Abstract
It is unclear which of four popular contemporary diet patterns is best for weight maintenance among postmenopausal women. Four dietary patterns were characterised among postmenopausal women aged 49–81 years (mean 63·6 (SD 7·4) years) from the Women’s Health Initiative Observational Study: (1) a low-fat diet; (2) a reduced-carbohydrate diet; (3) a Mediterranean-style (Med) diet; and (4) a diet consistent with the US Department of Agriculture’s Dietary Guidelines for Americans (DGA). Discrete-time hazards models were used to compare the risk of weight gain (≥10 %) among high adherers of each diet pattern. In adjusted models, the reduced-carbohydrate diet was inversely related to weight gain (OR 0·71; 95 % CI 0·66, 0·76), whereas the low-fat (OR 1·43; 95 % CI 1·33, 1·54) and DGA (OR 1·24; 95 % CI 1·15, 1·33) diets were associated with increased risk of weight gain. By baseline weight status, the reduced-carbohydrate diet was inversely related to weight gain among women who were normal weight (OR 0·72; 95 % CI 0·63, 0·81), overweight (OR 0·67; 95 % CI 0·59, 0·76) or obese class I (OR 0·63; 95 % CI 0·53, 0·76) at baseline. The low-fat diet was associated with increased risk of weight gain in women who were normal weight (OR 1·28; 95 % CI 1·13, 1·46), overweight (OR 1·60; 95 % CI 1·40, 1·83), obese class I (OR 1·73; 95 % CI 1·43, 2·09) or obese class II (OR 1·44; 95 % CI 1·08, 1·92) at baseline. These findings suggest that a low-fat diet may promote weight gain, whereas a reduced-carbohydrate diet may decrease risk of postmenopausal weight gain.
Keywords: Weight gain, Diets, Obesity prevention, Mediterranean diet, Low-fat diets, Reduced-carbohydrate diets, Postmenopausal women
Many women gain weight during menopause(1,2), which can increase the risk of obesity and related chronic diseases such as diabetes, cancer and CVD(3,4). Identifying one or more diet patterns that may prevent weight gain could reduce the burden of obesity and related diseases among women in this age group. Although the US Department of Agriculture (USDA) issues the Dietary Guidelines for Americans (DGA) every 5 years, a number of conflicting dietary patterns continue to be investigated for their ability to induce weight-loss(5–9). Despite their popularity, diets such as a Mediterranean-style diet, a low-fat diet and a reduced-carbohydrate diet, have not been compared with the USDA DGA for their role in prevention of weight gain in free-living postmenopausal women. Moreover, in this area of research, where the majority of studies aim to achieve an energetic deficit, how diet influences weight maintenance when individuals are not asked to reduce their energy intake is largely unexplored. Thus, it remains unclear what overall dietary advice should be provided to this population for the maintenance of weight.
In this study, the relationship between four common diet patterns and weight gain in a heterogeneous sample of US postmenopausal women was examined in order to inform population-level dietary guidelines for the prevention of weight gain among free-living postmenopausal women in the USA. Using data from the Women’s Health Initiative Observational Study (WHI/OS), four diet patterns were characterised: (1) a low-fat diet; (2) a reduced-carbohydrate diet; (3) a Mediterranean-style (Med) diet; and (4) a diet consistent with the USDA’s DGA. In separate models, hazard ratios were computed by comparing the risk of weight gain in high and low adherers of each diet pattern. Overall hazards by diet pattern and stratified hazards by category of baseline weight status were computed.
Methods
Sample
Data were included from women who participated in the WHI/OS, a longitudinal study of postmenopausal women aged 49–81 years who were enrolled between 1994 and 1998, and followed for up to 8 years (n 93 676). Details regarding the sample and design of WHI/OS have been published elsewhere(10). Respondents with a BMI < 18·5 kg/m2 (n 1107), or those who reported following a diabetic diet at baseline (n 3764), were excluded, leaving 88 805 respondents in the final analytic sample. All procedures were conducted in accordance with the Declaration of Helsinki. This study (no. PA16-0039) is exempt from approval by internal review board (reviewed by University of Texas MD Anderson Cancer Center Office of Human Research Ethics).
