Key Points
The diagnosis of TTP requires clinical judgment in addition to measurement of ADAMTS13 activity.
Patients with TTP may not seem to be seriously ill; they may have no or only mild neurologic and kidney function abnormalities.
Abstract
Our objective was to describe new observations from the Oklahoma Thrombotic Thrombocytopenic Purpura (TTP) Registry experience (November 1995 through December 2015) on the diagnosis of TTP along with patients’ clinical features and their outcomes. Among 363 patients with an initial episode of clinically suspected TTP, the diagnosis of TTP was supported by both ADAMTS13 activity <10% and clinical features in 78 patients (21%). ADAMTS13 activity was measured in all 363 patients by 2 methods: fluorescence resonance energy transfer (FRET) and immunoblotting (IB). Sixty patients had ADAMTS13 activity <10% by both methods, 15 had ADAMTS13 <10% only by FRET, and 3 had ADAMTS13 <10% only by IB. Five patients with ADAMTS13 activity <10% by 1 method had an alternative clinical diagnosis, not TTP. Two patients with characteristic clinical features of TTP (microangiopathic hemolytic anemia and thrombocytopenia, no alternative diagnosis) and multiple relapses initially had ADAMTS13 activity >10% by both measurements. ADAMTS13 inhibitor titers were not associated with presenting features or outcomes. Microangiopathic hemolytic anemia and thrombocytopenia were not severe in all patients. Forty-seven percent of patients had no or minor neurologic abnormalities; 95% had no or minor serum creatinine abnormalities. Ten patients (13%) died, 2 before completing 1 plasma exchange (PEX); 3 deaths were attributed to PEX complications. For patients presenting after we began using rituximab in some patients (December 2003), fewer PEX treatments were required and fewer relapses occurred. Patients with their first relapse presented with higher platelet counts and hematocrits and lower lactate dehydrogenase levels and required fewer PEX treatments compared with their initial episodes.
Visual Abstract
Introduction
During the past 20 years, thrombotic thrombocytopenic purpura (TTP) has become defined by a severe deficiency of ADAMTS13 activity,1-4 changing the way we diagnose and manage patients. Also during the past 20 years, the Oklahoma Thrombotic Thrombocytopenic Purpura Registry (hereafter, the Registry) has enrolled all consecutive patients diagnosed with their initial episode of acquired TTP. In previous reports, we described the demographics, presenting clinical features, and long-term outcomes of these patients.5-9 This article focuses on new observations that have not been previously reported. We describe the limitations of ADAMTS13 activity measurements for the diagnosis of TTP. These limitations emphasize that clinical evaluation continues to be an essential component for diagnosis. We also document the limitations of functional inhibitor measurements for anticipating the severity of patients’ clinical courses and outcomes. We note that many patients are not critically ill when they present with their first episode of TTP and describe how patients’ outcomes have changed since we began using corticosteroids in all patients and rituximab in selected patients. Finally, we note that patients’ clinical presentations at their first relapse episodes were less severe than at their initial episodes.
Methods
Patient identification and enrollment
The Registry has enrolled all consecutive patients for whom the Oklahoma Blood Institute was asked to provide plasma exchange (PEX) treatment for patients with clinically suspected TTP (or other thrombotic microangiopathies10) since January 1, 1989.5,6 The Oklahoma Blood Institute is the sole provider of transfusion services, including PEX, for all hospitals in 58 of the 77 Oklahoma counties. Therefore, the Registry includes all consecutive patients without selection or referral bias within this defined geographic region (population, 2.4 million) in whom a diagnosis of TTP was suspected and for whom a decision to initiate PEX treatment was made. All identified patients have been enrolled and no patients have been excluded. One of the authors (J.N.G.) began seeing Registry patients at the time of their hospitalization and enrollment in the Registry in 1995; he has seen 385 (89%) of the 434 patients enrolled in the Registry from 1995 through 2015. The Registry has been approved by the institutional review boards of the University of Oklahoma Health Sciences Center and participating hospitals. All patients provided informed consent to be enrolled in the Registry and for continuing long-term follow-up.
Diagnosis of TTP
Measurement of ADAMTS13 activity and functional inhibitors.
Serum samples are sent to Bern, Switzerland, once per year. ADAMTS13 activity has been measured in samples collected immediately before the first PEX since November 13, 1995.6 Measurements were performed in all samples by both a fluorogenic assay using fluorescence resonance energy transfer [FRET assay] with a VWF73 peptide substrate, a common commercial method, and a quantitative immunoblotting (IB) assay, a method used for the initial documentation of ADAMTS13 deficiency in patients with TTP.2 ADAMTS13 functional inhibitor activity was measured on samples with ADAMTS13 activity of ≤20% by determining residual ADAMTS13 activity of normal human plasma after 1:1 (v/v) incubation for 2 hours at 37°C with heat-inactivated patient’s serum by the FRET method. Inhibitor titers of ≤0.4 Bethesda units (BU/mL) were considered to be negative, and actual inhibitor titers up to 2 BU/mL were reported. Titers are reported as >2 BU/mL when residual ADAMTS13 activity is ≤25%.
Interpretation of ADAMTS13 activity and functional inhibitor measurements.
Because serum samples are analyzed only once per year, we did not know the results of our ADAMTS13 activity measurements at the time of the patients’ initial hospitalization; therefore diagnostic decisions were based on clinical evaluations. When ADAMTS13 activity measurements became available, patients were initially diagnosed as having TTP if their ADAMTS13 activity was <10% by either assay method. The activity level of <10% was selected because this included all but 2 patients who had relapsed.6 Patients with ADAMTS13 activity <10% were excluded from our cohort of TTP patients if an alternative diagnosis was confirmed. When ADAMTS13 activity was ≥10% by both measurements, patients were described as not having TTP, even if their clinical features and disease course suggested the diagnosis of TTP. This decision excluded some patients in whom TTP would have been an appropriate diagnosis.
Patients with TTP were diagnosed as having an acquired etiology if they had functional ADAMTS13 inhibitor activity or their ADAMTS13 activity increased during remission, or if a functional inhibitor was documented at the time of relapse.6 In some patients who did not meet these criteria, an acquired etiology was suspected on the basis of their clinical features.
Patient evaluation
Patients were evaluated for a diagnosis of TTP if they had microangiopathic hemolytic anemia characterized by schistocytes on the peripheral blood smear, thrombocytopenia, and no evidence for disseminated intravascular coagulation.
Neurologic abnormalities.
Severe neurologic abnormalities were defined in 4 categories: coma, stroke, generalized seizures, or transient focal signs, such as motor or sensory abnormalities, diplopia, and aphasia.5 When no severe abnormalities were present, patients were assessed for minor abnormalities that included headache, blurred vision, mild ataxia, and minor mental status changes. Determination of minor abnormalities was inherently imprecise.
Laboratory data.
