Abstract
Contributory risk factors to premature coronary artery disease (CAD) in premenopausal women are poorly understood and data on this subset of women is lacking. There is growing evidence that the process of inflammation is a part of the atherosclerotic process. Mechanistic insights from animal work suggest that the profile of circulating cytokines reflects both endothelial integrity and the presence of immune and progenitor cells. Significant differences in pro- and anti-inflammatory cytokine concentrations between patients with and without CAD exist. Young women with obstructive CAD may experience differences in pro-inflammatory cytokines and the recruitment of reparative cells that secrete T-Helper (TH2 cytokines compared to women without CAD. Thus, cytokine balance may play a role in obstructive CAD in young women. In this pilot study we set out to identify an array of circulating inflammatory marker profiles which could be useful for the development of risk assessment and preventive strategies. We tested the hypothesis that an increase in serologic TH1 cytokines relative to TH2)/hematopoietic regulatory (HR) cytokines is related to premature coronary atherosclerosis in premenopausal women.
INTRODUCTION
Contributory risk factors to premature coronary atherosclerosis in premenopausal women are poorly understood. Over the last two decades, inflammation has been confirmed as a key mediator of atherogenesis.(1–3) In addition, an evolving hypothesis of atherosclerosis is a disease of failed endogenous vascular repair.(4) Serologic T helper 1 (TH1-type pro-inflammatory) and hematopoietic/regulatory (HR) cytokines and their counteraction by TH2-type (anti-inflammatory) appear to relate with the degree of atherosclerosis in animal models.(5) Consistent with this hypothesis, cytokine balance is related to endothelial integrity and is likely mediated by circulating cells.(4) Mechanistic insights from animal work suggest that the profile of circulating cytokines reflects both endothelial integrity and the presence of immune and progenitor cells.(4) Significant differences in pro- and anti-inflammatory cytokine concentrations between human subjects with and without obstructive coronary artery disease (CAD) have been reported.(6)
Vascular injury generates an inflammatory response that triggers the recruitment of “detrimental” cells, including dendritic cells, T lymphocytes and type 1 macrophages, leading to tissue damage. (7) In response to this inflammatory state, and the secretion of pro-inflammatory chemo/cytokines, hematopoietic stem cells (HSCs), type 2 macrophages, and other cells that mediate repair, and renew the endothelium are mobilized ((4). Although these cells secrete anti-inflammatory chemokines such as interleukin 3 (IL-3) and IL-8, if the TH1 secreting cells predominate, ensuing tissue damage leads to lesion progression.(4) However, as reparative cells are recruited they secrete anti-inflammatory chemo/cytokines that counteract the pro-inflammatory status and can augment repair in activated segments of the endothelium.(8) (4)
Atherosclerosis is apparent at a relatively later age in women compared to similarly aged men.(9) In women, obstructive CAD is more frequently associated with autoimmune diseases, which suggests that the immune mechanisms for the development of CAD are different in men and women.(10) Furthermore women have more non-obstructive coronary atherosclerosis than men, which may suggest that key differences in inflammatory processes between obstructive and non-obstructive CAD may exist. (11) This has not been investigated to date.
The hypothesis
An increase in TH1 cytokines relative to TH2/HR cytokines is related to premature coronary atherosclerosis in premenopausal women. The objective of this pilot study was to compare the levels of TH1, TH2 and HR cytokines in premenopausal women with and without obstructive CAD using core laboratory determinations.
METHODS
The study was approved by the Cedars-Sinai Medical Center and the University of California Los Angeles Institutional Review Boards. All patients gave informed consent. Premenopausal women with chest pain undergoing coronary angiography for suspected cardiac ischemia were included. Pre-menopause was determined by published criteria established by the Women’s Ischemia Syndrome Evaluation (WISE) based on hormone levels and cycling history (12). Exclusion criteria were: 1) pregnancy, 2) currently using hormone replacement therapy, oral contraceptives, estrogen, or phytoestrogen treatments and 3) the presence of any co-morbidity (i.e., cardiomyopathy, valvular disease).
