Key Points
Increased human B-cell reconstitution is seen in female compared to male mice in multiple humanized mouse models.
The PI mouse model recapitulates HSC-intrinsic autoimmune defects from T1D and RA bone marrow donors.
Abstract
B cells play a major role in antigen presentation and antibody production in the development of autoimmune diseases, and some of these diseases disproportionally occur in females. Moreover, immune responses tend to be stronger in female vs male humans and mice. Because it is challenging to distinguish intrinsic from extrinsic influences on human immune responses, we used a personalized immune (PI) humanized mouse model, in which immune systems were generated de novo from adult human hematopoietic stem cells (HSCs) in immunodeficient mice. We assessed the effect of recipient sex and of donor autoimmune diseases (type 1 diabetes [T1D] and rheumatoid arthritis [RA]) on human B-cell development in PI mice. We observed that human B-cell levels were increased in female recipients regardless of the source of human HSCs or the strain of immunodeficient recipient mice. Moreover, mice injected with T1D- or RA-derived HSCs displayed B-cell abnormalities compared with healthy control HSC-derived mice, including altered B-cell levels, increased proportions of mature B cells and reduced CD19 expression. Our study revealed an HSC-extrinsic effect of recipient sex on human B-cell reconstitution. Moreover, the PI humanized mouse model revealed HSC-intrinsic defects in central B-cell tolerance that recapitulated those in patients with autoimmune diseases. These results demonstrate the utility of humanized mouse models as a tool to better understand human immune cell development and regulation.
Visual Abstract
Introduction
Humanized mouse models have been used as tools for studying the development and function of human immune cells1-3 in a controlled system in which environmental factors (eg, food, microorganisms) are the same for all mice. We recently modified a humanized mouse model that involves transplanting human fetal thymus tissue and fetal liver (FL)–derived hematopoietic stem cells (HSCs) to immunodeficient mice4-9 to allow the study of immune development in HSCs from adults.10 We have termed this the personalized immune (PI) mouse model. By reconstituting immunodeficient mice with bone marrow (BM)–derived HSCs from adults with established disease, we aimed to identify HSC-intrinsic abnormalities in immune regulation and development because some autoimmune diseases are transferable by HSCs.11-16 Therefore, underlying immunoregulatory defects could potentially be dissected in this model.
The development and role of autoantibodies in disease progression has highlighted the central involvement of B cells in autoimmune disease.17 Type 1 diabetic (T1D) patients demonstrate impaired B-cell homeostasis in peripheral blood (PB),18 and both T1D and rheumatoid arthritis (RA) patients show defects in central and peripheral B-cell tolerance.19,20 The central role of B cells has become especially clear since the introduction of B-cell depletion by rituximab therapy,21 which was beneficial in the treatment of RA,22 reduced diabetes in CD20 Tg-NOD mice,23 and preserved β-cell function in newly diagnosed humans for 1 year.24 We now report on our use of the PI mouse model to determine whether abnormalities in B-cell development can be identified and therefore could provide a model to understand the HSC-intrinsic underpinnings of disease.
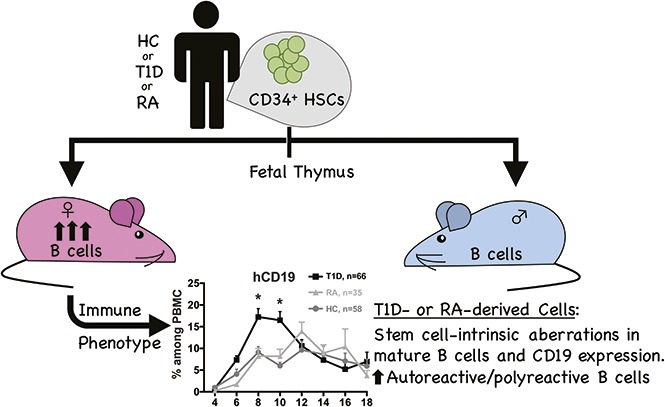
Several autoimmune diseases show increased prevalence in females compared with males. For example, RA is more common in females before the age of 50 years25 with autoantibodies directed against immunoglobulin G (IgG) regions (rheumatoid factor) and citrullinated proteins appearing well before disease onset.26 For T1D, which has similar incidence in males and females,27 female patients show higher glutamic acid decarboxylase antibody levels compared with age-matched male patients.28 Thus, we examined the impact of the sex of recipient mice on B-cell development and function in humanized mice. Furthermore, we have compared B-cell development and autoreactivity in PI mice constructed from HSCs of healthy, T1D, and RA donors and evaluated the impact of BM donor and recipient sex. Our data indicate that female mice support higher human immune cell production than males, largely because of increased B-cell reconstitution. Similar effects were seen in humanized NS (NOD.CB17- Prkdcscid/J) and NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice injected with BM- or FL-derived CD34+ cells. Adjusting CD34+ cell numbers commensurate with weight did not abolish the decrease in B-cell production in males. Importantly, peripheral B-cell reconstitution in PI mice was greater when the HSCs were obtained from patients with T1D than from healthy controls (HCs). Finally, defects in central tolerance in T1D and RA were recapitulated in BM of PI mice, demonstrating the utility of this model for dissecting genetically predetermined HSC-intrinsic abnormalities in B-cell development.
Methods
Animals and human tissues and cells
NS and NSG mice were obtained from The Jackson Laboratory. NSG pig cytokine transgenic (NSG-pct) mice that produced porcine cytokines (interleukin-3, granulocyte-macrophage colony-stimulating factor, and stem cell factor) were developed by crossing NOD/SCID-pct mice29 (The Jackson Laboratory) with NSG mice. On the basis of studies to be reported elsewhere that demonstrated improved myeloid but not B-cell reconstitution in these animals, NSG-pct recipients were used in some PI mouse experiments and were internally controlled by using the same type of recipients for HC- and autoimmune patient-derived cohorts in each experiment. All mice were maintained under specific pathogen-free conditions, and all experiments were performed under approved protocols from the Columbia University Institutional Animal Care and Use Committee.