We described presenting laboratory data as the most abnormal values within a period defined as the 7 days preceding the day of diagnosis of TTP, which was the day of the first PEX, and also the 7 days after the day of diagnosis. We established this method to identify the lowest platelet count and hematocrit, which may have been affected by transfusions, and the highest serum creatinine concentration, which may have increased after diagnosis.5 To compare lactate dehydrogenase (LDH) values from different hospitals, values were adjusted to an upper limit of normal of 200 U/L. Stages of acute kidney injury have been previously defined.5 Chronic kidney disease was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2 measured by the Chronic Kidney Disease Epidemiology Collaboration method.11
Definitions of clinical outcomes
Response was defined as the achievement of a platelet count of ≥150 000/µL. Exacerbation was defined by recurrent thrombocytopenia after a response with resumption of PEX or if PEX was continued because the platelet count decreased from ≥150 000/µL to <100 000/µL. Remission was defined as a sustained response for ≥30 days after PEX was stopped. Relapse was defined as the recurrence of an acute episode of TTP after remission.5
Statistical analysis
Descriptive statistics were calculated by using medians and proportions. Comparisons between patients with low vs high inhibitor titers were made by using the Wilcoxon Mann-Whitney U test with normal approximation (continuous variables) or the χ2 test (or Fisher’s exact test) (categorical variables). Similar analyses were conducted to compare patients in the pre-rituximab vs rituximab eras and separately to compare PEX complications in patients with a supported diagnosis of TTP vs patients without a supported diagnosis of TTP. To compare characteristics between first and second episodes in the subset of patients in the Registry region who had second episodes (n = 23), Wilcoxon signed rank test (continuous variables) or the McNemar test (dichotomous variables) was performed. All analyses were conducted by using SAS, Version 9.4 (SAS Institute, Cary, NC). An α of .05 was used.
Results
Patients
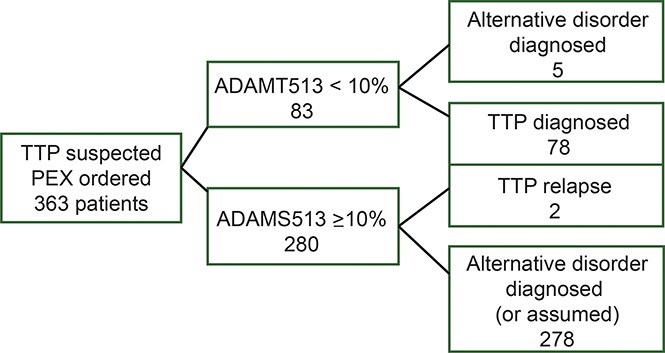
From November 13, 1995, when systematic collection of serum samples began, through December 31, 2015, serum samples were collected immediately before the first PEX, and ADAMTS13 activity was measured on 389 (94%) of all 412 enrolled patients. Because this article focuses on patients with their first episode of acquired TTP, 26 of the 389 patients were excluded: 14 because they were enrolled at the time of a relapse episode (their initial episode had occurred before 1989 or outside the Registry region), 10 because thrombotic microangiopathy (TMA) was initially observed on a kidney biopsy (some did not have microangiopathic hemolytic anemia or thrombocytopenia, and none had ADAMTS13 activity <10%), and 2 because they had hereditary TTP (1 was previously reported12). Therefore our report includes 363 patients.
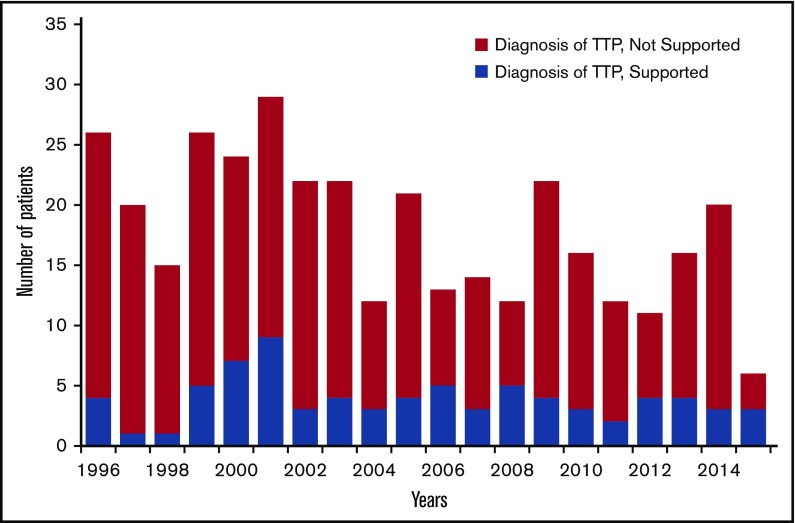
Figure 1 shows the annual number of all patients enrolled in the Registry and the number of patients in whom the diagnosis of TTP was supported by ADAMTS13 deficiency and by clinical criteria beginning in 1996, the first complete year of ADAMTS13 measurements, through 2015. Four patients were enrolled from November 13 to December 31, 1995; 1 was diagnosed with TTP. Common alternative diagnoses for patients in whom the diagnosis of TTP was not supported by ADAMTS13 activity <10% were systemic infections and other disorders with disseminated intravascular coagulation; transplant-associated TMA; drug-induced TMA; severe preeclampsia and hemolysis, elevated liver function tests, and low platelets syndrome; systemic lupus erythematosus; systemic cancer; and malignant hypertension. In addition, some patients with Shiga toxin–induced hemolytic-uremic syndrome were treated with PEX, and 5 patients treated with PEX were subsequently diagnosed with complement-mediated TMA.
Figure 1.
All patients enrolled in the Oklahoma TTP Registry each year from 1996 through 2015. (Blue) Patients in whom the diagnosis of TTP was supported by ADAMTS13 activity <10% at the time of their initial episode and by their clinical features. (Red) Patients in whom the diagnosis of TTP was not supported by ADAMTS13 activity <10% or by their clinical features.
Incidence and prevalence of TTP.
We have previously reported the standardized incidence rate of TTP in the Registry region: 2.17 patients per 1 000 000 people per year.7 When all episodes of TTP are included (along with those for patients who were first enrolled at the time of a relapse and those for previously enrolled patients who relapsed), the estimated annual incidence of TTP is 3.10 episodes of TTP per 1 000 000 per year. Among the 78 patients, 2 were lost to follow-up, 25 died, and 45 are living within the Registry region. Therefore the estimated current prevalence of patients in remission from acquired TTP in the Registry region is 19 patients per 1 000 000.
Diagnosis of TTP
ADAMTS13 activity and inhibitor measurements.
The diagnosis of TTP was supported by both ADAMTS13 activity <10% and clinical criteria in 78 patients (Table 1). ADAMTS13 activity was <10% by both FRET and IB assays in 60 patients (77%). In 15 patients, the FRET assay measured ADAMTS13 activity as <10% whereas the IB assay measured ADAMTS13 activity at 10% to 68%. Among these 15 patients, the 3 with the highest ADAMTS13 activity by IB measurements (patients 73-75; 35% to 68%) all had high-titer functional inhibitors (≥2 BU/mL). All 3 have relapsed; in 2 patients, both assays were <10% at the time of relapse; in 1 patient, the FRET measurement was <10% and the IB measurement was 18%. In 3 other patients, the IB assay measured ADAMTS13 activity as <10% whereas the FRET assay measured ADAMTS13 activity of 11% to 23%. All 3 patients had documented ADAMTS13 functional inhibitors by FRET or IB measurements. Only 1 of the 78 patients (described below) was initially diagnosed with an alternative disorder at the time of her initial episode. These data suggest that both methods of measuring ADAMTS13 activity may be susceptible to falsely high values.
