Key Points
Three independent association signals at ADAMTS13 and smoking were identified as major predictors of plasma ADAMTS13 levels.
Evidence was presented that 2 nonsynonymous ADAMTS13 variants were driving the variation of plasma ADAMTS13 concentrations.
Abstract
The metalloprotease ADAMTS13 cleaves von Willebrand factor (VWF) in circulating blood, limiting the size of VWF multimers and regulating VWF activity. Abnormal regulation of VWF contributes to bleeding and to thrombotic disorders. ADAMTS13 levels in plasma are highly variable among healthy individuals, although the heritability and the genetic determinants of this variation are unclear. We performed genome-wide association studies of plasma ADAMTS13 concentrations in 3244 individuals from 2 independent cohorts of healthy individuals. The heritability of ADAMTS13 levels was between 59.1% (all individuals) and 83.5% (siblings only), whereas tobacco smoking was associated with a decrease in plasma ADAMTS13 levels. Meta-analysis identified common variants near the ADAMTS13 locus on chromosome 9q34.2 that were significantly associated with ADAMTS13 levels and collectively explained 20.0% of the variance. The top single nucleotide polymorphism (SNP), rs28673647, resides in an intron of ADAMTS13 (β, 6.7%; P = 1.3E-52). Conditional analysis revealed 3 additional independent signals represented by rs3739893 (β, −22.3%; P = 1.2E-30) and rs3124762 (β, 3.5%; P = 8.9E-9) close to ADAMTS13 and rs4075970 (β, 2.4%; P = 6.8E-9) on 21q22.3. Linkage analysis also identified the region around ADAMTS13 (9q34.2) as the top signal (LOD 3.5), consistent with our SNP association analyses. Two nonsynonymous ADAMTS13 variants in the top 2 independent linkage disequilibrium blocks (Q448E and A732V) were identified and characterized in vitro. This study uncovered specific common genetic polymorphisms that are key genetic determinants of the variation in plasma ADAMTS13 levels in healthy individuals.
Visual Abstract
Introduction
von Willebrand factor (VWF) plays a central role in hemostasis by tethering platelets to areas of endothelial injury and by serving as a carrier for coagulation factor VIII. As a large multimeric glycoprotein, VWF requires specialized assembly, posttranslational modification, and pH-dependent storage conformations to facilitate the regulated secretion of VWF into flowing blood.1 The mature VWF complex varies from dimers to concatamers of more than 250 monomers (ultralarge VWF), and the size of VWF is correlated with its prothrombotic activity in circulation.2 The homeostatic distribution of VWF multimer size is modulated by ADAMTS13, a metalloprotease that cleaves circulating VWF into smaller concatamers.3 Previous studies have reported loss of higher-molecular-weight multimers in the bleeding disorder von Willebrand disease type 2A, and conversely, an accumulation of ultralarge VWF monomers4 in thrombotic thrombocytopenic purpura,5 demonstrating the importance of the regulation of VWF activity by ADAMTS13. Recent reports have also linked lower ADAMTS13 plasma concentrations with an increased risk of ischemic stroke,6,7 coronary artery disease, and all-cause mortality.8,9 Unlike many proteases, ADAMTS13 is secreted in an active form and, to date, no specific inhibitor of ADAMTS13 has been identified. Recent reports have suggested that the regulation of ADAMTS13 activity is primarily allosteric and is dependent on specific exosite-substrate interactions.10 Therefore, ADAMTS13 activity is primarily dependent on its concentration in circulation and its ability to interact with VWF. In a healthy population, VWF levels vary up to fivefold and are determined by multiple environmental and genetic factors.11 ADAMTS13 activity levels seem to be less variable than VWF concentrations in healthy populations and decrease with age after 30 years.12 ADAMTS13 antigen levels exhibit less variability than VWF when measured serially.13 To date, the amount of variation in ADAMTS13 antigen levels determined by genetic factors is unknown. Recently, de Vries et al14 reported a genome-wide association study (GWAS) of ADAMTS13 activity toward a synthetic VWF substrate in an adult Dutch population, that uncovered an association of single nucleotide variants at the ADAMTS13 locus that explained ∼6% of the variance.
In this study, we measured ADAMTS13 antigen levels in 2 independent young healthy cohorts (n = 3244) and reported heritability, genetic associations, and the impact of smoking status. Consistent with the previously published GWAS of ADAMTS13 activity, our analyses identified a strong influence of variants at ADAMTS13, our study identified a new top association signal for ADAMTS13 concentrations, and stepwise conditional analyses demonstrated the presence of a previously unreported association signal on chromosome 21q2.3. In vitro analyses of 2 common nonsynonymous variants of ADAMTS13 support their causal role in plasma ADAMTS13 variation.