Human fetal thymus and liver tissues (gestational age, 17 to 20 weeks) were obtained from Advanced Biosciences Resources. Fetal thymus fragments were cryopreserved in 10% dimethyl sulfoxide (Sigma-Aldrich) and 90% human AB serum (Gemini Bio Products).10 FL fragments were treated for 20 minutes at 37°C with 100 μg/mL of Liberase (Roche) to obtain a cell suspension. Human CD34+ cells were purified from FL or BM by density gradient centrifugation (Histopaque-1077, Sigma) followed by positive immunomagnetic selection using anti-human CD34 microbeads (Miltenyi Biotec) according to the manufacturer’s instructions. Cells were either frozen or injected immediately. BM aspirates from T1D, RA, and HC volunteers were recruited by the Center for Translational Immunology Human Studies Core, the Naomi Berrie Diabetes Center, or the Division of Rheumatology at Columbia University Medical Center. All human samples were collected under protocols approved by the Institutional Review Board of Columbia University Medical Center in accordance with the Declaration of Helsinki.
Human tissue transplantation
Six- to 12-week old mice were sublethally irradiated (1.2 Gy for NSG-pct, 1.5 Gy for NSG, and 2.4 Gy for NS mice) with X-rays. Human fetal thymus fragments of about 1 mm3 were implanted beneath the kidney capsule and 1 × 105 to 2.6 × 105 human CD34+ cells were injected either IV or directly into the bone cavity. For the latter, a 26-gauge needle was used to create a hole in the tibia, followed by injection of 15 to 25 μL of cells using a 27-gauge needle (Hamilton). Recipient mice were treated IV with 400 μg of anti-human CD2 monoclonal antibody BTI-322 or LO-CD230 (produced at Bio X Cell, Inc) on days 0, 7, and 14. NS mice received a total of 8 injections of anti-asialo GM1 serum (Wako Pure Chemicals), given every 5 days. Human HC and T1D or RA BM donors were matched with fetal thymus for autoimmune disease-associated class II HLA DR3, DR4, or DQ8 alleles (supplemental Table 1).
Cell staining and sorting and B-cell tolerance analysis
B cells were purified from PB of human donors and from the spleens of PI mice. Single CD19+CD21loCD10+IgMhiCD27– new emigrant/transitional B cells were sorted on a FACSAria cell sorter (BD Biosciences) into 96-well polymerase chain reaction plates. Expression cloning and antibody reactivity was tested as previously described.31
Statistical analysis
Statistical analysis and comparisons were performed with Graph-Pad Prism 6.0 (GraphPad Software). All data are expressed as average ± standard error of mean. The nonparametric Mann-Whitney U test was used to compare groups at individual time points for each cell population. One-way analysis of variance (ANOVA) with post hoc Tukey test was performed to compare 3 groups. Two-way ANOVA was used to resolve overall effects between transplant groups over time. When 2-way ANOVA was significant, Sidak’s multiple comparison test was run. P < .05 was considered to be statistically significant.
Results
Female mice injected with adult BM CD34+ cells showed higher levels of PB B-cell reconstitution than male recipients
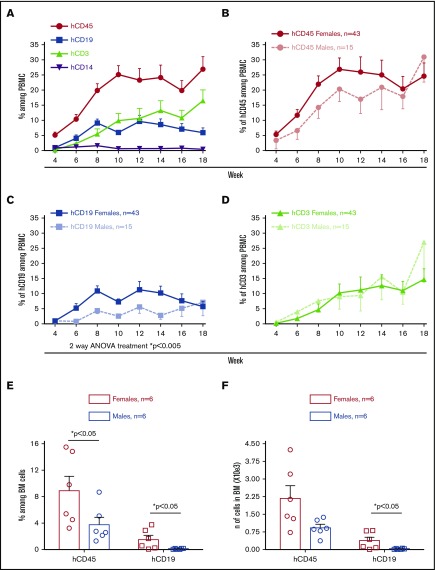
To study the B-cell compartment in the PI mouse model, we injected NSG mice with adult BM CD34+ cells obtained from 10 different HC donors. On the same day, a piece of a partially HLA-matched cryopreserved and thawed fetal thymus was implanted under the kidney capsule of the animals. As previously shown,10 human immune cells were detectable in PB 4 weeks after transplantation (Figure 1A). B and T cells were the main human cell populations observed, with the former peaking around 10% of peripheral blood mononuclear cells (PBMCs) at week 12 and then slowly declining. In contrast, T cells increased over time. Female recipient mice displayed higher total human chimerism than males (Figure 1B) because of a greater percentage of CD19+ B cells in female than male recipient PBMCs (Figure 1C). Although peripheral B-cell reconstitution was faster and peaked at higher levels in female than in male mice, the 2 groups became similar after week 14. T cells were similar between female and male animals throughout the course of the experiment (Figure 1D).
Figure 1.
Female NSG mice reconstituted with adult BM CD34+cells show higher B-cell chimerism than male recipients. Sublethally irradiated NSG and NSG-pct mice were injected with 1.5 × 105 to 2 × 105 BM CD34+ cells and grafted under the kidney capsule with a partially HLA-matched human fetal thymus. (A-D) Human peripheral reconstitution was measured by flow cytometry in PB every other week (10 HC donors; n = 58 mice). Means of multilineage chimerism among total (mouse plus human) PBMCs are shown over time. Bars represent standard error of the mean (SEM). (E-F) Mice were euthanized at week 3 after transplantation and BM (1 leg) was collected. Mean + SEM of human CD45 and CD19 percentage and number are shown. Each symbol represents a single mouse.
In mice euthanized 3 weeks after transplantation, the percentage and absolute number of B cells was greater in BM of females than males (Figure 1E-F), but no significant difference was observed among splenocytes (supplemental Figure 1A-B). In contrast, no difference was detected in B-cell levels of male vs female BM or spleens at later time points (weeks 14-18) (supplemental Figure 2A-D).
