Key Points
Enveloped AAV vectors are able to transduce the liver highly efficiently, driving superior correction of hemophilia B in mice.
Enveloped AAVs are less susceptible to antibody-mediated neutralization, allowing for liver transduction in preimmunized animals.
Abstract
Results from clinical trials of liver gene transfer for hemophilia demonstrate the potential of the adeno-associated virus (AAV) vector platform. However, to achieve therapeutic transgene expression, in some cases high vector doses are required, which are associated with a higher risk of triggering anti-capsid cytotoxic T-cell responses. Additionally, anti-AAV preexisting immunity can prevent liver transduction even at low neutralizing antibody (NAb) titers. Here, we describe the use of exosome-associated AAV (exo-AAV) vectors as a robust liver gene delivery system that allows the therapeutic vector dose to be decreased while protecting from preexisting humoral immunity to the capsid. The in vivo efficiency of liver targeting of standard AAV8 or AAV5 and exo-AAV8 or exo-AAV5 vectors expressing human coagulation factor IX (hF.IX) was evaluated. A significant enhancement of transduction efficiency was observed, and in hemophilia B mice treated with 4 × 1010 vector genomes per kilogram of exo-AAV8 vectors, a staggering ∼1 log increase in hF.IX transgene expression was observed, leading to superior correction of clotting time. Enhanced liver expression was also associated with an increase in the frequency of regulatory T cells in lymph nodes. The efficiency of exo- and standard AAV8 vectors in evading preexisting NAbs to the capsid was then evaluated in a passive immunization mouse model and in human sera. Exo-AAV8 gene delivery allowed for efficient transduction even in the presence of moderate NAb titers, thus potentially extending the proportion of subjects eligible for liver gene transfer. Exo-AAV vectors therefore represent a platform to improve the safety and efficacy of liver-directed gene transfer.
Visual Abstract
Introduction
Adeno-associated virus (AAV) vectors have emerged as one of the most efficient delivery systems for in vivo gene transfer.1 In particular, in the setting of liver-directed gene transfer, recent data from clinical trials of gene transfer for hemophilia demonstrated the therapeutic potential of the AAV vector platform, with published results showing sustained multiyear correction of the bleeding phenotype in hemophilia B patients after a single vector administration.2
However, although there is no doubt that in vivo gene transfer with AAV vectors has the potential of becoming a curative treatment of bleeding disorders, vector-directed immune responses remain an important limitation to the widespread use of this gene-delivery tool in the clinic.3 Recombinant AAV vectors are derived from their wild-type counterpart,4 which naturally infects humans. Because AAV infection in nature occurs in the context of a helper virus, humans develop both neutralizing antibodies (NAbs)5,6 and memory T-cell reactivity7,8 directed against the capsid. As a consequence, depending on the AAV vector serotype, up to two-thirds of humans are seropositive to AAV,9 thus ineligible for enrollment in gene transfer clinical trials.10 Additionally, the vector dose-dependent (re)activation of T cells directed against the AAV capsid can result in clearance of transduced cells, transient elevation of liver enzymes, and loss of transgene expression.2,10-12 Thus, strategies to develop AAV vectors resistant to antibody-mediated neutralization and highly efficient in targeting hepatocytes (to achieve therapeutic efficacy at lower vector doses) are highly needed.
Exosomes or microvesicles are natural cell-derived extracellular vesicles with a diameter of 20 nm to 1 μm that originate from internal endocytic compartments, multivesicular bodies, as well as plasma membrane budding, and participate in intercellular communication.13 Exosomes (as well as artificial nanoparticles) are currently being explored as systems for the delivery of nucleic acids and proteins, among several other uses.13 The natural release of a fraction of recombinant AAV vectors associated with exosomes (exosome-associated AAVs [exo-AAVs]) in the cell medium during vector production has been recently described.14 The use of exo-AAV vectors has been demonstrated to enhance transduction in the setting of gene transfer to the retina,15 the nervous system,16 and the inner ear.17
In the present study, we tested the ability of exo-AAV vectors to transduce the liver and drive efficient correction of hemophilia B. Our results indicate that exo-AAV vectors can efficiently transduce a variety of liver cell lines in vitro and drive high levels of transgene, expressing at low vector doses in vivo. exo-AAV vectors also exhibited a lower susceptibility to neutralization by preexisting anti-AAV8 NAbs, thus allowing for expansion of the number of subjects potentially eligible for in vivo gene transfer.
Material and methods
See supplemental Methods for a complete description of materials and methods.
AAV and exo-AAV vector production
Standard AAV and exo-AAV vectors were produced using the adenovirus helper-free transfection method as described previously.18 exo-AAV vectors were isolated by differential centrifugation as described earlier.17 From the same production, standard AAV vectors were purified from cell lysates using 2 successive ultracentrifugation rounds in cesium chloride density gradient.19 The AAV genome in both standard AAV and exo-AAV vectors was quantified using quantitative real-time polymerase chain reaction (qPCR).
Size characterization of exo-AAV vectors was performed using Nanosight NS300 (Malvern Instruments, Malvern, United Kingdom) following the manufacturer’s protocol.
Western blot analysis
exo- and standard AAV vectors were denatured and protein concentration was determined using the BCA Protein Assay (Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s instructions. Western blot analysis was performed using an anti–viral protein (VP) capsid protein antibody (clone B1; Progen, Heidelberg, Germany), an anti-human TSG101 antibody (Abcam, Cambridge, MA), and an anti-human CD9 antibody (Cell Signaling Technology, Leiden, The Netherlands). For autophagy quantification, HUH-7 cells were transduced with either standard or exo-AAV8 vectors. Total cellular proteins were extracted using radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% NP-40, 0.5% sodium deoxycholate, 1% sodium dodecyl sulfate, protease inhibitor cocktail; Sigma-Aldrich, St. Louis, MO). An anti-LC3B (Novus Biological, Littleton, CO) and anti-actin antibody (Sigma-Aldrich) were used for the western blot. Secondary antibodies and detection system were from Li-Cor Biosciences (Lincoln, NE).
Cryotransmission electron microscopy
Cryotransmission electron microscopy was performed as described by Hudry and colleagues.16
Mouse experiments
Mice were handled according to the French and European legislation of animal care and experimentation. Hemostatically normal male or female C57BL/6 mice and hemophilia B mice were injected IV via the tail vein with AAV or exo-AAV vectors encoding for human coagulation factor IX (hF.IX). For passive immunization experiments, mice were injected intraperitoneally with human immunoglobulin (IV immunoglobulin [IVIg]) and 24 hours later they were infused with vector.
