Key Points
Haplo-Cord is an effective strategy to quicken neutrophil and platelet recovery.
In specific treatment platforms, sUCBT and Haplo-Cord offer similar long-term outcomes.
Abstract
We retrospectively compared the clinical outcomes of adults with acute leukemia who received single-unit umbilical cord blood (UCB) transplantation (sUCBT) (n = 135) or stem cell transplant using coinfusion of a UCB graft with CD34+ cells from a third-party donor (Haplo-Cord) (n = 72) at different institutions within the Grupo Español de Trasplante Hematopoyético. In multivariable analysis, patients in the Haplo-Cord group showed more rapid neutrophil (hazard ratio [HR], 2.3; 95% confidence interval [CI], 1.5-3.3; P < .001) and platelet recovery (HR, 1.6; 95% CI, 1.2-2.3; P = .015) and lower incidence of chronic graft-versus-host disease (GVHD) (relative risk, 0.5; 95% CI, 0.3-0.8; P = .01). Nonrelapse mortality, relapse, disease-free survival (DFS), and GVHD/relapse-free survival were similar in the 2 groups. Regarding disease-specific outcomes, DFS in both acute myeloid leukemia (AML) and acute lymphoblastic leukemia patients was not significantly different; however, a significantly higher relapse rate was found in patients with AML treated with Haplo-Cord (HR, 2.3; 95% CI, 1-5.4; P = .04). Our study confirms that Haplo-Cord was an effective strategy to accelerate neutrophil and platelet recovery and shows that, in the context of specific treatment platforms, sUCBT and Haplo-Cord offer similar long-term outcomes.
Visual Abstract
Introduction
Umbilical cord blood (UCB) transplantation (UCBT) offers curative potential for patients with high-risk acute leukemia in need of allogeneic stem cell transplantation (SCT). However, a major drawback of the procedure is the fear of delayed neutrophil recovery due to the low progenitor cell content of the graft, especially in adults.
Clinical research has focused on enhancing engraftment in an effort to improve outcomes and expand the use of UCBT, mainly with infusion of double cord blood (CB) units1 and ex vivo expansion.2 The Hospital Puerta de Hierro group of Madrid pioneered the coinfusion of a UCB graft with CD34+ cells from a third-party donor (TPD), so-called Haplo-Cord stem cell transplant (Haplo-Cord). This approach elegantly showed that fast neutrophil recovery originated from the third-party graft, which was subsequently substituted by cord blood–derived hematopoiesis.3-5 The procedure has been shown to be feasible and reproducible,6 but it has not yet been compared with single-unit UCBT (sUCBT).
The aim of this study was to compare retrospectively the clinical outcomes of adults with acute leukemia undergoing Haplo-Cord or sUCBT within the Grupo Español de Trasplante Hematopoyético (GETH).
Patients and methods
Eligibility criteria
Eligibility criteria were: (1) adult patients over 15 years of age; (2) diagnosis of acute leukemia; (3) first allogeneic SCT; (4) myeloablative conditioning regimen. All consecutive patients transplanted from January 2005 until December 2012 were included in the study. The Haplo-Cord platform was carried out at 3 institutions. The sUCBT strategy was performed at 9 institutions participating in 2 subsequent prospective trials: GETH-2005 and GETH/Gruppo Italiano Trapianto Midollo Osseo (GITMO)-2008 (EudraCT with code 2008-000927-24).
Informed consent was obtained from all patients. Each center’s institutional review boards approved treatment protocols according to the Declaration of Helsinki.
Transplant procedures
UCB unit selection, conditioning regimen, immune suppression, and supportive care have been previously reported in detail for both the sUCBT7,8 and Haplo-Cord platforms,9,10 and are summarized in the following sections. All patients received granulocyte colony-stimulating factor (G-CSF), starting on day +1 and +7 in the Haplo-Cord and sUCBT platform, respectively, until neutrophil recovery.
UCB unit selection
The graft selection algorithm required that UCB units be ≥4 of 6 HLA matched with the recipient (HLA class I antigens [A and B] considering the antigen level and class II antigen [DRB1] considering allele-level resolution DNA typing).
Single-unit platform.
In the GETH-2005 protocol, the following cell doses were required: total nucleated cells (TNCs) > 2 × 107/kg and CD34+ cells > 0.6 × 105/kg or TNCs > 1.5 × 107/kg and CD34+ cells > 1 × 105/kg. In the GETH/GITMO-2008 protocol, the recipient’s body weight was not taken into account, with the minimum required dose being: TNC > 150 × 107 and CD34+ cells > 70 × 105. When the UCB units had a similar cell dose, a higher degree of HLA match was preferred.
Haplo-Cord transplant platform.
UCB units with a minimum of 2 × 107 TNCs per kilogram and 1 × 105 CD34+ cells per kilogram were preferable. ABO compatibility was used as secondary selection criteria. Donors of the HLA-mismatched CD34+ cells were sought among patients’ first-degree relatives. If no relatives were available, an unrelated individual was selected as donor. G-CSF 10 µg/kg per day was administrated for 4 consecutive days to all donors, and cells were collected with a continuous flow apheresis device. Selection of CD34+ cells was performed by positive immunomagnetic procedures (CliniMACS; Miltenyi Biotec) to obtain a final product with 2.5 × 106 to 3 × 106 CD34+ cells per kilogram and <1 × 104 CD3+ cells per kilogram of recipient body weight, as previously described.4
Conditioning regimen and GVHD prophylaxis
Single-unit platform.
All patients received thiotepa-busulfan-fludarabine (TBF) conditioning: thiotepa (10 mg/kg), busulfan (9.6 mg/kg IV), fludarabine (150 mg/m2), and antithymocyte globulin (ATG) (Thymoglobulin; Genzyme Transplant, Cambridge, MA; 8 mg/kg in the GETH-2005 and 6 mg/kg in the GETH/GITMO-2008 trial).
For graft-versus-host disease (GVHD) prophylaxis, all patients received cyclosporine starting on day −1 at a dose of 1.5 mg/kg per 12 hours IV, followed by 3 mg/kg to 5 mg/kg per 12 hours orally when oral intake was possible. A slow tapering started between day +90 and +180 to discontinue on day +180 or before if feasible. In the GETH-2005 protocol (66 patients), cyclosporine was combined with a long-course prednisone that was replaced by mycophenolate mofetil in the GETH/GITMO-2008 trial (69 patients).
Haplo-Cord transplant platform.