Analysis of the effect of BM donor sex on human chimerism showed no difference in total human CD45 percentage (supplemental Figure 3A) and increased B-cell reconstitution in female compared with male recipients of male BM HSCs (supplemental Figure 3B). Although early B-cell reconstitution tended to be higher in female mice injected with female vs male donor HSCs, this difference did not achieve statistical significance. Unfortunately, we did not have a group of male mice injected with female HSCs to permit further comparison.
Because age-matched male mice normally weigh more than females, we considered the possibility that the observed difference in B-cell reconstitution could reflect the lower number of CD34+ cells injected on a per weight basis into male mice. We tested this hypothesis in 1 experiment comparing females and males injected with 2 × 105 BM CD34+ cells with males receiving 2.6 × 105 cells (males weighed ∼30% more than females). In this pilot experiment, females showed a significantly higher frequency of human CD45+ (hCD45+) cells in PB compared with males receiving either 2 × 105 or 2.6 × 105 CD34+ cells because of an increase in the proportion of B cells (supplemental Figure 4A-C). B-cell percentages (supplemental Figure 4D) and numbers (supplemental Figure 4E) were similar between females and both male groups in BM and spleen at week 20 after transplantation. This preliminary study suggests that the increased percentage of B cells in female compared with male mice is not the result of injection of greater number of CD34+ cells per gram in females.
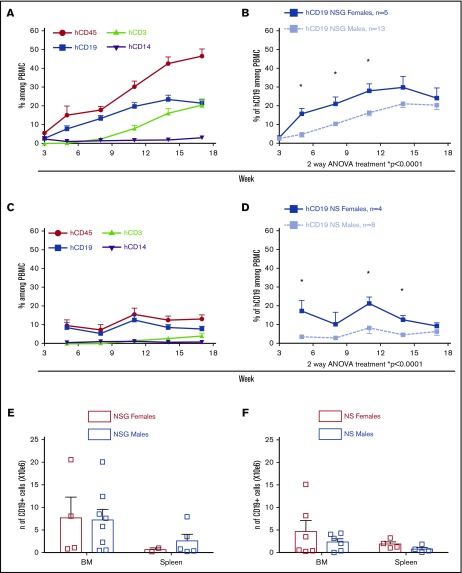
Similar male-female disparity in human B-cell reconstitution after transplantation of FL CD34+ cells
To determine whether increased peripheral B-cell chimerism in female mice was generalizable, we compared male and female recipient mice injected with human FL CD34+ cells and grafted with an autologous fetal thymus fragment using both NS and NSG recipients. Consistent with previous studies,32,33 NSG mice showed higher levels of human PB chimerism than NS mice (Figure 2A vs 2C), peaking at 50% vs 12% hCD45+ cells among PBMCs respectively, at week 18. B cells in the NSG mice receiving FL CD34+ cells were greater than those in mice reconstituted with adult BM CD34+ cells (Figure 1A). Nevertheless, for both NSG (Figure 2B) and NS (Figure 2D) recipients of FL CD34+ cells, the percentage of circulating B cells was increased in female compared with male mice. As in the PI mouse model, B-cell frequencies converged in females and males after week 12. BM and spleen analysis at weeks 20 to 22 revealed no difference in B-cell number (Figure 2E-F) or percentage (supplemental Figure 5A-B) between female and male recipients. Human IgM was present in serum of both NSG and NS mice 20 weeks after transplantation at similar level in females and males (supplemental Figure 5C-D).
Figure 2.
B-cell levels in PB are higher in NSG and NS female vs male recipient mice injected with FL CD34+cells. Sublethally irradiated NSG or NS mice were injected with 1.5 × 105 to 2.5 × 105 FL CD34+ cells and grafted with autologous human fetal thymus under the kidney capsule. (A-D) Human peripheral chimerism was measured by flow cytometry in PB every 3 weeks (2 FL samples; n = 18 NSG and n = 12 NS mice). Means of multilineage chimerism among total (mouse plus human) PBMCs are shown over time. Bars represent + SEM. (E-F) Mice were euthanized between weeks 20 and 22 after transplantation, and BM and spleen were collected and analyzed. Mean + SEM of CD19 number are shown. Each square represents a single mouse. *P < .05 (statistically significant) calculated by Sidak’s multiple comparison test.
Similar B-cell composition in hemato-lymphoid tissues of female and male recipient mice
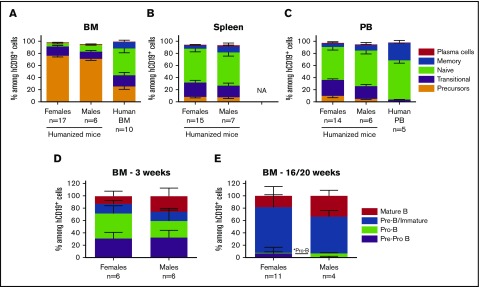
To further investigate the disparity in B-cell reconstitution in male vs female recipients, we analyzed developing B-cell populations in BM, spleen, and PB of the mice. As shown in supplemental Figure 6, we analyzed expression of CD24, CD38, and surface IgM (sIgM) on gated CD19+ cells by flow cytometry. This strategy distinguishes mature naïve B cells (CD24lo CD38lo)34 from transitional and precursor populations (CD24hi CD38hi), which are further resolved by high expression of surface IgM on transitional B cells. BM CD19+ cells of both female and male mice receiving adult BM CD34+ cells were comprised of almost 80% B-cell precursors (Figure 3A) and few naïve and memory B cells compared with the BM of the human donors. Few plasma cells were detected using a combination of CD138 and CD27 markers (supplemental Figure 7A-D). BM B cells in NSG and NS mice reconstituted with FL CD34+ cells showed a lower percentage (∼50% to 60%) of precursors (supplemental Figure 8A-B) with equal percentages of transitional and mature naïve B cells. Spleen and PB contained 60% naïve B cells (Figure 3B-C; supplemental Figure 8C-D), demonstrating increased maturity of peripheral B cells. However, at least 20% remained at the transitional stage, which is higher than that in normal human PBMCs (<5%; Figure 3C). Overall, no major differences in B-cell subsets were observed between females and males among the different tissues.