Materials and methods
Genes and Blood Clotting (GABC) study
A cohort of 1189 healthy siblings representing 507 sibships between 14 and 35 years old was collected between 2006 and 2009 at the University of Michigan (Ann Arbor, MI). Participants who reported that they were pregnant or had a known bleeding disorder or chronic illness requiring regular medical care were excluded. All participants gave informed consent before participating.15
Trinity Student Study (TSS)
Healthy Irish individuals age 18 to 28 years who attended Trinity College of the University of Dublin (Dublin, Ireland) were recruited between 2003 and 2004 for genetic analysis of nutrition and diet-related traits. Ethical approval was obtained from the Dublin Federated Hospitals Research Ethics Committee and was reviewed by the Office of Human Subjects Research at the US National Institutes of Health. Participants provided written informed consent before recruitment.11
ADAMTS13 phenotyping and data processing
ADAMTS13 levels were determined by AlphaLISA (Perkin-Elmer, Waltham, MA) from platelet-poor plasma for the 2 cohorts and cell culture media or lysates for the in vitro characterization. A polyclonal anti-ADAMTS13 antibody, raised in rabbit against a recombinant human ADAMTS13 variant with C1r-C1s urinary epidermal growth factor bone morphogenetic protein (CUB) domain deleted and recombinant murine full-length ADAMTS13 (9:1 ratio) (Open Biosystems, Huntsville, AL) was obtained from the laboratory of X. Long Zheng (University of Alabama at Birmingham, Birmingham, AL). Monoclonal anti-ADAMTS13 antibody (GMA-360) was purchased from Green Mountain Antibodies (Burlington, VT). This antibody pair was used in an ADAMTS13-specific AlphaLISA assay. ADAMTS13 antigen levels were calculated by using a standard dilution curve of standard plasma (FACT; George King Biomedical, Overland Park, KS). Each plasma sample was independently assayed at least 4 times. The mean replicate sample coefficients of variation were 2.15% (GABC) and 2.87% (TSS).
We noted a difference in the mean and median ADAMTS13 concentrations in the GABC and TSS samples. To detect a difference in assay performance between samples collected in acid citrate dextrose solution (GABC) and samples collected in EDTA (TSS), we measured ADAMTS13 levels from a single blood donor collected in citrate or EDTA with and without additions of 0.8 mM CaCl2 to the assay buffer. Consistent with the differences between the GABC and TSS samples, we noted a 20% reduction in the AlphaLISA signal from plasma collected in an EDTA-coated tube compared with the same plasma collected in a 10% acid citrate dextrose solution (Sigma, St. Louis, MO) that was not corrected by CaCl2 additions. Therefore, we mean-centered the distributions to allow for comparisons between the 2 cohorts.
Three GABC and 8 TSS participants did not have ADAMTS13 measurements and were excluded. The raw ADAMTS13 levels were log2-transformed and mean-centered within each cohort. Potential covariates, including age, sex, height, weight, body mass index, smoking status, and principal components (PCs) of genotype data were evaluated for their influence on ADAMTS13 levels by stepwise linear regression with forward selection. As a result, log-transformed ADAMTS13 levels were adjusted by smoking and PC1 in the GABC cohort, and by sex, smoking status, body mass index, and PC2 in the TSS cohort. After covariate adjustment, 9 outlier samples (defined as 3 times the standard deviation from mean) were removed in GABC, and 34 outliers were removed in TSS. We also separately analyzed the effect of smoking.
Genotyping and imputation
GABC and TSS samples were genotyped by using the Illumina HumanOmni1-Quad_v1-0_B array. Details of genotyping and the data-cleaning process have been previously published.11,16 Nine hundred forty of the 1152 GABC participants and all 2304 TSS participants had verified European ancestry.11,16 The final cleaned genotype data set contained 795 544 single nucleotide polymorphisms (SNPs) for 1152 GABC participants and 755 451 SNPs for 2304 TSS participants. After combining imputed genotypes from both cohorts,17 the final data set contained ∼5.82 million SNPs common to both cohorts. All the genotyping data from SNP arrays has been uploaded to dbGaP (https://www.ncbi.nlm.nih.gov/gap accession number phs000304.v2.p1).
Heritability estimation using SNP genotypes and pedigree data
The proportions of variance of adjusted ADAMTS13 levels explained by all genotyped SNPs or the top associated SNPs were estimated by using a restricted maximum likelihood method with Genome-Wide Complex Trait Analysis, v1.25 (GCTA).18 We used 557 sibships (1139 GABC and 138 TSS sibs) and also used 2 pedigree-based methods to estimate the narrow-sense heritability of ADAMTS13 levels: (1) intraclass correlation of sibpairs16 using the irr package19 in R20 and (2) pedigree-wide regression analysis using MERLIN-REGRESS (v1.1.2).21
Association analyses
SNP quantitative trait association analysis for the adjusted ADAMTS13 level was performed in the European subset of GABC (n = 940) and in TSS (n = 2304) using a variance component model implemented in EMMAX (Efficient Mixed-Model Association Expedited).22 The genome-wide significance level was set at P = 5 × 10−8 based on a Bonferroni correction for 1 million independent tests. To identify independent signals that could be masked by the top-associated SNPs, we performed stepwise conditional analysis using GCTA-COJO23 by adding the SNP with the strongest evidence of association as a new covariate iteratively until there was no significantly associated SNP.