Outcome
Height and weight were measured at baseline to classify respondents as normal weight (BMI: 18·5–24·9 kg/m2), overweight (BMI: 25·0–29·9 kg/m2), obese class I (BMI: 30·0–34·9 kg/m2), obese class II (BMI: 35·0–39·9 kg/m2) or obese class III or more (BMI ≥40·0 kg/m2). Respondents’ self-reported highest weight since last follow-up, assessed at years 1, 3, 4, 5, 6, 7 and 8, was used to compute weight change from baseline. Sensitivity analyses were conducted to examine the correlation between measured weight at baseline, and highest reported weight in the time since last follow-up at year 1 (Pearson’s r: 0·87; P < 0·001). Participants were identified as having experienced the ‘outcome’ if their reported highest weight since last follow-up was ≥10 % higher than baseline weight. In sensitivity analyses comparing 3, 5 and 10 % weight gain, and the average BMI at baseline (27·4 kg/m2), a 10 % increase in weight was found to be the smallest increment to shift the average BMI to the obese range (30·1 kg/m2). Thus, ≥10 % was the threshold used to define the outcome, which was modeled as a binary variable to accommodate a time-to-event analysis. Respondents were censored after developing the outcome, or when lost to follow-up. A sensitivity analysis was also performed using continuous weight change as the outcome of interest.
Dietary data
At baseline and year 3, dietary data were ascertained using a FFQ comprising 112 items. Dietary intake data from the baseline FFQ was used to assign respondents to a diet pattern. Food, beverage and nutrient intake was computed utilising the University of Minnesota Nutrition Coding Center nutrient database(11,12). The 2010 Healthy Eating Index (2010-HEI)(13) (available from http://appliedresearch.cancer.gov/hei/tools.html), and the MyPyramid Equivalents Database 2.0(14), were used to characterise adherence to the DGA diet. Baseline total 2010-HEI scores and component scores were computed for total vegetables; dark green vegetables, peas and beans; total fruit; whole fruit; whole grains; total dairy products; seafood and plant proteins; fatty acids; Na; and refined grains. The Alternate Mediterranean Diet (aMed) score was used to evaluate adherence to a Mediterranean-style diet(15). In brief, the aMed assigns 1 point for each of the following categories if intake is above the sample median: (1) vegetables; (2) legumes; (3) fruit; (4) nuts; (5) whole grains; (6) fish; and (7) ratio of monounsaturated fat:saturated fat. Before computing aMed component scores, intakes were adjusted for total energy, and thus component scores were based on the resulting ‘relative’ sample medians. In addition, 1 point is given if intake of total red and processed meats is below the median, or if alcohol (ethanol) intake is in the range of 5–25 g/d(15). The aMed gives a score of 0–9, which we rescaled to a 100-point scale for congruence with the 2010-HEI. For the 2010-HEI and aMed, a higher score indicates greater adherence with the DGA diet and the Mediterranean-style diet patterns, respectively. Quintile of total score was used to delineate high (top quintile) and low adherers (bottom quintile) of the Mediterranean-style and the DGA diet patterns. Below, we use ‘DGA diet’ to refer to those in the highest quintile of 2010-HEI score, and ‘Mediterranean-style diet’ to reference those in the highest quintile of aMed score. Quintiles of ‘percentage of total energy from fat’ and ‘percentage of total energy from carbohydrates’ were used to delineate high and low in the low-fat diet and the reduced-carbohydrate diet, respectively. Accordingly, ‘low-fat diet’ and ‘reduced-carbohydrate diet’ are used below to refer to those in the lowest quintile of intake fat and carbohydrates, respectively. To accommodate their continuous nature, the four diet patterns were compared using estimates from separate models (one for each diet pattern), rather than from a single combined model.
