Abstract
Objective
Although there is an overall reduction in underfive mortality rate, the progress in reducing neonatal mortality rate has been very slow. Over the last 20 years, preterm births have steadily increased in low-income and medium-income countries (LMICs) particularly in sub-Saharan Africa and South Asia. Preterm birth is associated with increased mortality and morbidity, particularly in LMICs. Based on systematic reviews of randomised controlled trials (RCTs), many neonatal units in high-income countries have adopted probiotics as standard of care for preterm neonates. We aimed to systematically review the safety and efficacy of probiotics in reducing mortality and morbidity in preterm neonates in LMICs.
Design
Systematic review and meta-analysis of RCTs.
Data sources
Medline, Embase, Cochrane Central Register of Controlled Trials, Cumulative Index of Nursing and Allied Health Literature and E-abstracts from Pediatric Academic Society meetings and other paediatric and neonatal conference proceedings were searched in January 2017.
Eligibility criteria
RCTs comparing probiotics versus placebo/no probiotic in preterm neonates (gestation<37 weeks) conducted in LMICs.
Results
Total 23 (n=4783) RCTs from 4 continents and 10 LMICs were eligible for inclusion in the meta-analysis using fixed effect model. The risk of necrotising enterocolitis (NEC greater than or equal to stage II) (risk ratio (RR) 0.46 (95% CI 0.34 to 0.61), P<0.00001, numbers needed to treat (NNT) 25 (95% CI 20 to 50)), late-onset sepsis (LOS) (RR 0.80 (95% CI 0.71 to 0.91), P=0.0009, NNT 25 (95% CI 17 to 100)) and all-cause mortality (RR 0.73 (95% CI 0.59 to 0.90), P=0.003, NNT 50 (95% CI 25 to 100)) were significantly lower in probiotic supplemented neonates. The results were significant on random effects model analysis and after excluding studies with high risk of bias. No significant adverse effects were reported.
Conclusion
Probiotics have significant potential to reduce mortality and morbidity (eg, NEC, LOS) in preterm neonates in LMICs.
Keywords: infant, newborn, necrotising enterocolitis, preterm neonates, sepsis, probiotics, review, surgical, supplementation, developing countries
Strengths and limitations of this study.
The strengths of our systematic review include its robust methodology, comprehensive nature, large sample size and exclusive focus on randomised controlled trials (RCTs) of probiotics in preterm neonates in low-income and medium-income countries.
The limitations include variations in the probiotic protocols in the included RCTs. Furthermore, nearly 40% of the included trials carried a high risk of bias in many domains of assessment.
Introduction
The Unicef 2010 report showed that the global burden of underfive mortality was reduced by one-third compared with 1990s; however progress in reducing neonatal mortality has been slow.1–3 Almost 40% of underfive deaths occur during the neonatal period and majority of these deaths occur in sub-Saharan Africa, South Asia and Oceania. An estimated 98% of all neonatal deaths occur in low-income and medium-income countries (LMICs).4–6 Out of 135 million births each year, 3.1 million have died within the neonatal period and nearly 35% of these deaths occur in preterm neonates.2 5 It may be perceived that prematurity is not a problem of LMICs. However, it is important to note that only 8.6% of preterm births occur in developed countries.5 Over the last 20 years, the number of preterm births has steadily increased to 9.1 million as of 2010 in the regions of sub-Saharan Africa and South Asia. Preterm birth is associated with increased risk of mortality and morbidity including late-onset sepsis (LOS), necrotising enterocolitis (NEC), feeding difficulties and long-term neurodevelopmental impairment.6–8 Although survival of preterm neonates has improved in some LMICs, morbidities such as NEC and LOS are still a major issue.5 9–12 Considering the United Nation’s (UN’s) millennium developmental goal and the UN Secretary-General’s Global Strategy for Women’s and Children’s Health (2010) and its accompanying ‘Every Woman, Every Child initiative, Every Newborn Action plan’(ENAP), it is important to develop cost-effective simple strategies to reduce the mortality and morbidity associated with prematurity in LMICs.13
WHO defines probiotics as ‘live micro-organisms which when administered in adequate amounts confer a health benefit on the host’.14 Probiotics have been shown to significantly reduce the risk of NEC, all-cause mortality, LOS and facilitate feed tolerance in preterm very low birth weight (VLBW) neonates.15–17 The mechanisms of benefits of probiotics include gut barrier enhancement, immune response modulation (eg, TLR4 receptor, nuclear factor-B, inflammatory cytokines) and direct inhibition of gut colonisation by pathogens.18–22 Many developed countries are already using probiotics routinely in preterm neonates for prevention of NEC.23–32 It has been suggested that probiotics may have a role in LMICs for prevention, treatment of acute gastrointestinal diseases, particularly in children with HIV infection.33–36 Given their simplicity and affordability, we aimed to systematically review the safety and efficacy of probiotics in reducing the risk of mortality and morbidity in preterm neonates in LMICs.
Methods
Guidelines from the Cochrane Neonatal Review Group (http://neonatal.cochrane.org/resources-review-authors),37 Centre for Reviews and Dissemination (http://www.york.ac.uk/crd/guidance/)38 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement39 were followed for undertaking and reporting this systematic review and meta-analysis. Ethics approval was not required.
Eligibility criteria
Types of studies
Only randomised controlled trials (RCTs) were included in the review. Observational studies, narrative/systematic reviews, case reports, letters, editorials and commentaries were excluded but read to identify potential additional studies.
Types of participants
Preterm neonates born at a gestational age (GA) <37 weeks or LBW (<2500 g) or both (same criteria as the Cochrane review, 2014).15
Setting
Only RCTs from LMICs were included. LMICs were defined as per the World Bank guidelines which include countries with gross national income per capita of under US$12 736/year.40
Intervention and comparison
Enteral administration of probiotic supplement versus control (placebo/no probiotic).
Outcomes
All-cause mortality, LOS (positive blood/cerebrospinal fluid (CSF) culture on a sample collected 48–72 hours after birth), definite NEC (stage ≥II modified Bell staging)41 and time to full enteral feeds (TFEF: 120 mL/kg/day).
Search strategy
The databases Medline searched via PubMed (https://www.ncbi.nlm.nih.gov 1966–2017), Embase (Excerpta Medica dataBASE) via Ovid (http://ovidsp.tx.ovid.com, 1980–2017), Cochrane Central Register of Controlled Trials (http://www.thecochranelibrary.com, through January 2017), Cumulative Index of Nursing and Allied Health Literature via OVID (http://ovidsp.tx.ovid.com, 1980–January 2017) and E-abstracts from the Pediatric Academic Society meetings (https://www.pas-meeting.org/about/#past, 2000–January 2017) were searched in January 2017. Abstracts of other conference proceedings such as European Academy of Paediatric Societies and the British Maternal and Fetal Medicine Society were searched in Embase. ‘Google Scholar’ was searched for articles that might not have been cited in the standard medical databases. Grey literature was searched using the national technical information services (http://www.ntis.gov/), Open Grey (http://www.opengrey.eu/), and Trove (http://trove.nla.gov.au/). We have also searched Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS) and Caribmed via the BIREME/PAHO/WHO—Latin American and Caribbean Center on Health Sciences Information; PAHO, Pan American Health Organization (http://lilacs.bvsalud.org/en/) using broad terminologies Probiotics OR Probiotic Or Bifidobacterium OR Bifidobacteria OR Lactobacillus OR Lactobacilli OR Saccharomyces. We also searched ClinicalTrials.gov (https://clinicaltrials.gov), International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/) and BioPortfolio (https://www.bioportfolio.com) for ongoing RCTs. The reference lists of eligible studies and review articles were searched to identify additional studies. Reviewers SR, GJ and GD conducted the literature search independently. No language restriction was applied. The non-English studies were identified by reading the recent systematic reviews of probiotic supplementation for reducing the risk of NEC42 43 and from cross references of individual studies. Full texts of all non-English studies were obtained via University of Sydney and Department of New South Wales (NSW) health library. A research officer from the NSW Health, University of Sydney translated the articles. Attempts were made to contact the authors for additional data and clarification of methods. Only published data were used for those studies where available.
PubMed was searched using the following terminology: (((‘Infant, Newborn’ [Mesh]) OR (‘Infant, Extremely Premature’ [Mesh] OR ‘Infant, Premature’ [Mesh])) OR (‘Infant, Low Birth Weight’ [Mesh] OR ‘Infant, Extremely Low Birth Weight’ [Mesh] OR ‘Infant, Very Low Birth Weight’ [Mesh])) AND ‘Probiotics’ [Majr]. It was also searched using ((‘Infant, Extremely Premature’ [Mesh] OR ‘Infant, Extremely Low Birth Weight’ [Mesh] OR ‘Infant, Very Low Birth Weight’ [Mesh] OR ‘Infant, Small for Gestational Age’ [Mesh] OR ‘Infant, Premature, Diseases’ [Mesh] OR ‘Infant, Premature’ [Mesh] OR ‘Infant, Newborn, Diseases’ [Mesh] OR ‘Infant, Newborn’ [Mesh] OR ‘Infant, Low Birth Weight’ [Mesh])) AND (((‘Bifidobacterium’ [Mesh]) OR ‘Lactobacillus’ [Mesh]) OR ‘Saccharomyces’ [Mesh]). The other databases were searched using similar terminologies. The detailed search terminology is given in online supplementary appendix 1.
bmjopen-2017-017638supp001.pdf (56KB, pdf)
Study selection
The abstracts of citations obtained from the initial broad search were read independently by reviewers SR, GJ and GD to identify potentially eligible studies. Full-text articles of these studies were obtained and assessed for eligibility by reviewers SR, GJ and GD independently, using the predefined eligibility criteria. Differences in opinion were resolved by group discussion to reach consensus. Care was taken to ensure that multiple publications of the same study were excluded to avoid data duplication.