Meta-analysis of TSS and GABC
Meta-analysis was carried out by using a fixed effect, sample size–weighted approach implemented in METAL24 using EMMAX association results for European GABC and TSS from a common set of ∼5.82 million SNPs. The genomic control factor25 was 0.991 for GABC and 0.995 for TSS for single-marker test results in EMMAX. Regional plots of top-associated SNPs were generated by LocusZoom.26 SNP annotation was obtained from SNPnexus.27 Damaging status of nonsynonymous coding variants was predicted by PolyPhen-2 (Polymorphism Phenotyping v2).28
Linkage analysis using GABC and TSS sibling data sets
Linkage analyses were performed by MERLIN-REGRESS, v1.1.221 for the 557 sibships using 35 356 linkage disequilibrium (LD) clusters as previously described.11
Recombinant ADAMTS13
ADAMTS13 variants Q448E and A732V were synthesized by site-directed mutagenesis of a stable mammalian expression vector containing wild-type ADAMTS13 complementary DNA (cDNA). Vector sequence was generated by amplification of pDEST-pcDNA5-BirA-FLAG C-term29 in which BirA and FLAG tags were excluded in the final Gibson assembly. Plasmids were fully sequenced to ensure that no polymerase chain reaction–generated mutations were present. Plasmids were transfected into flip-in T-REx-293 cells by using Lipofectamine-2000 (Life Technologies, Carlsbad CA), and drug-resistant cells were selected with hygromycin and maintained with 5 μg/mL blasticidin. To compare synthesis and secretion rates, cells were plated into Dulbecco’s modified Eagle medium media with 10% fetal bovine serum at a cell density of 5 × 105 cells per well and incubated at 37°C in 5% CO2. Conditioned media was collected after 48-hour induction with 1 μg/mL tetracycline and clarified by centrifugation, and cells were washed in phosphate-buffered saline and harvested to obtain cell lysates. Western blots using mouse anti-ADAMTS13 antibodies (Green Mountain Antibodies) confirmed synthesis of all wild-type and ADAMTS13 variants at expected molecular weights. Antigen levels were determined by AlphaLISA as described above, substituting conditioned media and cell lysates for plasma. As an estimate of cell numbers per well, levels of glyceraldehyde-3-phosphate dehydrogenase were determined according to the AlphaLISA kit protocol (Perkin-Elmer). Activity of the recombinant ADAMTS13 variants was determined by FRETS-VWF73 assays according to the manufacturer’s specifications (Peptides International, Louisville, KY) with the following modifications: 25 µL recombinant wild-type or variant ADAMTS13 in serum-free conditioned OptiMEM media (Life Technologies, Carlsbad, CA) was used as the enzyme source at reaction concentrations of 0.1 to 3.2 nM and a volume of 25 µL. FRETS-VWF73 substrate was used at final concentrations of 0.125 to 4.0 µM for a total reaction volume of 50 µL. We measured signal generation over 60 minutes at varying enzyme and substrate concentrations to calculate specific activity by using a Molecular Devices Spectramax M2 plate reader. Differences in synthesis and expression among the ADAMTS13 variants were tested by using Mann-Whitney U tests (Prism 7; GraphPad Software, San Diego, CA).
Results
Study cohorts and ADAMTS13 antigen levels
The demographic characteristics and raw ADAMTS13 antigen levels of the GABC and TSS cohorts are summarized in Table 1. The 5th and 95th percentiles of raw ADAMTS13 levels spanned a 2.0-fold range for GABC (81.8-165.6 IU/dL) and a 1.8-fold range for TSS (56.0-99.6 IU/dL). There was no significant difference in the distribution of mean-centered log-transformed ADAMTS13 levels between GABC and TSS (Kolmogorov-Smirnov test, P = .06; Mann-Whitney U test, P = .61; supplemental Figure 1). Mean-centered levels were adjusted for covariates as described in “Materials and methods.”
Table 1.
Characteristics of study cohorts
| Cohort | GABC | TSS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sibship size (no. of sibships) | n/N | % | Q1 | Q3 | Sibship size (no. of sibships) | n/N | % | Q1 | Q3 | |
| No. of participants | 1152 | 2304 | ||||||||
| Age, y | 21 | 19 | 23 | 22 | 21 | 24 | ||||
| Females | 721 | 62.6 | 1353 | 58.7 | ||||||
| Weight, lbs (kg) | 145 (65.8) | 125 (56.7) | 165 (74.8) | 148 (67.1) | 133 (60.3) | 167 (75.7) | ||||
| Height, inches (cm) | 67 (170) | 64 (163) | 70 (178) | 68 (173) | 65 (165) | 71 (180) | ||||
| BMI, kg/m2 | 22.5 | 20.7 | 25.0 | 22.6 | 21.0 | 24.5 | ||||
| Current smoker | 53/1151* | 4.6 | 723/2299 | 31.4 | ||||||
| Sibships | 2 (366) 3 (94) 4 (22) 5 (5) 6 (2) |
2 (66) 3 (2) |
||||||||
| ADAMTS13, IU/dL | 115.4* | 101.8 | 131.6 | 76.3* | 68.0 | 85.2 | ||||
Values are medians for age, height, weight, body mass index (BMI), and raw ADAMTS13 levels.
N or n, number of individuals in the indicated study cohort(s); Q1, first quartile; Q3, third quartile.
Previously unreported data. All other tabulated data are adapted from Ma et al.14
ADAMTS13 levels are highly heritable
The narrow-sense heritability (h2) of the ADAMTS13 antigen levels derived from all SNP genotyping data from GABC and TSS combined was 59.1% using GCTA. The upper bound of h2 in the sibling subset of GABC and TSS combined was 83.5% estimated by interclass correlation, which was consistent with the pedigree-based estimate of 79.5% by MERLIN-REGRESS (supplemental Table 1).
Linkage analysis for ADAMTS13 in 1139 GABC and 138 TSS sibs
Linkage analysis for ADAMTS13 in 489 sibships (1139 individuals) from GABC and 68 sibships (138 individuals) from TSS identified 1 independent region of linkage with LOD score greater than 3. The highest signal was at ∼136 Mb onchromosome 9q34.2, close to the ADAMTS13 gene (LOD, 3.5; MERLIN-REGRESS P = 2.9E-5; simulation-based empirical P = .10) (supplemental Figure 2). No other independent regions of linkage were identified.