Figure 3.
Comparison of B-cell subpopulations and precursors in female and male recipient mice. Sublethally irradiated NSG and NSG-pct mice were injected with 1.5 × 105 to 2 × 105 BM CD34+ cells and grafted under the kidney capsule with a partially HLA-matched human fetal thymus. (A-C) Mice were euthanized between weeks 16 and 20 after transplantation and BM (n = 17 females and 6 males), spleen (n = 15 females and 7 males), and PB (n = 14 females and 6 males) were collected and analyzed for their B-cell composition. Mean ± SEM of indicated B-cell subpopulation percentages among total CD19+ cells are shown. Animals with less than 1% CD19+ cells were excluded from the analysis. Females in the analysis were reconstituted from 5 to 7 BM donors whereas males were reconstituted from 2 to 3 BM donors. (A) Ten human BM and (C) 5 human PBMC samples were plotted for comparison. (D-E) Mice were euthanized (D) at week 3 (n = 6 females and 6 males) or (E) between weeks 16 and 20 (n = 11 females and 4 males) after transplantation. BM cells were collected, and early B-cell progenitors were analyzed by flow cytometry. Mean + SEM of indicated BM B-cell progenitor percentages among total CD19+ cells are shown. Animals with less than 1% CD19+ cells were excluded from the analysis. *Statistical significance calculated by the nonparametric Mann-Whitney U test used for each B-cell population. NA, not available.
We looked at earlier stages of B-cell differentiation in the BM using CD34 and CD10 markers35 (supplemental Figure 9). At week 3 following reconstitution with adult CD34+ cells, the recipients’ BM contained more than 50% early B-cell precursors (Pre-Pro B and Pro-B cells) (Figure 3D), whereas at later time points, the main population was Pre-B/immature B cells (Figure 3E). Female mice showed a trend toward greater percentages of Pro-B cell precursors at week 3 (40.47% ± 5.1% vs 27.06% ± 7.31%; P = .197) and lower percentages of mature B cells (12.84% ± 7.67% vs 25.83% ± 12.57%; P = .065) (Figure 3D). At weeks 16 to 20, Pro-B cell precursors were significantly decreased in females compared with males (1.46% ± 0.46% vs 4.9% ± 0.75%; P = .018) with corresponding increases in Pre-B/immature B-cell frequencies (73.76% ± 7.17% vs 59.93% ± 4.89%; P = .18) (Figure 3E).
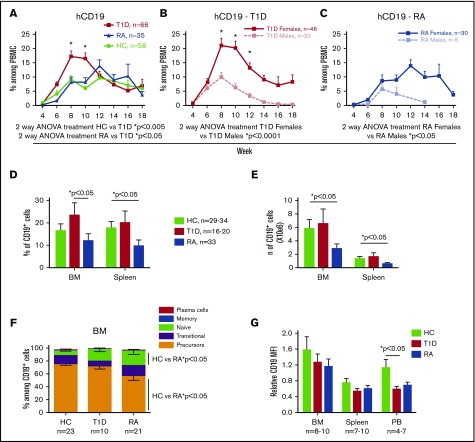
Mice injected with T1D-derived BM CD34+ cells show higher peripheral B-cell reconstitution than HC and RA-derived recipients
B cells have been implicated in the development of autoimmune diseases such as T1D and RA. We were therefore interested in B-cell reconstitution in PI mice constructed with CD34+ cells from patients with autoimmune disease compared with PI mice derived from HC BM CD34+ cells. Human peripheral B-cell reconstitution was faster and reached a higher peak in T1D-derived than in HC-derived mice in the same experiments. In contrast, B-cell reconstitution in RA-derived PI mice was similar to that of HC-derived animals (Figure 4A). PBMCs of T1D-derived mice contained almost 20% human B cells at week 8 and then declined so that levels were similar in all groups by week 12. Analysis by recipient sex revealed much higher B-cell chimerism in female than in male recipients of both T1D (Figure 4B) and RA (Figure 4C) HSCs. Males lost B-cell chimerism by week 14, whereas females from all groups maintained B-cell reconstitution at later time points (∼5% to 10%). Analysis of BM and spleen revealed significantly lower B-cell percentages (Figure 4D) and numbers (Figure 4E) in RA-derived mice compared with controls and T1D-derived mice. Overall, RA mice showed low human chimerism in all hemato-lymphoid organs (data not shown). B-cell subpopulation analysis revealed significantly increased naïve and decreased B-cell precursors in the BM of RA-derived compared with HC-derived PI mice (Figure 4F). Plasma collected from all 3 groups contained very low concentrations of human IgG (supplemental Figure 10). No statistically significant correlation was observed between IgG level and percentage of B cells or between degree of HLA matching of fetal thymic tissue and BM donor and the percentage of B cells in female recipients (data not shown).
Figure 4.
Peripheral B-cell reconstitution is higher in T1D than in HC-and RA-derived PI humanized mice. Sublethally irradiated NSG and NSG-pct mice were injected with 1.5 × 105 to 2.7 × 105 BM CD34+ cells and grafted under the kidney capsule with a partially HLA-matched human fetal thymus. (A-C) Human peripheral CD19 levels were measured by flow cytometry in PBMCs every other week (10 HC donors, n = 58 mice; 9 T1D donors, n = 66 mice; 5 RA donors, n = 35 mice). Mean of CD19+ cells among total (mouse plus human) PBMCs is shown over time. Bars represent SEM. *Statistical significance calculated by Sidak’s multiple comparison test. (D-E) Mice were euthanized between weeks 14 and 18 after transplantation and BM and spleen were collected. Mean + SEM of CD19 percentage and number are shown. (F) Mean ± SEM of indicated B-cell subpopulation percentage among total BM CD19+ cells are shown. Animals with less than 1% CD19+ cells were excluded from the analysis. (G) Mean + SEM of relative CD19 median fluorescence intensity (MFI) was calculated as follows: CD19 MFI of the sample divided by CD19 MFI of human PBMC run on the flow cytometer in the same analysis.