Data extraction
Reviewers GD, SR and GJ extracted the data independently using a data collection form designed for this review. Information about the study design and outcomes was verified by all reviewers. Discrepancies during the data extraction process were resolved by group discussion. We contacted authors for additional information/clarifications.
Assessment of risk of bias
Risk of bias (ROB) was assessed using the Cochrane ‘Risk of Bias Assessment Tool’.44 Authors GD, SR and GJ independently assessed the ROB in all domains including random number generation, allocation concealment, blinding of intervention and outcome assessors, completeness of follow-up, selectivity of reporting and other potential sources of bias. For each domain, the ROB was assessed as low, high or unclear risk based on the Cochrane Collaboration guidelines.
Data synthesis
Meta-analysis was conducted using Review Manager 5.3 (Cochrane Collaboration, Nordic Cochrane Centre). Fixed effects model (FEM) (Mantel-Haenszel method) was used. Random effects model (REM) analysis was conducted to recheck the results if there was significant heterogeneity on FEM. Effect size was expressed as risk ratio (RR) and 95% CI.
Statistical heterogeneity was assessed by the χ2 test, I2 statistic and visual inspection of the forest plot (overlap of CIs). A P value <0.1 on χ2 statistic was considered to indicate heterogeneity. I2 statistic values were interpreted as per the Cochrane handbook guidelines as follows: 0% to 40%—might not be important; 30% to 60%—may represent moderate heterogeneity; 50% to 90%—may represent substantial heterogeneity; 75% to 100%—considerable heterogeneity.37 The risk of publication bias was assessed by visual inspection of the funnel plot.45
Subgroup analysis
(1) Low ROB: random sequence generation and allocation concealment; (2) preterm neonates less than 34 weeks gestation or birth weight less than 1500 g; (3) where Bifidobacterium was part of the supplementation; (4) where Lactobacillus was part of the supplementation; (5) single strain probiotic were used and (6) multiple strain probiotics were used.
Summary of findings table
The key information concerning the quality of evidence, the magnitude of effect of the intervention and the sum of available data on the main outcome was presented in the ‘summary of findings table’ as per the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) guidelines.44
Results
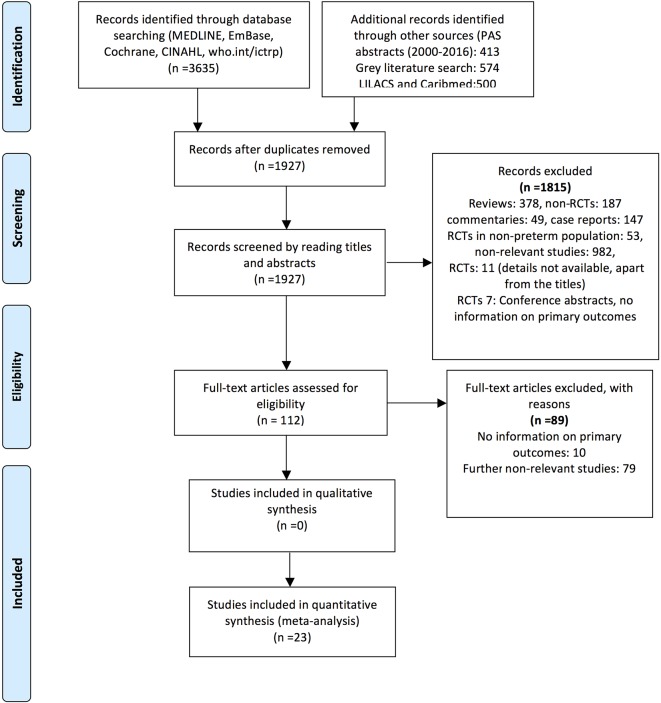
The literature search retrieved 1926 potential relevant citations. After carefully reviewing the abstracts, 1814 studies were excluded: reviews: 378; observational studies: 187; commentaries: 49; case reports: 147; RCTs in adult and paediatric population: 53 and non-relevant studies: 982. Finally, 23 RCTs (n=4783) conducted in 10 different LMICs in 4 continents were included in the meta-analysis.12 46–67 The search strategy results are given in online supplementary appendix 1. The flow diagram of study selection process is given in figure 1. The characteristics of the included studies are given in table 1. Out of the 23 included studies, single-strain probiotics were used in 11 studies, whereas 12 used multiple strains. Lactobacillus was part of the supplementation in 13 studies; Bifidobacterium was part of the supplementation in 11 studies and saccharomyces in 3 studies (table 1).
Figure 1.
Flow diagram of search strategy and study selection (January 2017). CINAHL, Cumulative Index of Nursing and Allied Health Literature; LILACS, Literatura Latino-Americana e do Caribe em Ciências da Saúde; PAS, Pediatric Academy Society; RCT, randomised controlled trial.
Table 1.
Characteristics of included studies
| Study ID | Location | Study characteristics |
| Awad et al 46 | Egypt |
Participants: all neonates admitted to nursery, 28–41 weeks and weight 1.1–4.3 kg Intervention and dose: KP (L. acidophilus, 6×109 CFU) versus LP (L. acidophilus, 6×109 CFU) versus placebo Duration of supplementation: commenced on D1, duration NA n=150 (60 vs 60 vs 30), Preterm: 89 (37 vs 36 vs 16) Type of milk: details NA; Type of delivery: preterm CS: KP (57%) versus LP (56%) versus placebo (75%) Primary outcome: all outcomes for LP versus KP versus Controls: incidence of neonatal sepsis (18/36, 50% vs 25/37, 68% vs 12/16, 75%; P=0.251) and NEC (0/36 vs 1/37 vs5/16; P=0.000) neonates and evaluation of efficacy of a KP Other outcome: mortality: 4/36 (11.1%) versus 12/37 (32.4%) versus 5/16 (31.3%), P=0.076 |
| Braga et al 47 | Brazil |
Participants: preterm infants 750–1499 g Intervention and dose: (L. casei + B. breve: 3.5×107 to 3.5×109 CFU) versus no probiotic Duration of supplementation: once daily from the second day of life until day 30 n=231 (probiotics: 119; controls: 112) Type of milk: EBM/PDHM; Type of delivery: CS 53.8% vs 49.1% Primary outcome: ≥Stage II NEC (0/119, 0% vs 4/112, 3.6%) Other outcomes: LOS: 40/119 (33.6%) versus 42/112 (37.5%); Mortality: 26/119 (21.8%) versus 27/112 (24.1%) |
| Dashti et al 48 | Iran |
Participants: preterm infants 700–1800 g Intervention and dose: (L. acidophilus, L. rhamnosus, B. longum, L. bulgaricus, L. casei, S. thermophilus, B. breve and Bifidobacterium: total 1×109 CFU/sachet) versus placebo powder Duration of supplementation: once daily from first feed of life until discharge n=136 (probiotics: 69; controls: 67) Type of milk: EBM/formula milk; Type of delivery: CS 82.4% versus 17.6% Primary outcome: ≥Stage II NEC (2/69, 2.9% vs 1/67, 1.5%) Other outcomes: mortality: 8/69 (11.6%) versus 4/67 (5.97%) |
| Demirel et al 49 | Turkey |
Participants: preterm infants ≤32 weeks and ≤1500 g Intervention and dose:S.boulardii, 5×109 CFU versus no probiotic Duration of supplementation: NA n=271 (probiotic: 135; controls: 136) Type of milk: EBM/formula; Type of delivery: CS 77.7% versus 83% Primary outcome: NEC ≥Stage II (6/135, 4.4% vs 7/136, 5.1%), P=1; mortality: (5/135, 3.7% vs 5/136, 3.7%), P=1 Other outcomes: LOS: 20/135 (14.9%) versus 21/136 (15.4%) P=0.906; feed intolerance: 30/135 (22.2%) versus 62/136 (46%), P<0.001 |
| Deng and Chen50 | China |
Participants: 125 preterm infants, <37 weeks, <2500 g at birth Intervention and dose: B. longum, L. acidophilus, Enterococcus faecalis, triple viable powder oral or nasal Bifico plus powder/capsules. For birth weight <1500 g: 0.33×107 CFU of each probiotic two times per day and >1500 g: 0.5×107 of each probiotic two times per day; control: sterile warm water Duration of supplementation: commenced from first feed until 14 days of life n=125 (62 controls 33.2±2.3 weeks vs 63 probiotic group 32.4±2.8 weeks), Type of milk: EBM/preterm formula; Type of delivery: NA Primary outcome: NEC: controls: Bell Stage I (1/62, 1.6%), Bell Stage II (4/62, 6.5%), Bell Stage III (4/62, 6.5%) versus Treatment Bell Stage I (1/63,1.6%), Bell Stage II (1/63, 1.6%) Other outcomes: LOS, mortality: NA |
| Dilli et al 51 | Turkey |