Patients received fludarabine 30 mg/m2 (days −8 to −5), cyclophosphamide 60 mg/kg (days −4 and −3), IV busulfan 3.2 mg/kg (in 43 patients, days −6 and −5) or 10 Gy of fractionated total body irradiation (TBI; in 29 patients) and rabbit ATG (Timoglobulin; Genzyme, Marcy L’Étoile, France) 2 mg/kg on days −2 and −1. CB cells were infused on day 0 followed by the TPD cells either the same day or on day +1. As GVHD prophylaxis, patients received cyclosporine A from day −5, first IV and then orally, and methylprednisolone 1 mg/kg to 2 mg/kg from day −2, tapered until suspension on day +14. In the absence of GVHD manifestations, cyclosporine A was tapered when full CB engraftment was achieved or from day +50.
Definitions
Myeloid recovery was defined as the first day of an absolute neutrophil count of 0.5 × 109/L lasting for 3 or more consecutive days. Platelet recovery was defined as the first day of a platelet count of 20 × 109/L or higher, without transfusion support for 7 consecutive days. In the sUCBT platform, patients who failed to achieve myeloid engraftment at any time point were considered as primary graft failure except for those patients with early death (before day 21) who could not be evaluated for engraftment and were considered as competing events. In the Haplo-Cord platform, graft failure was defined as the absence of myeloid engraftment of the CB graft that required subsequent salvage transplantation, regardless of the neutrophil recovery from the TPD. Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were defined and graded according to standard criteria. Disease stage at the time of transplantation was classified as follows: (1) early stage (first complete remission [CR1]); (2) intermediate stage (second or further CR); and (3) advanced stage (primary refractory or relapsed/refractory patients with active disease). Nonrelapse mortality (NRM) was defined as death from any cause without evidence of relapse. Leukemia-free survival (LFS) was defined as survival from the time of transplantation without evidence of leukemia relapse or graft failure.
Chimerism analysis
Chimerism was determined by quantitative analysis of informative microsatellite DNA polymorphisms for both cohorts as previously described.5,11,12 In the Haplo-Cord cohort, peripheral blood samples were analyzed weekly from day +14 until full CB chimerism. In the sUCBT cohort, unmanipulated bone marrow samples were analyzed at time of hematologic recovery. Full donor chimerism was defined as the presence of ≥95% of donor hematopoiesis.
Statistical analysis
Categorical and continuous variables from different series were compared using the χ2 test and the Wilcoxon rank-sum test, respectively. The probabilities of engraftment, NRM, GVHD, and relapse were estimated by the cumulative incidence method.13,14 For cumulative incidence analyses of engraftment, GVHD, and relapse, death in CR and graft failure was considered as a competing cause of failure, whereas relapse was the competing event for NRM. Unadjusted time-to-event analyses were performed using the Kaplan-Meier estimate,15 and, for comparisons, the log-rank tests.16 LFS was calculated from the date of UCBT. In the analysis of LFS, relapse, death in CR, and graft failure, whichever occurred first, was considered an uncensored event. For the analysis of GVHD/relapse-free survival (GRFS), grade III-IV aGVHD, chronic extensive GVHD, relapse, graft failure, and death were considered uncensored events. The follow-up was updated on January 1, 2015. The Cox proportional hazards model17 or the Fine and Gray method for competing events18 was used for multivariable analysis. Variables included in the models were: treatment platform, age, sex, recipient body weight, recipient cytomegalovirus serostatus, diagnosis (acute myeloid leukemia [AML] vs acute lymphoblastic leukemia [ALL]), disease stage at transplantation, conditioning regimen (TBI vs non-TBI), GVHD prophylaxis, CB unit cell content per kilogram of recipient body weight (TNC and CD34+ cells), and HLA compatibility. Statistical analysis was performed using R (The CRAN project).19
Results
Patient, UCB unit and transplant characteristics
Table 1 summarizes the characteristics of the 135 patients from the sUCBT platform and the 72 patients from the Haplo-Cord platform. Patient and disease characteristics were similar in both groups, except for higher patient’s age in the Haplo-Cord platform (median 35 years vs 33 years; P = .004). The median follow-up for surviving patients was 51 months (range, 15-116 months) in the Haplo-Cord platform and 66 months (range, 9-127 months) in the sUCBT platform (P = .4). Table 2 summarizes the characteristics of the CB units.
Table 1.
Characteristics of patients
| sUCBT platform | Haplo-Cord platform | P | |
|---|---|---|---|
| No. of patients | 135 | 72 | |
| Age, y | .004 | ||
| Median | 33 | 35 | |
| Range | 16-53 | 16-64 | |
| Age group, y, no. (%) | .1 | ||
| 16-20 | 26 (19) | 7 (10) | |
| 21-30 | 37 (27) | 17 (24) | |
| 31-40 | 38 (28) | 20 (28) | |
| >40 | 34 (25) | 28 (39) | |
| Sex, no. (%) | 1 | ||
| Male | 78 (58) | 42 (58) | |
| Female | 56 (42) | 30 (42) | |
| Weight, kg | .3 | ||
| Median | 70 | 72 | |
| Range | 37-105 | 42-111 | |
| Diagnosis, no. (%) | 1 | ||
| AML | 74 (55) | 45 (63) | |
| ALL | 61 (45) | 27 (37) | |
| Disease stage at transplant, no. (%) | .1 | ||
| First complete remission | 73 (54) | 44 (61) | |
| Second or beyond complete remission | 43 (32) | 14 (20) | |
| Relapsed or refractory | 19 (14)* | 14 (20)† | |
| CMV serologic status before transplantation, no. (%) | .4 | ||
| Positive | 108 (80) | 55 (86) | |
| Negative | 27 (20) | 9 (14) | |
| Time of follow-up of surviving patients, mo | .4 | ||
| Median | 66 | 51 | |
| Range | 9-127 | 15-116 |
Percentages may not sum to 100 because of rounding.
CMV, cytomegalovirus.
Primary refractory = 12; first refractory relapse = 4; and second refractory relapse = 3.
Primary refractory = 11; first refractory relapse = 2; and second refractory relapse = 1.
Table 2.
Graft- and transplantation-related characteristics
| sUCBT | Haplo-Cord | P | |
|---|---|---|---|
| HLA compatibility, no. (%)* | .1 | ||
| 6 of 6 | 8 (6) | 5 (8) | |
| 5 of 6 | 38 (28) | 19 (29) | |
| 4 of 6 | 89 (66) | 42 (63) | |
| ABO blood group mismatch, no. (%) | .2 | ||
| Major | 31 (23) | 12 (19) | |
| Minor | 43 (32) | 15 (23) | |
| None | 60 (45) | 37 (58) | |
| No. of nucleated cells infused × 107/kg, median (range) | 2.5 (1.2-5.8) | 2.4 (1.2-6) | .2 |
| CB units according to nucleated cells × 107/kg, no. (%) | .2 | ||
| <1.5 | 8 (6) | 7 (10) | |
| 1.6-2.5 | 59 (47) | 31 (43) | |
| 2.6-3.5 | 35 (28) | 27 (37) | |
| >3.5 | 24 (19) | 7 (10) | |
| No. of CD34+ cells infused × 105/kg, median (range) | 1.3 (0.1-10) | 1.5 (0.4-4) | 1 |
Percentages may not sum to 100 because of rounding.
