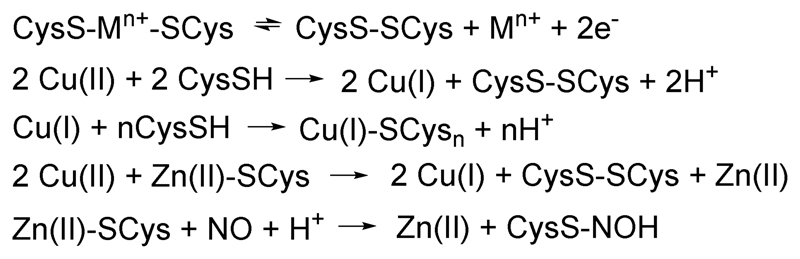Abstract
Cu and Zn ions are essential in most living beings. Their metabolism is critical for health and mis-metabolism can be lethal. In the last two decades, a large body of evidence has reported the role copper, zinc and iron, and oxidative stress in several neurodegenerative diseases like Alzheimer, Parkinson, Prion, etc. To what extend this mis-metabolism is causative or a consequence of these diseases is still a matter of research. In this context metallothioneins (MTs) appear to play a central gate-keeper role in controlling aberrant metal-protein interactions. MTs are small proteins that can bind high amounts of Zn(II) and Cu(I) ions in metal-cluster arrangements via their cysteines thiolates. Moreover, MTs are well known antioxidants. The present tutorial outlines the chemistry underlying the interconnection between copper(I/II) and zinc(II) coordination to amyloidogenic proteins and MTs, and their redox properties in generation and/or silencing reactive oxygen species (overproduced in oxidative stress) and other reactants. These studies have revealed the coordination chemistry involved in neurodegenerative diseases and the interactions between MTs with amyloidogenic proteins metal-complexes (like amyloid-β, α-synuclein and prion-protein). Overall, a protective role of MTs in neurodegenerative processes is emerging, serving as a foundation for exploring MTs chemistry as inspiration for therapeutic approaches.
1. Structure and reactivity of mammalian metallothioneins
1.1. General introduction to MT
Metallothioneins (MTs) are cysteine-rich proteins of low molecular weight (about 7 kDa for mammalian MTs) with a high metal ion content. Under physiological conditions, MTs bind mainly Zn(II) and Cu(I). Upon environmental exposure, MTs can also bind other non-essential toxic metal ions, in particular Cd(II). These metal ions are coordinated by the thiolate function of the cysteine (Cys) residues from the MTs.
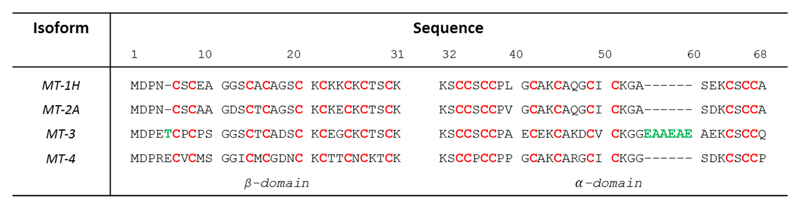
In mammals, four MTs designated MT1 to 4 are known. Several isoforms are known for MT1, one for MT2, MT3 and MT4. Sequences of MT1-4 are highly similar, consisting of 61-68 amino acids and a conserved array of 20 Cys (Figure 1).
Figure 1.
Amino acid sequences of the four human MT isoforms. The metal-binding cysteines are highlighted in red, green amino acids highlight the two inserts of MT3 compared to M1/2. The numbering refers to MT3. The boundary between α- and β-domain is between amino acid 31 and 32.
MT3 sequence contains 7 additional amino acids when compared to the canonical MT1 and MT2: a Thr insert at position 5 and a Glu/Ala-rich insert towards the C-terminus.1 MT4 has 62 amino acids with an insert of Glu at position 5 compared to MT-1/2 (Figure 1). MT4 seems to be expressed exclusively in cornified and stratified squamous epithelia.2 (and ref. therein) The present article is thus focused on MT1-3. MT1-2 isoforms are ubiquitously expressed while MT3 is more specifically expressed. The tissue with one of the highest MT3 expression is the central nervous system.
Functionally, MTs have been reported to play roles in the regulation of metal ion homeostasis, detoxification of heavy metals, cellular defence against oxidative stress, modulation of neuronal growth and amelioration of inflammatory cascades (for recent review see 3). In the current review, we will focus on chemical aspects of mammalian MT metal binding and redox reactivity, which are relvant for understanding their role in the brain and their potential as therapeutic targets in neurodegenerative diseases.
1.2. Coordination chemistry of MT1-3
1.2.a. Metal ion content
MT1-3 mainly bind Zn(II) and Cu(I). MT1-2 is classically isolated with seven Zn(II) ions, but some Zn(II) can be easily lost. Cd(II) or Cu(I) can become the dominant metal in MT1-2, but only under exposure to high concentrations of these metal ions. MT3 is different: it was isolated with a mixed Zn(II) and Cu(I) content, with about 4 Cu(I) ions and 3-4 Zn(II) ions.1 Whether Cu(I) is bound during purification or is present natively is not clear.4 Indications for Cu(I) being present natively comes from the classification suggested by Capdevila, Atrian and co-workers, who showed that MT3 has more Cu(I)-binding character compared to MT1/2.5 This means that although both MT1-2 and MT3 bind preferentially Cu(I) over Zn(II), the difference in affinity Cu(I) over Zn(II) is larger for MT3 than MT1/2, and hence in vivo Cu(I)-binding of MT3 is more likely compared to MT1-2. They also proposed that the isolated Cu(I)4,Zn(II)3-4 species contains disulphide bond(s) and that under reducing conditions a species containing Cu(I)6,Zn(II)4 is formed, which converts to a Cu(I)10 species at higher Cu(I) concentrations. In general, the preference for discrete species with distinct stoichiometries for Cu(I)-binding is based on cooperativity due to the formation of Cu(I)4/6-thiolate clusters. The more Cu(I)-binding character of MT3 compared to MT1-2 was assigned to the β-domain. Indeed, in the heterometallic species, Cu(I) is bound to the β-domain.2,6 (and ref. therein)
For Zn(II) (and Cd(II)) seven binding sites have been classically described for MT1-3, although less well defined for MT3. Additional binding sites have even been found, but with a much lower affinity. Although there is no consensus in the literature, it seems that not all the 7 Zn(II) ions have the same affinity (for discussion see ref 7 and 15). Hence it is likely that MTs with less than 7 Zn(II) occur in vivo to fulfill a Zn buffering function at the picomolar level by MTs in response to dynamic changes to intracellular zinc concentrations (Table 1).7 As a result of this flexible binding stoichiometry towards Zn(II), MTs are involved in controlling subcellular Zn(II) re-distribution and Zn(II) signaling.7
Table 1.