Statistical analyses
Data are shown as mean ± standard error of the mean. Statistical analysis was assessed using GraphPad Prism (GraphPad Software Inc, La Jolla, CA) version 6.0. Analysis of statistical significance between 2 groups was assessed using the unpaired Student t test. For multiple group comparisons, 1-way analysis of variance (ANOVA) with Bonferroni multiple comparison test was used.
Results
exo-AAV vectors display superior in vitro transduction efficiency via enhanced cell and nuclear uptake
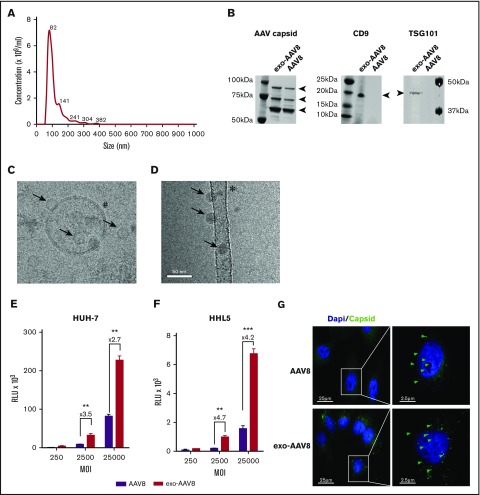
To study the in vitro transduction efficiency of exo-AAV8 vectors in several cell lines, exo- and standard AAV8 vectors were produced. In agreement with several reports showing efficient release of AAV vectors in culture media during production,19-21 yields in production were similar for exo- and standard AAV vector preparations across several serotypes, with the exception of AAV6, which gave much higher yields in the exo-AAV fraction (supplemental Table 1). Analysis of the exosome profile of exo-AAV8 vectors showed an average size of ∼115 nm (Figure 1A). Western blot analysis confirmed the presence of the 3 AAV8 capsid proteins (VP1, VP2, VP3; Figure 1B) in both exo- and standard AAV preparations, although upon loading an identical amount in vector genomes (vg) of exo- and standard AAV8 vectors, higher intensity bands were noted for the exo-AAV8 vector, suggesting the presence of an approximately sixfold excess of empty capsids. Analysis of the typical exosomal markers TSG101 and CD9 confirmed the presence of an envelope in the exo-AAV8 vector preparation (Figure 1B). The exo- and standard AAV8 preparations were then visualized by cryo-transmission electron microscopy The exo-AAV8 vector sample contained exosomes of the expected size (∼100 nm), which appeared to carry capsids inside (Figure 1C). AAV particles not associated with any membrane were also visualized (Figure 1C), which were either copurified with exo-AAV or were released upon exo-AAV membrane disruption during purification. The conventional AAV sample presented well-preserved viruses, as expected (Figure 1D), with no detectable exosomes, reflecting the use of a detergent step during purification.19 In addition to isolated AAV and empty exosomes, vesicular structures containing several exosomes, which ranged in size from 150 to 250 nm and contained a large number of viruses, were also observed (supplemental Figure 1A).
Figure 1.
Characterization of exo-AAV8 vector preparations and in vitro transduction efficiency. (A) Concentration and mean particle size of an exo-AAV8 vector expressing luciferase. Representative plot generated from the average of three 90-second videos. (B) Western blot for the AAV8 capsid proteins VP1, VP2, and VP3, and for the exosomal markers TSG101 and CD9. (C-D) Cryo-transmission electron microscopy of exo-AAV8 (C) and standard AAV8 (D) vectors. Arrows indicate AAV particles; #, membrane of the exosome with the characteristic lipid bilayer; *, carbon support film. (E-F) In vitro transduction of HUH-7 and HHL5 liver cell lines. Analysis performed 24 hours posttransduction. Data are shown as mean of 3 independent experiments ± standard error of the mean (SEM). **P < .01; ***P < .001; unpaired Student t test. (G) HUH-7 cells transduced with exo- or standard vectors at an MOI of 25 000 and stained with the ADK8/9 antibody (intact AAV particles, in green; Alexa-Fluor 488) 1 hour postinfection. Green arrows show representative positive signal for AAV particles. Dapi, 4′,6-diamidino-2-phenylindole; MW, molecular weight marker; RLU, relative light unit.
Next, we evaluated the transduction efficiency of exo-AAV8 vectors encoding for luciferase in different cell lines. Human hepatoma cell lines (HUH-7, HepG2, and HHL5) and murine muscle cell lines (C2C12 and NIH 3T3) were transduced with either exo- or standard AAV8 vectors encoding for luciferase at increasing multiplicities of infection (MOI). A significant increase in transduction efficiency with exo-AAV8 vectors compared with standard AAV8 was observed in all cell lines tested (Figure 1E-F; supplemental Figure 1B-D).
To further investigate the mechanism of increase in transduction, we tested whether the nuclear localization of the vector was increased with exo-AAV8 vectors. To this end, we transduced HUH-7 cells with either AAV8 or exo-AAV8 vectors at a MOI of 25 000 and measured capsid uptake and nuclear internalization by imaging flow cytometry. Compared with standard AAV8, a stronger staining for capsid was evidenced in the cytoplasm of cells treated with exo-AAV8 vectors. Nuclear localization also showed a stronger signal with exo-AAV8 vectors, with almost ∼40% of the nuclei containing capsid after 1 hour of incubation compared with ∼20% with standard AAV8 vectors (supplemental Figure 1E-F). This result confirmed findings obtained with conventional microscopy (Figure 1G) in HUH-7 after 1 hour of treatment at an MOI of 25 000.
Additional in vitro studies showed that exo-AAV, unlike their standard counterpart, do not seem to rely on autophagy to transduce hepatocytes,22 as levels of LC3-II were unchanged in cells treated with exo-AAV vectors (supplemental Figure 2A-B). Furthermore, receptor saturation experiments with excess empty capsids showed inhibition of transduction of standard but not exo-AAV vectors (supplemental Figure 2C).
These results suggest that exo-AAV8 vectors enhance transduction efficiency in vitro by increasing the cell uptake and nuclear trafficking kinetics of the vector. The mechanism of enhancement of transduction seems to be AAV receptor independent and does not rely in conventional pathways of intracellular trafficking of AAV.