Participants: VLBW infants with a gestation of <32 weeks and birth weight <1500 g Intervention and dose: B.lactis (5×109 CFU) versus placebo (maltodextrin) Duration of supplementation: from day 8 of life, once daily until discharge or a maximum of 8 weeks n=200 (probiotic 100; placebo: 100) Type of milk: EBM/formula; Type of delivery: CS: 35/100 (35%) versus 37/100 (37%) Primary outcome: NEC (≥stage 2): 2/100 (2%) versus 18/100 (18%), P<0.001 Other outcomes: LOS: 8/100 (8%) versus 13/100 (13%), P=0.6; mortality: 3/100 (3%) versus 12/100 (12%), P=0.003; time to full enteral feeds* (150 mL/kg/day): 18 (14–23) days versus 25 (15–37) days, P<0.001 |
| Dutta et al 52 | India |
Participants: preterm infants 27–33 weeks gestation Intervention: high dose (10 billion CFU: L. acidophilus, L. rhamnosus, B. longum, S. boulardii) versus low dose (1 billion CFU: L. acidophilus, L. rhamnosus, B. longum, S. boulardii) versus placebo (potato starch, maltodextrin) Duration of supplementation: probiotic groups: (A): high dose for 21 days, (C): low dose for 21 days, (B): high dose short course (D1–D14 and D15–D21) N: probiotic (114) versus placebo (35) Type of milk: EBM/formula; Type of delivery: probiotic group versus placebo: SVD (69% vs 60%), CS: data NA Primary outcome: stool colonisation rates on D14, D21, D28 with three different probiotic regimens (Lactobacillus and Bifidobacterium colonisation was significantly higher in groups A, B and C vs placebo, respectively. Groups A, B and C did not differ from each other. There were trends towards more CFU of Lactobacillus and Bifidobacterium per millilitre of stool in group A versus B and B versus C. Groups A and B and SPL independently predicted high Lactobacillus counts on day 28; groups A, B and C and SPL predicted high Bifidobacterium counts) Other outcomes: LOS: 10/114 (8.8%) versus 6/35 (17.1%), P=0.14, mortality: 8/114 (7%) versus 2/35 (12.7%), P=0.85; NEC (≥stage 2): 6/114 (5.3%) versus 0/35 (0%), P=0.35 |
| Fernández- Carrocera et al 53 | Mexico |
Participants: preterm infants <1500 g Intervention and dosage: multispecies probiotic product (L. acidophilus+L. rhamnosus+L. casei+L. plantarum+B. infantis+S. thermophilus) versus no probiotic Duration of supplementation: from the day of commencement of enteral feeds, once daily. Actual duration: NA n=150 (probiotics:75; controls: 75) Type of milk: EBM/formula; Type of delivery: data not available Primary outcome: ≥ Stage 2 NEC: 6/75 (8%) versus 12/75 (16%), P=0.142 Other outcomes: LOS: 42/75 (56%) versus 44/75 (58.7%), P=NA; mortality: 1/75 (1.3%) versus 7/75 (9.3%), P=0.063 |
| Hua et al 54 | China |
Participants: preterm infants <37 weeks Intervention and dosage: probiotic Jin Shuang Qi (L. acidophilus, S. thermophilus, Bifidobacterium) 5×107 CFU/day versus no probiotic Duration of supplementation: from the day of commencement of enteral feeds, once daily. Duration of supplementation: not clear n=257 (probiotics:119, controls: 138) Type of milk: EBM/formula; type of delivery: CS 55.5% versus 64.5% Primary outcome: stool colonisation by drug-resistant bacteria (no difference in both groups, P>0.05) Other outcome: LOS: 2/119 (1.7%) versus 8/138 (5.8%); P=0.168, NEC (stage NS): 0/119 versus 2/138; P=0.501; Mortality: 2/119 versus 2/138 |
| Huang et al 55 | China |
Participants: preterm infants 28–32 weeks and <1500 g Intervention and dosage: Bifidobacterium (50 million live bacteria/capsule) 0.25×108 live bacteria oral/nasally two times per day versus non-treatment (control) Duration of supplementation: From 7 days until 14 days of age n=183 (probiotic: 95, control: 88) Type of milk: Not stated; type of Delivery: NA Primary outcomes: NEC: 2/95 (2.1%), both Bell’s stage 1 versus 9/88 (10.23%): Bell’s stage 1:6, stage 2:2, stage 3:1 (P<0.01), body mass changes/weight gain†: probiotic group: 8.109±2.127 g versus control group 6.489±2.327 g (P<0.01) Other outcomes: LOS, death; TFEF: NA, gut colonisation: after 7 days of treatment, the two groups’ intestinal bacteria and bacteria ratio of the total number of cocci and rods, the differences were statistically significant (P<0.01). Rod bacteria ratio before and after preventive treatment groups showed no significant difference (P>0.05); in the control group rod bacteria ratio difference was statistically significant (P<0.01) |
| Oncel et al 56 | Turkey |
Participants: preterm infants ≤32 weeks and <1500 g Intervention and dosage:L. reuteri DSM 17938 in oil-based suspension, 1×108 CFU/day vs placebo (oil-based suspension without probiotics) Duration of supplementation: from the time of first enteral feeds until discharge n=400 (probiotics: 200; placebo: 200) Type of milk: EBM/preterm formula; type of delivery: CS 75% versus 76% Primary outcome: probiotics versus controls: ≥ Stage 2 NEC or death: 20/200 (10%) versus 27/200 (13.5%); P=0.27, NEC (≥ stage 2):8/200 (4%) versus 10/200 (5%); P=0.63 Other outcomes: late-onset sepsis: 13/200 (6.5%) versus 25/200 (12.5%); P=0.041; time to full feeds†:9.1±3.2 versus 10.1±4.3 days; P=0.006; hospital stay*:38 (10–131) versus 46 (10–180) days; P=0.022; feed intolerance: 56/200 (28%) versus 79/200 (39.5%); P=0.015 |
| Qiao et al 57 | China |
Participants: preterm 28–34 weeks GA, >1000 g, <72 hours life Intervention: Bifidobacterium, Lactobacillus, Streptococcus thermophilus, 0.5 g per bag Duration of supplementation: 0.5 bag three times daily for 3 days after admission to hospital n=287 (probiotic: 149 versus control 138) Type of milk: not stated; type of delivery: no stats on CS/type of delivery Primary outcomes: time to full oral feeds (7.3 days vs 16.9 days); P<0.05, time to full enteral nutrition (9.8 days vs 16.9 days); P<0.05, LOS (6.7% vs 15.2%); P<0.05, NEC (3.4% vs 10.9%); P<0.05, hospitalisation time (25.0 days vs 30.8 days); P: NA; mortality†: (6.0±4.0)% and (9.0±6.5)%; P>0.05 |
| Rojas et al 58 | Columbia |
Participants: preterm infants ≤2000 g Intervention and dosage:L. reuteri DSM 17938, 1×108 CFU, once daily versus placebo (oil-based suspension without probiotics) Duration of supplementation: commenced within 48 hours of life. Duration: NA n=750 (probiotics: 372; placebo: 378) Type of milk: EBM/formula; type of delivery: VD non-instrumental: 16% (study) versus 17% (placebo), VD instrumental: 0% (study) versus 0.5% (placebo), elective CS: 18% (study) versus 17% (placebo), non-elective CS 65% (study) versus 65% (placebo) Primary outcome: nosocomial infection and mortality: 57/372 (15.3%) versus 67/378 (17.7%); P=0.38; death: 22/372 (5.9%) versus 28/378 (7.4%); P=0.41 Other outcomes: LOS: 24/372 (6.5%) versus 17/378 (4.5%); P=0.24; duration of hospitalisation*: 20 (11–33) versus 20 (11–38) days; P=0.53 |
| Roy et al 59 | India |
Participants: preterm infants <37 weeks and birth weight <2500 g Intervention and dosage: half of the 1-gram sachet that contained L. acidophilus 1.25×109 + B. longum 0.125×109 + B. bifidum 0.125×109 + B. lactis 1×109 versus sterile water Duration of supplementation: commenced within 72 hours of birth for 6 weeks or until discharge n=112 (probiotics: 56; placebo: 56) Type of milk: EBM; type of delivery: CS 83.9% versus 76.8% Primary outcome: enteric fungal colonisation†: 3.03±2.33 ×105 CFU versus 3±1.5×105; P=0.03 and LOS (bacterial and fungal): 31/56 (55.4%) versus 42/56 (75%); P=0.02 Other outcome: TFEF†:11.22±5.04 versus. 15.41±8.07 days; P=0.016 |
| Saengtawesin et al 60 | Thailand |