Variants at ADAMTS13 are associated with ADAMTS13 levels
GWAS of the European subset of GABC (n = 940) revealed 46 significantly associated SNPs for ADAMTS13 levels (P < 5.0E-8) in an additive model (supplemental Figure 3A). All 46 SNPs resided in a 200-kb region on 9q34.2 surrounding the ADAMTS13 gene (supplemental Table 2). The Q-Q plot of the observed vs expected –log10(P) demonstrated a deviation from expectation, entirely because of the significant signals on 9q34.2 (supplemental Figure 3B). The minor allele G of the top SNP, rs28673647, was associated with ADAMTS13 with a β coefficient of 6.9% ± 1.0% (minor allele frequency [MAF], 0.36; P = 8.1E-13), equivalent to a 6.9% increase in ADAMTS13 level per allele (supplemental Figure 3C).
GWAS of TSS revealed 162 significantly associated SNPs for ADAMTS13 residing in the same 200-kb region on 9q34.2 containing ADAMTS13 (supplemental Table 3; supplemental Figure 4A-B). The top SNP in GABC, rs28673647, was associated with a 6.6% increase in ADAMTS13 level in TSS (MAF, 0.39; P = 9.3E-42). The top SNP in TSS, rs28680325, had a β of 6.7% (MAF, 0.38; P = 1.3E-42) (supplemental Figure 4C). The top SNPs discovered in TSS showed similar allelic effect sizes and directions (r, 0.98; P < 2.2E-16) as well as P values (r, 0.92; P < 2.2E-16) as in GABC and vice versa (supplemental Figure 5).
Meta-analysis in GABC and TSS confirms ADAMTS13 signals
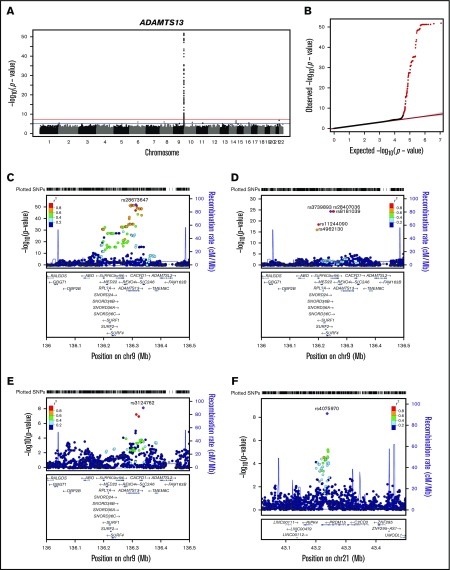
To increase statistical power, a meta-analysis of the GABC and TSS cohorts using a common set of ∼5.82 million SNPs was performed, revealing 153 SNPs significantly associated with ADAMTS13 levels (P < 5.0E-8) (Figure 1A-B; supplemental Table 4). These SNPs collectively explained 20.0% ± 5.9% of ADAMTS13 level variation in the combined GABC and TSS data. All significant SNPs were close to the ADAMTS13 gene on 9q34.2.
Figure 1.
Meta-analysis of ADAMTS13 levels in GABC and TSS. (A) Genome-wide plot of –log10(P) for ∼5.82 million SNPs. The red line marks the 5.0E-8 threshold of genome-wide significance. (B) Quantile-quantile plot of observed vs expected –log10(P) for ADAMTS13 meta-analysis. The observed P < 5.0E-8 are shown in red. (C) Regional plot for the associated region near ADAMTS13 on Chr9. (D) Regional plot for ADAMTS13 and STKLD1 (C9orf96) genes on chromosome 9 (chr9) conditioned by the top SNP rs28673647 from meta-analysis. (E) Regional plot for ADAMTS13 conditioned by the top 2 independent SNPs, rs28673647 and rs3739893. (F) Regional plot for PRDM15 on chr21 when using the top 2 independent SNPs, rs28673647 and rs3739893, as covariates.
Stepwise conditional analysis in GABC and TSS combined was performed to identify other association signals masked by the strong primary signal on 9q34.2. In conditional analysis, 3 independent signals on 9q34.2 represented by top SNPs rs28673647 (MAF, 0.38; β, 7.1% ± 0.5%; P = 1.2E-57), rs3739893 (β, −22.3% ± 1.8%; P = 1.2E-30), and a borderline-significant SNP rs3124762 (β, 3.5% ± 0.6%; P = 8.9E-9) were uncovered (Table 2; Figure 1C-E). The second independent SNP on 9q34.2, rs3739893 (MAF, 1.6%) was located in an intron of the STKLD1 (C9orf96) gene and had a larger effect size (β, −15.6% in GABC and −23.3% in TSS) than that of the top SNP, rs28673647. An additional signal in an intron of PRDM15 on 21q22.3 represented by the SNP rs4075970 (MAF, 0.45; β, 2.4% ± 0.4%; P = 6.8E-9) was masked by the first 2 independent 9q34.2 signals but revealed by conditional analysis (Table 2; Figure 1F). The individual genotypes of these 4 SNPs decreased ADAMTS13 levels independently (supplemental Figure 6), which supports the findings in the conditional analyses.
Table 2.
Significant SNPs in conditional meta-analysis in individual cohorts
| CHR | GABC + TSS (N = 3238) | GABC (n = 934) | TSS (n = 2304) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | MAF | βJ (%) | SE (%) | PJ | MAF | β (%) | SE (%) | P | MAF | β (%) | SE (%) | P | |
| 9q34.2 | rs28673647 | 0.38 | 7.1 | 0.5 | 1.2E-57 | 0.36 | 6.9 | 1.0 | 8.1E-13 | 0.39 | 6.6 | 0.5 | 9.3E-42 |
| rs3739893 | 0.02 | −22.3 | 1.8 | 1.2E-30 | 0.01 | −15.6 | 4.7 | 1.7E-3 | 0.02 | −23.3 | 1.9 | 2.0E-28 | |
| 21q22.3 | rs4075970 | 0.45 | 2.4 | 0.4 | 6.8E-9 | 0.48 | 5.0 | 1.0 | 1.4E-7 | 0.44 | 1.4 | 0.5 | 4.6E-03 |
| 9q34.2 | rs3124762 | 0.16 | 3.5 | 0.6 | 8.9E-9 | 0.15 | −1.2 | 1.4 | .38 | 0.17 | 0.9 | 0.7 | .18 |
β, percentage change per allele; CHR, chromosome; J, joint effects of independently associated SNPs; N or n, number of individuals in the indicated study cohort(s); SE, standard error.