HLA class I antigens (A and B) considering the antigen level and class II antigen (DRB1) considering allele level resolution DNA typing.
Hematopoietic engraftment
Neutrophil engraftment.
In the sUCBT cohort, 5 patients (4%) died day 9 to day 19 after UCB infusion without evidence of myeloid engraftment. Eight patients (6%) experienced primary graft failure, of whom 3 died of graft failure–related complications. The remaining 5 patients achieved hematopoietic recovery after salvage haploidentical transplantation in 3 cases or autologous backup infusion in 2 additional patients. Three of these patients remain alive and disease-free after 44, 48, and 60 months. The remaining 122 patients (90%) achieved stable myeloid engraftment.
In the Haplo-Cord cohort, 1 patient (1%) had primary graft failure of both the TPD and the UCB graft and died of graft failure–related complications. Five additional patients (7%) experienced engraftment and neutrophil recovery from the TPD, but failed to engraft the UCB unit. Of these, 4 patients received salvage Haplo-Cord and 1 haploidentical transplant from day 31 to day 68 after the first transplant. One of these patients remained alive and disease-free after 98 months. The remaining 66 patients (92%) achieved stable myeloid recovery mainly from the UCB graft.
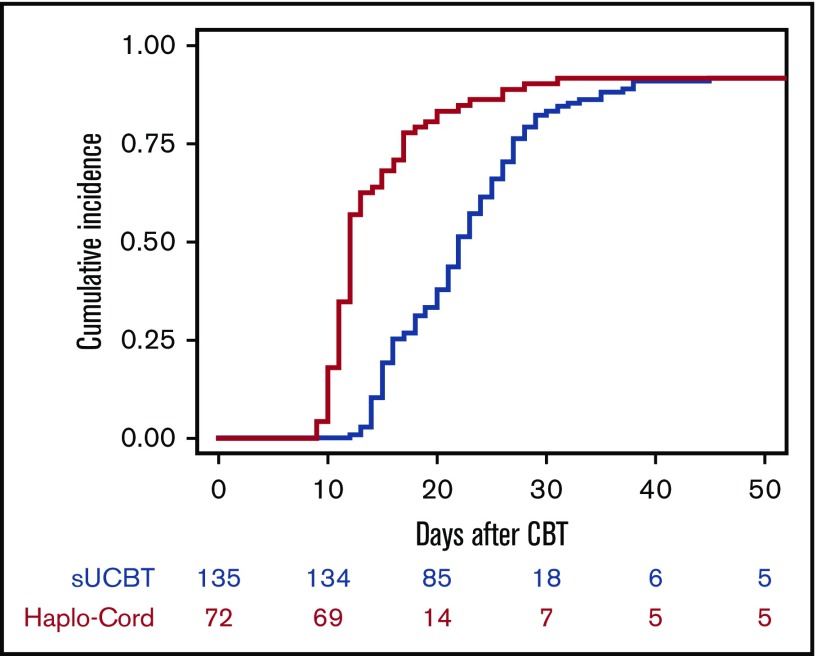
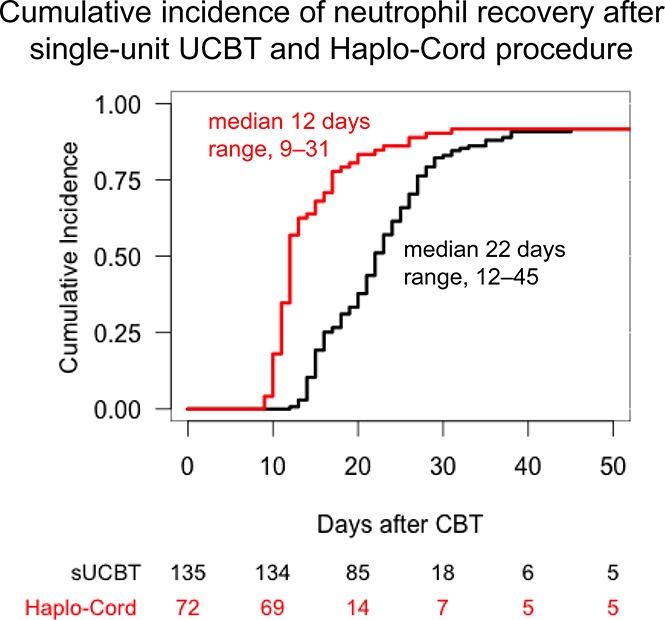
The cumulative incidence of sustained myeloid recovery at 45 days was 92% in both the sUCBT and Haplo-Cord cohorts, at a median time of 22 days (range, 12-45 days) and 12 days (range, 9-31 days), respectively (P < .001) (Figure 1). In multivariable analysis, the Haplo-Cord platform was associated with faster neutrophil recovery (P < .001) (Table 3). Time to neutrophil recovery and engraftment correlated with CD34+ cell dose in the sUCBT cohort (hazard ratio [HR], 1.2; 95% confidence interval [CI], 1.1-1.3; P = .002), but not in the Haplo-Cord (HR, 1; 95% CI, 0.8-1.2; P = .7).
Figure 1.
Cumulative incidence of neutrophil recovery after UCB transplantation with either single-unit or Haplo-Cord platforms.
Table 3.
Multivariable analysis of short- and long-term outcomes for all patients according to transplant platform
| Outcome | Relative risk (95% CI) | P | |
|---|---|---|---|
| sUCBT | Haplo-Cord | ||
| Myeloid engraftment | 1 | 2.3 (1.5-3.3) | <.001 |
| Platelet engraftment | 1 | 1.6 (1.2-2.3) | .015 |
| aGVHD, grade II-IV | 1 | 0.5 (0.2-1.1) | .08 |
| cGVHD | 1 | 0.5 (0.3-0.8) | .01 |
| NRM | 1 | 1 (0.6-1.6) | 1 |
| Relapse | 1 | 1.2 (0.7-2.1) | .6 |
| LFS | 1 | 1 (0.7-1.4) | .8 |
| GVHD-free/relapsed-free survival | 1 | 1 (0.8-1.2) | 1 |
Platelet engraftment.
Of the 122 patients with myeloid engraftment in the sUCBT cohort, 15 patients (11%) died between 28 and 250 days after transplantation without platelet recovery. The remaining 107 patients (79%) had platelet engraftment. Of the 66 patients with stable myeloid engraftment of UCB origin in the Haplo-Cord cohort, 7 patients died between 27 and 129 days after transplantation without platelet recovery. The remaining 59 patients had platelet engraftment.