Participants: preterm (<34 weeks) and VLBW (<1500 g) infants Intervention and dosage: probiotic mixture (L. acidophilus+B. bifidum each 1×109 CFU/250 mg), 125 mg/kg two times per day versus Nn Duration of supplementation: NA n=60 (probiotics: 31, controls:29) Type of milk: EBM/preterm formula; type of delivery: CS 67.7% versus 62% Primary outcome: NEC ≥stage 2: 1 (3.2%) versus 1 (3.4%); P=0.74 Other outcomes: LOS: 2 (6.45%) versus 1 (3.44%); P=0.53, TFEF†: 12.03±5.49 days versus 13.76±8.25 days (P=0.64) |
| Samanta et al 12 | India |
Participants: preterm(<32 weeks) and VLBW (<1500 g) infants Intervention and dosage: probiotic mixture (B. infantis+B. bifidum+B. longum+L. acidophilus, each 2.5×109 CFU), administered two times per day versus no probiotic Duration of supplementation: NA n=186 (probiotics: 91; controls: 95) Type of milk: EBM; type of delivery: CS 46.15% versus 49.47% Primary outcomes: Incidence of NEC (≥ stage 2): 5/91 (1.1%) versus 15/95 (15.8%); P=0.042, death due to NEC: overall death: 4/91 (4.4%) versus 14/95 (14.7%); P=0.032; feed tolerance: time to full feeds†: 13.76±2.28 versus 19.2±2.02 days; P<0.001 Other outcomes: LOS: 13/91 (14.3%) versus 28/95 (29.5%); P=0.02; hospital stay†: 17.17±3.23 versus 24.07±4 days; P<0.001 |
| Sari et al 61 | Turkey |
Participants: preterm infants <33 weeks or birth weight <1500 g Intervention and dosage: L. sporogenes, 0.35×109 CFU, once a day versus no probiotic Duration of supplementation: from first enteral feed until discharge n=221 (probiotics: 110, controls: 111) Type of milk: EBM/formula; type of delivery: CS 67.3% versus 75.7% Primary outcomes: NEC ≥ Stage II: 6/110 (5.5%) versus 10/111 (9%); P=0.447, death/NEC: 9/110 (8.2%) versus 13/111 (11.7%); P=0.515 Other outcomes: LOS: 29/110 (26.4%) versus 26/111 (23.4%); P=0.613, hospital stay: 34.5 versus 30 days; P=0.919, †: 17.3±8.7 versus 18.3±9.8 days, P=0.438, feed intolerance: 49/110 (44.5%) versus 70/111 (63.1%); P=0.006 |
| Serce et al 62 | Turkey |
Participants: preterm infants <32 weeks and <1500 g Intervention and dosage: Sacch. boulardii 0.5×109 CFU two times per day versus placebo (distilled water) Duration of supplementation: from the first enteral feed until discharge n=208 (probiotic: 104; placebo: 104) Type of milk: EBM/formula; type of delivery: CS 80.8% versus 88.5% Primary outcomes: stage ≥2 NEC: 7/104 (6.7%) versus 7/104 (6.7%); P=1 LOS: 19/104 (18.3%) versus 25/104 (24.3%); P=0.29 Other outcomes: death: 5/104 (4.8%) versus 4/104 (3.8%); P=0.74, hospital stay*: 39 (28–60) days versus 43 (29–60) days; P=0.62 |
| Shadkam et al 63 | Iran |
Participants: preterm infants 28 to 32 weeks and 1000–1800 g Intervention and dose: (L. reuteri DSM 17938: 2.0×107 CFU) versus distilled water Duration of supplementation: two times per day started once infant reached 40 mL/kg/day of feed until 120 mL/kg/day of feed n=60 (probiotics: 30; controls: 30) Type of milk: EBM/formula milk; type of delivery: details NA Primary outcome: (Stage NS) NEC (2/30, 6.7% vs 11/30, 36.7%); P=0.005 Other outcomes: LOS: 4/30 (13.3%) versus 10/30 (33.4%); P=0.109, TFEF†: 12.83±4.26 versus 16.78±6.66 days; P=0.01; mortality: 1/30 (3.3%) versus 2/30 (6.7%); P=0.5 |
| Tewari et al 64 | India |
Participants: preterm infants <34 weeks (two groups: EPT: 27–30+6 weeks and VPT: 31–33+6 weeks) Intervention: Bacillus clausii (2.4×109 spores per day) versus placebo Duration of supplementation: commenced D5 in asymptomatic and D10 in symptomatic neonates and continued for 6 weeks/discharge/death/occurrence of LOS whichever was earlier n=244 (study: EPT: 61 and VPT: 62) versus(placebo:121) Type of milk: EBM/PDHM; type of delivery: CS: EPT: 66% versus 59% and VPT: 58% versus 60% Primary outcome: incidence of definite and probable LOS: definite LOS: EPT: 6/61 (10%) versus 8/59 (14%); P=0.26; VPT: 2/62 (3%) versus 3/62 (5%); P=0.39; probable LOS: EPT: 8/61 (12%) versus 9/59 (15%); VPT: 4/62 (6%) versus 5/62 (7%) Other outcomes: death: EPT: 8/61 (13%) versus 9/59 (15%); P=0.84, VPT: 4/62 (7%) versus 5/62 (8%); P=0.79; NEC (≥ stage 2): EPT: 0/61 versus 0/59; VPT: 0/62 versus 0/62 |
| Van Niekerk et al 65 | South Africa |
Participants: preterm infants <34 weeks and birth weight 500 to 1250 g Intervention and dosage: Pro-52 (L. rhamnosus GG and B. infantis), 0.35×109 CFU of each daily versus placebo (MCT oil) Duration of supplementation: from the first enteral feed until day 28 of life n=184 (probiotic: 91; placebo: 93) Type of milk: EBM/formula; type of delivery: CS 80.8% versus 88.5% Primary outcome: impact of probiotic supplementation on the incidence and severity of NEC in premature VLBW infants that are exposed to HIV. NEC: 3/91 (3.3%) versus 6/93 (6.45%) Other outcomes: LOS: 15/91 (16.5%) versus 10/93 (10.8%); death: 5/91 (5.5%) versus 6/93 (6.45%); TFEF†: HIV exposed: 10.19±4.055 versus 9.68±3.46 days, P=0.56 and HIV non-exposed: 9.63±2.42 versus 11.14±4.15 days, P=0.022 |
| Yang et al 66 | China |
Participants: 62 preterm infants <37 weeks Intervention: B. longum, L. acidophilus, Enterococcus faecalis triple viable powder oral or nasal Bifico plus powder/capsules (probiotics powder/capsules), Shanghai Xinyi Pharmaceutical), 0.5×107 CFU two times per day of each Duration of supplementation: from commencement of feeds until 14 days of life n=62 (controls: 31; probiotics: 31) Type of milk: EBM/preterm formula; type of delivery: NA Primary outcomes: NEC incidence: 2/31 (6.45%) versus 3/31 (9.68%) versus (no mention of criteria for NEC used) Other outcomes: sepsis, mortality, TFEF: NA |
| Xu et al 67 | China |
Participants: 125 neonates with a GA of 30–37 weeks and birth weight 1500–2500 g. Intervention:S. boulardii CNCM I-745 at a dose of 50 mg/kg (109 CFU) two times per day Duration of supplementation: 9–28 days (mean 25.3 days) n=125 (probiotic: 63; control: 62); analysis (probiotic: 51; control: 49) Type of milk: EBM/formula; type of delivery: NA Primary outcome: weight gain was 16.14±1.96 g/kg/day versus 10.73±1.77 g/kg/day; P<0.05 and linear growth was 0.89±0.04 cm/week versus 0.87±0.04 cm/week; P=0.17 Other outcome: TFEF: 0.37±0.13 versus 1.70±0.45; P<0.01, maximal enteral feeding volume tolerated: 128.44±6.67 versus 112.29±7.24 mL/kg/day: P=0.03 and duration of hospitalisation: 23.3±1.6 versus 28.0±1.8; P=0.035 |
For all outcomes, results in the study/probiotic group are given first.
*Median and IQR (25%–75%).
†Mean and SD.
CFU, colony forming unit; CS, caesarean section; EBM, expressed breast milk; EPT, extremely preterm; GA, gestational age; KP, killed probiotic; LGG, Lactobacillus rhamnosus GG (ATCC 53103) Gorbach and Goldin; LOS, late-onset sepsis; LP, living probiotic; MCT, medium chain triglycerides; NA, not available; NEC, necrotising enterocolitis; NS, not specified; PDHM, pasteurised donor human milk; SPL, spontaneous preterm labour; SVD, spontaneous vaginal delivery; TFEF, time to full enteral feed; VD, vaginal delivery; VLBW, very low birth weight; VPT, very preterm.
ROB of included studies
A total of 14/23 (60%) included studies were judged to have low ROB for the domain of ‘random sequence generation’, and (56%) were considered to have low ROB for ‘allocation concealment’ (table 2).
Table 2.