Procedures
A WISE core laboratory (Rhode Island Hospital, Providence, RI, USA), blinded to clinical data, analyzed all angiograms to characterize the presence and extent of CAD. Obstructive CAD was defined as ≥50% stenosis in any epicardial coronary artery, non-obstructive CAD was defined as ≥20% but <50%, and no CAD was <20% (13).
Menopausal status was confirmed by baseline and second visit hormone levels that were timed to coincide with the luteal phase of the menstrual cycle. Blood was collected at visit two to evaluate plasma levels of 22 chemokines/cytokines and endothelial function markers. Chemo/cytokines were measured at a core lab at the University of Minnesota as previously reported by using a cytokine multiplex platform (Human Fluorokine MAP Base Kit; R&D Systems).(4) The detectable chemo/cytokines in our analysis were grouped as TH1 or TH2 as follows: TH1 (pro-inflammatory) interleukin-1 receptor antagonist (IL-1ra), Regulated on activation, normal T expressed and secreted (RANTES), macrophage inducible protein 1 (MIP-1b) and tumor necrosis factor alpha (TNFα); TH2 monocyte chemoattractant protein 1 (MCP-1). The regulatory cytokines and mobilizing chemo/cytokines detected were interleukin 6.
(IL-6), Granulocyte colony-stimulating factor (G-CSF), Vascular endothelial growth factor (VEGF), thrombopoietin (TPO) and epithelial-derived neutrophil-activating peptide-78 (ENA-78). IL-6 was included in the HR category although it is sometimes considered to be in the TH 2 (anti-inflammatory) group. RANTES was included because it is often associated with a TH1 environment.
Group comparisons of demographic and clinical variables between women with and without CAD were made using nonparametric Kruskal-Wallis for continuous measures and Fischer exact tests for frequencies. Median z-scores for each cytokine were used to create comparative cytokine profiles of women with and without obstructive CAD. Z-Scores were used because they are unit-non-specific and allowed for a comparison across cytokines. Nonparametric Kruskal-Wallis was used because of the small N and the high variability in the cytokines (Table 2). For a visual comparison, a Rose-of-Wind diagram was created depicting the differences in cytokine concentrations. All analyses were performed with SAS 9.3 (SAS Institute, Cary, North Carolina. The significance level was set at .05.
Table 2.
Cytokine Ratios by CAD Status
| Cytokine Ratio T1/T2: |
No CAD (n=17) |
CAD (n=7) |
P (K-W) | P (GLM)* unadjusted |
P (GLM)* adjusted |
|---|---|---|---|---|---|
| IL-1ra/MCP-1 | 10.6 ± 7.4 | 15.9 ± 18.6 | 0.72 | 0.67 | 0.69 |
| IL-8/MCP-1 | 0.07 ± 0.07 | 0.02 ± 0.02 | 0.004 | 0.0005 | 0.0007 |
| ENA-78/MCP-1 | 15.4 ± 13.7 | 4.0 ± 2.6 | 0.007 | 0.006 | 0.008 |
| MIP-1b/MCP-1 | 0.31 ± 0.16 | 0.22 ± 0.14 | 0.24 | 0.28 | 0.29 |
| RANTES/MCP-1 | 193 ± 87 | 120 ± 80 | 0.028 | 0.020 | 0.021 |
| TNFa/MCP-1 | 0.06 ± 0.04 | 0.05 ± 0.03 | 0.59 | 0.24 | 0.25 |
Proinflammatory
IL-1ra = interleukin-1 receptor antagonist
RANTES = regulated on activation, normal T expressed and secreted
MIP-1 = macrophage inducible protein 1
TNFa = tumor necrosis factor alpha
Anti-inflammatory
MCP-1 = monocyte chemoattractant protein 1
Hematopoietic Regulatory
ENA-78 = epithelial-derived neutrophil-activating peptide 78
IL-= interleukin
K-W = Kruskal-Wallis (nonparametric test)
GLM = General Linear Models regression (parametric test)
RESULTS
Twenty-four women (mean age 44 ± 5 years) met the study inclusion criteria and comprised the final sample. Patient demographics, clinical characteristics and traditional risk factors of women with versus without obstructive CAD are displayed in Table 1. Of note, some of the differences between the two groups are quite large, although they were not statistically significant.
Table 1.