Table 1.
ADAMTS13 activity measurements and final clinical diagnosis in patients with suspected TTP (n = 85)
| Patient | ADAMTS13 | Comment | Functional inhibitor (BU/mL) | |
|---|---|---|---|---|
| FRET (%) | IB (%) | |||
| Diagnosis of TTP by FRET and IB | ||||
| 1-60 | <10 | <10 | 20 (38%) of 52 survivors relapsed | |
| Diagnosis of TTP by FRET only | ||||
| 61 | <10 | 10 | No relapse | 0.5 |
| 62 | <10 | 12 | 1 relapse: both <10% | 1.2 |
| 63 | <10 | 12 | No relapse | 1.2 |
| 64 | <10 | 13 | No relapse | 0 |
| 65 | <10 | 13 | No relapse | 0.8 |
| 66 | <10 | 15 | No relapse | 0.6 |
| 67 | <10 | 18 | Died before PEX started | 0 |
| 68 | <10 | 20 | No relapse | 0.5 |
| 69 | <10 | 20 | 3 relapses: 2, both <10%; 1, IB 10% | 0 |
| 70 | <10 | 20 | No relapse | 0.5 |
| 71 | <10 | 25 | 2 relapses: 1, both <10%; 1, IB 18% | 0 |
| 72 | <10 | 30 | No relapse | 0.5 |
| 73 | <10 | 35 | 1 relapse: FRET <10%, IB 18 | 2 |
| 74 | <10 | 40 | 1 relapse: both <10% | >2 |
| 75 | <10 | 68 | 2 relapses, both <10% | >2 |
| Diagnosis of TTP by IB only | ||||
| 76 | 11 | <10 | No relapse | 1.3 |
| 77 | 16 | <10 | 2 relapses (no ADAMTS13 measurements) | >2 |
| 78 | 23 | <10 | Died with first episode | >2 |
| Diagnosis of TTP not supported by clinical features; alternative etiology established | ||||
| 79 | 6 | 13 | HIV, died; autopsy: systemic Kaposi sarcoma, no evidence of TTP | 0.9 |
| 80 | 9 | 20 | Bilirubin 24 mg/dL; died; autopsy: hepatic necrosis, no evidence of TTP | 1.3 |
| 81 | 9 | 25 | After unrelated donor HSCT, GVHD, systemic aspergillosis, bilirubin 64 mg/dL; died | >2 |
| 82 | 12 | 9 | Sepsis (Group A Streptococcus), hypotension, rhabdomyolysis, lactic acidosis; survived | 0 |
| 83 | 28 | 8 | Sepsis (Candida), hypotension, DIC, after liver transplant; died | 1.2 |
| Diagnosis of TTP supported by occurrence of relapses; neither FRET nor IB <10% | ||||
| 84 | 53 | 60 | 6 episodes: second, no measurements; third, FRET 15% with inhibitor, IB 50%; fourth through sixth: both measurements <10%, with inhibitors. | 0.8-1.4 |
| 85 | 100 | 16 | 4 episodes: PEX performed only for third episode; ADAMTS13 measured for episodes 3 and 4. | 1.9 |
| 100 | 29 | 1.3 | ||
The term “both” in the Comments column refers to measuring ADAMTS13 activity by both FRET and IB assays. The patients are presented in 5 categories. In patients 1-78, the diagnosis of TTP was supported by both ADAMTS13 activity <10% and clinical criteria (no alternative diagnosis was recognized as the cause of the clinical features). In patients 1-60, ADAMTS13 activity was <10% with both assays; in patients 61-75, the IB assay reported ADAMTS13 activity as 10%-68%; in patients 76-78, the FRETS assay reported ADAMTS13 activity as 11%-23%. These observations suggest that in vitro ADAMTS13 measurements may not always reflect in vivo ADAMTS13 activity. In patients 79-83, 1 of the 2 ADAMTS13 activity measurements was <10%, but another disorder was diagnosed as the cause of the clinical features. In patients 80 and 81, high bilirubin levels may have caused falsely low ADAMTS13 measurements by the FRET assay.13 In patients 84 and 85, TTP was suspected by clinical criteria but the diagnosis of TTP was not supported by measurements of ADAMTS13 <10%. Patients 79-85 are described individually in the text. Functional inhibitor measured at the time of the initial episode of TTP was expressed in Bethesda units (BU/mL). Titers of ≤0.4 BU/mL were considered to be within the normal range. Titers of ≥0.5 BU/mL are reported and are considered to be clinically important. Inhibitors were measured by the FRET assay unless noted. For patients enrolled from November 13, 1995, through August 1, 2005, functional inhibitor activity was also measured by the quantitative IB assay. IB inhibitor titers are described only if an inhibitor was not measured by the FRET assay. Five of the 60 patients in whom ADAMTS13 activity was <10% by both assays had no demonstrable inhibitor by the FRET assay; an IB inhibitor assay was not performed. In patients 69 and 71, the IB assay showed no inhibitor activity. In patient 67, a FRET inhibitor assay was not performed; no inhibitor was documented by IB. Patient 78 did not have a demonstrable inhibitor by the FRET assay; the IB inhibitor was >2. Patient 84 had demonstrable inhibitors at the time of episodes 3-6. In patient 85, inhibitor activity was not measured with the FRET assay but was documented by the IB assay.
DIC, disseminated intravascular coagulation; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplant.
Although we describe all 78 patients as having acquired TTP, 13 did not have an ADAMTS13 functional inhibitor by FRET measurement; 4 of these patients had a functional inhibitor by IB measurement. In 4 of the remaining 9 patients, ADAMTS13 activity recovered to normal during remission. The other 5 patients did not have ADAMTS13 measurements during remission. In 3 of these 5 patients, acquired TTP was suspected because they had other autoimmune disorders and an otherwise normal past medical history. The remaining 2 women died during their initial episode; they both had normal past medical histories, including uncomplicated pregnancies.
Functional inhibitor titers.
The distribution of functional inhibitor titers determined by FRET measurements is presented in Table 2. No association was demonstrated between the inhibitor titer and presenting features or clinical outcomes (Table 3).
Table 2.
ADAMTS13 functional inhibitor strength measured by FRETS (n = 77)
| Inhibitor titer (BU/mL) | Patients | |
|---|---|---|
| No. | % | |
| None | 13 | 17 |
| 0.5-0.9 | 14 | 18 |
| 1-2 | 20 | 26 |
| >2 | 30 | 39 |
In one of the 78 patients, inhibitor activity was measured only by IB. Among the 13 patients with no inhibitor activity measured by the FRET method, IB inhibitor activity was >2 in 1 patient, <1 to 1 in 4 patients, no inhibitor was documented in 2 patients, and an IB inhibitor measurement was not performed in 6 patients.