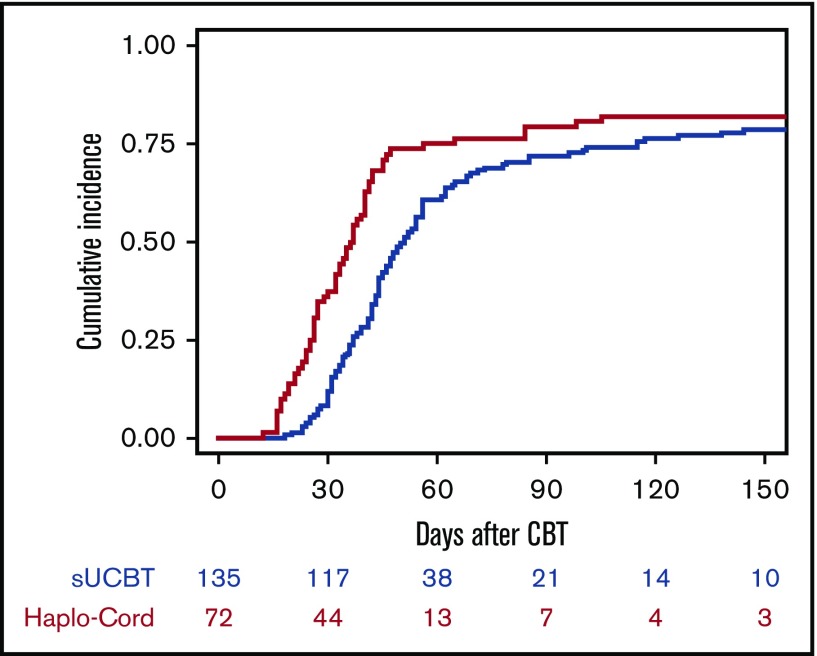
The cumulative incidence of sustained platelet engraftment at 180 days was 79% in the sUCBT at a median time of 44 days (range, 18-179 days) and 82% in Haplo-Cord cohorts at a median time of 32 days (range, 12-105 days) (P = .005) (Figure 2). In multivariable analysis, the Haplo-Cord platform was associated with faster platelet recovery (P = .015) (Table 3). Time to platelet recovery and engraftment correlated with TNC dose in the sUCBT cohort (HR, 1.2; 95% CI, 1.1-1.9; P = .03) but not in the Haplo-Cord (HR, 1.2; 95% CI, 1-1.5; P = .07).
Figure 2.
Cumulative incidence of platelet recovery after UCB transplantation with either single-unit or Haplo-Cord platforms.
Chimerism analysis.
All 122 sUCBT patients with myeloid engraftment showed full donor chimerism at time of reconstitution. In the Haplo-Cord procedure, 68% of patients with myeloid engraftment showed peripheral blood mixed chimerism at day +14, with variable percentages of CB and TPD cells, superseded by stable CB engraftment. Median percentages of TPD and CB cells at day +14 were 69% (interquartile range, 20-82) and 31% (interquartile range, 11-80), respectively. The cumulative incidence of full CB chimerism was 70% at 180 days (95% CI, 60%-82%), achieved at a median time of 73 days. All patients who achieved full CB chimerism showed full sustained engraftment, and all patients who achieved initial neutrophil recovery showed full sustained engraftment, including those with prolonged mixed chimerism.
Transfusion support and days of hospitalization.
The median number of random donor pooled platelets transfused was 28 (range, 1-149) in the sUCBT group and 21 (range, 5-142) in the Haplo-Cord group (P = .1). The median number of packed red blood cells transfused was 13 (range, 0-80) in the sUCBT group and 15 (range, 2-48) in the Haplo-Cord group (P = .2). The median days of hospitalization within the first 100 days after transplant was 40 (range, 17-100) and 47 (range, 14-100) in the sUCBT and Haplo-Cord cohorts, respectively (P = .1).
GVHD
aGVHD.
In the sUCBT cohort, 50 patients (37%) developed aGVHD: grade I in 22 (16%), grade II in 13 (10%), grade III in 10 (7%), and grade IV in 5 patients (4%). The median time to the development of aGVHD grade II to IV was 26 days (range, 10-124 days). In the Haplo-Cord cohort, 25 patients (33%) developed aGVHD: grade I in 16 (22%), grade II in 6 (8%), grade III in 2 (3%), and grade IV in 1 patient (1%). The median time to the development of aGVHD grade II to IV was 43 days (range, 23-62 days).
The cumulative incidence of aGVHD at 100 days in the sUCBT and the Haplo-Cord cohorts was 21% and 11% for grade II-IV, respectively (P = .08), whereas for grade III-IV it was 11% and 4%, respectively (P = .09) (Table 3). No factor was associated with the risk of grades II-IV or III-IV aGVHD in multivariable analysis.
cGVHD.
Forty-five of 106 patients at-risk (42%) in the sUCBT cohort developed cGVHD, limited in 18 patients (17%) and extensive in 27 patients (25%), at a median time of 147 days (range, 65-876 days). In the Haplo-Cord cohort, 13 of 55 patients at-risk (24%) developed cGVHD, limited in 7 patients (13%) and extensive in the remaining 6 patients (11%), at a median time of 184 days (range, 99-540 days).
The 3-year cumulative incidence of cGVHD and extensive cGVHD in the sUCBT and Haplo-Cord cohorts was 43% and 24% (P = .01) and 25% and 11% (P = .02), respectively. In multivariable analysis, the only factor associated with increased risk of cGVHD was the sUCBT platform (P = .01) (Table 3).
NRM and causes of death
Forty-nine patients (36%) in the sUCBT cohort died without prior relapse at a median time of 181 days after transplantation (range, 9-1352 days), whereas in the Haplo-Cord cohort, 25 patients (35%) died at a median time of 88 days after transplantation (range, 27-530 days). Causes of death in the different transplant platforms are shown in Table 4.
Table 4.
Causes of death according to transplant platform
| sUCBT, no. (%) | Haplo-Cord, no. (%) | |
|---|---|---|
| Relapse | 29 (37) | 13 (34) |
| Infections | 31 (40) | 8 (21) |
| Viral | 10 (13) | 3 (8) |
| Bacterial | 12 (15) | 3 (8) |
| Fungal | 5 (6) | 0 |
| Parasitic | 3 (4) | 1 (1) |
| Unknown | 1 (1) | 1 (1) |
| GVHD | 7 (9) | 4 (10) |
| Secondary malignancy* | 5 (6) | 0 (0) |
| Graft failure | 2 (3) | 4 (10) |
| Cerebrovascular event | 2 (3) | 1 (1) |
| Veno-occlusive disease | 1 (1) | 2 (5) |
| Pulmonary toxicity | 0 (0) | 3 (8) |
| Multiorgan failure of unknown cause | 1 (1) | 3 (8) |
*Epstein-Barr virus–associated posttransplant lymphoproliferative disorder in 3 cases; donor-derived AML in 2 cases.