Robust enhancement of liver-mediated gene transfer with exo-AAV vectors in vivo
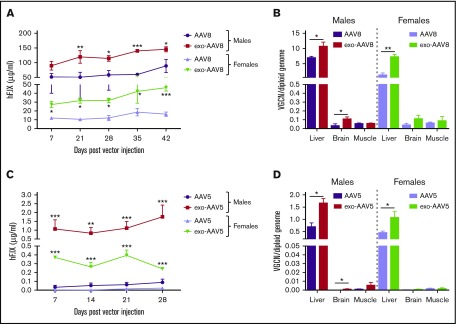
We next hypothesized that exo-AAV vectors would allow for enhanced liver-directed gene transfer in vivo. We IV injected naive male and female C57BL/6 mice with an equivalent vector dose (5 × 1010 vg) of AAV8 or exo-AAV8 vectors encoding for hF.IX under the control of a liver-specific promoter.23 Plasma samples were collected to monitor circulating hF.IX transgene expression levels for 6 weeks. In male mice, treatment with exo-AAV8 resulted in sustained and approximately twofold higher expression of circulating hF.IX compared with standard AAV8 vectors (Figure 2A). Female mice were also treated with exo- or standard AAV8 vectors to provide a more stringent test to the technology, as several studies indicate that the liver of female mice is refractory to AAV vector transduction.24-26 Also in this case, higher (approximately fourfold) circulating hF.IX transgene plasma levels were observed with exo-AAV8 vectors (Figure 2A). Interestingly, female mice treated with exo-AAV8 vectors showed comparable hF.IX transgene expression levels to male mice treated with standard AAV8 vectors (46 ± 4 μg/mL vs 89 ± 22 μg/mL at week 6, respectively, P = .5317; Mann-Whitney U test; Figure 2A).
Figure 2.
Efficiency of liver transduction with exo-AAV vectors in vivo. (A-B) Naive male and female C57BL/6 mice (n = 5) treated with exo- or standard AAV8-hF.IX vectors at 5 × 1010 vg per mouse. (A) Plasma hF.IX levels measured by enzyme-linked immunosorbent assay (ELISA). (B) Vector genome copy number (VGCN) per diploid genome at sacrifice (day 42) in liver and other tissues. (C-D) Naive male and female C57BL/6 mice treated with exo- or standard AAV5-hF.IX vectors at 1 × 109 vg per mouse. (A) Plasma hF.IX levels measured by ELISA. (B) VGCN per diploid genome at sacrifice (day 42) in liver and other tissues. Data are shown as mean ± SEM. For hF.IX plasma levels, a 2-way ANOVA with Bonferroni posttest was used to determine statistical significance; for VGCN, an unpaired Student t test was used. *P < .05; **P < .01; ***P < .001.
When euthanized, 6 weeks postvector infusion, we determined the biodistribution profile of exo- and standard AAV8 vectors. exo-AAV8 vectors showed preferential tropism and superior transduction efficiency in the liver compared with standard AAV8 vectors (approximately twofold and approximately fourfold increase in male and female animals, respectively; Figure 2B). In agreement with previous publications,16 an increase in transduction of brain was observed in mice treated with exo-AAV8 (Figure 2B); skeletal muscle also seemed to be transduced at higher levels in exo-AAV8–treated mice (supplemental Figure 3).
To test whether our approach would work with different AAV serotypes, exo-AAV5 vectors containing the same liver-specific expression cassette as the AAV8 vectors were produced, titered (supplemental Table 1), and characterized (supplemental Figure 4). Naive male and female C57BL/6 mice were treated with an IV injection of either standard AAV5 or exo-AAV5 vectors at a dose of 109 vg per mouse. Remarkably, at this low vector dose, all animals treated with exo-AAV5 vectors showed detectable hF.IX transgene circulating levels, which were ∼20- and ∼30-fold higher than those in male and female animals treated with standard AAV5 vectors, respectively (Figure 2C). In particular, although almost no expression was detectable in female animals treated with standard AAV5-hF.IX vectors, exo-AAV5–treated animals showed circulating hF.IX levels of about 400 ng/mL. Similarly, high levels of transgene expression, compatible with full correction of the bleeding phenotype of hemophilia, were achieved in male animals treated with the exo-AAV5-hF.IX vector but not with the standard AAV5 vector. In agreement with the transgene expression results, in the liver of animals treated with exo-AAV5 vectors, a significantly higher vector genome copy number was observed compared with standard AAV5 vectors in both male and female mice (Figure 2D). Together, these data indicate that exo-AAV vectors represent a powerful and feasible alternative to greatly enhance liver gene transfer with virtually any AAV serotypes.
exo-AAV vectors drive widespread enhanced targeting of hepatocytes
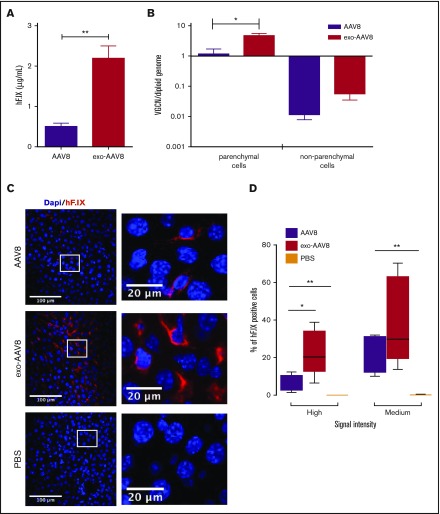
To better characterize the distribution of transduced cells in the liver of mice treated with exo- or standard AAV8 vectors, we treated naive male C57BL/6 mice with a low vector dose (109 vg per mouse). Also at this dose, we observed enhanced transgene expression (approximately fivefold higher) with exo-AAV8 vectors (Figure 3A). When euthanized, 2 weeks postgene transfer, livers were collected and lobes were digested with collagenase for isolation of parenchymal and nonparenchymal cells. As expected, gene copy number was higher in parenchymal cells isolated from animals treated with exo- vs standard AAV vectors (4.65 ± 0.9 vg copy per diploid genome vs 1.15 ± 0.4 vg copy per diploid genome, respectively; P = .0317; Mann-Whitney U test; Figure 3B). No statistical difference in vector genome copy number was observed in nonparenchymal cells (0.06 ± 0.05 vg copy per diploid genome vs 0.02 ± 0.008 vg copy per diploid genome for exo- vs standard AAV vectors, respectively; P = .056; Mann-Whitney U test; Figure 3B). Importantly, vector genome copy numbers were ∼2 log lower than parenchymal cells (Figure 3B). The additional liver lobes collected at euthanization were stained for hF.IX with an antibody that recognizes specifically the human protein. Interestingly, and in agreement with the other results obtained, mice infused with exo-AAV8 vectors showed a widespread distribution of hepatocytes expressing the hF.IX transgene, similar to that found in animals treated with standard AAV8 vectors (Figure 3C). However, the intensity of staining appeared to be significantly higher in animals treated with exo-AAV8 vectors (Figure 3D), in which a larger proportion of hepatocytes expressing high levels of hF.IX transgene was found (Figure 3D).