Functional annotation of associated variants in 9q24.3
There were 153 SNPs in the 9q24.3 region associated with ADAMTS13 antigen levels identified through meta-analysis. To identify causal variants, we looked for nonsynonymous variants in ADAMTS13 or in SNPs at cis expression quantitative loci (eQTLs) that were in LD with the top independent association signals. Of the 153 significant SNPs, we did not identify eQTL SNPs in our analysis, which used 2 different eQTL browsers,30,31 but we did detect 2 missense SNPs in ADAMTS13 that were associated with ADAMTS13 levels. The top missense variant, rs2301612, encoding Q448E, was in high LD with the top meta-analysis SNP rs28673647 (r2, 0.74) and was associated with a 5.4% increase (P = 4.9E-36) in ADAMTS13 levels. The second strongest missense SNP (rs41314453 encoding A732V) was in high LD with the top conditional analysis SNP (rs3739893; r2, 0.84) and was associated with a 26.5% decrease (P = 2.3E-34) in ADAMTS13 levels. Variants encoding Q448E and A732V were not in LD with each other (r2, 0.02).
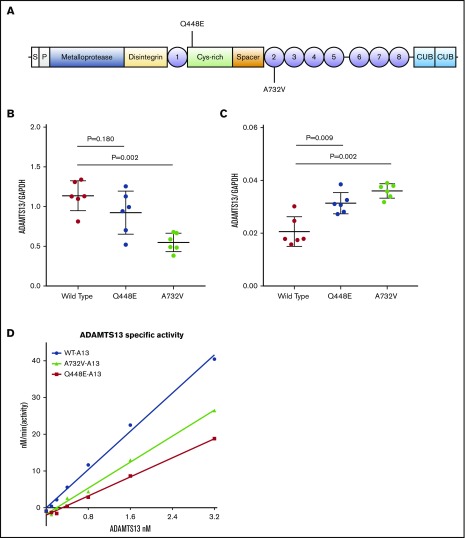
The Q448E amino acid substitution occurs in the cysteine-rich domain of ADAMTS13, whereas A732V alters the second thrombospondin-1 repeat domain (Figure 2A). We hypothesized that these missense variants were driving the direction of associations in the top 2 LD blocks, so we compared the synthesis and secretion of wild-type ADAMTS13 to Q448E and A732V mutants in vitro. We generated stable HEK293 Flp-In T-REx cell lines expressing these 3 forms of ADAMTS13 and measured the concentration of ADAMTS13 (μg/μL) in conditioned media and cell lysates, adjusting concentrations for the estimated number of cells per well with glyceraldehyde-3-phosphate dehydrogenase (μg/μL) levels from cell lysates. This cell culture system, in which a transposase is used to insert cDNA into a specific genomic locus, facilitated equally controlled transgene expression under a tet promotor and allowed for a more precise quantification of the differences in synthesis and secretion due only to the missense variants. Mean media concentrations of adjusted ADAMTS13 were highest in wells plated with wild-type ADAMTS13 and Q448E-producing cells (1.14 and 0.92, respectively; P = .180). Cell lysates from Q448E variants had higher concentrations compared with wild-type cells (0.03 and 0.02 respectively, P = .009) (Figure 2B-C). Conditioned media from cells expressing A732V had lower adjusted ADAMTS13 concentrations compared with wild-type media (0.55 and 1.14, respectively; P = .002) and higher concentrations in cell lysates compared with wild-type (0.04 and 0.02, respectively; P = .009) (Figure 2B-C). Specific activity was 13.0 ± 0.44 nM/min/nM for wild-type, 6.492 ± 0.23 nM/min/nM for Q448E, and 8.849 ± 0.30 nM/min/nM for A732V (Figure 2D).
Figure 2.
Functional tests of ADAMTS13 variant expression secretion and activity. (A) Cell lines expressing wild-type, Q448E, and A732V ADAMTS13 were generated by using an FLP-recombinase system in 293 cells. Q448E is rs2301612 and A732V is rs41314453. (B-C) Concentrations of ADAMTS13 in (B) conditioned media and (C) cell lysate were measured by using AlphaLISA and adjusted by cell lysate glyceraldehyde-3-phosphate dehydrogenase levels to account for variations in cell number. Mean and standard deviations are plotted as well as individual values. (D) Recombinant enzyme-specific activity was measured with FRETS-VWF73 substrate. Differences in group levels were determined by the Mann Whitney U test. 1-8, thrombospondin type 1 repeats; CUB, C1r-C1s urinary epidermal growth factor bone morphogenetic protein domains; Cys-rich, cysteine-rich domain; P, propeptide; S, signal peptide.