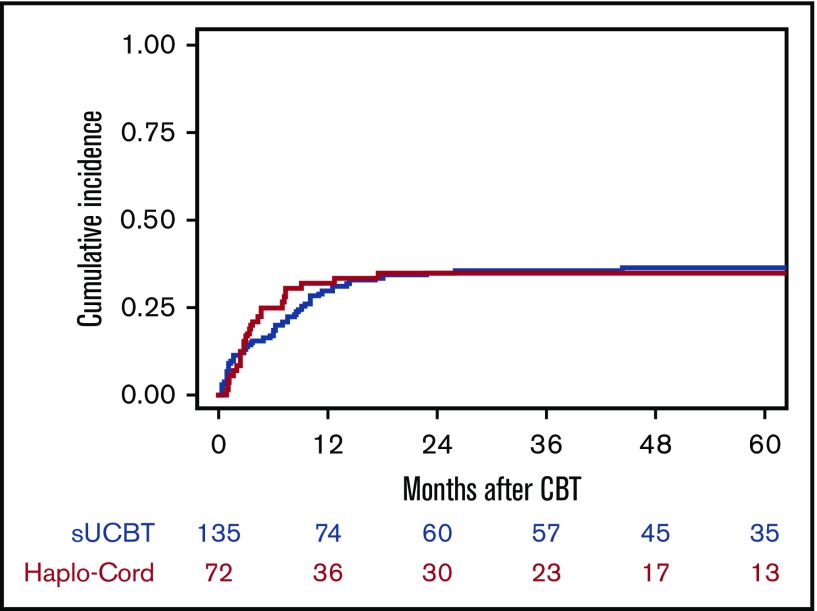
The cumulative incidence of NRM was similar in both groups (P = 1.0) (Figure 3; Table 3). For patients in the sUCBT cohort, the incidence of NRM at 30 days, 100 days, 180 days, and 5 years was 7%, 13%, 18%, and 36%, respectively, whereas for patients in the Haplo-Cord cohort was 3%, 17%, 25%, and 35%, respectively. In multivariable analysis, early disease stage at time of transplantation (HR, 1.7; 95% CI, 1.1-2.7; P = .02) and non-TBI conditioning regimens (HR, 2.5; 95% CI, 1.4-4.3; P = .001) were associated with improved NRM (HR, 1.7; 95% CI, 1.1-2.6; P = .03). The 5-year cumulative incidence of NRM was 30% vs 32% (P = .6) for patients in early stage, and 44% vs 39% (P = .7) for patients in more advanced phase for the sUCBT and Haplo-Cord cohorts, respectively. Five-year NRM was 59% vs 32% (P = .03) for patients conditioned with TBI- or busulfan-based regimens, respectively.
Figure 3.
Cumulative incidence of NRM after UCB transplantation with either single-unit or Haplo-Cord platforms.
For patients with AML, the 5-year NRM was 39% for patients in the sUCBT cohort and 24% in the Haplo-Cord (P = .1) and 29% vs 23% (P = .7) for patients in first CR, respectively. In multivariable analysis, more advanced disease stage (P = .03) was the only variable independently associated with increased NRM (Table 5).
Table 5.
Multivariable analysis of disease-specific outcomes: NRM, relapse risk, and LFS
| Outcome | Variable | AML | Variable | ALL | ||
|---|---|---|---|---|---|---|
| Relative risk (95% CI) | P | Relative risk (95% CI) | P | |||
| NRM | Transplant platform | .4 | Transplant platform | .5 | ||
| sUCBT | 1 | sUCBT | 1 | |||
| Haplo-Cord | 0.7 (0.2-1.7) | Haplo-Cord | 1.4 (0.5-3.8) | |||
| Disease status | .003 | Conditioning regimen | .03 | |||
| Complete remission | 1 | TBI-based | 1 | |||
| Relapse/refractory | 2.1 (1.1-4.2) | Busulfan-based | 0.5 (0.2-0.9) | |||
| Relapse | Transplant platform | .04 | Transplant platform | .5 | ||
| sUCBT | 1 | sUCBT | 1 | |||
| Haplo-Cord | 2.3 (1.1-5.4) | Haplo-Cord | 0.6 (0.1-2.8) | |||
| Disease status | .007 | Disease status | .001 | |||
| Complete remission | 1 | Complete remission | 1 | |||
| Relapse/refractory | 3.2 (1.4-7.4) | Relapse/refractory | 4.3 (1.8-10.3) | |||
| HLA match* | <.001 | |||||
| Matched | 1 | |||||
| Mismatched | 0.2 (0.1-0.5) | |||||
| LFS | Transplant platform | .7 | Transplant platform | .7 | ||
| sUCBT | 1 | sUCBT | 1 | |||
| Haplo-Cord | 0.9 (0.7-1.8) | Haplo-Cord | 0.9 (0.6-1.9) | |||
| Disease status | <.001 | Disease status | <.001 | |||
| Complete remission | 1 | Complete remission | 1 | |||
| Relapse/refractory | 0.6 (0.4-0.8) | Relapse/refractory | 0.3 (0.1-0.5) | |||
| HLA match* | <.001 | |||||
| Matched | 1 | |||||
| Mismatched | 2.5 (1.2-5.3) | |||||
| CD34+ cell dose | .004 | |||||
| <1 × 105/kg | 1 | |||||
| ≥1 × 105/kg | 2.1 (1.3-3.5) | |||||
HLA class I antigens (A and B) considering the antigen level and class II antigen (DRB1) considering allele-level resolution DNA typing.
For patients with ALL, the 5-year NRM was 33% for patients in the sUCBT cohort and 52% in the Haplo-Cord (P = .05), and 32% vs 44% (P = .2) for patients in first CR, respectively. In multivariable analysis, non-TBI conditioning regimens were associated with improved NRM (P = .03) (Table 5). Five-year NRM was 58% vs 33% (P = .04) for patients conditioned with TBI- or busulfan-based regimens, respectively.
Relapse
Overall, 33 patients (24%) in the sUCBT cohort relapsed at a median time of 202 days (range, 25-1480 days) and 19 patients (26%) in the Haplo-Cord cohort relapsed at a median time of 194 days (range, 60-1618 days). The cumulative incidence of relapse at 5 years in the sUCBT and Haplo-Cord cohorts was 23% and 28%, respectively (P = .5) (Table 3). In multivariable analysis, active disease at time of transplantation (HR, 3.5; 95% CI, 1.9-6.5; P < .001) and 6 of 6 HLA match between donor and recipient (HR, 3; 95% CI, 1.3-6.7; P = .007) were associated with higher relapse risk. The 5-year cumulative incidence of relapse was 20% vs 22% (P = .6) for patients in complete remission, and 47% vs 54% (P = .9) for patients with active disease at time of transplantation for the sUCBT and Haplo-Cord cohorts, respectively. Five-year cumulative incidence of relapse was 46% vs 23% (P = .05) for patients transplanted with HLA-matched or HLA-mismatched grafts, respectively.