Figure 3.
Analysis of hepatocyte targeting with exo-AAV vectors. Male C57BL/6 mice (n = 5) injected IV with 109 vg per mouse of exo- or standard AAV8- hF.IX vectors. (A) Plasma levels of hF.IX measured by ELISA 14 days posttreatment. (B) VGCN per diploid genome in parenchymal and nonparenchymal cells isolated 14 days posttreatment. (C) Immunostaining for hF.IX in livers from mice treated with standard (top) or exo-AAV8 (middle) vectors, or phosphate-buffered saline (PBS; bottom) performed 14 days posttreatment. hF.IX is shown in red and nuclear staining (Dapi) in blue. Representative sections were acquired at a magnification of ×20 (insets at ×300). (D) hF.IX quantification after staining in liver. Analysis was performed using the Cellprofiler software and the percentage of positive hepatocytes was determined based on high (200-5000 arbitrary units), or medium (130-199 arbitrary units) signal intensity. Background signal (1-129 arbitrary units) was not calculated. Data are shown as mean ± SEM (for details of analysis, see supplemental Methods). For hF.IX plasma levels and VGCN, statistical significance was determined with the Mann-Whitney U test; for signal intensity, a 1-way ANOVA was used to determine statistical significance; for VGCN, an unpaired Student t test was used. *P < .05; **P < .05.
These results confirm the superior efficiency of liver targeting of exo-AAV vectors, which results in widespread high-level distribution of F.IX-expression hepatocytes.
exo-AAV vectors display similar immunogenicity to standard vectors while they enhance regulatory T-cell expansion
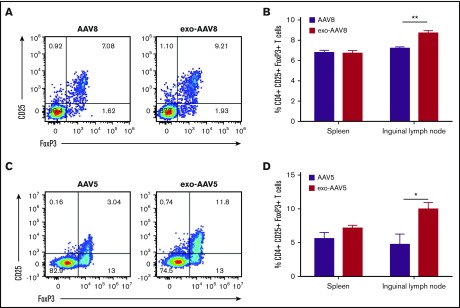
Anti-AAV capsid antibody analysis was performed in all animals injected IV with exo- and standard AAV vectors. All animals developed robust anti-capsid antibodies shortly after vector administration (supplemental Figure 5A-B), whereas no animal developed a detectable T-cell response directed against the AAV capsid or the hF.IX transgene (supplemental Figure 5C-D). qPCR analysis of livers also revealed no significant increase in T-cell infiltrates in exo-AAV–treated animals (supplemental Figure 5E-H). None of the animals treated with high vector doses of vector (5 × 1010 vg per mouse) developed anti-hF.IX transgene antibody responses (supplemental Figure 6A). At low vector doses (1 × 109 vg per mouse), 1 animal treated with standard AAV8-hF.IX developed an anti-hF.IX transgene antibody (supplemental Figure 6B), whereas none of the animals treated with exo-AAV8 vectors developed anti-transgene antibodies (supplemental Figure 6). Accordingly, as previous studies showed that high expression levels promote better transgene-specific immune tolerance,27,28 we evaluated the frequency of CD4+CD25+FoxP3+ regulatory T cells (Tregs) in animals treated with exo- or standard AAV-hF.IX vectors at a dose of 1 × 109 vg per mouse. At this dose, no differences were noted in the spleens, however, a significant enhancement in the frequency of Tregs was noticeable in the lymph nodes of exo-AAV–treated animals. in AAV8-treated animals (Figure 4A-B), the frequency of Tregs in lymph nodes was 8.76% ± 0.2% vs 7.2% ± 0.13% for exo-AAV8 and standard AAV8, respectively (n = 5 per group, P = .0079; Mann-Whitney U test). In AAV5-treated animals (Figure 4C-D), an even greater frequency of Tregs in lymph nodes was induced by exo-AAV5 (10.03% ± 0.9%) vs standard AAV5 (4.7% ± 1.5%) (n = 5 per group, P = .0202; Mann-Whitney U test). These data suggest that, because of the higher transduction efficiency, exo-AAV vectors can mediate a more efficient induction of antigen-specific liver immune tolerance, thus providing better protection from antitransgene immune responses and enhancing the overall safety profile of the approach.
Figure 4.
Frequency of regulatory T cells in spleen and lymph nodes. (A-B) Male C57BL/6 mice (n = 5) injected with 1 × 109 vg of exo- or standard AAV8-hF.IX and euthanized 2 weeks later. (A) Representative flow cytometry plot showing the frequency of CD25+FoxP3+ Tregs gated on CD3+CD4+ T cells. (B) Frequency of Tregs in spleens and inguinal lymph nodes. (C-D) Analysis of frequency of Tregs in male C57BL/6 mice (n = 5 per group) treated with 1 × 109 vg of exo- or standard AAV5-hF.IX and euthanized 28 days later. Data are shown as mean ± SEM. *P < .05, **P < .01; Mann-Whitney U test.