Demographic and Clinical Characteristics by Obstructive CAD Status
| Variable | Obstructive CAD (n = 7) | No Obstructive CAD (n = 17) | P* |
|---|---|---|---|
| Age (Medians [IQR]) | 44 [43, 47] | 43 [41, 47] | 0.63 |
| White race (%) | 43 | 76 | 0.17 |
| BMI (Medians [IQR]) | 34.4 [24.2, 39.4] | 29.0 [24.2, 38.1] | 0.69 |
| Education: HS or more (%) | 86 | 100 | 0.29 |
| Married (%) | 71 | 65 | >0.99 |
| Income > 35K (%) | 40 | 88 | 0.06 |
| Hx Hypertension (%) | 83 | 38 | 0.15 |
| Hx Dyslipidemia (%) | 71 | 53 | 0.65 |
| Diabetes (%) | 57 | 18 | 0.13 |
| Family History of CAD (%) | 71 | 41 | 0.37 |
| Current smoker (%) | 0 | 12 | >0.99 |
| DASI (Medians [IQR]) | 18.7 [17.0, 35.0] | 30.7 [13.2, 50.7] | 0.84 |
p-values based on Kruskal-Wallis test (continuous) and Fisher exact test (frequencies) %= Per cent
DASI- Duke Activity Status Index
IQR= Interquartile Range
Among the six TH1/TH2 ratios available for comparison, three ratios ENA/MCP, IL6/MCP, and RANTES/MCP) were lower in women with obstructive CAD compared to women without obstructive CAD (Table2), indicating higher ratios of anti-inflammatory/reparative cytokines. Further, an examination of TH1(pro-inflammatory) and TH2/HR (anti-inflammatory/reparative) patterns in women with and without obstructive CAD demonstrated that lower TH1 cytokines predominated in women with obstructive CAD, while both groups had similar levels of TH2/HR cytokines (Figure 1, p<0.05).
FIGURE 1. COMPARATIVE CYTOKINE PROFILES OF WOMEN WITH VS WITHOUT OBSTRUCTIVE CAD.
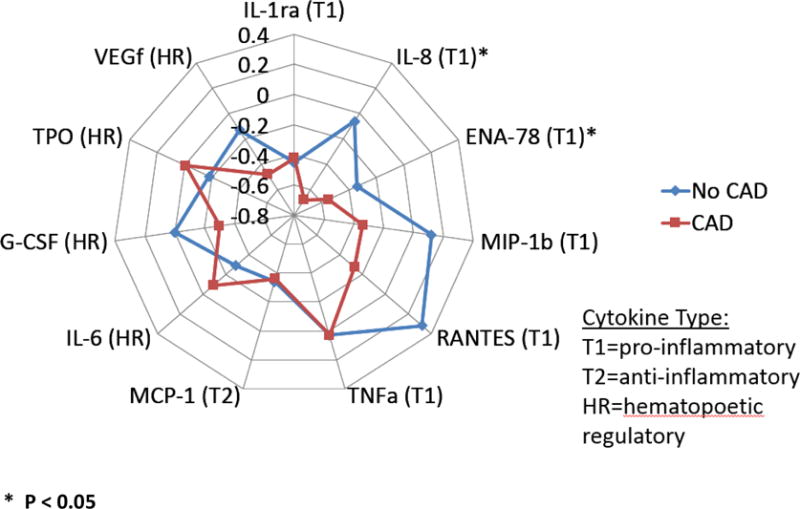
The display of the median z-scores for each cytokine, where Th-1 Cytokines are presented clockwise from IL-1ra to TNF alpha, Th-2 Cytokine MCP-1, Hematopoietic/Regulatory cytokines (left side of diagram) IL-6-VEGf, IL-1ra = interleukin-1 receptor antagonist, RANTES = Regulated on Activation, Normal T Expressed and Secreted, TNFa = tumor necrosis factor alpha, MCP-1 = monocyte chemoattractant protein 1,IL-8 = interleukin 8
DISCUSSION of the HYPOTHESIS
This pilot study suggests that women with obstructive CAD have lower pro-inflammatory and anti-inflammatory (TH1/TH2) ratios compared to women without CAD – the opposite of our hypothesis. These data suggest the alterative hypotheses that young women without obstructive CAD have better “remodeling” responses or that they have subclinical non-obstructive atherosclerosis and coronary microvascular dysfunction(13) reflecting latent inflammation, which may lead to future obstructive CAD.(14)
These results may extend recent work of cytokine patterns differing by age, sex and hormone levels which contribute to cardiac remodeling, with TH2 cytokines believed to play a predominant reparative role.(11) Furthermore, prior data showed higher ENA-78/MCP ratios in young women after an ischemic event.(14) There is extensive knowledge of inflammatory phenomenon and atherosclerosis.(15, 16) Our current findings differ from previous longitudinal studies, in which investigators reported higher TH1 levels in CAD patients, particularly those evaluated early after an acute event. (17) This may be attributable to several factors. First, women with obstructive CAD in our sample had not experienced recent acute events. As a result, they may have been experiencing a protective immune response in which a significant rise in TH2/HR cytokines counters levels of TH1 cytokines. Second, this finding may also be related to statin administration. Evidence exists as to the reversion of pro-inflammatory oxidative functional responses after treatment with statins in patients with dyslipidemia.(18, 19) As expected, in our sample, more women with obstructive CAD were on statins than women without CAD. But even when statin administration was controlled statistically, a significant difference remained. Third, we have a small sample with only seven women with obstructive CAD. Thus, our sample may not be representative of the population of women with and without obstructive CAD.