Table 3.
Comparison of patients with no demonstrable inhibitor with those who had strong inhibitors
| Outcome | No inhibitor (<1 BU/mL) (n = 27) | >2 BU/mL (n = 30) | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | Median | Range | No. | % | Median | Range | ||
| Platelets × 103/µL* | 9 | 4-101 | 11 | 5-63 | .12 | ||||
| Hematocrit, %* | 20 | 14-33 | 22 | 13-28 | .69 | ||||
| Creatinine, mg/dL | 1.3 | 0.7-6.8 | 1.35 | 0.8-5.5 | .86 | ||||
| LDH, U/L | 1207 | 432-2962 | 1500 | 274-3909 | .36 | ||||
| Severe neurologic abnormalities | 12 | 44 | 18 | 60 | .29 | ||||
| PEX | 12 | 2-71 | 11 | 1-79 | .79 | ||||
| Exacerbation | 9 | 33 | 16/29* | 55 | .12 | ||||
| Survival | 25 | 93 | 23 | 77 | .15 | ||||
| Relapse | 8/25 | 32 | 10/23 | 44 | .55 | ||||
Functional inhibitor activity was measured by the FRET assay. The 20 patients with an intermediate-strength inhibitor titer (1-2 BU/mL) were not included in this analysis.
One patient failed to respond and therefore could not be evaluated for exacerbation.
Patients with ADAMTS13 reported as <10% in whom the diagnosis was not supported by clinical data and who were diagnosed with an alternative disorder.
In 5 patients, ADAMTS13 activity was reported to be <10% by 1 measurement but they were diagnosed with disorders other than TTP (patients 79-83; Table 1). All had severe systemic disorders, and 4 died. Two patients had autopsies with no evidence of TTP, 2 had high serum bilirubin levels (24 and 64 mg/dL), which may have interfered with ADAMTS13 measurements by the FRET assay,13 and 2 had sepsis with hypotension.
One additional patient had bacterial endocarditis with bacteremia, aortic valve vegetation, myocardial infarction, and stroke. TTP was also suspected, and PEX treatment was requested. Her ADAMTS13 activity was <10% by both measurements, and no functional inhibitor was detected. However, we assumed that the correct diagnosis was bacterial endocarditis. During her subsequent remission, ADAMTS13 activities were 100% (IB) and 92% (FRET). She moved to another state and then had 2 relapses. With her second relapse, ADAMTS13 activity was <10% with a functional inhibitor of 1.2 BU/mL. Therefore she was reclassified as having acquired TTP, initially concurrent with bacterial endocarditis, and she was included in our cohort of 78 patients.
Patients in whom ADAMTS13 was reported to be ≥10% who were diagnosed with TTP.
One patient (patient 84; Table 1) had normal values of ADAMTS13 reported by both measurements at the time of his initial presentation with characteristic clinical features of TTP, including transient focal neurologic abnormalities.14 He then had 5 relapses. With each episode, his reported ADAMTS13 activity decreased and became <10% by both measurements. Anti-ADAMTS13 antibodies were present with his first episode when his reported ADAMTS13 activity was normal. These antibodies may have dissociated from ADAMTS13 during the in vitro incubation, which allowed the observation of ADAMTS13 activity.14 Patient 85 (Table 1) had 4 episodes of microangiopathic. hemolytic anemia and thrombocytopenia without neurologic abnormalities or increased serum creatinine. Her first 2 episodes were diagnosed as primary immune thrombocytopenia, and they responded to corticosteroids. Her third episode, when she was enrolled in the Registry, was diagnosed as TTP and was treated with PEX. A fourth episode responded to corticosteroids without PEX. Although ADAMTS13 activity was not reported to be <10% with episodes 3 and 4, she had a functional inhibitor.
Presenting clinical features of initial episodes and the basis for clinical criteria used for an initial diagnosis of TTP
Fever.
Eight patients (10%) had fever associated with chills preceding their admission.
Neurologic abnormalities.
Forty-one patients (53%) had severe neurologic abnormalities; in 9, severe neurologic abnormalities occurred only after PEX began (Table 4). The most common severe abnormalities were transient focal deficits occurring in 31 patients (40%). Twenty-one patients (27%) had mild neurologic abnormalities, and 16 (20%) had no neurologic abnormalities.
Table 4.
Neurologic abnormalities at presentation and after PEX began in 78 patients with their initial episode of TTP
| Number of patients with neurologic abnormalities | ||||||
|---|---|---|---|---|---|---|
| Neurologic abnormalities | At presentation | After PEX began | Total neurologic abnormalities | |||
| No. | % | No. | % | No. | % | |
| None | 26 | 33 | 60 | 77 | 16 | 20 |
| Minor | 20 | 26 | 2 | 3 | 21 | 27 |
| Severe | 32 | 41 | 16 | 20 | 41 | 53 |
| Coma | 2 | 3 | 4 | 5 | 6 | 8 |
| Stroke | 6 | 8 | 4 | 5 | 9 | 12 |
| Seizure | 6 | 8 | 7 | 9 | 12 | 15 |
| Transient focal abnormalities | 26 | 35 | 10 | 10 | 31 | 40 |
Minor neurologic abnormalities were not recorded in patients who had severe neurologic abnormalities. Neurologic abnormalities documented after PEX began were distinct abnormalities. One patient had a recurrent minor abnormality after PEX began. Seven patients had distinct severe abnormalities both at presentation and after PEX began. Among the 4 categories of severe abnormalities, 17 of the 41 patients had abnormalities in multiple categories. One patient had an extension of her stroke after PEX began; 12 had seizures; 1 had seizures before PEX began and another seizure after her initial PEX; 31 had transient focal neurologic abnormalities; 5 had recurrent abnormalities (diplopia, aphasia, face/hand weakness/numbness) after PEX began; 19 had transient focal abnormalities as their only severe abnormality; and 12 had transient focal abnormalities in addition to seizures, stroke, and/or coma.
Hematologic abnormalities.
All 78 patients had thrombocytopenia and microangiopathic hemolytic anemia. The median platelet count was 10 000/µL. Only 11 patients (14%) had platelet counts ≥20 000/µL; only 3 patients (4%) had platelet counts ≥30 000/µL. The median hematocrit was 21%; only 2 patients (3%) had hematocrits ≥30%. The median LDH was 1288 U/L, and the lowest value was 274 U/L.
Kidney function.
In 37 patients, serum creatinine concentrations were <1.5 mg/dL; in another 37 patients, serum creatinine concentrations were ≥1.5 mg/dL, but criteria for severe acute kidney injury were not met (Table 5). Only 11 patients (14%) had serum creatinine concentrations ≥2.5 mg/dL. Four patients (5%) had severe acute kidney injury, and 3 required dialysis. Each of these 4 patients had additional complicating features at the time of their TTP episode, such as severe pancreatitis, eclampsia with severe hypertension, cardiac arrest with hypotension, or stroke with hypotension.