Covariates
Sociodemographic information was collected at baseline using a standard questionnaire. This information included annual family income, race/ethnicity (American Indian or Alaskan Native, Asian or Pacific Islander, Black or African American, Hispanic/Latino, White (not of Hispanic Origin), or other), age and highest education level completed. Alcohol intake was assessed by self-report at baseline, with possible responses ranging from ‘none to <1/month’ to ‘ ≥3 each day’. Lifetime smoking status at the time of survey was also ascertained at baseline (current, former and never). Physical activity was assessed at baseline using a standard questionnaire previously shown to have acceptable validity and reliability(16–18). Mild activity was defined as walking. Moderate activity was defined as ‘not exhausting’ and included biking outdoors, callisthenics, easy swimming and dancing. Strenuous or very hard exercise was defined as activities during which ‘You work up a sweat and your heart beats fast’. Waist circumference was measured at baseline using a standard protocol.
Statistical analysis
All analyses were conducted in SAS (version 9.4; SAS Institute Inc.) and Stata (version 14; StataCorp). Discrete-time hazards models were used to model the relationship between diet and weight gain. This approach is appropriate for estimating the hazard when the time to event is represented by a small number of wide intervals such that there are a preponderance of individuals with tied event times(19).
In separate models, the hazard for ≥10 % weight gain from baseline was compared among quintiles of a single dietary pattern of interest. All adjusted models controlled for baseline total energy intake (continuous) in order to adjust for potential measurement error in the ascertainment of dietary variables(20). In addition, adjusted models included diet type at year 3 to control for instability of diet class and associated measurement error over time, as well as the following potential confounders(8,15,21–26): (1) age (continuous); (2) baseline total mild, moderate and hard physical activity as metabolic equivalents of task (MET)-h/week (three continuous variables); (3) race/ethnicity; (4) annual family income; and (5) baseline smoking status. All categorical variables were modeled using disjoint indicator variables. Completes case analysis was used, whereby respondents with missing data for one or more covariates were excluded from the analyses.
Sensitivity analyses
We repeated our unadjusted and adjusted analyses specifying the hazard for weight gain from baseline to be ≥5 %.
Results
Sample characteristics are given in Table 1 for the eligible sample (n 88 805) and by level of weight gain at last follow-up. In all, 11 % of respondents (n 10 109) were missing data for one more covariates. At baseline, women were aged 49–81 years (mean: 63·6 (SD 7·4) years). Respondents were followed an average of 6·9 (SD 1·8), during which 19·5 % (n 17 290) of the sample gained ≥10 % of baseline weight. Degree of weight gain was significantly related to age, baseline weight status, waist circumference, education level, household income level, race/ethnicity, weekly MET-h of mild physical activity, smoking status and alcohol use (P < 0·01). In addition, baseline total energy intake, 2010-HEI score, aMed score, percent of total energy intake from fat, and percent of total energy from carbohydrates, were related to degree of weight gain over time (P < 0·01).