Conditional dissociation constants (at pH 7.4) for Zn(II) sites in MT2 10
| Kd (M) | |
|---|---|
| Zn7-MT2 | |
| 1st-4th sites | 1.6 10-12 |
| 5th site | 3.5 10-11 |
| 6th site | 1.1 10-10 |
| 7th site | 2.0 10-8 |
| Cd7-MT2 | 5 10-15 |
| Cu(I)-MT estimation | ~10-17 (ref. 6) |
The selectivity of MTs for Zn(II), Cu(I) and Cd(II) metal ions is directly linked to the Hard and Soft Acid Base (HSAB) theory developed by Pearson according to which soft bases react preferentially with soft acids. Hence thiolate is expected to bind preferentially to Cu(I) > Cd(II) > Zn(II) according to the softness of the overall metal ions, corresponding to Kd of 10-19 M, 10-15 M and 10-12 M, respectively. 8 Despite the higher affinity of Cu(I) compared to Zn(II) (several orders of magnitude), MT can bind Zn(II) in vivo, because Zn(II) is more bioavailable than Cu(I). Thus if MT bind Zn(II) and/or Cu(I) in vivo, depends on the affinity of Cu(I) and Zn(II) to the MT and the bioavalability of these metals. Latter, can depend on cell type, localization and physiological conditions.9 Note, that these considerations are limited to thermodynamics. They do not include kinetics, which could also influence the metal content.
In conclusion, mammalian MTs have versatile metal binding properties. Under normal conditions, Zn(II) and Cu(I) are the most prevalent metal ions bound. While all MTs prefer thermodynamically Cu(I) over Zn(II), each MT has a relative preference for Zn(II) or Cu(I). For instance, MT3 is more prone to bind Cu(I) (than Zn(II)) compared to MT1. Depending on the availability, the metal ion content can be different, not only by the nature of the metal ion (Cu(I) vs Zn(II)), but also by stoichiometry, as the affinities of each site are, at least for Zn(II), not the same. Note, that these considerations are limited to thermodynamics. They do not include kinetics, which could also influence the metal content.
1.2.b. Structure
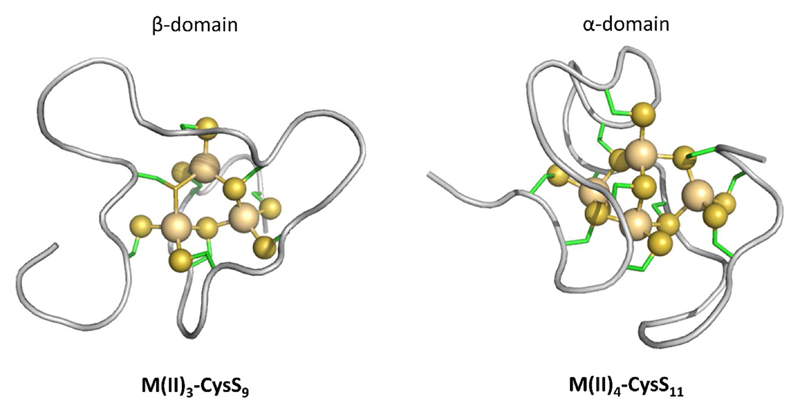
While unstructured in their apo-form, MTs acquire defined 3D structures upon metal ion binding. Several 3D structures of MTs have been determined, mainly by NMR but also by X-ray. For NMR analyses, 11 Cd(II) was often used to replace Zn(II), as Cd(II) possess isotopes with a nuclear spin I=½. MT1-2 structure is characterized by two domains in the form of a dumbbell. Each domain contains a metal-thiolate cluster, M3-S9 in the N-terminal β-domain and a M4-Cys11 in the C-terminal α-domain (Figure 2). For MT3 only the structure of the C-terminal α-domain with Cd(II) has been solved, showing a similar M4-Cys11 cluster. Spectroscopic data indicate a M3-Cys9 cluster in the β-domain, but with greater dynamic exchange than in MT1-2. This highly dynamic process has been assigned to the presence of the TCPCP motif (amino acids 5-9, Figure 1), as engineering this motif into MT1 yielded a MT with dynamics and bioactivity similar to MT3.12 Zn(II) binding to MT-1/2 has recently been demonstrated to follow a non-cooperative pathway at physiological pH in which Zn(II) is initially bound terminally to the cysteine thiols of MTs, making bead-like structures, preceding the generation of the Zn(II)-thiolate clusters observed in Zn(II)7MTs.13,14
Figure 2.
Scheme of the divalent metal-thiolate clusters in mammalian MTs as exemplified by the structure of human Zn7MT2. Left: M3-Cys9 cluster in the N-terminal β-domain, right: M4-Cys11 cluster in the C-terminal α-domain, (PDB: 2MHU and 1MHU respectively). M(II) is Zn(II) or Cd(II) (gold spheres), S (yellow spheres) represents the sulphur of the thiolate function from Cys residues (side chains in green). Cluster structures have been determined by X-ray and/or NMR for MT1-2. For MT3, only the structure of the M4-Cys11 cluster has been determined by NMR. For the M3-Cys9 cluster the structure is hypothetical.
No structure including the Cu(I)-thiolate cluster of mammalian MTs has been reported.6 The only structures available that include Cu(I)-thiolate clusters are for yeast and N. crassa MT, which possess a different array of cysteine coordinating residues and different protein folds compared to mammalian MTs. These large differences hamper the prediction of mammalian Cu(I)-MT structures based on the known strucutres from yeast and N. crassa. However, in mammalian MT-1, the structure of the polypeptide chain (without the metal-thiolate connectivity) has been compared between the Cu(I) and the Zn(II) loaded form. Addition of Cu(I) to Zn(II)-MT1 induced a strong modification of the protein folding, due to Cu(I) binding and Zn(II) release in the α- and β-domain. Thus, the peptide fold of both domains is highly different between the Cu(I)-MT1 and Zn(II)-MT1, in line with spectroscopic evidence that Cu(I) is generally bound trigonally and digonally to MTs.8 Overall, biophysical studies demonstrated that Cu(I)-loaded MT can bind up to 12 Cu(I) ions organized in two separated Cu(I)6-thiolate clusters, with distinct stable Cu(I)8-MT intermediates formed in the pathway of cluster assembly. Two separated Cu(I)4-thiolate clusters with short Cu-Cu distances (< 2.8 Å) are present in Cu(I)8-MT. The first Cu(I)4-thiolate cluster is formed in the β-domain, followed by the second one in the α-domain. By further Cu(I) addition, these Cu(I)4-thiolate clusters can be expanded to the Cu(I)6-cores resulting in increased Cu-Cu distances and susceptibility to oxidation (at least in MT-3).6
1.2.c. Metal buffering capacity
As highlighted in table 1, MT affinity for Zn(II) corresponds to cellular pZn values typical of cells with free Zn buffered at pM levels.(7 and references therein) Thus, under physiological conditions MTs possess appropriate metal-binding properties for buffering fluctuations in the free Zn concentration in the cell, although other biomolecules may also participate in Zn-buffering.15 Based on the MT structure, this buffering capacity stems from the capability of MT to accommodate a dynamic transition from tetrathiolate coordination in partially metallated MTs (in which Zn can be coordinated exclusively by terminal thiolate ligands) to fully metallated forms (in which some of the thiolates are present as µ2-bridging ligands). Based on the Kds for Zn (table 1), MT-2 can exist in forms ranging from Zn4MT to Zn7MT indise cells. This allows efficient Zn buffering capacity to match the cellular concentrations of both resting and transient Zn concentrations. (7 and references therein).