Table 5.
Kidney function at presentation (n = 78) and at long-term follow-up
| Kidney function test | Results | |||
|---|---|---|---|---|
| No. | % | Median | Range | |
| At presentation | ||||
| Serum creatinine, mg/dL | 1.3 | 0.7-6.5 | ||
| Acute kidney injury stage | ||||
| 0 | 37 | 47.5 | ||
| 1 | 37 | 47.5 | ||
| 3 | 4 | 5 | ||
| Required dialysis | 3 | 4 | ||
| Long-term follow-up, y | 62* | 6.4 | 0.15-18.2 | |
| eGFR, mL/min/1.73 m2 | 97 | 30-154 | ||
| Chronic kidney disease (eGFR <60) | 4 | 6 | 47 | 30-57 |
| Required dialysis after initial hospitalization | 0 | |||
Serum creatinine at presentation was highest on the day of first PEX ±7 days.5 The upper limit of normal for serum creatinine at the patients’ hospitals was 1.1-1.5 mg/dL.
eGFR, estimated glomerular filtration rate.
Ten of 78 patients died with their first episode, 2 were lost to follow-up, and 4 other patients did not have a follow-up serum creatinine measurement. Therefore, 62 patients are included in long-term follow-up data.
Management and outcomes of initial episodes
Among all 78 patients, 74 achieved a response; four patients, who presented in 1999-2005, died before responding (Table 6). One patient died in the Emergency Department before a central venous catheter for PEX was inserted. One patient died during her first PEX.15 In both of these patients, autopsies confirmed TTP as the cause of death. One patient had persistent seizures and died following 3 PEX treatments. One patient had a cardiac arrest before PEX was begun, attributed in part to cocaine abuse; she remained comatose without a response throughout 12 days of PEX before she died. Six additional patients died following a response; 5 have been previously described.6 In the sixth patient, TTP and eclampsia occurred simultaneously following her third pregnancy; she also had postpartum cardiomyopathy and hemorrhagic pancreatitis.
Table 6.
Comparison of presentation, management, and outcomes in the pre-rituximab era (November 1995-November 2003) and the rituximab era (December 2003-December 2015)
| Outcome | Patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Enrolled November 1995-November 2003 | Enrolled December 2003-December 2015 | ||||||||||
| No. | % | Median | Range | No. | % | Median | Range | No. | % | Median | Range | |
| All patients | 78 | 34 | 44 | |||||||||
| Severe neurologic abnormalities* | 41 | 18 | 53 | 23 | 52 | |||||||
| Coma | 6 | 4 | 12 | 2 | 5 | |||||||
| Stroke | 9 | 3 | 9 | 6 | 14 | |||||||
| Seizure | 12 | 7 | 21 | 5 | 11 | |||||||
| Transient focal abnormalities | 31 | 10 | 29 | 21 | 48 | |||||||
| Laboratory data† | ||||||||||||
| Platelets × 103/µL | 10 | 2-101 | 11 | 2-101 | 10 | 4-63† | ||||||
| Hematocrit, % | 21 | 13-33 | 21 | 13-30 | 22 | 13-33† | ||||||
| Creatinine, mg/dL | 1.3 | 0.7-6.8 | 1.2 | 0.7-5.5 | 1.4 | 0.8-6.8† | ||||||
| LDH, U/L | 1522 | 343-12 078 | 1870 | 491-12 078 | 1369 | 343-5540 | ||||||
| Outcomes | ||||||||||||
| Death before completing 1 PEX | 2 | 0 | 2 | |||||||||
| Death before response | 2 | 2 | 0 | |||||||||
| Response | 74 | 95 | 32 | 94 | 42 | 95 | ||||||
| Exacerbation | 39 | 53 | 18 | 56 | 21 | 50 | ||||||
| Death after response | 6 | 3 | 3 | |||||||||
| Remission | 68 | 87 | 29 | 85 | 39 | 89 | ||||||
| No. of PEX and hospital days (patients who had ≥1 PEX) | 76 | |||||||||||
| PEX‡ | 14 | 2-79 | 20 | 3-74 | 11 | 2-79 | ||||||
| Days in hospital | 19 | 4-109 | 19 | 4-109 | 17 | 6-89 | ||||||
| Days in hospital before diagnosis | 1 | 0-37 | 1 | 0-14 | 1 | 0-37 | ||||||
| Additional treatments received | ||||||||||||
| Corticosteroids | 64 | 82 | 21 | 62 | 43 | 98 | ||||||
| Rituximab | 19 | 24 | 0 | 19 | 43 | |||||||
| Cyclophosphamide | 1 | 0 | 1 | |||||||||
| Vincristine | 4 | 1 | 3 | |||||||||
| Splenectomy | 1 | 1 | 0 | |||||||||
| Twice-daily PEX | 13 | 8 | 5 | |||||||||
| Surviving patients | 68 | 29 | 39 | |||||||||
| Patients who relapsed§ | 15 | 52 | 12 | 31 | ||||||||
| Total No. of relapses | 35 | 16 | ||||||||||
| No. of relapses per patient | ||||||||||||
| 1 | 6 | 8 | ||||||||||
| 2 | 2 | 4 | ||||||||||
| 3 | 3 | 0 | ||||||||||
| 4 | 4 | 0 | ||||||||||
The rituximab era is defined as beginning with the first patient treated with rituximab who was hospitalized and diagnosed in December 2003. Therefore patients are grouped as those who presented from November 1995 through November 2003 and those who presented from December 2003 through December 2015.
Neurologic abnormalities include abnormalities at presentation and after PEX began.
There was no difference in the presenting platelet count (P = .497), hematocrit (P = .094), or creatinine (P = .740) values. The LDH values in the rituximab era were less than the values in the pre-rituximab era (P = .047).
Patients in the rituximab era received significantly fewer PEX procedures than patients in the pre-rituximab era (P = .006)
Ten relapses in 6 patients occurred after December 2003. The frequency of relapses was not compared because patients in the earlier cohort had more time in which a relapse could occur.
Comparison of patients before and during the rituximab era.
Table 6 divides our patients into 2 time periods related to our use of rituximab: patients whose initial episode occurred before December 2003 and patients who presented from December 2003 through 2015, when we first used rituximab (375 mg/m2/wk for 4 weeks) for treatment of exacerbations. There was no difference in the occurrence of severe neurologic abnormalities, the frequency of responses and exacerbations, and death between the 2 time periods. After 2003, the number of PEX treatments required to achieve remission and the frequency of relapse decreased. Before 2003, only 62% of patients received corticosteroids; after 2003 only 1 patient (who died before PEX was begun) was not treated with corticosteroids. PEX twice per day has not been used since 2006.
PEX complications.