Enhanced correction of the hemophilia B phenotype with exo-AAV8 vectors
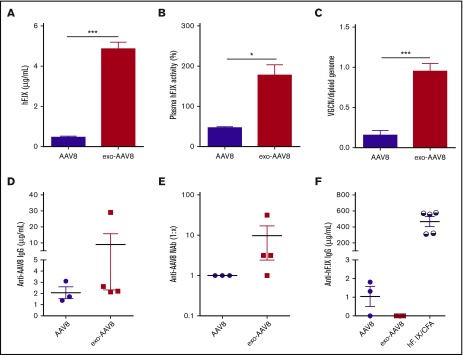
With the rationale of increasing the efficacy of hemophilia B gene therapy at lower vector doses, we assessed the therapeutic potential of exo-AAV8 vectors encoding for hF.IX in F9−/− mice. Male mice were IV injected with either exo- or standard AAV8-hF.IX at a vector dose of 1 × 109 vg per mouse. Plasma samples were collected for monitoring hF.IX antigen levels and clotting activity 1 month following gene transfer. We observed a striking ∼1 log increase in circulating hF.IX antigen levels in hemophilia B mice treated with exo-AAV8 (n = 4, 4.85 μg/mL ± 0.67 μg/mL, ∼97% of normal levels) compared with standard AAV8 (n = 3, 0.46 μg/mL ± 0.15 μg/mL, ∼10% of normal levels) (Figure 5A). Furthermore, activated partial thromboplastin time (aPTT) assay demonstrated that the hF.IX produced was functional, as animals treated with the exo-AAV8-hF.IX vector displayed clotting activity around 100% (177% ± 52%). Clotting activity was about fourfold above that measured in animals treated with standard AAV8-hF.IX vectors, and the difference lasted through the period of observation (Figure 5B). In agreement with these results, we also observed a significant approximately sixfold increase of vector copy number in livers of mice treated with exo-AAV8 vectors (0.95 copy per diploid genome ± 0.09 copy per diploid genome) compared with standard AAV8 vectors (0.15 copy per diploid genome ± 0.06 copy per diploid genome, Figure 5C). In all animals, anti-AAV8 antibodies were triggered following vector administration at similar levels between the 2 treatment groups (Figure 5D-E). Low-titer anti-hF.IX transgene immunoglobulin G (IgG) were detected in animals injected standard AAV8 vectors, although these were not neutralizing (Figure 5F; supplemental Table 2). Importantly, in agreement with the observed enhancement in the frequency of Tregs (Figure 4), none of the mice treated with exo-AAV8 vectors developed anti-hF.IX IgG antibodies or NAbs to the hF.IX transgene product (Figure 5F; supplemental Table 2). These results indicate that exo-AAV8 vectors can increase the efficacy and the therapeutic index of AAV-mediated liver gene therapy, resulting in complete correction of the clotting deficiency in hemophilia B mice at low vector doses.
Figure 5.
Treatment of hemophilia B mice with exo- and standard AAV8 vectors. Male C57BL/6 F9−/− mice were injected IV with 109 vg standard (n = 3) or exo- (n = 4) AAV8-hF.IX vectors (n = 4). (A) Plasma hF.IX expression levels measured by ELISA. (B) hF.IX activity measured by aPTT assay. (C) VGCN per diploid genome measured in liver at sacrifice. (D) Anti-AAV8 IgG antibodies determined by ELISA. (E) Anti-AAV8 NAb titer. (F) Anti-hF.IX IgG antibodies determined by ELISA. hF.IX/CFA, positive control mice injected subcutaneously with 100 μg of hF.IX protein in complete Freund adjuvant (n = 5). All data shown are at 30 days posttreatment. Shown are mean ± SEM. Statistical analysis was performed using the unpaired Student t test. *P < .05; ***P < .001.
Efficient protection from preexisting neutralizing anti-capsid antibodies with exo-AAV8 vectors
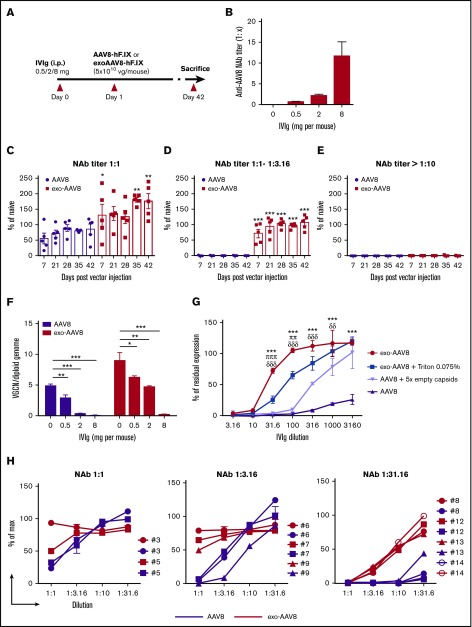
We hypothesized that the encapsulating exosome can shield AAV8 vectors from preexisting humoral immune responses. To address this, we used a passive immunization model in immunocompetent male C57BL/6 mice (Figure 6A).29 Animals were first injected intraperitoneally with human IVIg harboring anti-AAV8 antibodies at 3 different doses, resulting in anti-AAV8 NAb titers of about 1:1, 1:1 to 1:3.16, or >1:10 (Figure 6B). Mice were then IV injected with vector at a dose of 5 × 1010 vg of either exo- or standard AAV8-hF.IX. At a dose of 0.5 mg (anti-AAV8 NAb titer 1:1) and 2 mg (anti-AAV8 NAb titers ranging from 1:1 to 1:3.16) of IVIg, we observed complete protection from preexisting antibodies resulting in the rescue of hF.IX transgene expression with exo-AAV8 vectors (Figure 6C-D). In contrast, for mice treated with standard AAV8 vectors, we observed a partial neutralization (30%) in the presence of an anti-AAV8 NAb titer of 1:1 (Figure 6C), and full neutralization in the presence of a NAb titer ranging from 1:1 to 1:3.16 (Figure 6D). In the presence of Nab titers > 1:10, neither vector achieved efficient liver transduction (Figure 6E). Vector genome copy number measured in livers collected 42 days after gene transfer confirmed these findings (Figure 6F). These results further demonstrate that low titers of preexisting anti-AAV8 antibodies (titers ranging from 1:1 to 1:3.16) can block standard AAV8 vector transduction, a result in agreement with previous findings.29-31 They also indicate successful protection from low-level preexisting antibodies and rescue of transgene expression with exo-AAV8 vectors. To confirm that the rescue from antibody-mediated neutralization was mediated by a membrane envelope, we next treated exo-AAV8 with a detergent (Triton) to disrupt exosomes. This resulted in loss of ability to evade NAbs of the exo-AAV8 vector and made the transduction profile of exo-AAV8 in vitro similar to that of standard AAV8 vectors (Figure 6G). Notably, formulation of standard AAV8 vectors in a fivefold excess of empty capsid29 did not result in the same level of rescue from NAbs as exo-AAV8 vectors (Figure 6G).
Figure 6.
