Abstract
Gaucher’s disease is a rare autosomal recessive, potentially fatal disorder but most common type among lysosomal storage disorders. The disease’s incidence is around 1/40 000 to 1/60 000 births in the general population. A 32-year-old man, born out of non-consanguineous union, presented with generalised tonic–clonic seizures and myoclonus since 17 years of age. Seizures were noted to be resistant to multiple epileptic drugs. He developed gait imbalance, intentional tremor and dysarthria. Detailed examination revealed hepatosplenomegaly, bilateral pancerebellar signs with normal power, reflexes and sensory system. He had major cognitive impairment with impaired frontal and temporal lobar functions. Bone marrow evaluation revealed Gaucher cells, confirming the diagnosis.
Keywords: neurology, epilepsy and seizures
Background
Gaucher’s disease (GD), first described by Philippe Gaucher in 1882, is the most common sphingolipidosis. It is a rare, autosomal recessive disease caused by mutations in the GBA1 gene, located on chromosome 1q21, resulting in markedly decreased activity of lysosomal enzyme, glucocerebrosidase (GCase).1 General findings common of GD include hepatosplenomegaly, hypersplenism and skeletal disease, with the presence of foamy histiocytes (Gaucher cells) in these organs as well as in bone marrow.2 GD III is characterised by a milder neurological involvement along with visceral involvement. Neurological involvement is characterised by severe hypertonia, rigidity, opisthotonus, swallowing impairment, gaze impairment, cerebellar dysfunction, cognitive decline, myoclonus and seizures. Our report stresses on need to keep this rare disorder in mind while evaluating patients with cognitive decline and epilepsy especially in the presence of gaze palsy. Keeping this rare possibility in mind can help to avoid unnecessary diagnostic and therapeutic interventions.
Case presentation
A 32-year-old man was apparently normal until 15 years back when he started having presented with generalised tonic–clonic seizure (GTCS). The frequency of GTCS gradually increased from once every 3 weeks in beginning to current frequency of twice a week. There was history of gait imbalance, clumsiness and slow speech since last 7 years along with intention tremors since last 6 years and progressive cognitive decline since last 6 months. Since last 2 years, he developed jerky movements of all body parts (distal>proximal) which was sensitive to auditory stimulus. Birth, developmental and family histories were unremarkable. He was treated with multiple antiepileptic drugs without relief. General physical examination revealed hepatosplenomegaly. He has impaired frontal and temporal lobe functions, pancerebellar dysfunction, gaze palsy (horizontal more than vertical) with preserved vestibulo-ocular reflex and frequent myoclonic jerks (video 1).
Video 1.
Eye movements of the individual showing impaired vertical and horizontal gaze with preserved vestibulo-ocular reflex.’
Investigations and treatment
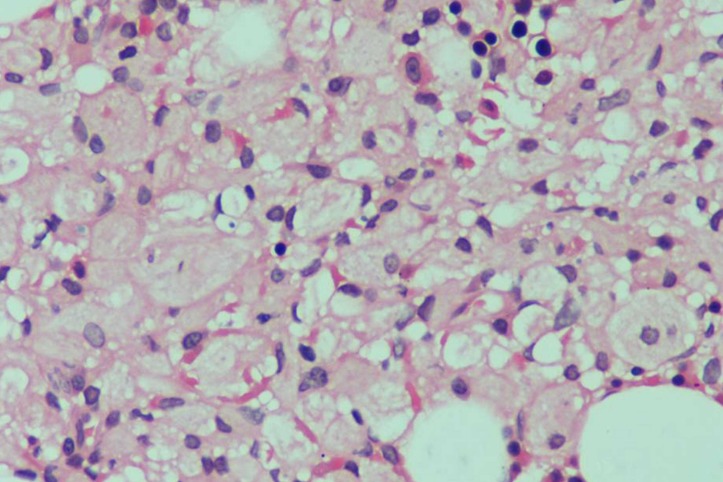
Routine haematological and biochemical investigations including arterial ammonia and serum lactate were normal. Electroencephalography revealed normal background with intermittent polyspike discharges, also seen during photic stimulation. Gadolinium-enhanced MRI scan of brain was normal except mild atrophy. Bone marrow examination revealed large, multinucleate cells with abundant bluish-grey cytoplasm on Giemsa staining (figure 1). The cytoplasm assumed an appearance of ‘crumpled tissue paper’ on periodic acid Schiff (PAS) staining, confirming diagnosis of of GD. He was counselled about enzyme replacement therapy (imiglucerase) and substrate reduction therapy (miglustat) of GD, but due to financial constraints, he refused, so he was discharged on antiepileptic drugs (levetiracetam 3 g/day, sodium valproate 1.5 g/day, topiramate 200 mg/day, phenobarbital 180 mg/day and clobazam 20 mg/day).