Table 1.
Baseline characteristics of women who participated in the Women’s Health Initiative Observational Study according to category of self-reported weight gain during the study* (Numbers and percentages; mean values and standard deviations)
| Full sample | Lost ≥5 % | Maintained weight within 5 % | Gained 5–10 % | Gained ≥10 % of baseline weight | Missing | P† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||
| n | % | n | % | n | % | n | % | n | % | ||||
| n | 88 805 | 1616 | 1·8 | 42 512 | 47·9 | 25 063 | 28·2 | 17 290 | 19·5 | 2324 | 2·6 | ||
| Weight status | <0·001 | ||||||||||||
| Normal weight (BMI: 18·5–24·9 kg/m2) | 35 994 | 234 | 0·7 | 17 396 | 48·3 | 10 570 | 29·4 | 7234 | 20·1 | 560 | 1·6 | ||
| Overweight (BMI: 25·0–29·9 kg/m2) | 30 316 | 333 | 1·1 | 14 709 | 48·5 | 8743 | 28·8 | 5958 | 19·7 | 573 | 1·9 | ||
| Obese class I (BMI: 30·0–34·9 kg/m2) | 13 616 | 259 | 1·9 | 6754 | 49·6 | 3622 | 26·6 | 2556 | 18·8 | 425 | 3·1 | ||
| Obese class II (BMI: 35·0–39·9 kg/m2) | 4920 | 130 | 2·6 | 2271 | 46·2 | 1351 | 27·5 | 982 | 20·0 | 186 | 3·8 | ||
| Obese class III (BMI ≥40·0 kg/m2) | 2902 | 272 | 9·4 | 1288 | 44·4 | 715 | 24·6 | 512 | 17·6 | 115 | 4·0 | ||
| Waist circumference (cm) | <0·001 | ||||||||||||
| <88 | 58 915 | 959 | 1·6 | 28 056 | 47·6 | 17 064 | 29·0 | 11 648 | 19·8 | 1188 | 2·0 | ||
| ≥88 | 29 518 | 633 | 2·1 | 14 336 | 48·6 | 7928 | 26·9 | 5593 | 19·0 | 1028 | 3·5 | ||
| Highest education completed | <0·001 | ||||||||||||
| Less than high school | 18 657 | 360 | 1·9 | 8645 | 46·3 | 5217 | 28·0 | 3684 | 19·8 | 751 | 4·0 | ||
| High school diploma or equivalent | 32 123 | 568 | 1·8 | 15 239 | 47·4 | 9015 | 28·1 | 6501 | 20·2 | 800 | 2·5 | ||
| Some college | 10 196 | 182 | 1·8 | 5085 | 49·9 | 2937 | 28·8 | 1780 | 17·5 | 212 | 2·1 | ||
| Baccalaureate degree or more | 27 097 | 485 | 1·8 | 13 212 | 48·8 | 7689 | 28·4 | 5182 | 19·1 | 529 | 2·0 | ||
| Household income | <0·001 | ||||||||||||
| <$20 000 | 12 772 | 307 | 2·4 | 5830 | 45·7 | 3350 | 26·2 | 2642 | 20·7 | 643 | 5·0 | ||
| $20 000–$49 999 | 35 709 | 620 | 1·7 | 17 181 | 48·1 | 10 090 | 28·3 | 6 999 | 19·6 | 819 | 2·3 | ||
| $50 000–$99 999 | 24 689 | 398 | 1·6 | 11 688 | 47·3 | 7207 | 29·2 | 4974 | 20·2 | 422 | 1·7 | ||
| ≥$100 000 | 9127 | 147 | 1·6 | 4538 | 49·7 | 2665 | 29·2 | 1636 | 17·9 | 141 | 1·5 | ||
| Race/ethnicity | <0·001 | ||||||||||||
| Non-Hispanic White | 74 516 | 1257 | 1·7 | 36 240 | 48·6 | 21 156 | 28·4 | 14 404 | 19·3 | 1459 | 2·0 | ||
| Non-Hispanic Black | 6889 | 192 | 2·8 | 2943 | 42·7 | 1815 | 26·4 | 1471 | 21·4 | 468 | 6·8 | ||
| Hispanic | 3362 | 90 | 2·7 | 1434 | 42·7 | 884 | 26·3 | 684 | 20·4 | 270 | 8·0 | ||
| Other | 2780 | 45 | 1·6 | 1322 | 47·6 | 859 | 30·9 | 477 | 17·2 | 77 | 2·8 | ||
| Smoking status | <0·001 | ||||||||||||
| Never | 44 508 | 841 | 1·9 | 21 760 | 48·9 | 12 779 | 28·7 | 7996 | 18·0 | 1132 | 2·5 | ||
| Former | 37 630 | 620 | 1·7 | 18 133 | 48·2 | 10 628 | 28·2 | 7354 | 19·5 | 895 | 2·4 | ||