1.2.d. Reactivity
Generally, the reactivity of the two clusters is similar, meaning that reaction mechanisms and products are often the same. However, they can differ in the detailed mechanisms and, in particular, in the kinetics of the reaction. Often the Zn3/Cd3-Cys9 cluster in the β-domain is more reactive, likely due to the higher flexibility and solvent exposure. This difference is even more pronounced in MT3 than MT1/2.
(i) Metal-binding and exchange
As stated above, apo-MT1-3 are intrinsically disordered and gain a defined 3D structure upon metal binding. Metal binding to apo-MTs is fast (sub seconds time scale) as little steric hindrance occurs in the disordered peptide chain. Furthermore, in line with the much stronger affinity of Cu(I) compared to Zn(II) previously detailed, addition of Cu(I) to Zn-MTs in vitro results in the binding of Cu(I) to the thiolates, thus replacing and inducing the release of several Zn(II) ions. Other metal ions can also bind, like Pt(II), Ag(I), Hg(II) etc. Despite the quite high affinity (see previous §), the exchange of metal ions between the domains and between two MTs can be relatively fast (faster than minutes16). This points to the importance of an associative metal ion transfer between the two partners, because a dissociative metal ions transfer would be determined by the off rate of the metal ion from the MT, which would be, at least for Cu(I), too slow (t1/2 of days to years, estimated based on a Kd of ~10-17 M and a diffusion controlled kon). This is conceivable because the metal-thiolate clusters are partially exposed to the surface in these relatively small proteins (compared to the high content of metal ions). This property makes MTs relatively unique in being a high affinity metalloprotein with kinetic lability, which would be in line with a physiological role in metal ions transport, exchange and/or buffering. Thus, Zn-MTs would react rapidly upon fluctuations in Zn concentrations, faster than regulation via synthesis of Zn transporters.
(ii) Redox-dependent activity
MTs bind metal ions through thiolate functions that can be easily oxidized to disulphide bridges. Maret and Vallee 17 reported a very low redox potential of the clusters (Eo′ < −340 mV), lower than well-known reducing biomolecules like glutathione, NADH or thioredoxin. Thus MTs can be oxidized by mild cellular oxidants and their redox properties depend on metal-load (type of metals and stoichiometry). Thus, the metal binding event is redox dependent even for the redox silent metal ions Zn(II) and Cd(II). Oxidation of the coordinating Cys ligands leads to the release of the coordinated metal ions. This reaction is reversible and is a very important connection, linking metal binding ability to redox reactions.17
There are several ways to oxidize the cysteines, including the reaction with Cu(II) (Scheme 1). Cu(II) reacts with MTs, first by reduction of Cu(II) to Cu(I) by Cys and then Cu(I) subsequently binds to the non-reduced Cys. This also occurs with Zn-MTs.8
Scheme 1.
Types of reactions of MTs as discussed in the text.
Other Cys oxidation reactions can be triggered by ROS, in particular H2O2, O2•- or HO•. Rate constants of the order of kHO•/MT around 1012 M-1 s-1 and kO2•- /MT around 5x105 M-1s-1 were found.18 This indicates that a ROS signal could be translated into metal ion, i.e. under oxidative stress in a cell, Zn(II) could be released from MTs.
(iii) Reactivity towards electrophiles
Cysteines/cysteinates are good nucleophiles and hence reactions with electrophiles consume the thiol/thiolates leading to the release of metal ions (as for cysteine oxidation described in the previous §). This can apply to metal ions that are better electrophiles than Cu(I) or Zn(II) including several organic reagents such as alkylating reagents or NO 4 (and ref. therein) (Scheme 1) and metal ions such as Pt(II), Ag(I), Hg(II), etc.
2. Zinc and copper homeostasis and relationship to MTs
We will give just a brief overview about role of MTs in biology and their implication in neurodegenerative disorders (ND). Several reviews can be found in the literature (for recent reviews see e.g.19–21)
2.1. Zinc homeostasis in cells
Several Zn transporters are known from the literature: ZIP (ZRT, IRT-like proteins) type transporters catalyse the translocation of Zn into cells across membranes, whereas ZnT transporters translocate Zn out of the cytosol using the proton motive force. There are several subtypes known, some are responsible for transporting Zn in or out of other cellular compartments (like synaptic vesicles or lysosomes).
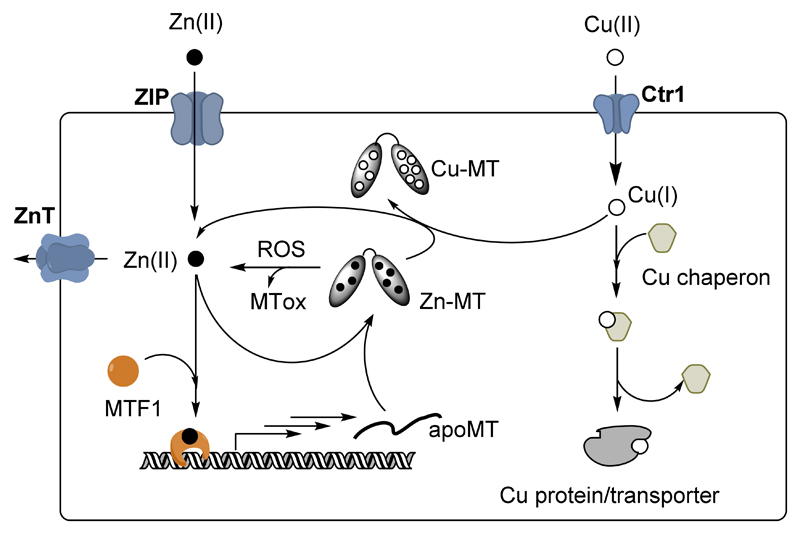
MT1-2 expression is under the control of the transcription factor MTF1.22 MTF1 is Zn dependent, i.e. if the Zn concentration rises in the cell, Zn binds to MTF1, which in turn induces the transcription of MT genes as well as several Zn transporters. The de novo synthesized apo-MT binds the Zn in the cell until the Zn concentration drops to the point that Zn is released from at least 2 of the 6 zinc fingers present in MTF1. This stops the synthesis of apoMT (see Figure 3). From this mechanism, it is clear that MTs are major components in the control of Zn concentrations in the cell and that the affinities of the Zn-binding sites in MTs and the MT concentration govern the level of free Zn in a cell. In light of this mechanism, it appears that the Zn and MT concentrations are inter-dependent. Chemical reactions such as those described in § 1.2.c.(iii) that result in Zn release induce the synthesis of apo-MTs. In other words, an increase in ROS, Cu concentration, presence of alkyl agent, etc. can result first in an increase of the Zn concentration and then in the synthesis of MTs.
Figure 3.
Schematic view of Zn and Cu metabolism in a cell focusing on the role of MTs (the scheme is restricted to the discussion here, for more details see e.g.25). Zn is imported by ZIP and exported by ZnT-type transporters. MT synthesis is regulated by the transcription factor MTF1. An increase in intracellular Zn activates MTF1 via Zn-binding. Activated MTF1 triggers the synthesis of apo-MT, which can coordinate the free Zn. Thus, free Zn concentration decreases and MTF1 become inactivated (release of Zn). Increase of free Zn can occur due to an imbalance between import and export or via Zn release from MTs. The latter can occur via Cu-binding to MTs, MT oxidation due to ROS or other compounds attacking the cysteines in MTs. Cu is imported via the transporter Ctr1 and shuttled to the target (protein or transporter) by Cu-chaperones.