Figure 1.
Bone marrow showing histiocytosis with abundant tissue paper-like wrinkled cytoplasm suggestive of Gaucher cells.
Outcome and follow-up
Two months after discharge, he was still having seizures, myoclonus decreased but still present and his antiepileptics were optimised further, but still, he is having seizures and myoclonus.
Discussion
About two-thirds of the patients with GD present before 20 years of age. Onset in childhood is predictive of severe and progressive phenotype. The most common signs and symptoms noted in GD include splenomegaly (95%), hepatomegaly (87%), radiological bone disease (81%), thrombocytopenia (50%), anaemia (40%), growth retardation (34%), bone pain (27%) and bone crisis (9%).3
Three types of GD have been described based on the clinical features, ethnicity and the natural history of the disease. GD type I occurs mainly in adults and is the most common lysosomal storage disorder. It does not affect the nervous system. Patients with GD type II (acute neuronopathic form) and type III (chronic neuronopathic form) have onset at <1 year of age and between 2 and 20 years of age, respectively. GD III is further subdivided into three subtypes—a, b and c depending on the clinical features.4
GD is characterised by decreased activity of GCase, resulting in accumulation of GCase substrate, glucocerebroside (GlcCer) into cells with resultant formation of Gaucher cells. Under light microscopy, Gaucher cells are enlarged cells, with eccentric nuclei, condensed chromatin and a cytoplasm with heterogeneous ‘crumpled tissue paper’ appearance. This appearance occurs due to the presence of GlcCer aggregates in characteristic twisted, fibrillar arrangements which can be visualised using electron microscopy.5 Gaucher cells mainly infiltrate bone marrow, spleen and liver, but can infiltrate other organs as well. These are considered the main protagonist factors in symptoms of the disease. The monocyte/macrophage lineage is preferentially affected as they eliminate erythroid cells and leucocytes, which contain large amounts of glycosphingolipids, a source of GlcCer. On microscopy, Gaucher cells are positive with PAS, Oil Red O and Prussian blue stains indicating the nature of accumulated material as glycolipids admixed with haemosiderin.6
GD I is characterised by substrate accumulation leading to organ damage, principally manifesting as enlargement of the liver, and often massive enlargement of the spleen and skeletal involvement including pathological fractures and bone pains. Fatigue, nose bleeds and easy bruising are a manifestation of the cytopenias, both as a result of bone marrow infiltration and splenomegaly.4 7 Although the disease is progressive in adults, it is compatible with long life. Individuals with this disorder have reduced but detectable levels of GCase activity. GD II is a neuronopathic form of disease with severe neurological disease and is usually fatal by 2 years of age.