Risk of bias of the included randomised controlled trials
| Author/reference | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
| Awad et al 46 | Unclear risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Braga et al 47 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Dashti et al 48 | Unclear risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Demirel et al 49 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Deng and Chen50 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk |
| Dilli et al 51 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Dutta et al 52 | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Fernández-Carrocera et al 53 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Hua et al 54 | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Huang et al 55 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk |
| Oncel et al 56 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Qiao et al 57 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk |
| Rojas et al 58 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Roy et al 59 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Saengtawesin et al 60 | Low risk | Unclear risk | High risk | High risk | Low risk | Low risk | Unclear risk |
| Samanta et al 12 | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Sari et al 61 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Shadkam et al 63 | Unclear risk | Low risk | Low risk | Low risk | Unclear risk | Unclear risk | Unclear risk |
| Serce et al 62 | Low risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| Tewari et al 64 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Van Niekerk et al 65 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Yang et al 66 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk |
| Xu et al 67 | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
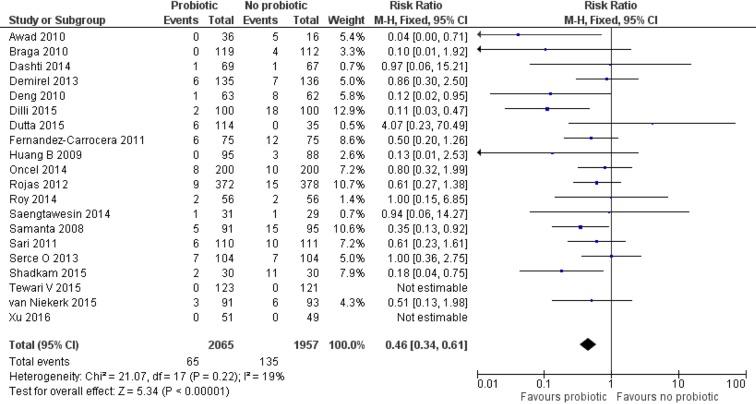
Effect of probiotics on ≥Stage II (definite) NEC
Data on definite NEC was reported by 20 trials (n=4022).12 46–53 55 56 58–65 67 A higher proportion of neonates in the control group developed definite NEC compared with the probiotic group (65/2065 (3.1%) vs 135/1957 (6.9%)). Meta-analysis using a FEM estimated a lower risk (RR 0.46 (95% CI 0.34 to 0.61), P<0.00001) of NEC in the probiotic group. There was no significant heterogeneity (I2=19%, P=0.22) among the trials. The numbers needed to treat (NNT) with probiotics to prevent one case of NEC was 25 (95% CI 20 to 50; figure 2).
Figure 2.
Forest plot: effect of probiotics on definite (≥Stage II) necrotising enterocolitis.
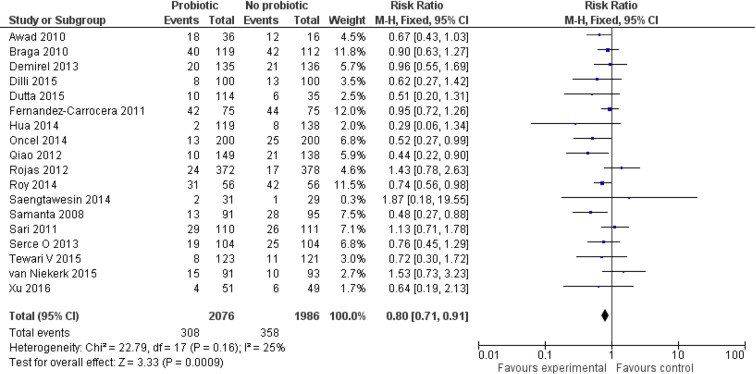
Effect of probiotics on LOS
Data from 18 trials12 46 47 49 51–54 56–62 64 65 67 (n=4062) showed that a higher proportion of neonates in the control group developed LOS compared with those in the probiotic group (308/2076 (14.5%) vs 358/1986 (18%)). Meta-analysis using a FEM estimated a lower risk (RR 0.80 (95% CI 0.71 to 0.91), P=0.0009) of LOS in the probiotic group. There was no significant heterogeneity (I2=25%; P=0.16) among the trials. The NNT with probiotics to prevent one case of LOS was 25 (95% CI 17 to 50; figure 3).
Figure 3.
Forest plot: effect of probiotics on late-onset sepsis.
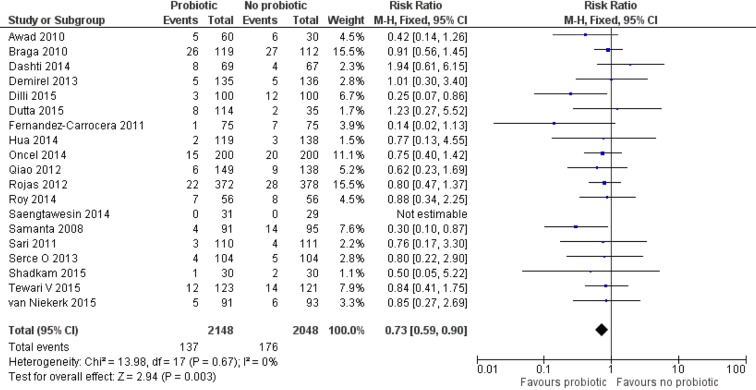
Effect of probiotics on all-cause mortality
Data from 19 trials (n=4196),12 46–49 51–54 56–65 showed reduced risk of death due to all causes in the probiotic versus control group (137/2148 (6.37%) vs 176/2048 (8.59%)). Meta-analysis using a FEM estimated a lower risk (RR 0.73 (95% CI 0.59 to 0.90), P=0.003) of death in the probiotic group. No significant heterogeneity was noted between the trials (I2=0%; P=0.67). The NNT to prevent one death by probiotic supplement was 50 (95% CI 25 to 100; figure 4).
Figure 4.
Forest plot: effect of probiotics on all-cause mortality.
Effect of probiotics on TFEF
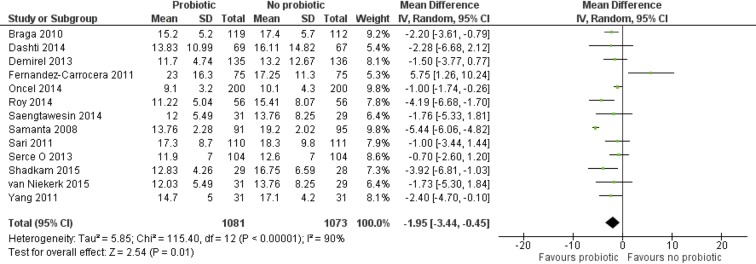
Meta-analysis of data (n=2154) from 13 trials12 47–49 53 56 59–63 65 66 showed significant reduction in TFEF in the probiotics versus control group (MD=−3.09 days (95% CI: −3.49 to –2.69), P<0.00001). However, there was significant heterogeneity (I2=90%, P<0.00001) among the trials. These results were hence checked by using REM and remained significant (MD=−1.95 days (95% CI: −3.44 to –0.45), P=0.01; figure 5). MD, mean difference.
Figure 5.
Forest plot: effect of probiotics on time to full enteral feeds.
Subgroup analysis
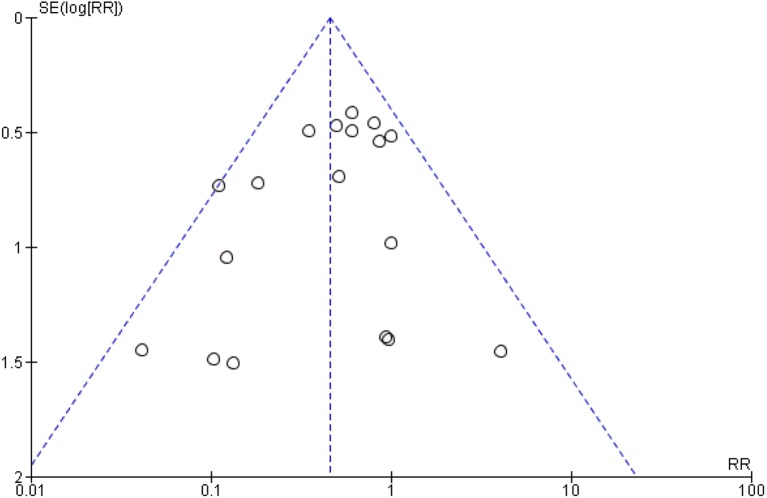
The beneficial effects continued to be observed in studies: (1) low ROB: random sequence generation and allocation concealment (table 3); (2) that only included infants with gestational age <34 weeks or birth weight <1500 g; (3) where Bifidobacterium was part of the supplementation; (4) where Lactobacillus was part of the supplementation; (5) single strain probiotics were used and (6) multiple strain supplements were used; however, on REM meta-analysis, statistical significance was lost for some of these analyses (table 4). The overall evidence according to GRADE guidelines is provided as a summary of findings table (table 5). The evidence was deemed high in view of the large sample size, low risk of bias in majority (14/20) of the included studies, narrow CIs around the effect size estimate, very low P value for effect size estimate and mild statistical heterogeneity. Visual inspection of the funnel plot suggested that there was no publication bias (figure 6).
Table 3.
Results of the subgroup analysis (ROB)
| Item | Number of studies | Sample size | RR (95% CI) (FEM) | RR (95% CI) (REM) | I2 statistic (%) |
| Definite NEC: studies with low ROB on random sequence generation | 14 | 3464 | 0.55 (0.40 to 0.74) | 0.58 (0.42 to 0.81) | 1 |
| Definite NEC: studies with low ROB on allocation concealment | 13 | 3035 | 0.48 (0.34 to 0.66) | 0.52 (0.33 to 0.80) | 29 |
| LOS: studies with low ROB on random sequence generation | 15 | 3466 | 0.85 (0.74 to 0.97) | 0.84 (0.72 to 0.98) | 18 |
| LOS: studies with low ROB on allocation concealment | 11 | 2839 | 0.86 (0.75 to 0.99) | 0.85 (0.74 to 0.97) | 6 |
| All-cause mortality: studies with low ROB on random sequence generation | 14 | 3366 | 0.72 (0.57 to 0.91) | 0.75 (0.60 to 0.95) | 0 |
| All-cause mortality: studies with low ROB on allocation concealment | 13 | 3073 | 0.76 (0.60 to 0.96) | 0.78 (0.62 to 0.99) | 0 |
FEM, fixed effect model; LOS, late-onset sepsis; NEC, necrotising enterocolitis; REM, random effects model; ROB, risk of bias; RR, relative risk.
