google Scholar]
- 2.Karasawa T, Steyger PS. Intracellular mechanisms of aminoglycoside-induced cytotoxicity. Integrative biology : quantitative biosciences from nano to macro. 2011;3(9):879–886. doi: 10.1039/c1ib00034a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiel H, Schamel A, Erre JP, Hayashida T, Dulon D, Aran JM. Cellular and subcellular localization of tritiated gentamicin in the guinea pig cochlea following combined treatment with ethacrynic acid. Hearing research. 1992;57(2):157–165. doi: 10.1016/0378-5955(92)90148-g. [DOI] [PubMed] [Google Scholar]
- 4.Imamura S-i, Adams JC. Distribution of Gentamicin in the Guinea Pig Inner Ear after Local or Systemic Application. JARO: Journal of the Association for Research in Otolaryngology. 2003;4(2):176–195. doi: 10.1007/s10162-002-2036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q, Steyger PS. Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells. J Assoc Res Otolaryngol. 2009;10:205–219. doi: 10.1007/s10162-009-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai CF, Steyger PS. A systemic gentamicin pathway across the stria vascularis. Hear Res. 2008;235:114–124. doi: 10.1016/j.heares.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SK, Choi D, Russell P, John EO, Jung TT. Protective effect of corticosteroid against the cytotoxicity of aminoglycoside otic drops on isolated cochlear outer hair cells. Laryngoscope. 2004;114(4):768–771. doi: 10.1097/00005537-200404000-00033. [DOI] [PubMed] [Google Scholar]
- 8.Himeno C, Komeda M, Izumikawa M, et al. Intra-cochlear administration of dexamethasone attenuates aminoglycoside ototoxicity in the guinea pig. Hear Res. 2002;167(1–2):61–70. doi: 10.1016/s0378-5955(02)00345-3. [DOI] [PubMed] [Google Scholar]
- 9.Sha SH, Schacht J. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hear Res. 2000;142(1–2):34–40. doi: 10.1016/s0378-5955(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 10.Campbell KC, Meech RP, Klemens JJ, et al. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226(1–2):92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Fetoni AR, Sergi B, Ferraresi A, Paludetti G, Troiani D. alpha-Tocopherol protective effects on gentamicin ototoxicity: an experimental study. International journal of audiology. 2004;43(3):166–171. doi: 10.1080/14992020400050023. [DOI] [PubMed] [Google Scholar]
- 12.Sha SH, Schacht J. Salicylate attenuates gentamicin-induced ototoxicity. Lab Invest. 1999;79(7):807–813. [PubMed] [Google Scholar]
- 13.Patten DA, Germain M, Kelly MA, Slack RS. Reactive oxygen species: stuck in the middle of neurodegeneration. Journal of Alzheimer's disease : JAD. 2010;20(Suppl 2):S357–367. doi: 10.3233/JAD-2010-100498. [DOI] [PubMed] [Google Scholar]
- 14.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annual review of pharmacology and toxicology. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 15.Antonenko YN, Roginsky VA, Pashkovskaya AA, et al. Protective effects of mitochondria-targeted antioxidant SkQ in aqueous and lipid membrane environments. The Journal of membrane biology. 2008;222(3):141–149. doi: 10.1007/s00232-008-9108-6. [DOI] [PubMed] [Google Scholar]
- 16.Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proceedings of the National Academy of Sciences. 1998;95(15):8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochimica et biophysica acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 18.Smith RA, Hartley RC, Murphy MP. Mitochondria-targeted small molecule therapeutics and probes. Antioxid Redox Signal. 2011;15(12):3021–3038. doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 19.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. European journal of biochemistry / FEBS. 1999;263(3):709–716. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A. 2003;100(9):5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jauslin ML, Meier T, Smith RA, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17(13):1972–1974. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 23.Smith RAJ, Murphy MP. Mitochondria-targeted Antioxidants as Therapies. Discovery Medicine. 2011;57:106–114. [PubMed] [Google Scholar]
- 24.Gane EJ, Weilert F, Orr DW, et al. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010;30(7):1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 25.Snow BJ, Rolfe FL, Lockhart MM, et al. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov Disord. 2010;25(11):1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 26.Ojano-Dirain CP, Antonelli PJ, Le Prell CG. Mitochondria-targeted antioxidant MitoQ reduces gentamicin-induced ototoxicity. Otol Neurotol. 2014;35(3):533–539. doi: 10.1097/MAO.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 27.Tate AD, Antonelli PJ, Hannabass KR, Dirain CO. Mitochondria-Targeted Antioxidant Mitoquinone Reduces Cisplatin-Induced Ototoxicity in Guinea Pigs. Otolaryngol Head Neck Surg. 2017;156(3):543–548. doi: 10.1177/0194599816678381. [DOI] [PubMed] [Google Scholar]
- 28.Spankovich C, Griffiths SK, Lobariñas E, et al. Temporary threshold shift after impulse-noise during video game play: Laboratory data. International journal of audiology. 2014;53(0 2):S53–S65. doi: 10.3109/14992027.2013.865844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jadidian A, Antonelli PJ, Ojano-Dirain CP. Evaluation of Apoptotic Markers in HEI-OC1 Cells Treated With Gentamicin With and Without the Mitochondria-Targeted Antioxidant Mitoquinone. Otol Neurotol. 2014;29:29. doi: 10.1097/MAO.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt HH, Stocker R, Vollbracht C, et al. Antioxidants in Translational Medicine. Antioxid Redox Signal. 2015 doi: 10.1089/ars.2015.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell RD, Swet JH, Kennedy KL, et al. MitoQ modulates oxidative stress and decreases inflammation following hemorrhage. J Trauma Acute Care Surg. 2015;78(3):573–579. doi: 10.1097/TA.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 32.McManus MJ, Murphy MP, Franklin JL. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31(44):15703–15715. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawkins JE. Histopathology of cochlear and vestibular ototoxicity in laboratory animals. Aminoglycoside Ototoxicity. 1981:175–195. [Google Scholar]
- 34.Huth ME, Ricci AJ, Cheng AG. Mechanisms of Aminoglycoside Ototoxicity and Targets of Hair Cell Protection. International Journal of Otolaryngology. 2011;2011:19. doi: 10.1155/2011/937861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng MRAV, Antonelli PJ, Joseph J, Dirain CO. Assessment of Mitochondrial Membrane Potential in HEI-OC1 and LLC-PK1 Cells Treated with Gentamicin and Mitoquinone. Otolaryngology -- Head and Neck Surgery. 2015;152(4):729–733. doi: 10.1177/0194599814564934. [DOI] [PubMed] [Google Scholar]
- 36.Ojano-Dirain CP, Antonelli PJ. Prevention of gentamicin-induced apoptosis with the mitochondria-targeted antioxidant mitoquinone. Laryngoscope. 2012;122(11):2543–2548. doi: 10.1002/lary.23593. [DOI] [PubMed] [Google Scholar]
- 37.Hämäläinen Riikka H, Ahlqvist Kati J, Ellonen P, et al. mtDNA Mutagenesis Disrupts Pluripotent Stem Cell Function by Altering Redox Signaling. Cell Reports. 11(10):1614–1624. doi: 10.1016/j.celrep.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]