The concerted actions of MTs with Zn transporters under the control of MTF-1 play central roles in two phenomena: “zinc buffering” and “zinc muffling”. MT dependent zinc buffering maintains free zinc cytosolic concentrations at pM levels, while muffling is responsible for the modulation of transient changes in zinc concentrations, in particular during signaling, by translocating Zn(II) out of the cells or into intracellular compartments.23
The MT buffering capacity of partially metallated MTs allows control of zinc concentration changes arising at least from three different cellular processes: i) signalling via zinc release caused by reactive oxygen species; ii) zinc signalling via release from the sarco/endoplasmic reticulum pools; iii) exocytosis of zinc filled vesicles upon membrane fusion (e.g. in zincergic neurons, see 2.3). (7 and references therein).
Moreover, recent work suggests that cooperative expression and activity of MT and ZnT transporters is necessary and responsible for the activation of zinc-dependent extracellular enzymes.23 (and ref. therein)
2.2. Copper homeostasis
Cu is imported into the cell mainly via Ctr1, which translocates it in the reduced state. Cu(I) is then shuttled to Cu-proteins (like Cytochrome-c oxidase in the mitochondria or superoxide dismutase in the cytosol) by specific Cu(I) chaperons. Together with Cu,Zn-SOD1, metallothioneins have the highest affinity for copper(I), but for kinetic reasons they cannot typically demetallate copper enzymes.24 As a result, under normal conditions, MT1-2 contain only Zn(II) and no significant amount of Cu(I) is bound. Under Cu overload conditions only, such as Wilson disease, Cu binds to MT1-2 inducing the release of Zn(II). Under these conditions, a different localization of Cu-MTs occurs in lysosomes compared to cytosolic Zn-MT. Whether Cu is bound to MT3 under normal conditions is not completely clear, but it is more likely than for MT1-2.1,5 It is emerging that MT-3 may play special roles in modulating cellular copper levels and potentially trafficking copper in specific organs and tissues, like the central nervous system.
2.3. Zn in zincergic neurons
A subset of glutamatergic neurons contains high amounts of Zn (mM) in their synaptic vesicles. Zn is transported via the ZnT3 transporter into these vesicles. Upon neuronal activation, these vesicles fuse with the cell membrane and Zn is released in the synaptic cleft. Locally, transient concentrations of up to several 100 µM have been detected. Zn is then rapidly taken up again by the neurons. It has been shown that this Zn-pool, released from neurons, plays a neuromodulatory role.26 MT3 is also particularly concentrated intraneuronally in Zn rich regions and it was suggested that MT3 is implicated in the metabolism of this Zn-pool. In addition, the extracellular Zn-pool may be able to bind to the amyloid-β peptide in Alzheimer’s disease.27 Similar events might also be observed with the prion protein or the α-synuclein, in Prion related disorders and Parkinson’s disease, respectively.
It is important to note that MT1-3 could also occur extracellularly. This indicates that MTs could play a role in the Zn metabolism around the synaptic cleft or could interact with extracellular proteins such as amyloid-β, α-synuclein or prion protein (Figure 4). Thus, the non-cooperative zinc buffering capacity of the partially metallated forms of MTs potentially modulate the available levels of free zinc in zincergic neurons and synaptic clefts.
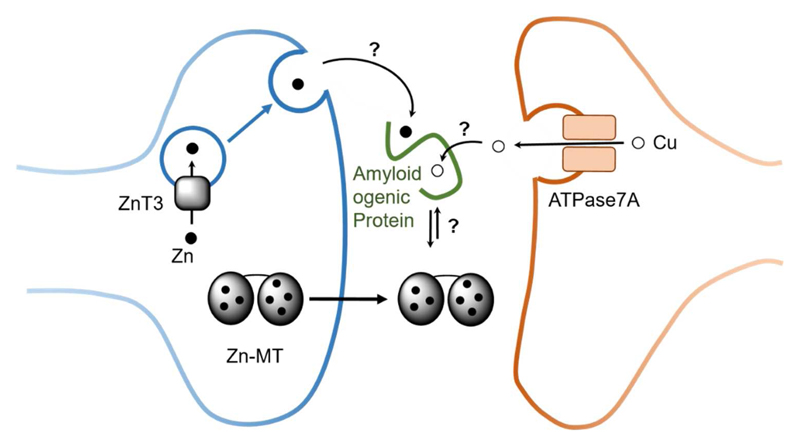
Figure 4.
Schematic view of Zn (black circle) and Cu (hollow circle) in certain synaptic cleft and the role of MTs (for more details see 26). Although MTs are mainly intracellular proteins, they can also occur extracellularly and hence potentially in the synaptic cleft. In such a case, they could encounter and interact with synaptic Zn and Cu, as well as with extracellular amyloidogenic proteins (Prion, amyloid-β, α-synuclein). Zn can be released in certain neurons into the synaptic cleft from vesicles in high concentration upon stimulation. Zn is rapidly taken up, thus there is a transitory increase in Zn concentration. Cu might be released into the synapse upon the translocation of the Cu-transporter ATPase7A upon stimulation of the glutamate receptor NMDA.
Evidence from the literature suggests that Cu that is pumped by the ATPase7A Cu-transporter into vesicles could be released into the synaptic cleft via vesicle fusion.28 The oxidation state of vesicular Cu and its ligand(s) is not known. Although not demonstrated, the Cu pool bound to amyloid-β in amyloid plaques in AD might stem from the Cu released into the synaptic cleft.
2.4. Zn, Cu and MTs under pathological conditions (in neurodegenerative disorders)
There is ample evidence that MT expression is affected in neurodegenerative diseases. Indeed, oxidative stress occurs in a vast majority of neurodegenerative diseases (including AD, PD and prion disease). This is due, among other, to an imbalance of ROS (O2•-, H2O2, HO•) production and defence, leading to an accumulation of ROS. MTs belong to the antioxidant defence system, as they are efficient ROS scavengers.18 This might explain the upregulation of MT1-2 in AD. In addition, astrocyte-released MTs protect dopaminergic neurons, which are degenerated in PD.3
Cu and Fe play an important role in oxidative stress, because they can efficiently catalyse the production of ROS. This happens with Fe enzymes in a controlled way such as with NADPH oxidase or by “loosely bound” Cu and Fe ions. The latter s catalyse Fenton-type reactions leading to the highly reactive HO• radical. Conversely, the same metal ions are also crucial for antioxidant defence, since Cu is the catalytic centre of SOD and Fe of catalase.
A large body of evidence suggests that metal dyshomeostasis occurs in neurodegenerative diseases. However, as for MTs, whether dysegulation of these metal ions and associated metalloproteins is a cause or a consequence is unclear. In particular, Zn and Cu metal ions have been suggested to bind to amyloidogenic peptides and proteins in several neurodegenerative diseases. This will be described for amyloid-β in AD, prion protein and α-synuclein in PD in the next paragraph.
2.5. Amyloidogenic proteins and neurodegeneration
2.5.a. General features
Neurodegenerative diseases are characterized by accumulation of misfolded/aggregated proteins in areas where neurodegeneration is found. These aggregates are formed by the misfolding of various proteins and peptides depending on the illness and accumulate in a variety of tissues.