For patients with AML, the 5-year cumulative incidence of relapse was 14% for patients in the sUCBT cohort and 37% in the Haplo-Cord cohort (P = .007), and 13% vs 26% (P = .1) for patients in CR, respectively. In multivariable analysis, 6 of 6 HLA match (P < .001), active disease at time of transplantation (P = .007), and Haplo-Cord procedure (P = .04) were associated with an increased risk of relapse (Table 5).
For patients with ALL, the 5-year cumulative incidence of relapse was 35% for patients in the sUCBT cohort and 15% in the Haplo-Cord cohort (P = .08), whereas for those transplanted in CR it was 28% vs 16% (P = .4), respectively. In multivariable analysis, the only variable associated with an increased risk of relapse was advanced disease stage (P = .001) (Table 5).
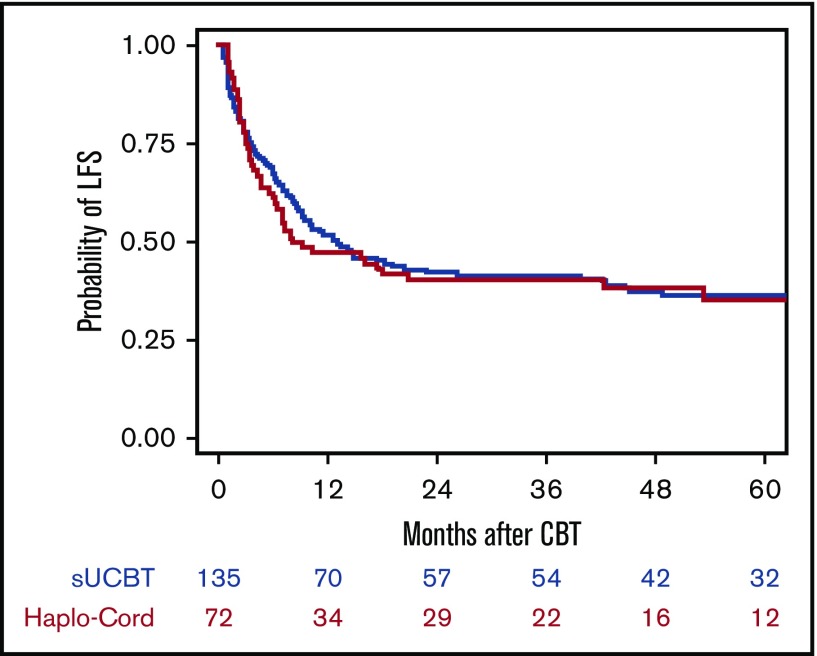
LFS
Fifty-three patients (39%) in the sUCBT cohort and 28 patients (39%) in the Haplo-Cord cohort are alive and leukemia free after UCBT at last follow-up. The overall LFS at 5 years was 37% and 36% for the sUCBT and Haplo-Cord cohorts, respectively (P = .8) (Table 3; Figure 4). We observed no impact of the transplant platform on LFS, regardless of UCB cell dose at any cutoff level, even in patients with CB units with TNC < 1.5 × 107/kg. In multivariable analysis, advanced disease status at time of transplantation (HR, 0.5; 95% CI, 0.3-0.7; P < .001) and diagnosis of ALL (HR, 0.8; 95% CI, 0.7-0.9; P = .04) were associated with a higher risk of treatment failure. The 5-year LFS was 46% vs 41% (P = .2) for patients in CR1, 30% vs 38% (P = .2) for patients in ≥CR2, and 16% vs 11% (P = .9) for those transplanted with active disease in the sUCBT and Haplo-Cord cohorts, respectively. The overall GRFS at 5 years was 35% and 34% for the sUCBT and Haplo-Cord cohorts, respectively (P = 1.0).
Figure 4.
Kaplan-Meier estimate of LFS after UCB transplantation with either single-unit or Haplo-Cord platforms.
The 5-year LFS for patients with AML was 44% in the sUCBT cohort and 37% in the Haplo-Cord cohort (P = .6), and 47% vs 47% (P = .9) for patients transplanted in first CR, respectively. In multivariable analysis, more advanced disease stage (P < .001), 6 of 6 HLA match (P = .02), and a lower dose of CD34+ cells (P = .01) were independently associated with a lower LFS (Table 5).
The 5-year LFS for patients with ALL was 27% in the sUCBT cohort and 33% in the Haplo-Cord cohort (P = 1.0), and 32% vs 36% (P = .9) for patients transplanted in CR, respectively. In multivariable analysis, only advanced disease stage (P < .001) was independently associated with a lower LFS (Table 5). The 5-year LFS was 29%, 39%, and 9% in patients transplanted in first CR, second or subsequent CR, and more advanced disease stage at time of transplantation, respectively.
Discussion
This study shows that the addition of CD34+ cells from a TPD to a single UCB unit in a myeloablative Haplo-Cord platform significantly enhances the speed of neutrophil and platelet recovery compared with unmanipulated sUCBT in adults with high-risk acute leukemia. Both platforms were valuable strategies that offered similar long-term outcomes. Prolonged LFS could be achieved in a substantial number of patients, especially if transplanted in an early stage of the disease. These data may provide clinically useful information for UCBT in adults with poor-risk acute leukemia.
This retrospective comparison of 2 relatively large series included patients who had also been included in prospective multicenter studies.7,8,10,20 These studies were carried out by highly experienced groups that have pioneered both transplant strategies within the Spanish GETH group and received a relatively homogeneous treatment strategy in terms of the conditioning regimen, GVHD prophylaxis, and donor selection.
A significant proportion of patients in both groups received a lower cell dose than the minimum required in most centers.21,22 This allowed donor availability for the vast majority of patients for whom donor search was initiated in both strategies. Whether the comparative arm of TBF sUCBT was adequate considering that half of patients received insufficient cell dose according to standard criteria could be a matter of concern. However, the TBF sUCBT has shown consistently high rates of engraftment and significant transplant outcomes using a lower cell dose content.7,8,23 In fact, it showed faster neutrophil recovery and comparable long-term outcomes in a head-to-head comparison with the double-unit UCBT platform from Minnesota.24 Similar findings were subsequently reported in a registry-based study from Eurocord.25 We therefore consider the TBF sUCBT as a valuable comparative arm allowing the use of lower cell dose units. Of note, the CD34+ cell dose was an important determinant of engraftment for sUCBT but had no impact in the Haplo-Cord arm. However, the TNC dose had no influence on any outcome in both platforms and regardless of the cell dose content at any cutoff level, even for lower cell doses, we found no benefit of any transplant platform.