A recent study in a humanized mouse model reported downmodulation of CD19 expression on autoreactive B cells compared with non-autoreactive B cells developing in the same mouse.36 In our study, the median fluorescence intensity of CD19 on T1D-derived PB B cells was significantly lower than that on HC-derived B cells in the same experiments (Figure 4G; supplemental Figure 11), suggesting the presence of more circulating autoreactive B cells in T1D mice. B cells from RA mice showed a similar trend (Figure 4G; supplemental Figure 11).
Defective central B-cell tolerance checkpoint in PI mice generated with HSCs from patients with RA and T1D
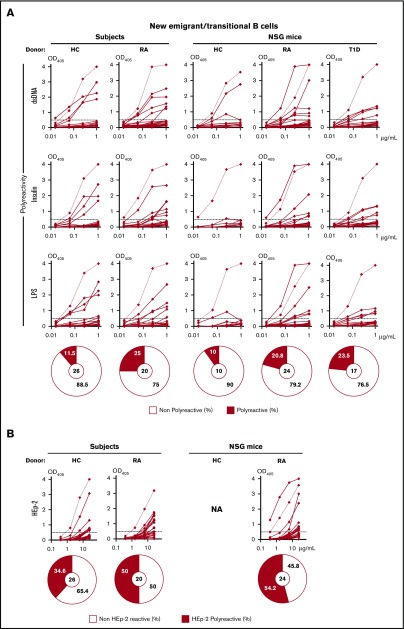
Because both RA and T1D patients have been shown to have defective central B-cell tolerance,19,20 we were interested in determining whether this defect was recapitulated in PI mice generated with HSCs from patients with RA or T1D. We isolated new emigrant/transitional B cells from the spleens of PI mice generated from BM of 1 HC, 1 RA, and 1 T1D donor and performed expression cloning of their B-cell antigen receptors as previously described.31 We compared them with the emigrant/transitional B cells isolated from the PB of 1 HC and 1 RA patient. Both the RA and the T1D NSG mouse generated more than 20% polyreactive (reactive with double-stranded DNA, insulin, and lipopolysaccharide) clones (Figure 5A). Both the HC donor and the HC NSG mouse contained only 10% autoreactive B cells in their spleens, consistent with previous results.37 For the RA donor, this defect was confirmed by the high percentage (50%) of B-cell clones making human epithelial type 2–binding antibodies in the RA donor and respective PI mouse compared with the HC patient (Figure 5B). These results indicate that PI mice might be able to recapitulate some defects in central B-cell tolerance present in autoimmune individuals.
Figure 5.
Impaired central B-cell tolerance in RA and T1D humanized mice. Single CD19+CD21loCD10+IgMhiCD27– new emigrant/transitional B cells were sorted from PB of 1 HC and 1 RA donor, and from the spleen of 1 HC, 1 RA, and 1 T1D humanized mouse. Expression cloning was carried out as described31 and antibodies from new emigrant/transitional B-cell clones were tested by enzyme-linked immunosorbent assay for reactivity against (A) double-stranded DNA (dsDNA), insulin, and lipopolysaccharide (LPS) or for (B) anti human epithelial type 2 (HEp-2) cell reactivity. Dotted lines show monoclonal polyreactive antinuclear antibody control (ED38+) and solid lines show binding for each cloned recombinant antibody. Horizontal lines define cutoff optical density at 405 nm (OD405) for positive reactivity. For each sample, the frequency of polyreactive (filled area) and nonpolyreactive (open area) clones is summarized in pie charts, with the total number of clones tested indicated in the centers.
Discussion
We report here that female NS and NSG immunodeficient mice show greater PB B-cell reconstitution than males regardless of donor sex or whether they were reconstituted with BM or FL CD34+ cells. Female recipients showed accelerated B-cell development in all 3 groups of PI mice (HC, T1D, and RA) compared with male recipients. Although this difference was not sustained at later time points for HC donor cohorts, female recipients of T1D- and RA-derived HSCs maintained higher B-cell frequencies compared with males over a longer period.
Notta et al38 reported increased overall human chimerism in female compared with male recipient BM and spleen, especially when limited numbers of human CD34+CD38– cord blood(CB) cells were transplanted. We did not detect any difference in human reconstitution in either BM or spleens of our mice at 16 to 20 weeks after transplantation. However, we injected a greater total number of progenitor cells than did Notta et al. Human CD45 reconstitution kinetics in NOD-Rag1−/−IL2rgamma−/− (NRG) mice injected with 1.5 × 105 to 2 × 105 CB-derived CD34+ cells trended toward higher chimerism in females than in males before week 16 and converged by week 20,39 consistent with our results. Higher human reconstitution from CB-derived HSCs was reported in female mice compared with males, especially at early time points.38-40 The cell type responsible for this difference was not identified. Our analysis of BM at week 3 after transplantation demonstrated higher percentages and numbers of CD19+ cells in females than in males, consistent with the difference in B-cell chimerism observed in PB at week 6. Data normalizing the number of HSCs injected to weight mitigated against the possibility that the greater weight of males compared with age-matched females was responsible for the difference. The kinetics of human T-cell reconstitution did not differ between female and male recipients, suggesting that sex does not significantly affect T-cell development in the human fetal thymic graft where these cells originate.