Protection from anti-capsid NAbs mediated by exo-AAV vectors. (A) Protocol outline. Naive male C57BL/6 mice (n = 5) were passively immunized with intraperitoneal (i.p.) injection of human IVIg at a dose of 0.5 mg, 2 mg, or 8 mg per mouse. Twenty-four hours later, animals received 5 × 1010 vg of exo- or standard AAV8-hF.IX vectors. (B) Anti-AAV8 NAb titer measured before vector administration (C-E) Plasma hF.IX levels over time assessed by ELISA in mice immunized with 0.5 mg (C), 2 mg (D), or 8 mg (E) of IVIg. hF.IX levels are reported as percentage of hF.IX levels in naive mice following gene transfer with standard AAV8-hF.IX vector. The symbols represent individual animals and the bars represent the mean ± SEM. *P < .05; **P < .01; ***P < .001; 2-way ANOVA with Bonferroni posttest. (F) VGCN per diploid genome in liver collected at euthanization from the animals in panels C-E and in naive controls. Data are shown as mean ± SEM. *P < .05; **P < .01; ***P < .001; 1-way ANOVA with Bonferroni posttest. (G) NAb assay performed at increasing IVIg dilutions with exo-AAV8 or exo-AAV8 vectors after treatment with 0.075% Triton-X-100, and standard AAV8 vectors alone or formulated in an excess of fivefold empty capsids. exo-AAV8 vectors were retitered after Triton treatment together with all other vectors used in the experiment. Shown are mean ± standard deviation of 2 independent experiments. ***P < .001; exo-AAV8 vs AAV8 vectors. πππP < .001; ππP < .01; exo-AAV8 vs exo-AAV8 after treatment with 0.075% Triton-X-100. δδδP < .001; δδP < .01; exo-AAV8 vs standard AAV8 vector formulated in an excess of fivefold empty capsids. Two-way ANOVA with Bonferroni posttest. (H) NAb assay performed with individual sera from healthy donors carry low (1:1), mid (1:3.16), or high (1:31.6) at increasing dilutions. Results are shown as percentage of max expression control (no inhibition). Each line represents a single donor.
Finally, to better understand the relevance of these findings to human trials, a survey of anti-AAV8 NAb titers was conducted in healthy donors, showing that the proportion of subjects harboring anti-AAV8 antibodies in the range of 1:1 to 1:3.16 range is ∼40% for AAV2 and 20% for AAV8 (Table 1). We then selected 3 groups of subjects with NAb titers to AAV8 of 1:1 (low positive), 1:3.16 (positive), and 1:31.6 (high positive) and incubated exo- or standard AAV8 vectors expressing luciferase with various dilutions of these serum samples. Residual luciferase expression was then measured after an in vitro transduction experiment (Figure 6H). Similar to the results of in vivo experiment in mice, complete rescue of transduction was observed at titers of 1:1 and 1:3.16, whereas at titers of 1:31.6 only a partial rescue of neutralization was measured (Figure 6H). Preliminary experiments in vivo in the setting of vector readministration support the in vitro findings, showing partial rescue of liver transduction following exo-AAV vector redosing (supplemental Figure 7). These results demonstrate that exo-AAV8 vectors can be used to allow for treatment of humans with some levels of preexisting antibodies to AAV, otherwise not eligible for enrollment in gene-therapy trials.
Table 1.
Anti-AAV2 and anti-AAV8 NAbs in adult healthy donors (n = 100)
| Serotype | NAb | |||
|---|---|---|---|---|
| <1:1 (naive) | 1:1 | 1:3.16 | ≥1:10 | |
| AAV2 | 1 | 35 | 10 | 54 |
| AAV8 | 42 | 7 | 13 | 38 |
Discussion
Recombinant AAV are the most clinically relevant vectors for in vivo gene transfer, as demonstrated in several trials and in particular in the context of bleeding disorders.1,2,10,12 Despite the excellent safety and efficacy profile, however, some important limitations of the technology remain, mainly associated with the need for more efficient vectors that can escape, to some extent, recognition by the host immune system.3
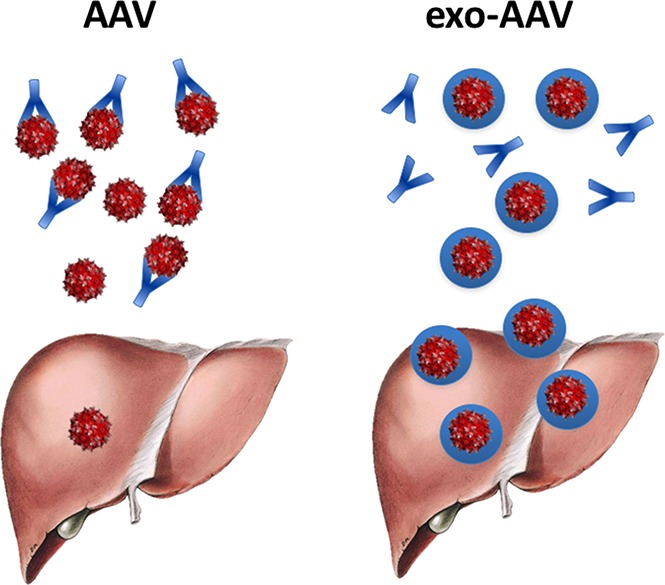
Here, we demonstrated that exosome-enveloped AAV vectors offer a feasible, relatively simple strategy to achieve superior correction of hemophilia via enhanced targeting of hepatocytes, at the same time providing protection from preexisting NAbs. Although the mechanism of enhancement of transduction is not completely understood, based on our in vitro results, exo-AAV vectors seem to present a faster nuclear translocation rate, which may be the result of more efficient and autophagy-independent22 intracellular trafficking. Additionally, competition experiments presented here suggest that exo-AAV entry occurs through a process dependent on the exosome membrane or protein components (eg, micropinocytosis, receptor-mediated endocytosis), which bypass the need of cell receptor binding of the AAV capsid.
To date, no experience with exo-AAV vectors is available in the context of liver gene transfer, particularly in the setting of hemophilia. In this study, we developed and tested 2 enveloped vectors, exo-AAV8 and exo-AAV5, 2 serotypes that have been tested in humans in the treatment of hemophilia A and B.32 Our data indicate that the use of enveloped AAV vectors results in a significant enhancement of transduction of hepatocytes, which was more evident at low vector doses. Accordingly, we observed up to a 40-fold increase in transduction with exo-AAV5 vs standard AAV5 vectors. This also translated in the ability to completely restore the clotting activity in hemophilia B mice with low doses of exo-AAV8 vectors but not with standard AAV8 vectors, resulting in a striking ∼1 log enhancement in transgene expression.