| Current | 5401 | 128 | 2·4 | 2020 | 37·4 | 1339 | 24·8 | 1695 | 31·4 | 219 | 4·1 | ||
| Alcohol use | <0·001 | ||||||||||||
| Non-drinker | 9577 | 220 | 2·3 | 4578 | 47·8 | 2629 | 27·5 | 1739 | 18·2 | 411 | 4·3 | ||
| Past drinker | 15 809 | 330 | 2·1 | 6946 | 43·9 | 4313 | 27·3 | 3629 | 23·0 | 591 | 3·7 | ||
| <1 drink/month | 10 198 | 171 | 1·7 | 4589 | 45·0 | 2947 | 28·9 | 2254 | 22·1 | 237 | 2·3 | ||
| <1 drink/week | 17 982 | 302 | 1·7 | 8425 | 46·9 | 5209 | 29·0 | 3633 | 20·2 | 413 | 2·3 | ||
| 1–6 drinks/week | 23 187 | 302 | 1·7 | 8425 | 46·9 | 5209 | 29·0 | 3633 | 20·2 | 413 | 2·3 | ||
| ≥7 drinks/week | 11 402 | 370 | 1·6 | 11 605 | 50·1 | 6607 | 28·5 | 4189 | 18·1 | 416 | 1·8 | ||
|
|
|||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
|
|
|||||||||||||
| Age | 63·6 | 7·4 | 64·8 | 7·6 | 64·9 | 7·2 | 62·9 | 7·2 | 61·0 | 7·2 | 0 | 0·0 | <0·001 |
| Physical activity (MET-h/week) | |||||||||||||
| Mild exercise | 1·4 | 3·1 | 1·3 | 3·1 | 1·4 | 3·2 | 1·4 | 3·1 | 1·2 | 3·0 | 1006 | 1·1 | <0·001 |
| Moderate exercise | 3·3 | 5·4 | 3·0 | 5·0 | 3·4 | 5·4 | 3·3 | 5·3 | 3·3 | 5·5 | 1006 | 1·1 | 0·327 |
| Hard exercise | 3·9 | 8·5 | 3·9 | 9·0 | 4·1 | 8·6 | 3·9 | 8·4 | 3·9 | 8·6 | 1006 | 1·1 | 0·160 |
| Diet | |||||||||||||
| Energy intake (kJ/d) | 6486·5 | 2881·5 | 6338·8 | 3233·4 | 6452·1 | 2753·9 | 6544·2 | 2880·7 | 6466·4 | 2997 | 381 | 0·003 | |
| Energy intake (kcal/d) | 1550·3 | 688·7 | 1515·0 | 772·8 | 1542·1 | 658·2 | 1564·1 | 688·5 | 1545·5 | 716·3 | 91 | 0·1 | |
| 2010 Healthy Eating Index score (out of 100) | 69·0 | 9·6 | 68·2 | 10·1 | 69·3 | 9·5 | 69·1 | 9·5 | 68·6 | 9·7 | 91 | 0·1 | <0·001 |
| Alternate Mediterranean Diet score (out of 9) | 4·3 | 1·8 | 4·1 | 1·8 | 4·3 | 1·8 | 4·3 | 1·7 | 4·2 | 1·7 | 91 | 0·1 | <0·001 |
MET, metabolic equivalents of task.
There were 77 393 respondents with complete data for all covariates of interest.
P corresponds to a χ2 test for categorical variables, and ANOVA (F test) for continuous variables.
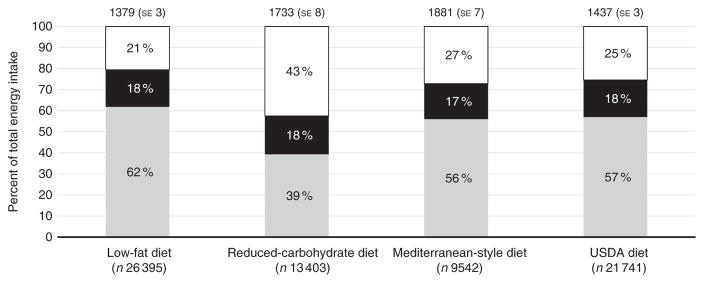
Selected dietary characteristics for high adherers of each dietary pattern are shown in Table 2. The Mediterranean-style diet was highest in energy content (7870 kJ/d (1881 kcal/d)), followed closely by the reduced-carbohydrate diet (7251 kJ/d (1733 kcal/d)). The low-fat diet was characterised by low dietary fat intake (32 (SD 14) g/d), low dietary cholesterol (132 (SD 72) mg/d) and moderate intake of total dietary fibre (18 (SD 8) g/d). The reduced-carbohydrate diet was characterised by low intake of carbohydrates (163 (SD 86) g/d), high intake of total fat (79 (SD 47) g/d) and high intake of dietary cholesterol (299 (SD 199) mg/d). The Mediterranean-style diet was highest in carbohydrate intake (258 (SD 86) g/d), total grains (6 (SD 3) servings/d) and alcohol intake (6 (SD 9) servings/week). The DGA diet was low in fat intake (42 (SD 20) g/d), moderate in carbohydrate intake (205 (SD 71) g/d) and highest in intake of total fibre (19 (SD 7) g/d). Macronutrient composition and mean total energy intake among high adherers of each diet pattern is given in Fig. 1. The proportion of total energy from carbohydrates was highest in the low-fat diet (61 %), whereas the proportion of total energy from fat was highest in the reduced-carbohydrate diet (41 %).