Immune responses have been reported to differ between females and males in both mice and humans.41-43 Females have stronger immune responses, as reflected in enhanced humoral immune responses,44-46 increased resistance to certain infections,47 and higher incidence of several autoimmune diseases.27,48 Different biological mechanisms have been proposed to explain this dimorphism, including genes found on X and Y chromosomes49 and autosomal genes regulated in a sex-specific manner.50 Most studies have emphasized the role of steroid hormones.51,52 Both estrogens and androgens reduce the numbers of immature T lymphocytes, leading to thymic involution during puberty53 and suppress B lymphopoiesis in BM.54-56 Interestingly, murine c-kithi cells, murine B cells, and adult human BM CD34+ cells express both estrogen and androgen receptors, whereas mouse FL and human CB progenitors do not.57,58 Estrogen signaling promotes cell cycling in mouse HSCs,59 which might accelerate B-cell development. Estradiol has also been shown to block apoptosis of immature B cells, causing the maintenance of autoreactive clones that would otherwise be deleted.60,61 In contrast, androgens suppressed humoral autoimmunity in the NZB/W F1 mouse model of systemic lupus erythematosus,62,63 and the absence of androgen receptors in mice enhanced B cells in both BM and blood.64 Thus, recipient sex hormones may play a major role in the accelerated human B-cell development in female vs male mice. Suppression of estrogen levels would help to elucidate the effect of sex hormones on B-cell homeostasis. Although comparisons of B-cell counts are limited, Yan et al65 reported similar percentages of peripheral B cells in women and men. However postmenopausal women have reduced numbers of B cells compared with premenopausal women,66 and hormone replacement therapy after menopause restores B lymphocyte counts.67,68 It is challenging to clearly distinguish the intrinsic factors that influence immune responses from environmental factors and exposures in humans. Our observation of an HSC-extrinsic impact of recipient sex on B-cell reconstitution suggests a unique opportunity to dissect the mechanisms of these differences.
Because sex hormones act on immature B cells in mice,60,61 we specifically looked at the B-cell differentiation stages in BM, spleen, and PB. No clear difference was observed between females and males in either BM or FL CD34+-injected mice. Our analysis confirmed the partial block in B-cell maturation described in other humanized mouse models,69-71 with 20% transitional B cells in spleen and PB and a very low percentage of memory B cells (<10%). Investigation of early B-cell precursors at week 3 showed a trend toward increased Pro-B-cell frequencies in females that in combination with the greater total CD19+ cell number might indicate an early expansion of this pool. Pre-Pro B-cell precursors disappeared at later time points, when the predominant B-cell population was represented by Pre-B/immature cells, possibly explaining the loss of B-cell peripheral chimerism observed in PI mice after week 12.
The PI mouse model allows us to investigate genetically determined HSC-intrinsic defects in patient BM CD34+ cells that may drive the immune dysregulation that promotes disease. Peripheral human B-cell levels were significantly higher in PB of T1D-derived compared with HC-derived PI mice until week 10. Although the greatest difference was seen in female T1D compared with female HC mice, even T1D males showed higher B-cell levels than HC-derived male mice at several time points. This difference may be related to the finding that transitional and naïve B cells are resistant to apoptosis in T1D patients.18 Increased circulating B cells and decreased surface CD19 levels in PB B cells might reflect the failure of central tolerance induction. Indeed, we observed defective negative selection of autoreactive/polyreactive B-cell clones in a T1D-derived compared with an HC-injected mouse, echoing the abnormality reported in patients with T1D.20 These results clearly show that PI mice provide an opportunity to elucidate abnormalities in B-cell development and homeostasis in patients with T1D.
Although RA-derived PI mice manifested the same increase in peripheral B-cell levels in female compared with male recipients, their human reconstitution levels were overall quite low. HSCs from RA patients have been reported to be defective.72 To overcome this, we increased the number of injected CD34+ cells per mouse to reliably achieve human immune reconstitution. Nevertheless, we also observed a failure of central B-cell tolerance induction in an RA-derived mouse, consistent with our observations in the donor used to reconstitute the mouse and with reported results in RA patients.19 In addition, RA-derived mice showed significantly increased percentages of mature naïve B cells and reduced percentages of immature B cells compared with HC mice in their BM, possibly reflecting the failed negative selection in these B-cell populations. A potential increase in B-cell levels in RA-derived PI mice could be masked by the overall poor HSC function and lymphopoiesis in these animals, which might reflect an impact of prior methotrexate or other cytotoxic therapy on hematopoietic progenitors in the human donor.
In conclusion, we describe here a highly reproducible effect of recipient sex on human B-cell reconstitution in humanized mice constructed with adult or fetal HSCs. These results set the stage for further in vivo studies to dissect the influence of sex dimorphism on the human immune system. Moreover, 2 PI mice generated from 1 T1D and 1 RA donor replicate the defect in the central B-cell tolerance checkpoint described in patients with autoimmune diseases, suggesting that PI mice might be able to recapitulate the defect in B-cell tolerance present in autoimmune individuals and thus provide an opportunity in the future to use this model to tease apart these defects.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank G. Zhao for animal husbandry; Siu-Hong Ho; L. Devine; and C. Wang for cell sorting.
This work was supported by National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases grants UC4 DK104207, R01 DK103585, R01 DK106436 (R.W.), and F32 DK095539 (N.M.D.); NIH, National Institute of Allergy and Infectious Diseases grants P01 AI045897 (M.S.), T32 AI106711 (G.N.), and AI071087 (E.M.); NIH, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R21 AR064473; Office of the Director awards S10RR027050 and S10OD020056 for research performed in the Center for Translational Immunology Flow Cytometry Core; by the American Diabetes Association grant 7-11-MN-62 (C.B.); and by a Naomi Berrie Diabetes Fellowship.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: C.B. designed and performed experiments, analyzed results, and wrote the paper; N.M.D. designed and performed experiments, analyzed results, and helped write the paper; G.N. performed experiments and analyzed results; M.A.H., C.F., E.C., and M.K.-M. performed experiments; S.G., F.R.D., and E.M. designed and carried out analyses of B-cell tolerance; D.G.S., S.R.C., and P.S. procured human bone marrow samples; R.G., E.G., and J. Bathon assisted with regulatory approval, volunteer selection, and recruitment; S.Y. assisted with regulatory approval and procured human bone marrow samples; J. Bi assisted with HLA typing; R.W. provided intellectual input and assisted with regulatory approval, HLA typing, volunteer selection, and recruitment; and M.S. oversaw the design, conduct, and interpretation of all experiments, and with C.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Megan Sykes, Columbia University Medical Center, 650 West 168th St, BB1512, New York, NY 10032; e-mail: megan.sykes@columbia.edu.