Focused analysis with ABO blood group serotypes
The ABO gene is located ∼200 kb 5′ to the ADAMTS13 locus. The ABO blood group serotypes (A1, A2, B, and O) were estimated by using haplotypes tagged by 3 ABO SNPs: rs8176749, rs8176704, and rs687289.32 Meta-analysis identified 15 SNPs 5′-upstream of ABO and 0 SNPs within ABO significantly associated with ADAMTS13 levels. None of the 3 ABO-serotype SNPs were in high LD with the 15 ABO 5′-upstream SNPs (r2, <0.2 in both GABC and TSS). Although several SNP haploblocks in ABO were associated with ADAMTS13 levels, ABO-serotype tagging alleles were not significantly associated with ADAMTS13 levels (minimum P = 4.14E-5 for A1 haplotype tagging SNP) (supplemental Table 5), suggesting that the associated SNPs upstream of ABO are in LD with functional SNPs in ADAMTS13 and not independently associated with ADAMTS13 levels. This is consistent with the minimal correlation between VWF levels, which are highly influenced by ABO serotypes, and ADAMTS13 levels (r, 0.04; P = .02) in the GABC and TSS cohorts (supplemental Figure 7). In addition, previous reports demonstrated no association between ADAMTS13 antigen levels33 or activity12 and ABO blood groups.
Smoking is associated with decreased ADAMTS13 levels
As a complex genetic trait, plasma ADAMTS13 concentrations are affected by both genetic and environmental factors such as tobacco smoking. ADAMTS13 levels were significantly lower for smokers compared with nonsmokers in both GABC (β, 8.5% ± 2.8%; P = 2.6E-3) and TSS (β, 3.3% ± 0.74%; P = 8.3E-6), despite the much smaller fraction of smokers in GABC (4.6%) than in TSS (31.4%) (supplemental Figure 8A). To explore gene-smoking interactions, we examined ADAMTS13 levels in current smokers and nonsmokers stratified by using the top 2 independent genetic signals at the ADAMTS13 locus (rs28673647 and rs3739893). ADAMTS13 levels in GABC and TSS combined were decreased by the GA and AA genotypes of the top SNP (rs28673647) and the TC genotype of rs3739893, as well as by smoking status (supplemental Figure 8B). The influence of smoking was observed in each stratum of rs28673647 and rs3739893 genotypes, demonstrating that the effect of smoking on ADAMTS13 antigen level is additive to and independent of the genetic effects.
Discussion
Plasma ADAMTS13 levels vary twofold in healthy populations33 but are tightly regulated within narrow ranges at the individual level. In this study, the estimated heritability of ADAMTS13 antigen levels was between 59.1% and 83.5%, suggesting that the majority of the population variance of plasma ADAMTS13 concentrations is the result of genetic factors. Even our lower-bound heritability estimates were higher than the lower-bound estimate of 35.2% reported by de Vries et al.14 This difference may have been a result of the younger age and the lack of age-related vascular diseases in our cohorts or the difference between the measurements of antigen levels in our study vs activity in de Vries et al. The reduction in the influence of environmental factors to ADAMTS13 variability may have also explained why our study accounted for a higher percentage of the variability of plasma ADAMTS13 by common genetic variants (∼20% in our study vs ∼6.0% in de Vries et al).
Tobacco smoking is known to be procoagulant and has a pleiotropic effect on multiple coagulation factors.34,35 Our results in European-ancestry cohorts demonstrated that smoking exposure was associated with a 3.6% decrease in ADAMTS13 levels. This finding is consistent with a previous study in Arab males that observed lower ADAMTS13 antigen levels in smokers.36 The effect of smoking on ADAMTS13 levels was independent of the genetic effects of significant signals at the ADAMTS13 locus. To date the correlation between ADAMTS13 and smoking has not been examined except in extreme deficiencies of ADAMTS13, such as those observed in patients with acute acquired or congenital thrombotic thrombocytopenic purpura.37
A genome-wide study has been conducted for plasma ADAMTS13 activity14 but not for ADAMTS13 protein concentrations. Although we expected these 2 traits to be highly correlated and identified similar associations, our top signal was not detected in the GWAS for ADAMTS13 activity. As a comparison, de Vries et al14 reported 4 common SNPs and 3 rare SNPs associated with ADAMTS13 activity at the ADAMTS13 locus. The top common SNP in their analyses, rs41314453, was in strong LD with the second strongest signal in our study. The second strongest SNP in the de Vries study was rs3118667, and it was also significantly associated with ADAMTS13 levels in our meta-analysis (β, 5.3%; P = 2.64E-36). Interestingly, our top SNP association, rs28673647, which is in high LD with the variant Q448E, was not reported in the de Vries et al study. The high minor allele frequency of this SNP (38%) indicated that the de Vries et al study was well powered to detect an association with rs28673647 and ADAMTS13 activity if it was present. The strong association with antigen levels and lack of association with activity suggests that the increased antigen levels must be balanced by decreased specific activity in vivo. Our experimental results are consistent with this finding with equivocal media levels of Q448E and decreased median activity of Q448E compared with wild-type.
When considering rare variants of 3 significant rare ADAMTS13 SNPs reported by de Vries et al, 2 SNPs had an MAF of 0% in GABC and the other, rs142572218, had an MAF of 0.05% in GABC with an association P = .72 compared with their MAF of 0.06% with P = 1.8E-5. The lack of replication in our study with these rare SNPs may have been secondary to reduced power in our study or to a more predominant effect of these rare variants on ADAMTS13 activity compared with plasma concentration. Outside the ADAMTS13 locus, 2 common SNPs in the SUPT3H and OBP2B genes, reported as significant in the de Vries study, were not significantly associated with ADAMTS13 antigen levels in our study (P = .44 and P = 0.75, respectively). In fact, only 1 SNP outside the ADAMTS13 locus was associated with a change in ADAMTS13 levels in our study: rs4075970 in the PRDM15 gene on chromosome 21q22. This signal has not been previously reported. PRDM15 encodes a transcription factor with zinc fingers that may play a role in the regulated expression of ADAMTS13. Experimental chromatin immunoprecipitation sequencing data from the Gene Transcription Regulation Database38 reveals that multiple transcription factors, including those with zinc fingers, bind to cis regulatory elements at ADAMTS13. However, this database had no direct evidence that the PRDM family transcription factor binds to ADAMTS13.