When we analyzed the distribution of expression in the liver, imaging data gathered suggested that exo-AAV vectors simply drive higher transgene expression levels in a larger number of cells in the liver compared with standard vectors. Aside from the treatment of hemophilia, this is a potentially attractive feature of exo-AAV, which may be useful for applications like promoterless AAV,33 or any application of gene transfer in which high levels of hepatocyte targeting are needed.34 Of note, one advantage of combining exosomes with AAV vectors, as opposed to using exosomes to carry naked plasmid DNA, resides in the long-lived transgene expression that can be obtained with AAV vectors in liver.2,35 Additionally, we have previously demonstrated that producing exo-AAV in the absence of capsid does not lead to detectable transduction, at least in cultured cells.14
The implications of these results for the development of safer and more effective gene therapies for bleeding disorders are multiple. First, as AAV vectors, and in particular AAV8, tend to target hepatocytes less efficiently in humans and nonhuman primates compared with mice,36 the potential benefit in terms of expression levels deriving from the use of exo-AAV vectors will be even more significant in large animals and in humans. Data emerging from clinical trials of AAV5 liver gene transfer for hemophilia A and B support this point, and indicate that high vector doses are needed to reach therapeutic levels of transgene expression in humans; hence, the use of exo-AAV5 vectors could potentially significantly lower the therapeutic dose in this setting. Second, the ability to lower the vector dose to achieve full therapeutic efficacy would confer a clear advantage in terms of avoidance of CD8+ T-cell responses directed to the capsid.11 This point can be extrapolated from a number of preclinical and clinical studies,12,37-40 suggesting that at low vector doses it is less likely to experience vector-related immunotoxicities.3 Last, as suggested here by the higher frequency of Tregs found in lymph nodes of exo-AAV–treated animals, higher transgene expression levels are likely to result in more robust induction of immunological tolerance.27
One additional potential advantage deriving from the use of highly efficient exo-AAV vectors is a decrease in terms of vector manufacturing needs. Although this from a theoretical point of view is an important point, it represents also a limitation of the technology, as manufacturing of exosomes is still in its infancy. Thus, future efforts will have to focus on the development of scalable methods for the purification of exo-AAV, based on the recent advances in the field of manufacturing of exosomes for clinical use.41 Additionally, careful characterization of the contents in exo-AAV vectors, mostly derived from the cells of origin, will be required in order to move forward toward the clinical use of these vectors.42 In particular, the role of contaminants such as proteins and nucleic acids, derived from the packaging cell lines,42,43 in the overall safety and immunogenicity profile of exo-AAV vectors will have to be extensively studied. Alternatively, the use of artificial liposome-based vesicles44 or exosome mimetics could address the issue of exosome contaminants. Similarly, to translate the current results to humans, careful assessment of exo-AAV vector biodistribution and genome integration profile45,46 will have to be performed, owing to the fact that these vectors enter the cell via receptor-independent pathways and traffic to the nucleus with high efficiency.
It is well established that a major limitation of AAV-mediated gene transfer is the high prevalence of anti-AAV antibodies in humans, which prevents enrollment in gene-therapy trials. In the context of natural humoral immunity to AAV, and depending on the AAV serotype, a significant proportion of subjects harbor anti-capsid neutralizing titers that are low to moderate.9,29,47 For example, results presented here suggest that ∼40% and ∼20% of healthy humans present NAbs ranging from 1:1 to 1:3.16 for AAV2 and AAV8, respectively. Although these titers are sufficient to block liver targeting of standard AAV vectors, here we showed that exo-AAV8 vectors are not affected by low to moderate levels of NAbs and retain the ability to transduce the liver. This is an important added advantage of exo-AAV vectors, as they can help ensure liver transduction in an extended population of prospective patients. Why exo-AAV do not efficiently escape neutralization at high antibody titers remains to be established. One possible explanation could reside in the role of antibodies in intracellular immunity to pathogens.48,49 Other purification methods that remove external AAV capsids (free or exosome surface-bound) may also increase resistance to neutralization. Future studies will have to better define the extent of exo-AAV’s ability to overcome NAbs; additionally, studies on the combination of exo-AAV with strategies aimed at lowering47,50 or removing circulating antibodies51,52 will help give access to in vivo gene therapy also to subjects with high-titer NAbs to AAV.
In conclusion, exo-AAV vector is a powerful tool to enhance liver-targeted gene transfer in the treatment of hemophilia. This technology could further pave the way to the widespread use of AAV vector-mediated gene therapy for the treatment of a variety of diseases affecting the liver.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by Genethon, by a grant from the European Research Council (ERC-CoG MoMAAV, grant agreement 617432) (F.M.), the European Union E-Rare2 funding scheme (SMART-Hemocare) (F.M.), the European Union Marie Slodowska Curie Career Integration Grant (NosMod, grant agreement 333628 ) (F.M.), and the Bayer Hemophilia Early Career Investigator Award (F.M.). A.M. was supported by a grant from the DIM-Biotherapies Ile-de-France. C.A.M. was supported by an American Brain Tumor Association Discovery Grant and a Cure Alzheimer’s Fund award.
Authorship
Contribution: A.M., Z.F., S.M., C.L., F.C., M.S.S., S.C., G.R., A.V., L.v.W., and B.M. performed the experiments; F.J., and O.B. assisted with the sourcing and analysis of human samples; O.C. assisted with the work in hemophilia B mice; A.R.B. and S.T performed the electron microscopy on the AAV vectors; and A.M., F.B., C.A.M., and F.M. designed the experiments, directed the studies, provided critical insight into the research project, and wrote the paper.
Conflict-of-interest disclosure: C.A.M. has filed patent applications related to the exo-AAV technology, and is a founder of, and scientific advisor for, Chameleon Biosciences, Inc, a gene therapy company. F.M. has consulted on topics related to the content of this manuscript. The remaining authors declare no competing financial interests.
Correspondence: Federico Mingozzi, Genethon, 1 Rue de l’Internationale, 91000 Evry, France; e-mail: fmingozzi@genethon.fr.