Table 4.
Results of the subgroup analysis
| Item | Definite NEC | Late-onset sepsis | All-cause mortality | ||||||
| Number of studies (sample size) | RR (95% CI) (FEM) | RR (95% CI) (REM) | Number of studies (sample size) | RR (95% CI) (FEM) | RR (95% CI) (REM) | Number of studies (sample size) | RR (95% CI) (FEM) | RR (95% CI) (REM) | |
| RCTs with gestational age <32 weeks or birth weight <1500 g | 14 (2886) | 0.51 (0.37 to 0.70) | 0.56 (0.40 to 0.78) | 11 (2470) | 0.84 (0.71 to 1.01) | 0.84 (0.68 to 1.04) | 12 (2591) | 0.75 (0.61 to 0.93) | 0.78 (0.61 to 0.99) |
| RCTs: Lactobacillus was part of the supplementation | 13 (2595) | 0.45 (0.32 to 0.64) | 0.48 (0.32 to 0.71) | 12 (2979) | 0.81 (0.70 to 0.93) | 0.79 (0.64 to 0.97) | 16 (3473) | 0.70 (0.56 to 0.89) | 0.73 (0.58 to 0.93) |
| RCTs: Bifidobacterium was part of the supplementation | 11 (1716) | 0.35 (0.22 to 0.55) | 0.38 (0.23 to 0.63) | 9 (1756) | 0.76 (0.64 to 0.89) | 0.75 (0.59 to 0.94) | 12 (2173) | 0.70 (0.52 to 0.93) | 0.71 (0.49 to 1.03) |
| Single-strain probiotic supplementation | 11 (2727) | 0.46 (0.32 to 0.66) | 0.46 (0.32 to 0.66) | 9 (2446) | 0.86 (0.7 to 1.04) | 0.83 (0.67 to 1.03) | 9 (2444) | 0.70 (0.52 to 0.94) | 0.71 (0.53 to 0.96) |
| Multistrain probiotic supplementation | 9 (1333) | 0.45 (0.28 to 0.73) | 0.47 (0.28 to 0.78) | 8 (1556) | 0.76 (0.65 to 0.90) | 0.75 (0.59 to 0.96) | 10 (1752) | 0.76 (0.56 to 1.03) | 0.78 (0.54 to 1.13) |
FEM, fixed effect model; NEC, necrotising enterocolitis; REM, random effects model; RR, relative risk.
Table 5.
Summary of findings as per GRADE guidelines38
| Outcome | Absolute risk | |||||
| Estimate without probiotic supplementation | Corresponding risk estimate with probiotic supplementation | Relative effect (RR) 95% CI |
Number of participants | Quality of evidence GRADE | Comment | |
| Late-onset sepsis | 358/1986 (18%) | 308/1986 (14.5%) | 0.80 (0.71 to 0.91); P=0.0009, I2=25% | 3902 | High | Refer note* |
| Mortality | 176/2048 (8.6%) | 137/2148 (6.4%) | 0.73 (0.59 to 0.9); P=0.003, I2=0% | 4196 | High | Refer note* |
| NEC | 135/1957 (6.9%) | 65/2065 (3.1%) | 0.46 (0.34 to 0.61); P<0.00001, I2=19% | 4022 | High | Refer note* |
*Note: The evidence was deemed high in view of the large sample size, low risk of bias in majority (14/20) of the included studies, narrow CIs around the effect size estimate, very low P Value for effect size estimate and mild statistical heterogeneity.
GRADE, Grades of Recommendation, Assessment, Development and Evaluation; RR, relative risk.
Figure 6.
Funnel plot assessing publication bias. RR, risk ratio.
Safety
None of the studies reported any significant adverse effects including probiotic sepsis.
Discussion
The results of our systematic review of 23 RCTs (n=4783) conducted in 10 LMICs across 4 continents show that probiotic supplementation in preterm neonates (born <37 weeks) significantly reduces the risk of all-cause mortality, LOS and NEC in such a set-up. The limitations of this review include variations in types of probiotics used in different studies and limitations of study qualities in few studies. The strengths of our systematic review include its robust methodology, comprehensive nature and exclusive focus on RCTs of probiotics in preterm neonates in LMICs. The limitations of our review include the variations in the probiotic protocols in the included RCTs, and the fact that nearly 40% of the included trials carried a high risk of bias in many domains of assessment.
To our knowledge, this is the first systematic review focusing on RCTs of probiotics in preterm neonates in LMICs. The summary findings as per GRADE guidelines confirm the high-quality evidence it provides (table 5). Our results are significant considering the UN’s MDG4 and UN Secretary-General’s Global Strategy for Women’s and Children’s Health (2010) and its accompanying Every Woman, Every Child initiative, ENAP and the burden of prematurity in LMICs.4 5 13
The incidence of prematurity is significantly increasing in LMICs compared with Europe or North America. There are issues related to reporting of preterm births and outcomes in LMICs.68 However, the studies funded by the WHO estimate 13 million preterm births/year in LMICs with 11 million (85%) of these being concentrated in Africa and Asia, ~0.5 million each in Europe and North America (excluding Mexico) and 0.9 million in Latin America and the Caribbean.69 The highest rates (11.9%) and number (seven million) of preterm births were in Africa and Asia, respectively. Mortality and morbidities such as LOS, NEC and feeding difficulties are major issues in preterm neonates. Although specific data from LMICs is not available, approximately one million preterm neonates die every year, predominantly due to sepsis, and long-term impairment in survivors is becoming an important issue.70
Consistent with our recent systematic review,71 our results show that probiotics reduced the risk of NEC and all-cause mortality and of LOS in preterm neonates. (RR 0.81 (95% CI 0.71 to 0.92), P=0.001). The reduction of LOS by probiotics is important considering that neonatal sepsis is responsible for nearly a third all neonatal deaths in LMICs.19 20 22 72–77
It is important to note that the burden of NEC is as significant in LMICs as in high-income countries. The incidence and severity of NEC is higher in LMICs and includes up to 15% cases of NEC totalis with ~100% mortality.9 12 It occurs in VLBW and ELBW neonates and in preterm neonates with higher birth weight. Lack of antenatal steroids and being small for gestational age (SGA) due to intrauterine growth restriction (IUGR) are known risk factors for NEC.78 The reason for higher incidence of NEC in LMICs could include the higher numbers of preterm ‘SGA-IUGR’ births and limited coverage of antenatal steroids.79 80 The NEC-related mortality and morbidity is almost entirely due to progression of the illness from stage II to stage III. Management of surgical NEC is difficult in LMICs considering the limited resources. Primary prevention of NEC is therefore an important strategy for reducing the health burden of the condition in LMICs. Considering the effect size with regards to reduced risk of NEC, the benefits of probiotics in LMICs could not be overemphasised.
The issue of implementing probiotics for preterm neonates in LMICs is complex. The options include either reconfirming their safety and efficacy in large definitive RCTs in LMICs or adopting their routine use based on current evidence. Conducting large multicentre trials and accessing proven safe and effective probiotics is difficult, especially in resource-limited set-ups.34 Apart from the significant budget, the difficulties include regulatory hurdles, logistics of importing a probiotic product, maintaining cold chain and providing ongoing independent safety and quality control. However, there are recent examples of large RCTs conducted successfully in community settings in LMICs.81–83 Neonatal demographic characteristics, such as gestation and IUGR, are an important issue in conducting RCTs in LMICs as they determine the risk of NEC, duration of probiotic supplementation and the cost-benefit ratio. It is also important to note that many RCTs have used different probiotic/s and probiotic activity could be strain specific.
Knowledge of the pattern of gut colonisation in preterm neonates in a given set-up is important before using probiotics for research or routine use. Dutta et al have reported abnormal intestinal colonisation patterns in the first week of life in VLBW neonates in their level III neonatal intensive care unit in India.52 On day 1, 45% neonates had sterile guts, and by day 3, all were colonised predominantly by Escherichia coli, Klebsiella pneumoniae and Enterococcus faecalis. Only one isolate had lactobacilli and bifidobacteria were not detected during the study period. Formula feeding was associated with E. coli colonisation. Results of completed82 and ongoing trials such as NCT02552706 will be important.83
Probiotic sepsis, antibiotic resistance and altered immune responses in the long run are the potential adverse effects of probiotics in preterm neonates. Availability of killed or inactivated probiotic strains with clinically proven benefits may help in avoiding such adverse effects and in avoiding the need to maintain the cold chain. Awad et al have compared the effect of oral killed (KP) versus living Lactobacillus acidophilus (LP) in reducing the incidence of LOS and NEC in neonates.46 Both LP and KP reduced the risk of NEC (absolute risk reduction (ARR): 16%, 15%, respectively) and LOS (ARR: 18%) significantly compared with placebo. LOS and NEC was reduced significantly in neonates colonised versus not colonised by Lactobacillus at day 7 (27.9 vs 85.9%, 0 vs 7.8%) and day 14 (48.7 vs 91.7% for LOS and 0 vs 20.8% for NEC). KP retained the benefits similar to LP on comparison between all groups. Given the global implications of these results, the benefits of inactivated/killed probiotics need to be assessed in further large definitive trials.