To more deeply understand the mechanisms of genetic control of complex phenotypes, GWASs would ideally lead to the identification of causal genetic variants. Our study used imputation to increase the number of variants tested and identified 2 missense variants in LD with the top 2 independent association signals at the ADAMTS13 locus. Although other unidentified regulatory elements near ADAMTS13 likely play additional roles in the genetic control of plasma ADAMTS13 levels, these 2 nonsynonymous mutations are strong candidate causal variants. Indeed, when expressed in cell culture, the A732V form of ADAMTS13 had decreased levels in the conditioned media and increased levels in cell lysate consistent with decreased secretion compared with wild-type. The Q448E variant of ADAMTS13 had equivalent concentrations in conditioned media compared with wild-type despite increased levels in the cell lysate, suggesting that the association with a modest increase in plasma ADAMTS13 may be the result of a combination of higher synthesis rates and/or decreased clearance rates in vivo (not tested). These 2 common ADAMTS13 variants have been examined in 2 previous studies. One study examined expression and secretion of Q448E in cell culture and found results similar to those of our study but an increased specific activity of this variant compared with wild-type,39 and another article examined both the Q448E and A732V variants expressed in cell culture and found similar decreases in secretion into culture media and ∼25% reduction in enzyme activity as measured by a collagen binding assay.40 However, both previous studies used transient transfection methods which may have altered patterns of synthesis and secretion compared with our experiments in cell lines with stable expression from the same locus.
In summary, this article described the results from genome-wide association and linkage studies of ADAMTS13 levels in 2 healthy young cohorts. We identified 153 SNPs at the ADAMTS13 locus that collectively explained 20.0% of ADAMTS13-level variation. We also identified and tested 2 common nonsynonymous ADAMTS13 variants with altered synthesis and/or secretion rates compared with reference ADAMTS13. Taken together, these findings suggest that common variants in ADAMTS13 are the major common genetic determinants of plasma ADAMTS13 levels and may be primarily driven by differences in secretion by 2 nonsynonymous variants on independent haplotypes.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the participants of the Genes and Blood Clotting study and the Trinity Student Study (TSS) for their contributions to this work; Lawrence Brody, James Mills, and Anne Molloy for their collaboration with the TSS; and X. Long Zheng for providing the polyclonal anti-ADAMTS13 antibodies.
This work was supported by the Intramural Research Programs of the National Human Genome Research Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Trinity Student Study); by grants R35HL135793 (K.C.D., A.B.O., and D.G.) and R01HL112642 (D.G., J.Z.L., A.B.O., and K.C.D.) from the National Institutes of Health, National Heart, Lung, and Blood Institute; by the Charles Woodson Accelerator grant at the University of Michigan (K.C.D. and P.M.J.); and by funds from the Center for Biologics Evaluation and Research, US Food and Drug Administration. D.G. is an investigator at the Howard Hughes Medical Institute.
Authorship
Contribution: Q.M. designed the research, analyzed the data, created tables and figures, and wrote the article; P.M.J., B.T.E., and B.M. performed the experiments; C.A.K. designed the activity experiments and analyzed the results; A.B.O. analyzed the data; C.K.-S. designed the research and performed the experiments; D.G. and J.Z.L. designed the research and wrote the article; and K.C.D. designed the research, performed the experiments, analyzed the data, created figures and tables, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karl C. Desch, Department of Pediatrics and Communicable Diseases, University of Michigan, 8-621 C&W Mott Hospital, SPC 4254, 1540 Hospital Dr, Ann Arbor, MI 48109; e-mail: kdesch@med.umich.edu.
References
- 1.Sadler JE. von Willebrand factor assembly and secretion. J Thromb Haemost. 2009;7(Suppl 1):24-27. [DOI] [PubMed] [Google Scholar]
- 2.Springer TA. Biology and physics of von Willebrand factor concatamers. J Thromb Haemost. 2011;9(Suppl 1):130-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong JF, Moake JL, Nolasco L, et al. . ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100(12):4033-4039. [DOI] [PubMed] [Google Scholar]
- 4.Moake JL, Rudy CK, Troll JH, et al. . Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;307(23):1432-1435. [DOI] [PubMed] [Google Scholar]
- 5.Budde U, Schneppenheim R. Interactions of von Willebrand factor and ADAMTS13 in von Willebrand disease and thrombotic thrombocytopenic purpura. Hamostaseologie. 2014;34(3):215-225. [DOI] [PubMed] [Google Scholar]
- 6.Sonneveld MA, de Maat MP, Portegies ML, et al. . Low ADAMTS13 activity is associated with an increased risk of ischemic stroke. Blood. 2015;126(25):2739-2746. [DOI] [PubMed] [Google Scholar]
- 7.Stoll M, Rühle F, Witten A, et al. . Rare Variants in the ADAMTS13 Von Willebrand Factor-Binding Domain Contribute to Pediatric Stroke. Circ Cardiovasc Genet. 2016;9(4):357-367. [DOI] [PubMed] [Google Scholar]
- 8.Bongers TN, de Bruijne EL, Dippel DW, et al. . Lower levels of ADAMTS13 are associated with cardiovascular disease in young patients. Atherosclerosis. 2009;207(1):250-254. [DOI] [PubMed] [Google Scholar]