The link between neurodegenerative diseases and amyloidogenic peptides/proteins lies in the neurotoxicity of the abnormal deposits of misfolded peptides and proteins comprising polymorphic oligomers and fibrils rich in β-sheet structure. Generally, soluble oligomeric species are considered to be the most toxic type of aggregates.29 Alzheimer’s, Parkinson’s and Prion diseases all share these common hallmarks. The accumulation of aggregates leads to the formation of extracellular amyloid or senile plaques in AD together with neurofibrillary tangles, intracellular Lewy’s bodies in PD and amyloid-like plaques in prion diseases.30 There is also evidence of oxidative stress in the affected areas of the CNS of the three diseases, where oxidative stress can produce neuronal death by many different pathways.31
2.5.b. Role of metal ions in neurodegenerative diseases
Metal ion dyshomeostasis has been linked to AD, PD and prion diseases,30 and could be a key factor in their development, as metals can greatly impact the aggregation and redox activity of the implicated peptides and proteins.
Several studies have found a relatively high concentration of metal ions (Zn, Cu and Fe) in aggregates such as senile plaques Lewy’s bodies, and PrP fibrils.30,32 We will focus on Cu and Zn, as both metal ions can be released from neurons and are found at relatively high concentration in the synaptic cleft (see section 2).
(i) Coordination of Cu and Zn to the amyloidogenic peptides/proteins
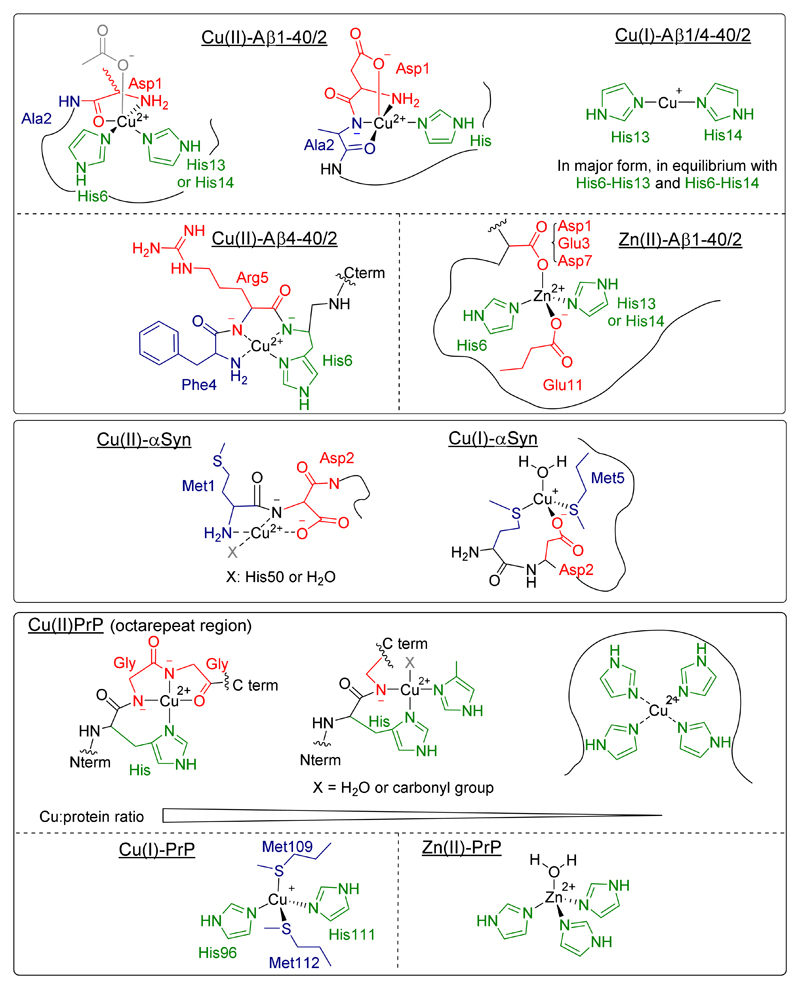
The coordination of metal ions to the different amyloidogenic peptides and proteins has been thoroughly studied in the test tube and, although there is still debate regarding some coordination modes, the most predominant structures at physiological conditions for amyloid-β peptide (full-length and the form truncated at position 4), α-synuclein and prion protein (PrP) (see Figure 5) are widely accepted.
Figure 5.
Most relevant coordination modes of Cu(II), Cu(I) and Zn(II) to different amyloidogenic peptides and proteins at physiological pH: (a) amyloid-β; (b) amyloid-β truncated (4-x); (c) α-synuclein; (d) prion protein (in the octarepeat region, the coordinating ligands depend on the Cu to peptide ratio).
(ii) How metal ions influence aggregation and oxidative stress
Metal ions such as Cu and Zn can impact the aggregation of intrinsically disordered peptides and proteins. In the case of the amyloid-β peptide the influence of metallic ions has been reviewed recently (e.g. 30,32). There is also in vitro evidence of the impact of metal ions on the fibrillation of α-synuclein. The influence on PrP misfolding is still not clear, as both promotion and inhibition of fibrils formation have been proposed for Cu(II) and Zn(II). There is strong evidence of oxidative stress affecting different areas of the brains of ND patients, such as lipid peroxidation, oxidized proteins and DNA oxidative damage.31 The metal-induced pathogenesis, and the consequent oxidative damage in ND, might arise from the production of ROS catalysed by loosely bound Cu(II) in the presence of physiological reductants such as ascorbate.
3. Interaction of MTs with amyloidogenic peptides and metallated counterparts
3.1. Interaction of MTs with Aβ
The interaction of Zn7-MT3 with apo-Aβ has been studied with respect to the H2O2-induced transfer of Zn(II) between the two proteins.33 H2O2 promotes Zn induced Aβ aggregation via slow cysteine oxidation and Zn(II) release from the Zn7-MT3. In addition, even in the absence of H2O2, a triggering of Aβ aggregation was observed, assigned to a minor leaking of Zn from MT3 (it has not been reported if it is due to a slow minor oxidation of Cys in MT3 or to Zn transfer to Aβ due to thermodynamic equilibrium, i.e. without Cys oxidation from the lower Zn(II) affinity sites). However, it is known that substoichiometric amounts of Zn(II) can have a strong impact on Aβ aggregation.
3.2. Interaction of MTs with Cu-complexes of Aβ, α-synuclein, prion and PolyQ
During the last decade, interest has arisen on the interaction of MTs and Cu complexes of amyloidogenic peptides and proteins. This will be illustrated below in details with the Aβ peptide.