References
- 1.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12(5):341-355. [DOI] [PubMed] [Google Scholar]
- 2.Nathwani AC, Reiss UM, Tuddenham EG, et al. . Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371(21):1994-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122(1):23-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muzyczka N, Berns KI. Parvoviridae: The Viruses and Their Replication. 4th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2001. [Google Scholar]
- 5.Erles K, Sebökovà P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol. 1999;59(3):406-411. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Narkbunnam N, Samulski RJ, et al. ; Joint Outcome Study Investigators. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 2012;19(3):288-294. [DOI] [PubMed] [Google Scholar]
- 7.Hui DJ, Edmonson SC, Podsakoff GM, et al. . AAV capsid CD8+ T-cell epitopes are highly conserved across AAV serotypes. Mol Ther Methods Clin Dev. 2015;2:15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veron P, Leborgne C, Monteilhet V, et al. . Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors. J Immunol. 2012;188(12):6418-6424. [DOI] [PubMed] [Google Scholar]
- 9.Boutin S, Monteilhet V, Veron P, et al. . Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704-712. [DOI] [PubMed] [Google Scholar]
- 10.Manno CS, Pierce GF, Arruda VR, et al. . Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response [published correction appears in Nat Med. 2006;12(5):592]. Nat Med. 2006;12(3):342-347. [DOI] [PubMed] [Google Scholar]
- 11.Mingozzi F, Maus MV, Hui DJ, et al. . CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13(4):419-422. [DOI] [PubMed] [Google Scholar]
- 12.Nathwani AC, Tuddenham EG, Rangarajan S, et al. . Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rezaie J, Ajezi S, Avci CB, et al. . Exosomes and their application in biomedical field: difficulties and advantages [published online ahead of print 11 May 2017]. Mol Neurobiol. doi:10.1007/s12035-017-0582-7. [DOI] [PubMed] [Google Scholar]
- 14.Maguire CA, Balaj L, Sivaraman S, et al. . Microvesicle-associated AAV vector as a novel gene delivery system. Mol Ther. 2012;20(5):960-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wassmer SJ, Carvalho LS, György B, Vandenberghe LH, Maguire CA. Exosome-associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Sci Rep. 2017;7:45329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudry E, Martin C, Gandhi S, et al. . Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther. 2016;23(11):819. [DOI] [PubMed] [Google Scholar]
- 17.György B, Sage C, Indzhykulian AA, et al. . Rescue of hearing by gene delivery to inner-ear hair cells using exosome-associated AAV. Mol Ther. 2017;25(2):379-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita T, Elliger S, Elliger C, et al. . Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5(7):938-945. [DOI] [PubMed] [Google Scholar]
- 19.Ayuso E, Mingozzi F, Montane J, et al. . High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 2010;17(4):503-510. [DOI] [PubMed] [Google Scholar]
- 20.Doria M, Ferrara A, Auricchio A. AAV2/8 vectors purified from culture medium with a simple and rapid protocol transduce murine liver, muscle, and retina efficiently. Hum Gene Ther Methods. 2013;24(6):392-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandenberghe LH, Xiao R, Lock M, Lin J, Korn M, Wilson JM. Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum Gene Ther. 2010;21(10):1251-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hösel M, Huber A, Bohlen S, et al. . Autophagy determines efficiency of liver-directed gene therapy with adeno-associated viral vectors. Hepatology. 2017;66(1):252-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miao CH, Ohashi K, Patijn GA, et al. . Inclusion of the hepatic locus control region, an intron, and untranslated region increases and stabilizes hepatic factor IX gene expression in vivo but not in vitro. Mol Ther. 2000;1(6):522-532. [DOI] [PubMed] [Google Scholar]
- 24.Guenzel AJ, Collard R, Kraus JP, Matern D, Barry MA. Long-term sex-biased correction of circulating propionic acidemia disease markers by adeno-associated virus vectors. Hum Gene Ther. 2015;26(3):153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidoff AM, Ng CY, Zhou J, Spence Y, Nathwani AC. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003;102(2):480-488. [DOI] [PubMed] [Google Scholar]
- 26.Pañeda A, Vanrell L, Mauleon I, et al. . Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders. Hum Gene Ther. 2009;20(8):908-917. [DOI] [PubMed] [Google Scholar]
- 27.Mingozzi F, Liu YL, Dobrzynski E, et al. . Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111(9):1347-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markusic DM, Hoffman BE, Perrin GQ, et al. . Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol Med. 2013;5(11):1698-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mingozzi F, Anguela XM, Pavani G, et al. . Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med. 2013;5(194):194ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scallan CD, Jiang H, Liu T, et al. . Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107(5):1810-1817. [DOI] [PubMed] [Google Scholar]
- 31.Jiang H, Couto LB, Patarroyo-White S, et al. . Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108(10):3321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nienhuis AW, Nathwani AC, Davidoff AM. Gene therapy for hemophilia. Mol Ther. 2017;25(5):1163-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barzel A, Paulk NK, Shi Y, et al. . Promoterless gene targeting without nucleases ameliorates haemophilia B in mice. Nature. 2015;517(7534):360-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Avola D, López-Franco E, Sangro B, et al. . Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J Hepatol. 2016;65(4):776-783. [DOI] [PubMed] [Google Scholar]
- 35.Niemeyer GP, Herzog RW, Mount J, et al. . Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113(4):797-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lisowski L, Tay SS, Alexander IE. Adeno-associated virus serotypes for gene therapeutics. Curr Opin Pharmacol. 2015;24:59-67. [DOI] [PubMed] [Google Scholar]
- 37.Finn JD, Hui D, Downey HD, et al. . Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther. 2010;18(1):135-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martino AT, Basner-Tschakarjan E, Markusic DM, et al. . Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood. 2013;121(12):2224-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pien GC, Basner-Tschakarjan E, Hui DJ, et al. . Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119(6):1688-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mingozzi F, Meulenberg JJ, Hui DJ, et al. . AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114(10):2077-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lener T, Gimona M, Aigner L, et al. . Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Chen X, Yi J, et al. . Identification and characterization of 293T cell-derived exosomes by profiling the protein, mRNA and microRNA components. PLoS One. 2016;11(9):e0163043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balaj L, Lessard R, Dai L, et al. . Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madni A, Sarfraz M, Rehman M, et al. . Liposomal drug delivery: a versatile platform for challenging clinical applications. J Pharm Pharm Sci. 2014;17(3):401-426. [DOI] [PubMed] [Google Scholar]
- 45.Chandler RJ, LaFave MC, Varshney GK, Burgess SM, Venditti CP. Genotoxicity in mice following AAV gene delivery: a safety concern for human gene therapy? Mol Ther. 2016;24(2):198-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandler RJ, LaFave MC, Varshney GK, et al. . Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J Clin Invest. 2015;125(2):870-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mingozzi F, Chen Y, Edmonson SC, et al. . Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther. 2013;20(4):417-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng Z, Hensley L, McKnight KL, et al. . A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496(7445):367-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foss S, Watkinson RE, Grevys A, et al. . TRIM21 immune signaling is more sensitive to antibody affinity than its neutralization activity. J Immunol. 2016;196(8):3452-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mingozzi F, Chen Y, Murphy SL, et al. . Pharmacological modulation of humoral immunity in a nonhuman primate model of AAV gene transfer for hemophilia B. Mol Ther. 2012;20(7):1410-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chicoine LG, Montgomery CL, Bremer WG, et al. . Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol Ther. 2014;22(2):338-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monteilhet V, Saheb S, Boutin S, et al. . A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther. 2011;19(11):2084-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.