Emerging information in relation to CAD progression now focuses on TH2 (anti-inflammatory) cytokines and the imbalance between pro-and anti-inflammatory cytokines, rather than the level of pro-inflammatory cytokines alone.(17) Given the relatively young age of this sample, the findings of unusual TH1/TH2 ratios calls out for further investigation. Having knowledge of the cytokine milieu may direct treatment to prevent the development or progression of CAD in young women. Further research conducted prior to and after statin initiation in women with multiple CAD risk factors may shed additional light on this phenomenon.
There are several limitations to this study. The pilot sample size is small, and participants had undergone clinically-indicated coronary angiography for suspected ischemic heart disease from one tertiary center in Los Angeles which may limit generalizability. Additionally, the number of cytokines detectable in the plasma of these women was limited. More sensitive cytokine assays are now available.(20) While statins are known to reduce inflammation (21), and our findings did not differ by statin use, we may have been underpowered to detect this. Finally, although samples were drawn with regard to menstrual cycling, the role of hormones is not considered in this analysis. Strengths of our study were the use of core labs for cytokine and coronary angiographic measurements.
Future Directions
These initial results suggest that premenopausal women with obstructive CAD have lower TH1:TH2/HR ratios compared to similar women without obstructive CAD. The findings suggest that young women with obstructive CAD may experience increased recruitment of reparative cells that secrete TH2 cytokines compared to women without CAD. Thus, cytokine balance may play a role in obstructive CAD in young women. Future work in larger samples of younger women with a wider array of circulating inflammatory marker profiles could be useful for development of risk assessment and preventive strategies.
Acknowledgments
The authors and coauthors would like to acknowledge Claudia Zierold PhD at the University of Minnesota for her work with the cytokine assays.
This work was supported by contracts from the National Heart, Lung and Blood Institutes nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, K23HL127262, T32HL69751, R01 HL090957, UL1TR000124, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women’s Health Research (SWHR), Washington, D.C., The Linda Joy Pollin Women’s Heart Health Program, and the Erika Glazer Women’s Heart Health Project, Cedars-Sinai Medical Center, Los Angeles, California and the University of California Los Angeles School of Nursing, Los Angeles, California.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: There are no relevant conflicts of interest of any of the authors to disclose.
Contributor Information
Jo-Ann Eastwood, University of California Los Angeles.
Doris A Taylor, Texas Heart Institute, Houston.
B. Delia Johnson, University of Pittsburgh.
Micheline Resende, Texas Heart Institute
Barry L. Sharaf, Rhode Island Hospital
Bina Ahmed, Dartmouth-Hitchcock Medical Center.
Margo Minissian, Barbra Streisand Women’s Heart Center, Cedars-Sinai Heart Institute.
Chrisandra Shufelt, Barbra Streisand Women’s Heart Center, Cedars-Sinai Heart Institute.