To our knowledge, this is the first comparative study in UCBT that has consistently demonstrated the benefit of an alternative strategy to accelerate hematopoietic recovery compared with the use of unmanipulated sUCBT. The reduction of the median time to neutrophil and platelet recovery in the Haplo-Cord platform (10 and 12 days, respectively) was achieved by unique engraftment kinetics obtained with a relatively simple, rapid, and affordable procedure. This kinetics is characterized by an initial engraftment of the TPD that is ultimately replaced by a permanent UCB engraftment. The fast neutrophil recovery and the presence of a hematopoietic backup from the TPD in case of failure of the UCB graft makes the Haplo-Cord procedure particularly appealing in patients with an underlying infection at time of transplant or with an anticipated high risk of graft failure. Future studies on strategies to improve engraftment, including double-cord SCT and ex vivo expansion techniques, should assess their value by comparing them with the Haplo-Cord platform.
Besides a faster hematopoietic recovery in the Haplo-Cord setting, both platforms offered similar outcomes in terms of engraftment rate, transfusion requirements, days of hospitalization, NRM, relapse, and survival rates. The lack of impact of a faster engraftment on other outcomes, particularly NRM, is probably multifactorial. NRM rates are comparable to most recent reports in adults in both single-center and registry data for both cohorts.26-28 It should be highlighted that around 40% of patients in the Haplo-Cord cohort received TBI-based conditioning that was associated with increased toxicity. In fact, the main cause of death in this cohort was organ toxicity, whereas infection was the main cause of death in the sUCBT cohort. However, severe infections that occur in the postengraftment period remain an important challenge in both platforms, as it has been previously noted.7,9,29 This may suggest a long-lasting impaired immune reconstitution in the setting of ATG-containing regimens that remains the biggest challenge in UCBT. Whether results can be improved by omitting ATG from the conditioning regimen is controversial. A better T-cell recovery with a lower incidence of viral reactivation and death from viral infections has been reported in patients not receiving ATG.30 The delayed T-cell recovery may not be inherent to the UCB graft, but rather due to in vivo T-cell depletion, which potentially does not differ so much from adult donor grafts. However, improved immune reconstitution without ATG is counterbalanced by an increased risk of GVHD.30,31 A randomized study is necessary to determine the real impact of ATG on survival for patients undergoing UCBT because retrospective studies have shown conflicting results.25,30-32 In addition, the optimal dose, preparation, and timing of ATG are not known. In our study, both cohorts had different exposures to ATG but their impact could not be assessed.
The observed increase of cGVHD in the sUCBT compared with the Haplo-Cord platform, as well as a trend toward higher rates of aGVHD, deserve some comment. Apart from a potential bias of assessment, given the multicenter nature of the study, the possibility of a protective effect of the TPD cells in the Haplo-Cord procedure facilitating immune tolerance cannot be ruled out. In this respect, CD34+ cells from the third-party graft may exert a veto effect, preventing the graft-versus-host–like reactions of CB lymphocytes.33 The higher incidence of cGVHD observed in the sUCBT cohort may explain, at least in part, the reduction in the relapse risk for patients with AML. A strong association of GVHD and relapse risk in UCBT in AML and ALL has been recently confirmed in a large study from the Japanese registry.34 Nevertheless, an alternative hypothesis could also be an underestimation of the cumulative incidence of relapse in AML patients in the sUCBT cohort due to the occurrence of more competing events.35
Overall, long-term LFS was encouraging for this high-risk population, with advanced disease stage and diagnosis of ALL being the variables associated with worse outcome. In patients with AML, the CD34+ cell dose and HLA mismatch were also strong predictors of survival, independent of the disease stage. This is especially interesting because these variables can be conveniently modified in the UCB unit selection process. The benefit of a higher number of CD34+ cells has been recognized from early studies with single-cord SCT.36 The higher relapse rate observed in AML recipients of HLA-matched units compared with those with HLA-mismatched grafts deserves some comment. This enhanced graft-versus-leukemia effect of HLA disparity has been reported in several AML studies with different platforms,37-40 which challenges the current recommendations for UCB unit selection. To address this important issue, disease-specific prospective trials are needed.
In conclusion, in the context of specific treatment platforms, sUCBT and Haplo-Cord are both valuable procedures for adult patients with high-risk acute leukemia with similar survival outcomes. Haplo-Cord shortened time to neutrophil and platelet recovery compared with sUCBT. Further studies are needed to improve immune reconstitution and other clinically significant outcomes using cost-effective approaches.
Supplementary Material
The full-text version of this article contains a data supplement.
Authorship
Contribution: J.S., M.K., G.F.S., M.A.S., J.L.D.-M., and R.C. conceived the study and interpreted the data; J.S. and M.K. wrote the paper; J.S. and P. Balsalobre performed the statistical analyses; and G.B., M.A.S., P. Balsalobre, I.L., J.L.P., C.S., R.D., C.F., J.G., C.M., P. Barba, I.B., M.J.P., R.M., J.M., C.R., A.d.l.I., J.L.D.-M., G.F.S., and R.C. reviewed the manuscript and contributed to the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the GETH appears in the supplemental appendix.
Correspondence: Jaime Sanz, Servicio de Hematología, Hospital Universitari i Politècnic La Fe, Avinguda Fernando Abril Martorell, 106, 46026 Valencia, Spain; e-mail: sanz_jai@gva.es.