Table 2.
Selected dietary characteristics among high adherers of a low-fat, reduced-carbohydrate, Mediterranean-style or Dietary Guidelines for Americans (DGA) diet pattern among women who participated in the Women’s Health Initiative Observational Study* (Mean values and standard deviations)
| Low-fat diet | Reduced-carbohydrate diet | Mediterranean-style diet | DGA diet | |||||
|---|---|---|---|---|---|---|---|---|
| Total energy intake (kJ/d) | 5770 | 2117 | 7251 | 3673 | 7870 | 3008 | 6012 | 2092 |
| Total energy intake (kcal/d) | 1379 | 506 | 1733 | 878 | 1881 | 719 | 1437 | 500 |
| Carbohydrates (g/d) | 211 | 80 | 163 | 86 | 258 | 86 | 205 | 71 |
| Added sugar (teaspoon equivalents) | 8 | 6 | 8 | 6 | 10 | 6 | 8 | 5 |
| Total dietary fat (g/d) | 32 | 14 | 79 | 47 | 59 | 36 | 42 | 20 |
| Saturated fat (g/d) | 10 | 5 | 27 | 17 | 18 | 12 | 13 | 6 |
| Monounsaturated fat (g/d) | 12 | 5 | 30 | 18 | 23 | 14 | 16 | 8 |
| Polyunsaturated fat (g/d) | 7 | 3 | 16 | 10 | 13 | 8 | 10 | 5 |
| Trans-fat (g/d) | 2 | 1 | 6 | 4 | 4 | 3 | 3 | 2 |
| Protein (g/d) | 60 | 25 | 74 | 40 | 79 | 32 | 64 | 25 |
| Total dietary fibre (g/d) | 18 | 8 | 12 | 6 | 24 | 7 | 19 | 7 |
| Soluble fibre (g/d) | 5 | 2 | 3 | 2 | 6 | 2 | 5 | 2 |
| Insoluble fibre (g/d) | 13 | 6 | 9 | 5 | 18 | 5 | 14 | 5 |
| Dietary cholesterol (mg/d) | 132 | 72 | 299 | 199 | 200 | 138 | 153 | 85 |
| Servings of fruit | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 1 |
| Servings of vegetables | 3 | 1 | 2 | 1 | 3 | 1 | 3 | 1 |
| Total grains (ounce equivalents) | 5 | 3 | 4 | 3 | 6 | 3 | 4 | 2 |
| Whole grains | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 |
| Non-whole grains | 3 | 2 | 4 | 3 | 4 | 2 | 3 | 1 |
| Alcohol intake (servings/week) | 3 | 6 | 6 | 9 | 4 | 4 | 2 | 3 |
High adherers to the low-fat and reduced-carbohydrate diet patterns were those in the bottom quintile of percentage of total energy intake from the nutrient of interest, whereas high adherers to the Mediterranean-style and DGA diets were those in the top quintile for Alternate Mediterranean Diet score and 2010 Healthy Eating Index score, respectively.
Fig. 1.
Total energy intake and percentage of total energy from carbohydrates (
 ), fat (■) and protein (□) among high adherers of a low-fat diet, a reduced-carbohydrate diet, a Mediterranean-style diet and a diet consistent with the US Department of Agriculture’s (USDA) Dietary Guidelines for Americans. Total energy intake is given as mean values with their standard errors. Percentages given represent the percent of mean total energy intake. Data are from the Women’s Health Observational Study.
), fat (■) and protein (□) among high adherers of a low-fat diet, a reduced-carbohydrate diet, a Mediterranean-style diet and a diet consistent with the US Department of Agriculture’s (USDA) Dietary Guidelines for Americans. Total energy intake is given as mean values with their standard errors. Percentages given represent the percent of mean total energy intake. Data are from the Women’s Health Observational Study.