C. Noel Bairey Merz, Barbra Streisand Women’s Heart Center, Cedars-Sinai Heart Institute.
References
- 1.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108(19):2317–22. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 4.Zenowich AG, Taylor DA. Atherosclerosis as a disease of failed endogenous repair. Frontiers in Bioscience. 2006;13:3621–36. doi: 10.2741/2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNeill E, Channon KM, Greaves DR. Inflammatory cell recruitment in cardiovascular disease: murine models and potential clinical applications. Clin Sci (Lond) 2010;118(11):641–55. doi: 10.1042/CS20090488. [DOI] [PubMed] [Google Scholar]
- 6.Martins TB, Anderson JL, Muhlestein JB, Horne BD, Carlquist JF, Roberts WL. Risk factor analysis of plasma cytokines in patients with coronary artery disease by a multiplexed fluorescent immunoassay. Am J Clin Pathol. 2006;125(6):906–13. doi: 10.1309/Q3E6-KF0Q-D3U3-YL6T. [DOI] [PubMed] [Google Scholar]
- 7.Hulsmans M, Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med. 2010;14(1–2):70–8. doi: 10.1111/j.1582-4934.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schächinger V, Zeiher AM. Atherogenesis—recent insights into basic mechanisms and their clinical impact. Nephrology Dialysis Transplantation. 2002;17(12):2055–64. doi: 10.1093/ndt/17.12.2055. [DOI] [PubMed] [Google Scholar]
- 9.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54(17):1561–75. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairweather D, Petri MA, Coronado MJ, Cooper LT. Autoimmune heart disease: role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev Clin Immunol. 2012;8(3):269–84. doi: 10.1586/eci.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairweather D. Sex differences in inflammation during atherosclerosis. Clin Med Insights Cardiol. 2014;8(Suppl 3):49–59. doi: 10.4137/CMC.S17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson BD, Merz CN, Braunstein GD, Berga SL, Bittner V, Hodgson TK, et al. Determination of menopausal status in women: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. Journal of women’s health. 2004;13(8):872–87. doi: 10.1089/jwh.2004.13.872. [DOI] [PubMed] [Google Scholar]
- 13.Mygind ND, Michelsen MM, Pena A, Frestad D, Dose N, Aziz A, et al. Coronary Microvascular Function and Cardiovascular Risk Factors in Women With Angina Pectoris and No Obstructive Coronary Artery Disease: The iPOWER Study. Journal of the American Heart Association. 2016;5(3) doi: 10.1161/JAHA.115.003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35(9):578–89. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lessner SM, Prado HL, Waller EK, Galis ZS. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am J Pathol. 2002;160(6):2145–55. doi: 10.1016/S0002-9440(10)61163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–97. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilic T, Ural D, Ural E, Yumuk Z, Agacdiken A, Sahin T, et al. Relation between proinflammatory to anti-inflammatory cytokine ratios and long-term prognosis in patients with non-ST elevation acute coronary syndrome. Heart. 2006;92(8):1041–6. doi: 10.1136/hrt.2005.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marino F, Guasti L, Cosentino M, Rasini E, Ferrari M, Maio RC, et al. Simvastatin treatment in subjects at high cardiovascular risk modulates AT1R expression on circulating monocytes and T lymphocytes. Journal of hypertension. 2008;26(6):1147–55. doi: 10.1097/HJH.0b013e3282f97dde. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Li WM, Gao C, Sun NL. Effects of atorvastatin on the Th1/Th2 polarization of ongoing experimental autoimmune myocarditis in Lewis rats. Journal of autoimmunity. 2005;25(4):258–63. doi: 10.1016/j.jaut.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Chen P, Chung MT, McHugh W, Nidetz R, Li Y, Fu J, et al. Multiplex serum cytokine immunoassay using nanoplasmonic biosensor microarrays. ACS Nano. 2015;9(4):4173–81. doi: 10.1021/acsnano.5b00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezaie-Majd A, Maca T, Bucek RA, Valent P, Muller MR, Husslein P, et al. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2002;22(7):1194–9. doi: 10.1161/01.atv.0000022694.16328.cc. [DOI] [PubMed] [Google Scholar]