REFERENCES
- 1.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12(11):786-798. doi: 10.1038/nri3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rongvaux A, Takizawa H, Strowig T, et al. . Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol. 2013;31(1):635-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cell Mol Immunol. 2012;9(3):208-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lan P, Wang L, Diouf B, et al. . Induction of human T-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood. 2004;103(10):3964-3969. [DOI] [PubMed] [Google Scholar]
- 5.Tonomura N, Habiro K, Shimizu A, Sykes M, Yang YG. Antigen-specific human T-cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood. 2008;111(8):4293-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onoe T, Kalscheuer H, Chittenden M, Zhao G, Yang YG, Sykes M. Homeostatic expansion and phenotypic conversion of human T cells depend on peripheral interactions with APCs. J Immunol. 2010;184(12):6756-6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onoe T, Kalscheuer H, Danzl N, et al. . Human natural regulatory T cell development, suppressive function, and postthymic maturation in a humanized mouse model. J Immunol. 2011;187(7):3895-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melkus MW, Estes JD, Padgett-Thomas A, et al. . Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12(11):1316-1322. [DOI] [PubMed] [Google Scholar]
- 9.Wege AK, Melkus MW, Denton PW, Estes JD, Garcia JV. Functional and phenotypic characterization of the humanized BLT mouse model. Curr Top Microbiol Immunol. 2008;324:149-165. [DOI] [PubMed] [Google Scholar]
- 10.Kalscheuer H, Danzl N, Onoe T, et al. . A model for personalized in vivo analysis of human immune responsiveness. Sci Transl Med. 2012;4(125):125ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minchinton RM, Waters AH, Kendra J, Barrett AJ. Autoimmune thrombocytopenia acquired from an allogeneic bone-marrow graft. Lancet. 1982;2(8299):627-629. [DOI] [PubMed] [Google Scholar]
- 12.Smith CI, Aarli JA, Biberfeld P, et al. . Myasthenia gravis after bone-marrow transplantation. Evidence for a donor origin. N Engl J Med. 1983;309(25):1565-1568. [DOI] [PubMed] [Google Scholar]
- 13.Aldouri MA, Ruggier R, Epstein O, Prentice HG. Adoptive transfer of hyperthyroidism and autoimmune thyroiditis following allogeneic bone marrow transplantation for chronic myeloid leukaemia. Br J Haematol. 1990;74(1):118-119. [DOI] [PubMed] [Google Scholar]
- 14.Sturfelt G, Lenhoff S, Sallerfors B, Nived O, Truedsson L, Sjöholm AG. Transplantation with allogenic bone marrow from a donor with systemic lupus erythematosus (SLE): successful outcome in the recipient and induction of an SLE flare in the donor. Ann Rheum Dis. 1996;55(9):638-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lampeter EF, McCann SR, Kolb H. Transfer of diabetes type 1 by bone-marrow transplantation. Lancet. 1998;351(9102):568-569. [DOI] [PubMed] [Google Scholar]
- 16.Goettel JA, Biswas S, Lexmond WS, et al. . Fatal autoimmunity in mice reconstituted with human hematopoietic stem cells encoding defective FOXP3. Blood. 2015;125(25):3886-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Browning JL. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat Rev Drug Discov. 2006;5(7):564-576. [DOI] [PubMed] [Google Scholar]
- 18.Habib T, Funk A, Rieck M, et al. . Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J Immunol. 2012;188(1):487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201(10):1659-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menard L, Saadoun D, Isnardi I, et al. . The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121(9):3635-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gürcan HM, Keskin DB, Stern JNH, Nitzberg MA, Shekhani H, Ahmed AR. A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol. 2009;9(1):10-25. [DOI] [PubMed] [Google Scholar]
- 22.Cohen MD, Keystone E. Rituximab for rheumatoid arthritis. Rheumatol Ther. 2015;2(2):99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu CY, Rodriguez-Pinto D, Du W, et al. . Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117(12):3857-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. ; Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kvien TK, Uhlig T, Ødegård S, Heiberg MS. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci. 2006;1069:212-222. [DOI] [PubMed] [Google Scholar]
- 26.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023-2038. [DOI] [PubMed] [Google Scholar]
- 27.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35(3):347-369. [DOI] [PubMed] [Google Scholar]
- 28.Lindholm E, Hallengren B, Agardh C-D. Gender differences in GAD antibody-positive diabetes mellitus in relation to age at onset, C-peptide and other endocrine autoimmune diseases. Diabetes Metab Res Rev. 2004;20(2):158-164. [DOI] [PubMed] [Google Scholar]
- 29.Chen AM, Zhou Y, Swenson K, Sachs DH, Sykes M, Yang YG. Porcine stem cell engraftment and seeding of murine thymus with class II+ cells in mice expressing porcine cytokines: toward tolerance induction across discordant xenogeneic barriers. Transplantation. 2000;69(12):2484-2490. [DOI] [PubMed] [Google Scholar]
- 30.Nizet Y, Chentoufi AA, de la Parra B, et al. . The experimental (in vitro) and clinical (in vivo) immunosuppressive effects of a rat IgG2b anti-human CD2 mAb, LO-CD2a/BTI-322. Transplantation. 2000;69(7):1420-1428. [DOI] [PubMed] [Google Scholar]
- 31.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374-1377. [DOI] [PubMed] [Google Scholar]
- 32.Ito M, Hiramatsu H, Kobayashi K, et al. . NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175-3182. [DOI] [PubMed] [Google Scholar]
- 33.McDermott SP, Eppert K, Lechman ER, Doedens M, Dick JE. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010;116(2):193-200. [DOI] [PubMed] [Google Scholar]
- 34.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179-191. [DOI] [PubMed] [Google Scholar]