We previously reported the frequency of death and major complications attributed to PEX in all patients treated with PEX for suspected TTP.16,17 In Table 7, we compare deaths and major complications attributed to PEX that occurred in 68 of the 78 TTP patients to 274 patients whose initial suspicion of TTP was not supported by ADAMTS13 activity <10%.16,17 Among the 68 TTP patients, 3 (4%) died and 36 (53%) had major complications. The 3 deaths were previously described.6 The frequency of all major complications and major catheter-related complications was significantly greater among patients with a supported diagnosis of TTP compared with patients whose diagnosis of TTP was not supported by ADAMTS13 activity <10%. We also compared PEX complications in the 32 patients diagnosed with TTP before December 2003 and the 42 patients diagnosed between December 2003 and December 2015. There were no differences in the frequency of death or in the frequency of any of the nonfatal major complications (P ≥ .08 for all comparisons; data not shown).
Table 7.
Death and major complications attributed to PEX treatment in patients with their initial episode of TTP from 1996 to 2014
| Description of complications | Diagnosis of TTP supported (n = 68) | Diagnosis of TTP not supported (n = 274) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Deaths (catheter-related) | 3 | 4.4 | 4 | 1.5 |
| Pulmonary hemorrhage caused by central venous catheter insertion for PEX | 1 | 3 | ||
| Systemic infection | 2 | 1 | ||
| Nonfatal major complications | 36 | 53 | 55 | 20 |
| Catheter-related complications | 28 | 41 | 41 | 15 |
| Systemic infection, documented bacteremia | 12 | 19 | ||
| Systemic infection, suspected bacteremia* | 2 | 2 | ||
| Localized infection at catheter insertion site† | 2 | 1 | ||
| Catheter obstruction‡ | 6 | 11 | ||
| Venous thrombosis requiring systemic anticoagulation | 3 | 4 | ||
| Catheter insertion site hemorrhage§ | 1 | 3 | ||
| Pneumothorax|| | 1 | 1 | ||
| Insertion of incorrect catheter‡ | 1 | 0 | ||
| Plasma-related complications | 8 | 12 | 14 | 5 |
| Anaphylaxis with cardiac arrest | 1 | 0 | ||
| Hypotension requiring vasopressor treatment | 3 | 6 | ||
| Hypoxia¶ | 1 | 8 | ||
| Hypocalcaemia, arrhythmia¶ | 1 | 0 | ||
| Serum sickness after PEX | 2 | 0 | ||
Registry patients with a supported diagnosis of TTP (ADAMTS13 <10% supported by clinical criteria) were compared with Registry patients whose initial suspected diagnosis of TTP was not supported by ADAMTS13 activity <10%. These data are derived from our previous analyses from 1996 to 2014.17 Major complications were previously defined.16 Ten of the 78 patients with TTP are not included in this comparison. One patient died before a catheter was inserted and PEX began (death caused by TTP, documented by autopsy). One patient died soon after her first PEX began (death caused by TTP, documented by autopsy).Two patients were diagnosed before active surveillance for PEX complications began in June 1996 (both had major complications: 1 had systemic infection with bacteremia; 1 had local infection at the catheter site requiring systemic antibiotics). Six patients were enrolled in 2014-2015 after our previous analysis of all patients treated with PEX had been completed in June 2014.17 Four of these 6 patients had no major complications; 2 had major complications: 1 had systemic infection with bacteremia; 1 had plasma allergic reaction with chest pain and dyspnea that required stopping PEX. Therefore, 40 (52%) of all 77 TTP patients treated with PEX had major nonfatal complications. The frequency of deaths (P = .144) and plasma-related major complications (P = .055) were not different between patients who had TTP and those who did not. The frequency of all major complications (P < .001), catheter-related major complications (P < .001), and systemic infections with documented bacteremia (P < .010) was significantly different between the 2 patient cohorts.
Blood cultures were negative but patient was treated with a full course of parenteral antibiotics for presumed sepsis.
Required hospitalization and systemic antibiotics.
Required removal of the catheter and placement of a new catheter.
Required red blood cell transfusion.
Required placement of a chest tube.
Required stopping PEX.
Long-term outcomes
Kidney function.
Among the 62 patients who survived their initial episode of TTP and had follow-up serum creatinine measurements, only 4 (6%) had chronic kidney disease; none required continuing dialysis (Table 5).
Comparison of the initial TTP episodes to the patients’ first relapse.
Table 8 compares the presenting features and outcomes of the initial episode and first relapse in 23 patients. Relapse episodes had less severe anemia and thrombocytopenia and lower LDH activity. They required fewer PEX treatments and fewer days in the hospital, probably related to more frequent use of rituximab. Two patients died at the time of their relapse episode. The circumstances of both deaths were unusual. One patient died unexpectedly on the sixth day after uncomplicated coronary artery bypass surgery; TTP was recognized only at autopsy. The patient had been deaf since birth, could not speak, and could not communicate effectively with his physicians. One patient died unexpectedly during a PEX procedure 9 days after her platelet count had returned to normal; the PEX frequency was being tapered, according to our practice in 1997. Myocardial infarction was confirmed by autopsy; there was no evidence of TTP.
Table 8.
Comparison of initial episode with first relapse in 23 patients
| Outcome | Patients who relapsed (n = 23)* | |||||||
|---|---|---|---|---|---|---|---|---|
| Initial episode | First relapse | |||||||
| No. | % | Median | Range | No. | % | Median | Range | |
| Severe neurologic abnormalities† | 12 | 8 | ||||||
| Coma | 3 | 0 | ||||||
| Stroke | 1 | 0 | ||||||
| Seizure | 3 | 1 | ||||||
| Transient focal abnormalities | 9 | 7 | ||||||
| Laboratory data‡ | ||||||||
| Platelets × 103/µL | 8 | 2-27 | 15 | 3-50 | ||||
| Hematocrit, % | 20 | 13-30 | 26 | 18-40 | ||||
| Creatinine, mg/dL | 1.2 | 0.7-3.9 | 1.1 | 0.6-2.4 | ||||
| LDH, U/L | 1428 | 436-3423 | 733 | 264-1944 | ||||
| Treatment | ||||||||
| PEX§ | 19.5 | 6-79 | 8 | 2-63 | ||||
| Days in hospital§ | 17 | 6-109 | 8.5 | 4-56 | ||||
| Days in hospital before diagnosis | 1 | 0-14 | 1.5 | 1-3 | ||||
| Rituximab | 2 | 9 | 7 | 30 | ||||
| Outcomes | ||||||||
| Died before PEX was performed | 0 | 1|| | ||||||
| Died after response | 0 | 1¶ | ||||||
| Response | 23 | 22 | ||||||
| Exacerbation | 14 | 61 | 9 | 41 | ||||
| Survived in remission | 23 | 100 | 21 | 91 | ||||
Four of the 27 patients who relapsed were treated in hospitals outside the Registry region, and data are not sufficient to calculate outcomes other than survival; all 4 survived. Ten of the initial episodes and 14 of the relapses occurred in December 2003 through December 2015; rituximab was used for some patients during this time.
The total number of patients with severe neurologic abnormalities was not different between the 2 groups (P = .234).