In summary, our results indicate that probiotics are effective in significantly reducing the risk of all-cause mortality, LOS and NEC in preterm VLBW neonates in LMICs. Considering the burden of death, disease (NEC, LOS) and suboptimal nutrition in preterm neonates in LMICs, cooperation between various stake holders (eg, industry, scientists, regulatory agencies) is warranted to either develop or to improve access to high-quality safe and effective probiotics in such set-ups. Support from organisations such as the WHO is important in providing access to probiotics for the countries (eg, sub-Saharan Africa) where most prematurity related deaths occur. Whether probiotics could be used for research and/or routine use in preterm neonates in LMICs will depend on the national health priorities, resources and ethics.
Supplementary Material
Footnotes
Contributors: GD conceptualised and designed the study, performed an independent literature search, selected studies for inclusion, extracted and interpreted data, assessed risk of bias of included studies, handled the meta-analysis software, oversaw translation of manuscripts in the Chinese language and wrote the first and final drafts of the manuscript. GJ performed an independent literature search, selected studies for inclusion, contacted authors for additional information where necessary, extracted and interpreted the data, checked the data entered by GD on the meta-analysis software, assessed the risk of bias of included studies and helped with the first and the final draft of the manuscript. SR performed an independent literature search, selected studies for inclusion, verified the extracted data, assessed risk of bias, interpreted data and helped with the first and the final draft of the manuscript. SP supervised the project, acted as referee author in case of differences of opinion between the first three authors, interpreted the data and supervised the first and approved the final versions of the manuscript. All authors approved the final manuscript as submitted.
Funding: Nepean Neonatal Intensive Care Parent Support Group (NNICUPS)
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379:2151–61. 10.1016/S0140-6736(12)60560-1 [DOI] [PubMed] [Google Scholar]
- 2. Oestergaard MZ, Inoue M, Yoshida S, et al. Neonatal mortality levels for 193 countries in 2009 with trends since 1990: a systematic analysis of progress, projections, and priorities. PLoS Med 2011;8:e1001080 10.1371/journal.pmed.1001080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. UNICEF. Committing to child survival: a promise renewed, progress report 2014. http://files.unicef.org/publications/files/APR_2014_web_15Sept14.pdf (accessed 26 Apr 2017).
- 4. Blencowe H, Cousens S. Addressing the challenge of neonatal mortality. Trop Med Int Health 2013;18:303–12. 10.1111/tmi.12048 [DOI] [PubMed] [Google Scholar]
- 5. Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 2013;10:S2 10.1186/1742-4755-10-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–72. 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- 7. Rüegger C, Hegglin M, Adams M, et al. Population based trends in mortality, morbidity and treatment for very preterm- and very low birth weight infants over 12 years. BMC Pediatr 2012;12:17 10.1186/1471-2431-12-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 2015;314:1039–51. 10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arnold M, Moore SW, Sidler D, et al. Long-term outcome of surgically managed necrotizing enterocolitis in a developing country. Pediatr Surg Int 2010;26:355–60. 10.1007/s00383-010-2583-8 [DOI] [PubMed] [Google Scholar]
- 10. Katz J, Lee AC, Kozuki N, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 2013;382:417–25. 10.1016/S0140-6736(13)60993-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramji S, Modi M, Gupta N. 50 years of neonatology in India, progress and future. Indian Pediatr 2013;50:104–6. 10.1007/s13312-013-0023-2 [DOI] [PubMed] [Google Scholar]
- 12. Samanta M, Sarkar M, Ghosh P, et al. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr 2009;55:128–31. 10.1093/tropej/fmn091 [DOI] [PubMed] [Google Scholar]
- 13. WHO, UNICEF. Every Newborn: an action plan to end preventable deaths. Geneva: World Health Organization, 2014. http://apps.who.int/iris/bitstream/10665/127938/1/9789241507448_eng.pdf (accessed 21 Jan 2017). [Google Scholar]
- 14. Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, 2002. http://www.fao.org/tempref/docrep/fao/009/a0512e/a0512e00.pdf (accessed 15 Jan 2017).
- 15. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014;4:CD005496. [DOI] [PubMed] [Google Scholar]
- 16. Deshpande G, Rao S, Patole S, et al. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 2010;125:921–30. 10.1542/peds.2009-1301 [DOI] [PubMed] [Google Scholar]
- 17. Lau CS, Chamberlain RS. Probiotic administration can prevent necrotizing enterocolitis in preterm infants: A meta-analysis. J Pediatr Surg 2015;50:1405–12. 10.1016/j.jpedsurg.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 18. Di Mauro A, Neu J, Riezzo G, et al. Gastrointestinal function development and microbiota. Ital J Pediatr 2013;39:15 10.1186/1824-7288-39-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 2008;295:G1025–34. 10.1152/ajpgi.90227.2008 [DOI] [PubMed] [Google Scholar]
- 20. Garrido D, Barile D, Mills DA. A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Adv Nutr 2012;3:415S–21. 10.3945/an.111.001586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci 2013;9:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walker A. Intestinal colonization and programming of the intestinal immune response. J Clin Gastroenterol 2014;48:S8–11. 10.1097/MCG.0000000000000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deshpande G, Rao S, Patole S. Probiotics in neonatal intensive care - back to the future. Aust N Z J Obstet Gynaecol 2015;55:210–7. 10.1111/ajo.12328 [DOI] [PubMed] [Google Scholar]
- 24. Härtel C, Pagel J, Rupp J, et al. Prophylactic use of Lactobacillus acidophilus/Bifidobacterium infantis probiotics and outcome in very low birth weight infants. J Pediatr 2014;165:285–9. 10.1016/j.jpeds.2014.04.029 [DOI] [PubMed] [Google Scholar]
- 25. Janvier A, Malo J, Barrington KJ. Cohort study of probiotics in a North American neonatal intensive care unit. J Pediatr 2014;164:980–5. 10.1016/j.jpeds.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 26. Li D, Rosito G, Slagle T. Probiotics for the prevention of necrotizing enterocolitis in neonates: an 8-year retrospective cohort study. J Clin Pharm Ther 2013;38:445–9. 10.1111/jcpt.12084 [DOI] [PubMed] [Google Scholar]
- 27. Patole SK, Rao SC, Keil AD, et al. Benefits of Bifidobacterium breve M-16V supplementation in preterm neonates: a retrospective cohort study. PLoS One 2016;11:e0150775 10.1371/journal.pone.0150775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Repa A, Thanhaeuser M, Endress D, et al. Probiotics (Lactobacillus acidophilus and Bifidobacterium infantis) prevent NEC in VLBW infants fed breast milk but not formula [corrected]. Pediatr Res 2015;77:381–8. 10.1038/pr.2014.192 [DOI] [PubMed] [Google Scholar]
- 29. Samuels N, van de Graaf R, Been JV, et al. Necrotising enterocolitis and mortality in preterm infants after introduction of probiotics: a quasi-experimental study. Sci Rep 2016;6:31643 10.1038/srep31643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Viswanathan S, Lau C, Akbari H, et al. Survey and evidence based review of probiotics used in very low birth weight preterm infants within the United States. J Perinatol 2016;36:1106–11. 10.1038/jp.2016.144 [DOI] [PubMed] [Google Scholar]
- 31. Luoto R, Isolauri E, Lehtonen L. Safety of Lactobacillus GG probiotic in infants with very low birth weight: twelve years of experience. Clin Infect Dis 2010;50:1327–8. 10.1086/651694 [DOI] [PubMed] [Google Scholar]
- 32. Sathoh Y, Shinohara K, Umeazki H, et al. Bifidobacteria prevents necrotising enterocolitis and infection. Int J Probiot Prebiot 2007;2:149–54. [Google Scholar]
- 33. Applegate JA, Fischer Walker CL, Ambikapathi R, et al. Systematic review of probiotics for the treatment of community-acquired acute diarrhea in children. BMC Public Health 2013;13:S16 10.1186/1471-2458-13-S3-S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Enos MK, Burton JP, Dols J, et al. Probiotics and nutrients for the first 1000 days of life in the developing world. Benef Microbes 2013;4:3–16. 10.3920/BM2012.0020 [DOI] [PubMed] [Google Scholar]
- 35. Noratto G. Probiotics as a strategy to Improve overall human health in developing countries. J Probiotics Health 2014;02 10.4172/2329-8901.1000118 [DOI] [Google Scholar]
- 36. Sleator RD, Hill C. Probiotics as therapeutics for the developing world. J Infect Dev Ctries 2007;1:7–12. [Google Scholar]
- 37. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011: The Cochrane Collaboration, 2011. [Google Scholar]
- 38. Dissemination CFRA. Systematic reviews: CRD’s guidance for undertaking reviews in health care, 2009. http://www.york.ac.uk/crd/guidance/ (accessed 21 Jan 2017).