3.2a. Aβ
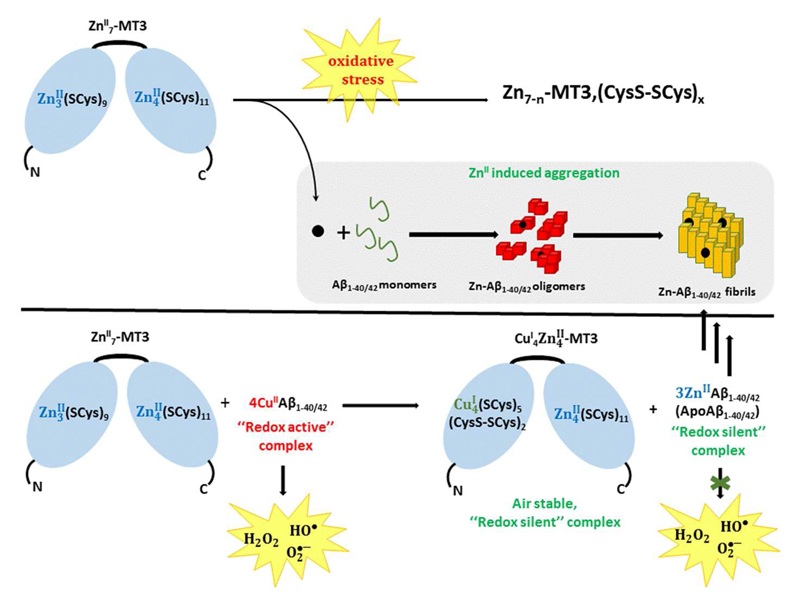
As described above, Aβ binds Zn and Cu ions in amyloid plaques in vivo. MTs bind either metal as well and are therefore a potential candidate to exchange Zn and Cu with Aβ. In vitro studies showed that Zn7-MT3 or Zn7-MT1/2 react rapidly with Cu(II)-Aβ species by a swap of the metal ions.34 This results in Cu binding to MT and Zn binding to Aβ (Figure 7). Cu(II) is first reduced to Cu(I) by cysteines of the MT. It could be shown that at stoichiometric ratios below 4 of Cu-Aβ per MT3 the reaction yielded a relatively defined species.34 (and ref. therein) It was proposed that the Zn3(SCys)9 cluster in the β-domain reacts leading to two disulphide bridges and fixation of four Cu(I) ions by the 5 remaining non-reduced Cys residues resulting in a Cu(I)4S5 cluster. Given that the adamantane-like M4S6 polyhedron is the most frequently observed species in copper-thiolate chemistry, the additional coordination by a disulphide sulphur cannot be excluded. 4 Zn ions remained bound, likely in an intact Zn4(SCys)11 of the α-domain. The reason for this specificity of the reaction towards the β-domain is not known yet.
As discussed above (§ 2.5.b.(ii)) Cu(II)-Aβ is able to catalyse the production of ROS in the presence of ascorbate and dioxygen. Addition of MT3 stopped this production of ROS, suggesting that Cu-bound to MT3 in the form of Cu(I)4Zn4-MT3 has no ROS production ability. In other words, MT can redox-silence Cu-Aβ species. This is quite remarkable for the often-unstable Cu(I)-thiolate bonds under aerobic conditions. In addition, tests in cell culture showed that MT3 can protect cells against toxicity of Cu(II)-Aβ in the presence of ascorbate and dioxygen, supporting its role as suppressor of Cu(II)-Aβ induced ROS.33
Further studies of the metal swap between Cu(II)-Aβ and Zn7-MT3 showed that it occurs via free/”loosely bound” Cu(II) and that a ternary Aβ–Cu–MT3 complex is not formed (see also above). This means that metal exchange does not require specific recognition between Aβ and Zn7–MT3. Furthermore, it was also found that the metal exchange between Zn7-MT3 and Cu(II)-Aβ aggregates was fast (< sec), but that it induces a slow structural and morphological change to Zn-Aβ fibrils.(2 and references therein)
Several forms of Aβ exist in vivo, including truncated forms. A relevant and relatively abundant form is the truncated form starting at position 4, i.e. Aβ4-40/42 (see § 2.5.b.(i)). This is relevant concerning Cu(II), because this peptide contains the so-called ATCUN motif that binds Cu(II) with relatively high apparent binding constant (conditional Kd=3.10-14 M at pH 7.4). Recently, the interaction of MT3 with Cu(II)-Aβ4-16 (mimic of the Aβ4-40/42) was studied. In contrast to what was observed for Cu(II)-Aβ1-40/42, no Cu(II) transfer occurred.35 This may be ascribed to the different Cu(II) binding site in Cu(II)-Aβ4-16 compared to Cu(II)-Aβ1-16 (see Figure 5) and to a lower off rate of Cu(II) from the truncated form compared to the Aβ1-16 peptide. However, cysteine and gluthathione are able to trigger the Cu-transfer from Cu(II)-Aβ4-16 to MT3.36
3.2.b. Other amyloidogenic peptides
Cu-αSyn is able to promote ROS production, dopamine oxidation and oligomer formation. Similarly to what was described above for Aβ, Zn7-MT3 was able to abolish these events by the same mechanism, i.e. Cu removal from α-synuclein, Cu(II) reduction by thiolate ligands of Zn7-MT3 and the formation of Cu(I)4Zn4MT3.37
Similarly, the Cu-Prion complex could be efficiently redox silenced by Zn7-MT3 by the same mechanism.38 This applied to different types of Cu(II)-coordination spheres known in PrP (See Figure 5).
MT3 also showed protection against the Huntington disease related polyQ aggregate. Hands et al.39 reported that overexpression of MT3 in mammalian cells significantly reduced polyQ aggregation and toxicity, which was proposed to be due to its Cu-binding properties.
Recently, MTs have been shown to be excellent target for directed modification of copper dyshomeostasis in mouse models of amyotrophic lateral sclerosis (ALS), characterized by aggregation of mutant forms of the Cu,ZnSOD. Aberrant copper binding/reactivity to Cu,Zn SOD in specific residues are implicated as a key process in the disease and a major cause for cellular toxicity.40,41
4. MTs as therapeutic lead in neurodegeneration
4.1. Biological background
As previously detailed, interaction between MTs and metal ions in ND context has been the subject of many studies. Most of them show a consensus on the upregulation of MT1-2 assigning them a neuroprotective role (for recent reviews see references19,20). The protection was assigned to binding of toxic Cu pools and MTs antioxidant properties. The deficiency of MT1-2 in Tg2576 mice, an animal model of AD, decreased the formation of amyloid plaques, in line with a more recent work reporting that the overexpression of MT1 increased the amyloid plaque burden in the hippocampus.42 (and ref therein) This seems to be the opposite of the protection of MT against Cu-induced aggregation. However, this is in line with reports showing that the amount of plaques are not directly correlated to the neurotoxicity and that aggregation intermediates (e.g. soluble oligomers) are more important.43
Meanwhile, the expression of MT3 in ND is more controversial. Some studies have found a down-regulation in AD brains, while others do not find altered MT3 levels.20 As detailed in section 3, in vitro studies showed a neuroprotective effect of Zn7-MT3 upon metal-swap with different Cu-protein/peptides connected to ND. The toxicity associated with Zn and Cu as well as the ability of MT3 to overcome it was verified in a transgenic Drosophila model.44 Meanwhile, studies in a transgenic mouse model of AD showed that MT3 deficiency rescued partially the mortality. In contrast, injection of exogenous Zn7-MT3 increased the concentration of soluble Aβ40 and Aβ42, as well as plaques, together with an improvement on behaviour and anxiety levels.42 (and ref. therein) Once again, this might reflect that amyloid plaques are not the best markers of the disease.
In general, in vivo data show often modest effect of MTs in ND models and data about their protective efficiency are not clear. Depending on the conditions and models, up- or downregulation of MTs can be protective. Further studies are needed to better define the effects, as in vivo, several other factors are important: localisation of MT, timing of expression, compensatory effects in transgenic models, etc.