Laboratory data, treatment, and the outcomes of response and exacerbation are described for 22 patients, omitting the patient who died suddenly before TTP was diagnosed at autopsy. For LDH values, only 21 patients were compared because 1 patient did not have LDH measured during her relapse episode. The platelet counts (P = .017) and hematocrits (P = .003) were significantly higher in these patients at the time of relapse; the LDH levels were significantly lower (P < .001). There was no different between the creatinine levels (P = .130).
The number of PEX treatments (P = .005) and days in hospital (P = .003) were significantly less for relapse episodes.
A 51-year-old man who was deaf from birth and could not speak had an acute myocardial infarction with hypotension requiring intra-aortic balloon pump circulatory assistance in 2004, 4 years after his initial TTP episode. His hematocrit was 25%, platelet count was 106 000/µL, and LDH was 283 U/L. One month earlier, his hematocrit was 36% and platelet count was 189 000/µL. Relapse of TTP was not suspected. He had coronary artery bypass graft surgery without complication. On the second day after surgery, his hematocrit was 32% and platelet count was 134 000/µL. He had no subsequent laboratory evaluations and was described as recovering well when he unexpectedly had a seizure, cardiac arrest, and died on the sixth day after surgery. Autopsy documented microvascular thrombi throughout the myocardium but no new myocardial infarction. Death was attributed to relapsed TTP.
A 59-year-old woman relapsed in 1997, 1 year after her initial episode, and was treated with PEX without corticosteroids (our practice at that time). After a response, she continued PEX once per day for 3 days, then every other day for 3 more treatments (also our practice at that time). Her platelet count remained normal (181 000/µL) on the day of her last PEX. Near the end of this PEX she had a sudden seizure and cardiac arrest. Autopsy documented a posterior left ventricle and septal infarction without evidence of TTP.
Discussion
Since the Registry began in 1989, important changes have occurred in the prevalence, diagnosis, management, and long-term outcomes of acquired TTP. After effective treatment with PEX was first reported in 1991,18 which allowed observation of patients in remission, relapses were noticed.19 The occurrence of relapses has increased the incidence of acute episodes of TTP. In addition, the prevalence of patients in remission after recovery from TTP is increasing. Our estimated current prevalence of patients in remission is 19 per 1 000 000, similar to the estimated 13 patients per 1 000 000 in France.20 These patients are at risk not only for relapse, but also for hypertension, depression, minor cognitive impairment, and premature death.8,9,21
Fifty years ago, before the era of effective treatment, TTP was defined and diagnosed by a pentad of clinical features (fever, thrombocytopenia, anemia, neurologic abnormalities, and kidney function abnormalities).22 For the Canadian randomized clinical trial that established the effectiveness of PEX (1982-1988), inclusion criteria were only thrombocytopenia and microangiopathic hemolytic anemia without an identifiable cause.18 The lack of specificity of these criteria was apparent from the eightfold increase in the number of patients treated for TTP in Canada after awareness of the benefit of PEX.23 Defining TTP by a severe deficiency of ADAMTS13 activity in 19982,3 provided greater specificity for the diagnosis.
However, the level of ADAMTS13 activity that defined a severe deficiency and the use of ADAMTS13 activity in the diagnosis of TTP remain uncertain. Some have suggested that ADAMTS13 activity <10% alone is sufficient for the diagnosis and treatment of TTP.24 Our experience, using 2 different methods of measurement in addition to clinical features, is that laboratory reports of ADAMTS13 activity <10% are neither absolutely necessary nor sufficient for the diagnosis of TTP. Only 60 (77%) of our 78 patients had ADAMTS13 activity <10% by both measurements. Patients with ADAMTS13 activity <10% may have an alternative clinical diagnosis with no evidence of TTP at autopsy. In addition, patients may have typical clinical features of TTP, including multiple relapses, without ADAMTS13 activity measurements reporting results <10%. Functional inhibitors of ADAMTS13 activity may not be documented in patients with acquired TTP and may be present in patients who do not have a diagnosis of TTP supported by clinical criteria or autopsy. The titer of functional inhibitors was not associated with presenting features or clinical outcomes. These limitations emphasize the importance of clinical judgment in the evaluation and management of patients with suspected TTP.25 Our current practice continues to treat patients with PEX if they have clinical features of TTP with no alternative diagnosis, even if the ADAMTS13 activity is not reported to be <10%.
Measurements of ADAMTS13 activity may not accurately reflect in vivo ADAMTS13 function. High levels of bilirubin may interfere with the FRET assay, causing the report of falsely low activity.13 Falsely high activity can occur when anti-ADAMTS13 immunoglobulin G antibodies, which inhibit ADAMTS13 function in vivo, dissociate from ADAMTS13 during the incubation required for in vitro measurements. This is suggested when increasing fluorescence evolution occurs over time of incubation in the FRET assay, as anti-ADAMTS13 antibodies dissociate from ADAMTS13 thereby restoring its activity. The IB assay may be especially vulnerable for dissociation of anti-ADAMTS13 antibodies from ADAMTS13 because the in vitro incubation time is 18 to 20 hours.14 These issues support the importance of clinical judgment in the diagnosis of TTP.
By using our criteria for the diagnosis of TTP, ADAMTS13 activity <10% by either of our 2 assays plus no alternative etiology for the patient’s clinical features, all of our 78 patients had thrombocytopenia and microangiopathic hemolytic anemia, although thrombocytopenia and anemia were not severe in a few patients. Thirty-seven patients (47%) had no or only minor neurologic abnormalities, such as headache, blurred vision, and minor mental status changes. Seventy-four patients (95%) had normal or minor transient increases of serum creatinine. Fever with chills occurred in only 8 patients (10%). These data show that patients with TTP may not initially seem to be seriously ill.15
Our data provide criteria for the initial clinical diagnosis of TTP and distinction of TTP from other disorders presenting the microangiopathic hemolytic anemia and thrombocytopenia. Only 10% of our patients had fever with chills. Therefore the presence of fever and chills suggests an alternative diagnosis, such as sepsis. Transient focal neurologic symptoms are characteristic of TTP, and they commonly occur. Eighty-six percent of our TTP patients had a platelet count <20 000/µL, 97% had a hematocrit <30%, and 86% had a serum creatinine concentration <2.5 mg/mL. Therefore, only mild or moderate thrombocytopenia and anemia or severe acute kidney injury suggest that the diagnosis is not TTP.
With the understanding that acquired TTP is an autoimmune disorder,2,3 corticosteroids (previously proven to be effective26) have become standard treatment. Since 2002, rituximab has been used to provide additional immunosuppression.27 We began using rituximab in December 2003 for treatment of exacerbations and as initial treatment of relapse episodes of TTP. Although the frequencies of severe neurologic abnormalities, exacerbations, and death have not changed since we began to use rituximab for these indications, the frequency of relapse has decreased.28 Some investigators have advocated the use of rituximab as routine initial treatment together with PEX and corticosteroids.29
During the past 20 years, the diagnosis of TTP has become more accurate and treatment has become more effective. Treatment of acute episodes of TTP may become even more effective with increasing use of rituximab and the addition of new agents, such as caplacizumab30 and recombinant ADAMTS13.31 With more effective treatments, the need for PEX and the risks for death and complications from PEX may decrease. We anticipate that more effective treatment will improve the quality and duration of life for patients in remission from TTP.