- 39. Moher D, Altman DG, Liberati A, et al. PRISMA statement. Epidemiology (Cambridge, Mass). 2011;22:128. [DOI] [PubMed] [Google Scholar]
- 40. The World Bank. Country and lending groups, 2015. http://data.worldbank.org/about/country-and-lending-groups#Lower_middle_income (accessed 16 Mar 2017).
- 41. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179–201. 10.1016/S0031-3955(16)34975-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg 2012;47:241–8. 10.1016/j.jpedsurg.2011.09.064 [DOI] [PubMed] [Google Scholar]
- 43. Yang Y, Guo Y, Kan Q, et al. A meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Braz J Med Biol Res 2014;47:804–10. 10.1590/1414-431X20143857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol 2013;66:158–72. 10.1016/j.jclinepi.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 45. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046–55. [DOI] [PubMed] [Google Scholar]
- 46. Awad H, Mokhtar H, Imam SS, et al. Comparison between killed and living probiotic usage versus placebo for the prevention of necrotizing enterocolitis and sepsis in neonates. Pak J Biol Sci 2010;13:253–62. 10.3923/pjbs.2010.253.262 [DOI] [PubMed] [Google Scholar]
- 47. Braga TD, da Silva GA, de Lira PI, et al. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 2011;93:81–6. 10.3945/ajcn.2010.29799 [DOI] [PubMed] [Google Scholar]
- 48. Dashti S, Seyyed A, Basiry A, et al. Prophylactic probiotics for prevention of necrotizing enterocolitis (nec) in low birth weight neonates. Arch Pediatr Infect Dis 2014;2:175–9. [Google Scholar]
- 49. Demirel G, Erdeve O, Celik IH, et al. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled study. Acta Paediatr 2013;102:e560–5. 10.1111/apa.12416 [DOI] [PubMed] [Google Scholar]
- 50. Deng J, Chen K. Early minimal feeding combined with probiotics to prevent necrotizing enterocolitis in preterm infant. Chinese Journal of Modern Drug Application 2010;4:13–14. [Google Scholar]
- 51. Dilli D, Aydin B, Fettah ND, et al. The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J Pediatr 2015;166:545–51. 10.1016/j.jpeds.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 52. Dutta S, Ray P, Narang A. Comparison of stool colonization in premature infants by three dose regimes of a probiotic combination: a randomized controlled trial. Am J Perinatol 2015;32:733–40. 10.1055/s-0034-1395473 [DOI] [PubMed] [Google Scholar]
- 53. Fernández-Carrocera LA, Solis-Herrera A, Cabanillas-Ayón M, et al. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2013;98:F5–9. 10.1136/archdischild-2011-300435 [DOI] [PubMed] [Google Scholar]
- 54. Hua XT, Tang J, Mu DZ. [Effect of oral administration of probiotics on intestinal colonization with drug-resistant bacteria in preterm infants]. Zhongguo Dang Dai Er Ke Za Zhi 2014;16:606–9. [PubMed] [Google Scholar]
- 55. Huang B, Yang H, Huang X. Probiotics supplementation for prevention of necrotizing enterocolitis in very low-birth-weight neonates: a randomized, controlled trial. J Guangdong Med Coll 2009;27:37–9. [Google Scholar]
- 56. Oncel MY, Sari FN, Arayici S, et al. Lactobacillus reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2014;99:F110–5. 10.1136/archdischild-2013-304745 [DOI] [PubMed] [Google Scholar]
- 57. Qiao L, Tang Y, Wang W, et al. The effects of probiotics supplementation on premature infants in NICU. Chin J Microbiol 2012;11:1011–3. [Google Scholar]
- 58. Rojas MA, Lozano JM, Rojas MX, et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 2012;130:e1113–20. 10.1542/peds.2011-3584 [DOI] [PubMed] [Google Scholar]
- 59. Roy A, Chaudhuri J, Sarkar D, et al. Role of enteric supplementation of probiotics on late-onset sepsis by candida species in preterm low birth weight neonates: a randomized, double blind, placebo-controlled trial. N Am J Med Sci 2014;6:50–7. 10.4103/1947-2714.125870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saengtawesin V, Tangpolkaiwalsak R, Kanjanapattankul W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J Med Assoc Thai 2014;97:S20–5. [PubMed] [Google Scholar]
- 61. Sari FN, Dizdar EA, Oguz S, et al. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr 2011;65:434–9. 10.1038/ejcn.2010.278 [DOI] [PubMed] [Google Scholar]
- 62. Serce O, Benzer D, Gursoy T, et al. Efficacy of Saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: a randomised controlled trial. Early Hum Dev 2013;89:1033–6. 10.1016/j.earlhumdev.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 63. Shadkam M, Jalalizadeh F, Nasiriani K. Effects of probiotic lactobacillus reuteri (DSM 17938) on the Incidence of Necrotizing Enterocolitis in Very Low Birth Weight Premature Infants. Iran J Neonatol 2015;6:15–20. [Google Scholar]
- 64. Tewari VV, Dubey SK, Gupta G. Bacillus clausii for prevention of late-onset sepsis in preterm infants: a randomized controlled trial. J Trop Pediatr 2015;61:377–85. 10.1093/tropej/fmv050 [DOI] [PubMed] [Google Scholar]
- 65. Van Niekerk E, Nel DG, Blaauw R, et al. Probiotics Reduce Necrotizing Enterocolitis Severity in HIV-exposed Premature Infants. J Trop Pediatr 2015;61:155–64. 10.1093/tropej/fmv004 [DOI] [PubMed] [Google Scholar]
- 66. Yang S, Yi H, Gan B, et al. The clinical application value of endangered preterm infants given earlier amounts of micro feedings and adding probiotics. J Pediat Pharmacy 2011;17:21–4. [Google Scholar]
- 67. Xu L, Wang Y, Wang Y, et al. A double-blinded randomized trial on growth and feeding tolerance with Saccharomyces boulardii CNCM I-745 in formula-fed preterm infants. J Pediatr 2016;92:296–301. 10.1016/j.jped.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 68. Gladstone M, Oliver C, Van den Broek N. Survival, morbidity, growth and developmental delay for babies born preterm in low and middle income countries - a systematic review of outcomes measured. PLoS One 2015;10:e0120566 10.1371/journal.pone.0120566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 2010;88:31–8. 10.2471/BLT.08.062554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lawn JE, Gravett MG, Nunes TM, et al. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth 2010;10:S1–22. 10.1186/1471-2393-10-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rao SC, Athalye-Jape GK, Deshpande GC Simmer KN, et al. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics 2016;137:1–16. 10.1542/peds.2015-3684 [DOI] [PubMed] [Google Scholar]
- 72. Sassone-Corsi M, Raffatellu M. No vacancy: how beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J Immunol 2015;194:4081–7. 10.4049/jimmunol.1403169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hammerman C, Kaplan M. Probiotics and neonatal intestinal infection. Curr Opin Infect Dis 2006;19:277–82. 10.1097/01.qco.0000224823.73223.29 [DOI] [PubMed] [Google Scholar]
- 74. Ashraf R, Shah NP. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr 2014;54:938–56. 10.1080/10408398.2011.619671 [DOI] [PubMed] [Google Scholar]
- 75. Hardy H, Harris J, Lyon E, et al. Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients 2013;5:1869–912. 10.3390/nu5061869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thaver D, Zaidi AK. Burden of neonatal infections in developing countries: a review of evidence from community-based studies. Pediatr Infect Dis J 2009;28:S3–9. 10.1097/INF.0b013e3181958755 [DOI] [PubMed] [Google Scholar]
- 77. Qazi SA, Stoll BJ. Neonatal sepsis: a major global public health challenge. Pediatr Infect Dis J 2009;28:S1–2. 10.1097/INF.0b013e31819587a9 [DOI] [PubMed] [Google Scholar]
- 78. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255–64. 10.1056/NEJMra1005408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mwansa-Kambafwile J, Cousens S, Hansen T, et al. Antenatal steroids in preterm labour for the prevention of neonatal deaths due to complications of preterm birth. Int J Epidemiol 2010;39:i122–33. 10.1093/ije/dyq029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lee AC, Katz J, Blencowe H, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health 2013;1:e26–36. 10.1016/S2214-109X(13)70006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sinha A, Gupta SS, Chellani H, et al. Role of probiotics VSL#3 in prevention of suspected sepsis in low birthweight infants in India: a randomised controlled trial. BMJ Open 2015;5:e006564 10.1136/bmjopen-2014-006564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Panigrahi P, Parida S, Nanda NC, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017;548:407–12. 10.1038/nature23480 [DOI] [PubMed] [Google Scholar]
- 83. ClinicalTrials.gov [Internet]. The Efficacy and Mechanisms of Oral Probiotics in Preventing Necrotizing Enterocolitis. Bethesda (MD): National Library of Medicine (US) 2000 Feb 29- Identifier NCT025552706, [about 4 screens], 2015. https://clinicaltrials.gov/ct2/show/record/NCT02552706?term=probiotics&recr=Open&rank=23 (accessed 21 Apr 2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-017638supp001.pdf (56KB, pdf)