- 35.LeBien TW. Fates of human B-cell precursors. Blood. 2000;96(1):9-23. [PubMed] [Google Scholar]
- 36.Lang J, Ota T, Kelly M, et al. . Receptor editing and genetic variability in human autoreactive B cells. J Exp Med. 2016;213(1):93-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schickel JN, Kuhny M, Baldo A, et al. . PTPN22 inhibition resets defective human central B cell tolerance. Sci Immunol. 2016;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Notta F, Doulatov S, Dick JE. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood. 2010;115(18):3704-3707. [DOI] [PubMed] [Google Scholar]
- 39.Volk V, Schneider A, Spineli LM, Grosshennig A, Stripecke R The gender gap: discrepant human T-cell reconstitution after cord blood stem cell transplantation in humanized female and male mice. Bone Marrow Transplant. 2016;51(4):596-597. [DOI] [PMC free article] [PubMed]
- 40.Chevaleyre J, Duchez P, Rodriguez L, et al. . Busulfan administration flexibility increases the applicability of scid repopulating cell assay in NSG mouse model. PLoS One. 2013;8(9):e74361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verthelyi D. Sex hormones as immunomodulators in health and disease. Int Immunopharmacol. 2001;1(6):983-993. [DOI] [PubMed] [Google Scholar]
- 42.Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev. 2012;11(6-7):A479-A485. [DOI] [PubMed] [Google Scholar]
- 43.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butterworth M, McClellan B, Allansmith M. Influence of sex in immunoglobulin levels. Nature. 1967;214(5094):1224-1225. [DOI] [PubMed] [Google Scholar]
- 45.Eidinger D, Garrett TJ. Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. J Exp Med. 1972;136(5):1098-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26(29-30):3551-3555. [DOI] [PubMed] [Google Scholar]
- 47.Lozano R, Naghavi M, Foreman K, et al. . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999;283(5406):1277-1278. [DOI] [PubMed] [Google Scholar]
- 49.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellegren H, Parsch J. The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet. 2007;8(9):689-698. [DOI] [PubMed] [Google Scholar]
- 51.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11(4):411-423. [DOI] [PubMed] [Google Scholar]
- 52.Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9(1):56-62. [DOI] [PubMed] [Google Scholar]
- 53.Tanriverdi F, Silveira LF, MacColl GS, Bouloux PM. The hypothalamic-pituitary-gonadal axis: immune function and autoimmunity. J Endocrinol. 2003;176(3):293-304. [DOI] [PubMed] [Google Scholar]
- 54.Medina KL, Smithson G, Kincade PW. Suppression of B lymphopoiesis during normal pregnancy. J Exp Med. 1993;178(5):1507-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viselli SM, Reese KR, Fan J, Kovacs WJ, Olsen NJ. Androgens alter B cell development in normal male mice. Cell Immunol. 1997;182(2):99-104. [DOI] [PubMed] [Google Scholar]
- 56.Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol. 1998;161(1):27-34. [PubMed] [Google Scholar]
- 57.Igarashi H, Kouro T, Yokota T, Comp PC, Kincade PW. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc Natl Acad Sci USA. 2001;98(26):15131-15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hill L, Jeganathan V, Chinnasamy P, Grimaldi C, Diamond B. Differential roles of estrogen receptors α and β in control of B-cell maturation and selection. Mol Med. 2011;17(3-4):211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakada D, Oguro H, Levi BP, et al. . Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bynoe MS, Grimaldi CM, Diamond B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proc Natl Acad Sci USA. 2000;97(6):2703-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109(12):1625-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roubinian JR, Papoian R, Talal N. Androgenic hormones modulate autoantibody responses and improve survival in murine lupus. J Clin Invest. 1977;59(6):1066-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978;147(6):1568-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Altuwaijri S, Chuang KH, Lai KP, et al. . Susceptibility to autoimmunity and B cell resistance to apoptosis in mice lacking androgen receptor in B cells. Mol Endocrinol. 2009;23(4):444-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan J, Greer JM, Hull R, et al. . The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing. 2010;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giglio T, Imro MA, Filaci G, et al. . Immune cell circulating subsets are affected by gonadal function. Life Sci. 1994;54(18):1305-1312. [DOI] [PubMed] [Google Scholar]
- 67.Kamada M, Irahara M, Maegawa M, et al. . B cell subsets in postmenopausal women and the effect of hormone replacement therapy. Maturitas. 2001;37(3):173-179. [DOI] [PubMed] [Google Scholar]
- 68.Porter VR, Greendale GA, Schocken M, Zhu X, Effros RB. Immune effects of hormone replacement therapy in post-menopausal women. Exp Gerontol. 2001;36(2):311-326. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe Y, Takahashi T, Okajima A, et al. . The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu-HSC NOG mice). Int Immunol. 2009;21(7):843-858. [DOI] [PubMed] [Google Scholar]
- 70.Lang J, Kelly M, Freed BM, et al. . Studies of lymphocyte reconstitution in a humanized mouse model reveal a requirement of T cells for human B cell maturation. J Immunol. 2013;190(5):2090-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Villaudy J, Schotte R, Legrand N, Spits H. Critical assessment of human antibody generation in humanized mouse models. J Immunol Methods. 2014;410(C):18-27. [DOI] [PubMed] [Google Scholar]
- 72.Papadaki HA, Kritikos HD, Gemetzi C, et al. . Bone marrow progenitor cell reserve and function and stromal cell function are defective in rheumatoid arthritis: evidence for a tumor necrosis factor alpha-mediated effect. Blood. 2002;99(5):1610-1619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.