Acknowledgments
This work was supported, in part, by Ablynx, N.V., Zwijnaarde, Belgium.
Ablynx, N.V., had no involvement in the data analysis or the manuscript preparation.
Authorship
Contribution: E.E.P. extracted and analyzed the data, created the tables and figure, and reviewed the manuscript; J.A.K.H. supervised the measuring of ADAMTS13 activity and functional inhibitors, analyzed the data, and reviewed the manuscript; D.R.T. organized the enrollment and follow-up of Registry patients and reviewed the manuscript; S.K.V. planned the project, supervised data analysis, and reviewed the manuscript; J.N.G. evaluated the patients enrolled in the Registry, supervised their treatment, followed their course through remission, planned the project, and wrote the first and final drafts of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James N. George, University of Oklahoma Health Sciences Center, 801 N.E. 13th St, Room CHB-358, Oklahoma City, OK 73104; e-mail: james-george@ouhsc.edu.
References
- 1.Furlan M, Robles R, Solenthaler M, Wassmer M, Sandoz P, Lämmle B. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood. 1997;89(9):3097-3103. [PubMed] [Google Scholar]
- 2.Furlan M, Robles R, Galbusera M, et al. . von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339(22):1578-1584. [DOI] [PubMed] [Google Scholar]
- 3.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339(22):1585-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy GG, Nichols WC, Lian EC, et al. . Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413(6855):488-494. [DOI] [PubMed] [Google Scholar]
- 5.Vesely SK, George JN, Lämmle B, et al. . ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102(1):60-68. [DOI] [PubMed] [Google Scholar]
- 6.Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(8):1500-1511. [DOI] [PubMed] [Google Scholar]
- 7.Reese JA, Muthurajah DS, Kremer Hovinga JA, Vesely SK, Terrell DR, George JN. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired Adamts13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer. 2013;60(10):1676-1682. [DOI] [PubMed] [Google Scholar]
- 8.Deford CC, Reese JA, Schwartz LH, et al. . Multiple major morbidities and increased mortality during long-term follow-up after recovery from thrombotic thrombocytopenic purpura. Blood. 2013;122(12):2023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han B, Page EE, Stewart LM, et al. . Depression and cognitive impairment following recovery from thrombotic thrombocytopenic purpura. Am J Hematol. 2015;90(8):709-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer SC, Sulzer I, Mäder G, George JN, Lämmle B, Kremer Hovinga JA. Congenital or acquired ADAMTS13 deficiency or both - that’s the question [abstract]. J Thromb Haemost. 2009;7(s2):959-960. Abstract PP-TH-079-959. [Google Scholar]
- 13.Meyer SC, Sulzer I, Lämmle B, Kremer Hovinga JA. Hyperbilirubinemia interferes with ADAMTS-13 activity measurement by FRETS-VWF73 assay: diagnostic relevance in patients suffering from acute thrombotic microangiopathies. J Thromb Haemost. 2007;5(4):866-867. [DOI] [PubMed] [Google Scholar]
- 14.Froehlich-Zahnd R, George JN, Vesely SK, et al. . Evidence for a role of anti-ADAMTS13 autoantibodies despite normal ADAMTS13 activity in recurrent thrombotic thrombocytopenic purpura. Haematologica. 2012;97(2):297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George JN. Clinical practice. Thrombotic thrombocytopenic purpura. N Engl J Med. 2006;354(18):1927-1935. [DOI] [PubMed] [Google Scholar]
- 16.Rizvi MA, Vesely SK, George JN, et al. . Complications of plasma exchange in 71 consecutive patients treated for clinically suspected thrombotic thrombocytopenic purpura-hemolytic-uremic syndrome. Transfusion. 2000;40(8):896-901. [DOI] [PubMed] [Google Scholar]
- 17.McClain RS, Terrell DR, Vesely SK, George JN. Plasma exchange complications in patients treated for thrombotic thrombocytopenia purpura-hemolytic uremic syndrome: 2011 to 2014. Transfusion. 2014;54(12):3257-3259. [DOI] [PubMed] [Google Scholar]
- 18.Rock GA, Shumak KH, Buskard NA, et al. ; Canadian Apheresis Study Group. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325(6):393-397. [DOI] [PubMed] [Google Scholar]
- 19.Shumak KH, Rock GA, Nair RC; Canadian Apheresis Group. Late relapses in patients successfully treated for thrombotic thrombocytopenic purpura. Ann Intern Med. 1995;122(8):569-572. [DOI] [PubMed] [Google Scholar]
- 20.Mariotte E, Azoulay E, Galicier L, et al. ; French Reference Center for Thrombotic Microangiopathies. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016;3(5):e237-e245. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy AS, Lewis QF, Scott JG, et al. . Cognitive deficits after recovery from thrombotic thrombocytopenic purpura. Transfusion. 2009;49(6):1092-1101. [DOI] [PubMed] [Google Scholar]
- 22.Amorosi EL, Ultmann JE. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine. 1966;45(2):139-159. [Google Scholar]
- 23.Clark WF, Garg AX, Blake PG, Rock GA, Heidenheim AP, Sackett DL. Effect of awareness of a randomized controlled trial on use of experimental therapy. JAMA. 2003;290(10):1351-1355. [DOI] [PubMed] [Google Scholar]
- 24.Shah N, Rutherford C, Matevosyan K, Shen YM, Sarode R. Role of ADAMTS13 in the management of thrombotic microangiopathies including thrombotic thrombocytopenic purpura (TTP). Br J Haematol. 2013;163(4):514-519. [DOI] [PubMed] [Google Scholar]
- 25.George JN. Measuring ADAMTS13 activity in patients with suspected thrombotic thrombocytopenic purpura: when, how, and why? Transfusion. 2015;55(1):11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325(6):398-403. [DOI] [PubMed] [Google Scholar]
- 27.Lim W, Vesely SK, George JN. The role of rituximab in the management of patients with acquired thrombotic thrombocytopenic purpura. Blood. 2015;125(10):1526-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Rituximab reduces risk for relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2016;127(24):3092-3094. [DOI] [PubMed] [Google Scholar]
- 29.Scully M, McDonald V, Cavenagh J, et al. . A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118(7):1746-1753. [DOI] [PubMed] [Google Scholar]
- 30.Peyvandi F, Scully M, Kremer Hovinga JA, et al. ; TITAN Investigators. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511-522. [DOI] [PubMed] [Google Scholar]
- 31.Scully M, Knoebl P, Kentouche K, et al. Pharmacodynamic profile of a recombinant ADAMTS13 (BAX930) in hereditary thrombotic thrombocytopenic purpura (Upshaw-Schulman Syndrome [USS]) [abstract]. Blood. 2016;128(22). Abstract 135. [Google Scholar]