References
- 1.Rocha V, Crotta A, Ruggeri A, et al. ; Eurocord Registry. Double cord blood transplantation: extending the use of unrelated umbilical cord blood cells for patients with hematological diseases. Best Pract Res Clin Haematol. 2010;23(2):223-229. [DOI] [PubMed] [Google Scholar]
- 2.Thompson PA, Rezvani K, Hosing CM, et al. . Umbilical cord blood graft engineering: challenges and opportunities. Bone Marrow Transplant. 2015;50(suppl 2):S55-S62. [DOI] [PubMed] [Google Scholar]
- 3.Fernández MN, Regidor C, Cabrera R, et al. . Cord blood transplants: early recovery of neutrophils from co-transplanted sibling haploidentical progenitor cells and lack of engraftment of cultured cord blood cells, as ascertained by analysis of DNA polymorphisms. Bone Marrow Transplant. 2001;28(4):355-363. [DOI] [PubMed] [Google Scholar]
- 4.Fernández MN, Regidor C, Cabrera R, et al. . Unrelated umbilical cord blood transplants in adults: early recovery of neutrophils by supportive co-transplantation of a low number of highly purified peripheral blood CD34+ cells from an HLA-haploidentical donor. Exp Hematol. 2003;31(6):535-544. [DOI] [PubMed] [Google Scholar]
- 5.Kwon M, Martínez-Laperche C, Balsalobre P, et al. . Early peripheral blood and T-cell chimerism dynamics after umbilical cord blood transplantation supported with haploidentical cells. Bone Marrow Transplant. 2014;49(2):212-218. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Rich ES, Godley L, et al. . Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118(24):6438-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanz J, Boluda JCH, Martín C, et al. ; Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH). Single-unit umbilical cord blood transplantation from unrelated donors in patients with hematological malignancy using busulfan, thiotepa, fludarabine and ATG as myeloablative conditioning regimen. Bone Marrow Transplant. 2012;47(10):1287-1293. [DOI] [PubMed] [Google Scholar]
- 8.Sanz J, Picardi A, Hernández Boluda JC, et al. ; GETH and GITMO. Impact of graft-versus-host disease prophylaxis on outcomes after myeloablative single-unit umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2013;19(9):1387-1392. [DOI] [PubMed] [Google Scholar]
- 9.Kwon M, Balsalobre P, Serrano D, et al. . Single cord blood combined with HLA-mismatched third party donor cells: comparable results to matched unrelated donor transplantation in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant. 2013;19(1):143-149. [DOI] [PubMed] [Google Scholar]
- 10.Bautista G, Cabrera JR, Regidor C, et al. . Cord blood transplants supported by co-infusion of mobilized hematopoietic stem cells from a third-party donor. Bone Marrow Transplant. 2009;43(5):365-373. [DOI] [PubMed] [Google Scholar]
- 11.Buño I, Nava P, Simón A, et al. . A comparison of fluorescent in situ hybridization and multiplex short tandem repeat polymerase chain reaction for quantifying chimerism after stem cell transplantation. Haematologica. 2005;90(10):1373-1379. [PubMed] [Google Scholar]
- 12.Moscardó F, Sanz J, Senent L, et al. . Impact of hematopoietic chimerism at day +14 on engraftment after unrelated donor umbilical cord blood transplantation for hematologic malignancies. Haematologica. 2009;94(6):827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695-706. [DOI] [PubMed] [Google Scholar]
- 14.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141-1154. [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 16.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163-170. [PubMed] [Google Scholar]
- 17.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187-220. [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 19.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 20.Kwon M, Bautista G, Balsalobre P, et al. . Haplo-cord transplantation using CD34+ cells from a third-party donor to speed engraftment in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant. 2014;20(12):2015-2022. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Kamani N, Confer DL. Principles and tools for selection of umbilical cord blood and unrelated adult donor grafts. Biol Blood Marrow Transplant. 2008;14(1 suppl 1):112-119. [DOI] [PubMed] [Google Scholar]
- 22.Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117(8):2332-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moscardó F, Sanz J, Carbonell F, et al. . Effect of CD8+ cell content on umbilical cord blood transplantation in adults with hematological malignancies. Biol Blood Marrow Transplant. 2014;20(11):1744-1750. [DOI] [PubMed] [Google Scholar]
- 24.Sanz J, Wagner JE, Sanz MA, et al. . Myeloablative cord blood transplantation in adults with acute leukemia: comparison of two different transplant platforms. Biol Blood Marrow Transplant. 2013;19(12):1725-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruggeri A, Sanz G, Bittencourt H, et al. ; Eurocord and Acute Leukemia Working Party of European Blood and Marrow Transplant Group. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia. 2014;28(4):779-786. [DOI] [PubMed] [Google Scholar]
- 26.Eapen M, Klein JP, Ruggeri A, et al. ; Center for International Blood and Marrow Transplant Research, Netcord, Eurocord, and the European Group for Blood and Marrow Transplantation. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123(1):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scaradavou A, Brunstein CG, Eapen M, et al. . Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121(5):752-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atsuta Y, Suzuki R, Nagamura-Inoue T, et al. ; Japan Cord Blood Bank Network. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009;113(8):1631-1638. [DOI] [PubMed] [Google Scholar]
- 29.Sanz J, Cano I, González-Barberá EM, et al. . Bloodstream infections in adult patients undergoing cord blood transplantation from unrelated donors after myeloablative conditioning regimen. Biol Blood Marrow Transplant. 2015;21(4):755-760. [DOI] [PubMed] [Google Scholar]
- 30.Lindemans CA, Chiesa R, Amrolia PJ, et al. . Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123(1):126-132. [DOI] [PubMed] [Google Scholar]
- 31.Ponce DM, Eapen M, Sparapani R, et al. . In vivo T cell depletion with myeloablative regimens on outcomes after cord blood transplantation for acute lymphoblastic leukemia in children. Biol Blood Marrow Transplant. 2015;21(12):2173-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pascal L, Mohty M, Ruggeri A, et al. . Impact of rabbit ATG-containing myeloablative conditioning regimens on the outcome of patients undergoing unrelated single-unit cord blood transplantation for hematological malignancies. Bone Marrow Transplant. 2015;50(1):45-50. [DOI] [PubMed] [Google Scholar]
- 33.van Besien K, Hari P, Zhang M-J, et al. . Reduced intensity haplo plus single cord transplant compared to double cord transplant: improved engraftment and graft-versus-host disease-free, relapse-free survival. Haematologica. 2016;101(5):634-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanda J, Morishima Y, Terakura S, et al. . Impact of graft-versus-host disease on outcomes after unrelated cord blood transplantation. Leukemia. 2017;31(3):663-668. [DOI] [PubMed] [Google Scholar]
- 35.Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. 2013;66(6):648-653. [DOI] [PubMed] [Google Scholar]
- 36.Wagner JE, Barker JN, DeFor TE, et al. . Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611-1618. [DOI] [PubMed] [Google Scholar]
- 37.Wagner JE Jr, Eapen M, Carter S, et al. ; Blood and Marrow Transplant Clinical Trials Network. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014;371(18):1685-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanz J, Jaramillo FJ, Planelles D, et al. . Impact on outcomes of human leukocyte antigen matching by allele-level typing in adults with acute myeloid leukemia undergoing umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2014;20(1):106-110. [PubMed] [Google Scholar]
- 39.Brunstein CG, Petersdorf EW, DeFor TE, et al. . Impact of allele-level HLA mismatch on outcomes in recipients of double umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2016;22(3):487-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atsuta Y, Kanda J, Takanashi M, et al. ; HLA Working Group of the Japan Society for Hematopoietic Cell Transplantation. Different effects of HLA disparity on transplant outcomes after single-unit cord blood transplantation between pediatric and adult patients with leukemia. Haematologica. 2013;98(5):814-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.