4.2. Therapeutic approaches inspired by MTs
Based on the putative protective role of MTs in neurodegenerative diseases, several therapy approaches could be proposed: (i) metallothionein mimics, (ii) metallothionein regulation, (iii) metal-swap between a Zn-loaded ligand and Cu(Aβ) species and (iv) Cu(I) chelators.
-
(i)
The administration of exogenous MTs is limited by its passage through the intact blood-brain barrier. To overcome this issue, a MT mimic peptide capable of passing the BBB, EmtinB, showed in vitro and in vivo protection against kainic acid-induced neurotoxicity, similar to that of MT1-2.45 To the best of our knowledge, the neuroprotective effect of this peptide has not been extrapolated to ND.
-
(ii)
MTs up-regulation by “activators” might also be a way to counteract AD, but to the best of our knowledge there is no report on that approach. This strategy might be more relevant for MT1/2, as they are inducible with a multitude of compounds, which is not the case for MT3. For example, dexamethasone potently induces MT biosynthesis and has been demonstrated to protect against copper dyshomeostasis in models of both ALS and α-synucleinopathy.3,46 Very recently, Roy et al. reported on benzothiazolone-2 that was able to enhanced MT-3 protein and mRNA levels in cell cultures and would be of interest to be tested in AD model, organisms.47
-
(iii)
Metal-swap between Zn7-MT and Cu(Aβ) species has shown success in rescuing amyloid neurotoxicity in vitro. Alternatively, chelation therapy has been proposed by Bush and co-workers who have investigated a series of chelators as effective inhibitors of amyloid aggregation.30 Taking into account the chelating capacity of MTs, a first approach could be proposed: use of Zn-loaded ligands which could promote the metal-swap with Cu(Aβ) species. This strategy has been recently considered by our group.48 It is important for this strategy to take into account the effect of Zn(II) on the chelation of Cu(II), as Zn can be found in much higher concentration in the synaptic cleft and could impede Cu removal from Aβ species.
-
(iv)
The second Cu-removal-based approach consist of Cu(I) chelation, since Cu is sequestered in MTs in this oxidation state. Most Cu(I) chelators have been studied for the treatment of Wilson’s disease. In the context of AD, almost exclusively Cu(II) chelators have been investigated.30,48,49 The first Cu(I) ligands were described by Cherny et al. and were further functionalized as nanoparticles.50 Our group demonstrated the effectiveness of 1,3,5-triaza-7-phosphaadamante (PTA) in vitro, a known water-soluble phosphine to remove Cu from Aβ peptide and, consequently, stop the production of ROS due to the formation of an stable Cu(I) complex.51
There are still many aspects to clarify for the function of MTs in the development of neurodegenerative diseases, it is clear that they play an important role, especially regarding their interaction with metal ions and oxidative stress. Thus, we would expect further studies on the use of these characteristic metalloproteins and their mimics therapeutically; either by mimicking their chemistry for the development of new chelators, or via the control of their expression for optimal regulation of metal ions in the brain.
Supplementary Material
Key learning points.
-
(1)
Metallothioneins (MTs) bind Zn(II) and Cu(I) tightly (thermodynamically) but exchange Zn(II) and Cu(I) rapidly (kinetically labile). MTs are important to buffer and muffle Zn concentrations.
-
(2)
Metallothioneins constitute a hub, which can connect Cu and Zn metabolism, as well as connecting oxidative stress to Zn release via cysteine oxidation.
-
(3)
MTs are involved in many neurodegenerative diseases and can have protective effects (as metal chelators and/or antioxidants).
-
(4)
How MTs could serve as an inspiration for metal-based therapeutic strategies.
Figure 6.
Top: How Zn-MT could impact aggregation of Aβ under oxidative stress conditions: ROS induces the release of Zn(II) ions that could bind to Aβ and modulate its aggregation behaviour. Bottom: Metal-swap mechanism: Zn-MTs can react rapidly with Cu(II)-Aβ to form –CuI4ZnII4-MT and Zn-Aβ. The Cu(II) is reduced by the Cys in MTs. Cu(I) bind preferentially to the β-domain, in which Cys oxidation also occurs.
Acknowledgements
Alice Santoro is supported by a PhD grant from the IDEX program of the University of Strasbourg. We acknowledge financial support of Menzies Health Institute Queensland and Griffith University (DLP), University of Strasbourg Institute for Advanced Study (USIAS) (PF and CH), and the Frontier Research in Chemistry Foundation (Strasbourg) (PF). Gabriele Meloni is supported by funds from the University of Texas at Dallas and by the Robert A. Welch Foundation (Grant: AT-1935-20170325). E.A.B And C.H. thank the ERC StG-638712 for financial support.
References
- 1.Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M. Neuron. 1991;7:337–347. doi: 10.1016/0896-6273(91)90272-2. [DOI] [PubMed] [Google Scholar]
- 2.Vašák M, Meloni G. Int J Sci Mol. 2017;18:1117. doi: 10.3390/ijms18061117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okita Y, Rcom-H’cheo-Gauthier AN, Goulding M, Chung RS, Faller P, Pountney DL. Front Neurosci. 2017;11:1–9. doi: 10.3389/fnins.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faller P. FEBS J. 2010;277:2921–2930. doi: 10.1111/j.1742-4658.2010.07717.x. [DOI] [PubMed] [Google Scholar]
- 5.Artells E, Palacios Ò, Capdevila M, Atrian S. FEBS J. 2014;281:1659–1678. doi: 10.1111/febs.12731. [DOI] [PubMed] [Google Scholar]
- 6.Calvo J, Jung H, Meloni G. IUBMB Life. 2017;69:236–245. doi: 10.1002/iub.1618. [DOI] [PubMed] [Google Scholar]
- 7.Krężel A, Maret W. Int J Mol Sci. 2017;18:1237. doi: 10.3390/ijms18061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vašák M, Meloni G. J Biol Inorg Chem. 2011;16:1067–1078. doi: 10.1007/s00775-011-0799-2. [DOI] [PubMed] [Google Scholar]
- 9.Palacios O, Pagani A, Pérez-Rafael S, Egg M, Höckner M, Brandstätter A, Capdevila M, Atrian S, Dallinger R. BMC Biol. 2011;9:4. doi: 10.1186/1741-7007-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krężel A, Maret W. J Am Chem Soc. 2007;129:10911–10921. doi: 10.1021/ja071979s. [DOI] [PubMed] [Google Scholar]