GD III is the chronic neuropathic form of GD and has a later onset than GD II. GD III is characterised by a milder neurological involvement compared with GD II along with visceral and bone marrow involvement. Neurological progression is marked by severe hypertonia, rigidity, opisthotonus, swallowing impairment, gaze impairment, cerebellar dysfunction, cognitive decline, myoclonus and seizures.4 Our patient had gaze impairment, pancerebellar dysfunction, myoclonus, seizures and cognitive decline. The basic pathology in neuronopathic GD is accumulation of glucosylceramide in neurons. Once a critical threshold of glucosylceramide storage is reached, the neurons become dysfunctional and trigger a signalling cascade activating surrounding microglia. The net result is activation of a neuroinflammatory cascade with release of cytokines, neurotoxic agents and reactive oxygen/nitrogen species and neuronal cell death.8 Sharma et al report a similar case of Gaucher’s disease presenting as horizontal gaze palsy with progressive myoclonic epilepsy.9
Clinical symptoms of seizure and cognitive decline are attributed to involvement of cortical layers 5 and 3 of temporal lobe, entorhinal cortex, parietal lobe, cingulate gyrus, posterior parietal lobule and occipital lobe. Involvement of midbrain, red nucleus, rostral interstitial nucleus of the medial longitudinal fasciculus and the paramedian pontine reticular formation accounts for impairment of gaze, one of the most common and earliest manifestations of neuropathic GD.10 Myoclonus seen in GD is cortical and associated with a marked increase in the amplitude of the somatosensory evoked potential, indicating a defect of inhibitory input into the cerebral cortex.11
Though there is no cure yet for GD, various treatment options are available which include enzyme replacement therapy (imiglucerase and velaglucerase alfa), substrate reduction therapy (miglustat and eliglustat) aimed at reducing the production of GlcCers and treatment of osteoporosis. Bone marrow transplantation can be used as a last resort.
Learning points.
Gaucher’s disease is a rare cause of progressive myoclonic epilepsy.
Proper clinical and laboratory evaluation is necessary for diagnosis.
Clinical pointers towards Gaucher’s disease III are horizontal gaze palsy, organomegaly and myoclonic epilepsy.
There are limited treatment options for this disease.
Early treatment may halt progression of the disease.
Footnotes
Contributors: RS: data collection, drafting of the manuscript and review of the literature. ASK: concept and revision of the manuscript. AC: drafting and revision of the manuscript. MKG: concept, drafting and revision of the manuscript.
Competing interests: None declared.
Patient consent: Guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hruska KS, LaMarca ME, Scott CR, et al. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum Mutat 2008;29:567–83. 10.1002/humu.20676 [DOI] [PubMed] [Google Scholar]
- 2.Beutler EGG : Scriver CR BA, Sly WS, Valle D, Glucosylceramide lipidoses. New York: McGraw-Hill, 1995. [Google Scholar]
- 3.Binesh F, Yousefi A, Ordooei M, et al. Gaucher’s disease, an unusual cause of massive splenomegaly, a case report. Iran J Ped Hematol Oncol 2013;3:173–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Nagral A. Gaucher disease. J Clin Exp Hepatol 2014;4:37–50. 10.1016/j.jceh.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee RE. The fine structure of the cerebroside occurring in Gaucher’s disease. Proc Natl Acad Sci U S A 1968;61:484–9. 10.1073/pnas.61.2.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mistry PK, Liu J, Yang M, et al. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc Natl Acad Sci U S A 2010;107:19473–8. 10.1073/pnas.1003308107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta A. Epidemiology and natural history of Gaucher’s disease. Eur J Intern Med 2006;17:S2–5. 10.1016/j.ejim.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 8.Vitner EB, Farfel-Becker T, Eilam R, et al. Contribution of brain inflammation to neuronal cell death in neuronopathic forms of Gaucher’s disease. Brain 2012;135:1724–35. 10.1093/brain/aws095 [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Lal V, Das R. Horizontal gaze palsy with progressive myoclonic epilepsy: rare presentation of Gaucher’s disease. Neurol India 2013;61:177–8. 10.4103/0028-3886.111136 [DOI] [PubMed] [Google Scholar]
- 10.Wong K, Sidransky E, Verma A, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab 2004;82:192–207. 10.1016/j.ymgme.2004.04.011 [DOI] [PubMed] [Google Scholar]
- 11.Park JK, Orvisky E, Tayebi N, et al. Myoclonic epilepsy in Gaucher disease: genotype-phenotype insights from a rare patient subgroup. Pediatr Res 2003;53:387–95. 10.1203/01.PDR.0000049515.79882.94 [DOI] [PubMed] [Google Scholar]