- 9.Sonneveld MA, Franco OH, Ikram MA, et al. . Von Willebrand Factor, ADAMTS13, and the Risk of Mortality: The Rotterdam Study. Arterioscler Thromb Vasc Biol. 2016;36(12):2446-2451. [DOI] [PubMed] [Google Scholar]
- 10.Muia J, Zhu J, Gupta G, et al. . Allosteric activation of ADAMTS13 by von Willebrand factor. Proc Natl Acad Sci USA. 2014;111(52):18584-18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desch KC, Ozel AB, Siemieniak D, et al. . Linkage analysis identifies a locus for plasma von Willebrand factor undetected by genome-wide association. Proc Natl Acad Sci USA. 2013;110(2):588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokame K, Sakata T, Kokubo Y, Miyata T. von Willebrand factor-to-ADAMTS13 ratio increases with age in a Japanese population. J Thromb Haemost. 2011;9(7):1426-1428. [DOI] [PubMed] [Google Scholar]
- 13.Kilercik M, Coskun A, Serteser M, Inan D, Unsal I. Biological variations of ADAMTS13 and von Willebrand factor in human adults. Biochem Med (Zagreb). 2014;24(1):138-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vries PS, Boender J, Sonneveld MA, et al. . Genetic variants in the ADAMTS13 and SUPT3H genes are associated with ADAMTS13 activity. Blood. 2015;125(25):3949-3955. [DOI] [PubMed] [Google Scholar]
- 15.Desch K, Li J, Kim S, et al. . Analysis of informed consent document utilization in a minimal-risk genetic study. Ann Intern Med. 2011;155(5):316-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Q, Ozel AB, Ramdas S, et al. . Genetic variants in PLG, LPA, and SIGLEC 14 as well as smoking contribute to plasma plasminogen levels. Blood. 2014;124(20):3155-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozel AB, McGee B, Siemieniak D, et al. . Genome-wide studies of von Willebrand factor propeptide identify loci contributing to variation in propeptide levels and von Willebrand factor clearance. J Thromb Haemost. 2016;14(9):1888-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamer M, Lemon J, Fellows I, Singh P. irr: Various Coefficients of Interrater Reliability and Agreement. 2012. https://cran.r-project.org/web/packages/irr/irr.pdf.
- 20.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015 [Google Scholar]
- 21.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30(1):97-101. [DOI] [PubMed] [Google Scholar]
- 22.Kang HM, Sul JH, Service SK, et al. . Variance component model to account for sample structure in genome-wide association studies. Nat Genet. 2010;42(4):348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Ferreira T, Morris AP, et al. . Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44(4):369-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devlin B, Roeder K, Wasserman L. Genomic control, a new approach to genetic-based association studies. Theor Popul Biol. 2001;60(3):155-166. [DOI] [PubMed] [Google Scholar]
- 26.Pruim RJ, Welch RP, Sanna S, et al. . LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26(18):2336-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chelala C, Khan A, Lemoine NR. SNPnexus: a web database for functional annotation of newly discovered and public domain single nucleotide polymorphisms. Bioinformatics. 2009;25(5):655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adzhubei IA, Schmidt S, Peshkin L, et al. . A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couzens AL, Knight JD, Kean MJ, et al. . Protein interaction network of the mammalian Hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci Signal. 2013;6(302):rs15. [DOI] [PubMed] [Google Scholar]
- 30.Pitchard JK. eQTL browser. 2013. http://eqtl.uchicago.edu/cgi-bin/gbrowse/eqtl/. Accessed 6 June 2017. [Google Scholar]
- 31.Westra HJ, Peters MJ, Esko T, et al. . Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45(10):1238-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbalic M, Dupuis J, Dehghan A, et al. . Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19(9):1863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chion CK, Doggen CJ, Crawley JT, Lane DA, Rosendaal FR. ADAMTS13 and von Willebrand factor and the risk of myocardial infarction in men. Blood. 2007;109(5):1998-2000. [DOI] [PubMed] [Google Scholar]
- 34.Semlali A, Witoled C, Alanazi M, Rouabhia M. Whole cigarette smoke increased the expression of TLRs, HBDs, and proinflammory cytokines by human gingival epithelial cells through different signaling pathways. PLoS One. 2012;7(12):e52614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barua RS, Ambrose JA. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler Thromb Vasc Biol. 2013;33(7):1460-1467. [DOI] [PubMed] [Google Scholar]
- 36.Al-Awadhi AM, Jadaon MM, Alsayegh FA, Al-Sharrah SK. Smoking, von Willebrand factor and ADAMTS-13 in healthy males. Scand J Clin Lab Invest. 2012;72(8):614-618. [DOI] [PubMed] [Google Scholar]
- 37.Balasubramaniyam N, Kolte D, Palaniswamy C, et al. . Predictors of in-hospital mortality and acute myocardial infarction in thrombotic thrombocytopenic purpura. Am J Med. 2013;126(11):1016.e1-1016.e7. [DOI] [PubMed] [Google Scholar]
- 38.Yevshin I, Sharipov R, Valeev T, Kel A, Kolpakov F. GTRD: a database of transcription factor binding sites identified by ChIP-seq experiments. Nucleic Acids Res. 2016;45(D1):D61-D67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards NC, Hing ZA, Perry A, et al. . Characterization of coding synonymous and non-synonymous variants in ADAMTS13 using ex vivo and in silico approaches. PLoS One. 2012;7(6):e38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plaimauer B, Fuhrmann J, Mohr G, et al. . Modulation of ADAMTS13 secretion and specific activity by a combination of common amino acid polymorphisms and a missense mutation. Blood. 2006;107(1):118-125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.