- 11.Armitage IM, Drakenberg T, Reilly B. Met Ions Life Sci. 2013;11:117–144. doi: 10.1007/978-94-007-5179-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero-Isart N, Jensen LT, Zerbe O, Winge DR, Vašák M. J Biol Chem. 2002;277:37023–37028. doi: 10.1074/jbc.M205730200. [DOI] [PubMed] [Google Scholar]
- 13.Irvine GW, Pinter TBJ, Stillman MJ. Metallomics. 2016;8:71–81. doi: 10.1039/c5mt00225g. [DOI] [PubMed] [Google Scholar]
- 14.Jayawardena DP, Heinemann IU, Stillman MJ. Biochem Biophys Res Commun. 2017 doi: 10.1016/j.bbrc.2017.08.137. [DOI] [PubMed] [Google Scholar]
- 15.Petering DH, Mahim A. Int J Mol Sci. 2017;18:1289. doi: 10.3390/ijms18061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nettesheim DG, Engeseth HR, Otvos JD. Biochemistry. 1985;24:6744–6751. doi: 10.1021/bi00345a003. [DOI] [PubMed] [Google Scholar]
- 17.Maret W, Vallee BL. Proc Natl Acad Sci USA. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornalley PJ, Vašák M. Biochim Biophys Acta (BBA)/Protein Struct Mol. 1985;827:36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- 19.Adam P, Křížková S, Heger Z, Babula P, Pekařík V, Vaculovičová M, Gomes CM, Kizek R, Adam V. J Alzheimer’s Dis. 2016;51:637–656. doi: 10.3233/JAD-150984. [DOI] [PubMed] [Google Scholar]
- 20.Bolognin S, Cozzi B, Zambenedetti P, Zatta P. J Alzheimer’s Dis. 2014;41:29–42. doi: 10.3233/JAD-130290. [DOI] [PubMed] [Google Scholar]
- 21.Szewczyk B. Front Aging Neurosci. 2013;5:1–12. doi: 10.3389/fnagi.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westin G, Schaffner W. EMBO J. 1988;7:3763–3770. doi: 10.1002/j.1460-2075.1988.tb03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura T, Kambe T. Int J Mol Sci. 2016;17:336. doi: 10.3390/ijms17030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banci L, Bertini I, Ciofi-Baffoni S, Kozyreva T, Zovo K, Palumaa P. Nature. 2010;465:645–648. doi: 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- 25.Maret W. Adv Nutr An Int Rev J. 2013;4:82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sensi SL, Paoletti P, Bush AI, Sekler I. Nat Rev Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 27.Lee J-Y, Cole TB, Palmiter RD, Suh SW, Koh J-Y. Proc Nat Acad Sci USA. 2002;99:7705–7710. doi: 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlief ML, Craig AM, Gitlin JD. J Neurosci. 2005;25:239–246. doi: 10.1523/JNEUROSCI.3699-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagel-Steger L, Owen MC, Strodel B. ChemBioChem. 2016;17:657–676. doi: 10.1002/cbic.201500623. [DOI] [PubMed] [Google Scholar]
- 30.Barnham KJ, Bush AI. Chem Soc Rev. 2014;43:6727–6749. doi: 10.1039/c4cs00138a. [DOI] [PubMed] [Google Scholar]
- 31.Tramutola A, Lanzillotta C, Perluigi M, Butterfield DA. Brain Res Bull. 2016:1–9. doi: 10.1016/j.brainresbull.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Nasica-Labouze J, Nguyen PH, Sterpone F, Berthoumieu O, Buchete N-V, Coté S, De Simone A, Doig AJ, Faller P, Garcia A, Laio A, et al. Chem Rev. 2015;115:3518–3563. doi: 10.1021/cr500638n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durand J, Meloni G, Talmard C, Vašák M, Faller P. Metallomics. 2010;2:741–4. doi: 10.1039/c0mt00027b. [DOI] [PubMed] [Google Scholar]
- 34.Meloni G, Sonois V, Delaine T, Guilloreau L, Gillet A, Teissié J, Faller P, Vašák M. Nat Chem Biol. 2008;4:366–372. doi: 10.1038/nchembio.89. [DOI] [PubMed] [Google Scholar]
- 35.Wezynfeld NE, Stefaniak E, Stachucy K, Drozd A, Płonka D, Drew SC, Krężel A, Bal W. Angew Chemie Int Ed. 2016;55:8235–8238. doi: 10.1002/anie.201511968. [DOI] [PubMed] [Google Scholar]
- 36.Santoro A, Wezynfeld N, Vašák M, Bal W, Faller P. Chem Commun. 2017 doi: 10.1039/C7CC06802. [DOI] [PubMed] [Google Scholar]
- 37.Meloni G, Vašák M. Free Radic Biol Med. 2011;50:1471–1479. doi: 10.1016/j.freeradbiomed.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Meloni G, Crameri A, Fritz G, Davies P, Brown DR, Kroneck PMH, Vašák M. ChemBioChem. 2012;13:1261–1265. doi: 10.1002/cbic.201200198. [DOI] [PubMed] [Google Scholar]
- 39.Hands SL, Mason R, Sajjad MU, Giorgini F, Wyttenbach A. Biochem Soc Trans. 2010;38:552–558. doi: 10.1042/BST0380552. [DOI] [PubMed] [Google Scholar]
- 40.Tokuda E, Watanabe S, Okawa E, Ono S-I. Neurotherapeutics. 2015;12:461–476. doi: 10.1007/s13311-015-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashimoto K, Hayashi Y, Watabe K, Inuzuka T, Hozumi I. Neuroscience. 2011;189:293–298. doi: 10.1016/j.neuroscience.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 42.Manso Y, Comes G, López-Ramos JC, Belfiore M, Molinero A, Giralt M, Carrasco J, Adlard P, Bush AI, Delgado-García JM, Hidalgo J. J Alzheimer’s Dis. 2016;51:81–95. doi: 10.3233/JAD-151025. [DOI] [PubMed] [Google Scholar]
- 43.Hardy J, Selkoe DJ. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 44.Hua H, Münter L, Harmeier A, Georgiev O, Multhaup G, Schaffner W. Biol Chem. 2011;392:919–926. doi: 10.1515/BC.2011.084. [DOI] [PubMed] [Google Scholar]
- 45.Sonn K, Pankratova S, Korshunova I, Zharkovsky A, Bock E, Berezin V, Kiryushko D. J Neurosci Res. 2010;88:1074–1082. doi: 10.1002/jnr.22281. [DOI] [PubMed] [Google Scholar]
- 46.McLeary FA, Rcom-H’cheo-Gauthier AN, Kinder J, Goulding M, Khoo TK, Mellick GD, Chung RS, Pountney DL. Neurotox Res. 2017 doi: 10.1007/s12640-017-9825-7. accepted Sep 19. [DOI] [PubMed] [Google Scholar]
- 47.Roy S, Gumulec J, Kumar A, Raudenska M, Baig MH, Polanska H, Balvan J, Gupta M, Babula P, Odstrčilík J, Choi I, et al. Brain Behav. 2017;7:e00799. doi: 10.1002/brb3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atrián-Blasco E, Conte-Daban A, Hureau C. Dalton Trans. 2017;46:12750–12759. doi: 10.1039/c7dt01344b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santos MA, Chand K, Chaves S. Coord Chem Rev. 2016;327–328:287–303. [Google Scholar]
- 50.Cherny RA, Barnham KJ, Lynch T, Volitakis I, Li Q-X, McLean CA, Multhaup G, Beyreuther K, Tanzi RE, Masters CL, Bush AI. J Struct Biol. 2000;130:209–216. doi: 10.1006/jsbi.2000.4285. [DOI] [PubMed] [Google Scholar]
- 51.Atrián-Blasco E, Cerrada E, Conte-Daban A, Testemale D, Faller P, Laguna M, Hureau C. Metallomics. 2015;7:1229–1232. doi: 10.1039/c5mt00077g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.