Pooled models
Risk of weight gain among high adherers of each diet was compared with that of low adherers in Table 3. In unadjusted models, high adherence to the low-fat (OR 0·86; 95 % CI 0·82, 0·91), Mediterranean-style (OR 0·68; 95 % CI 0·64, 0·73) and DGA (OR 0·77; 95 % CI 0·73, 0·81) diets was associated with decreased risk of weight gain. High adherence to the reduced-carbohydrate diet was weakly associated with increased risk of weight gain in unadjusted models (OR 1·05; 95 % CI 1·00, 1·11; P < 0·05). In adjusted models, high adherence to the low-fat (OR 1·43; 95 % CI 1·33, 1·54) and DGA (OR 1·24; 95 % CI 1·15, 1·33) diets was associated with increased risk of weight gain. There was no longer a significant relationship between diet pattern and risk of weight gain among high adherers to the Mediterranean-style diet (OR 0·95; 95 % CI 0·88, 1·03) in adjusted models. However, high adherence to the reduced-carbohydrate diet was associated with a sharply lower risk of weight gain in adjusted models (OR 0·71; 95 % CI 0·66, 0·76).
Table 3.
Relative odds of weight gain (≥10 % from baseline weight v. <10 %) by quintile (Q) of adherence to a low-fat, reduced-carbohydrate, Mediterranean-style or Dietary Guidelines for Americans (DGA) diet pattern among postmenopausal women who participated in the Women’s Health Initiative Observational Study* (Odds ratios and 95 % confidence intervals)
| Unadjusted (n 88 714) | Adjusted (n 70 177) | |||
|---|---|---|---|---|
|
|
|
|||
| OR | 95 % CI | OR | 95 % CI | |
| Low-fat diet | ||||
| Q1 (high adherence) | 0·86 | 0·82, 0·91 | 1·43 | 1·33, 1·54 |
| Q2 | 0·77 | 0·73, 0·81 | 1·14 | 1·06, 1·22 |
| Q3 | 0·79 | 0·74, 0·84 | 1·05 | 0·97, 1·13 |
| Q5 | 0·83 | 0·78, 0·89 | 0·99 | 0·93, 1·07 |
| Q5 (low adherence) | 1·00 | Ref. | 1·00 | Ref. |
| Ptrend† | <0·001 | <0·001 | ||
| Reduced-carbohydrate diet | ||||
| Q1 (high adherence) | 1·05 | 1·00, 1·11 | 0·71 | 0·66, 0·76 |
| Q2 | 0·92 | 0·88, 0·97 | 0·71 | 0·67, 0·76 |
| Q3 | 0·89 | 0·85, 0·93 | 0·77 | 0·73, 0·82 |
| Q5 | 0·91 | 0·87, 0·95 | 0·84 | 0·80, 0·88 |
| Q5 (low adherence) | 1·00 | Ref. | 1·00 | Ref. |
| Ptrend† | 0·516 | <0·001 | ||
| Mediterranean-style diet | ||||
| Q1 (low adherence) | 1·00 | Ref. | 1·00 | Ref. |
| Q2 | 0·89 | 0·85, 0·93 | 0·99 | 0·94, 1·05 |
| Q3 | 0·85 | 0·81, 0·89 | 1·01 | 0·96, 1·07 |
| Q5 | 0·78 | 0·74, 0·82 | 1·00 | 0·94, 1·07 |
| Q5 (high adherence) | 0·68 | 0·64, 0·73 | 0·95 | 0·88, 1·03 |
| Ptrend† | <0·001 | 0·513 | ||
| DGA diet | ||||
| Q1 (low adherence) | 1·00 | Ref. | 1·00 | Ref. |
| Q2 | 0·89 | 0·84, 0·94 | 1·04 | 0·97, 1·11 |
| Q3 | 0·82 | 0·78, 0·87 | 1·07 | 1·00, 1·14 |
| Q5 | 0·80 | 0·76, 0·84 | 1·14 | 1·07, 1·22 |
| Q5 (high adherence) | 0·77 | 0·73, 0·81 | 1·24 | 1·15, 1·33 |
| Ptrend† | <0·001 | <0·001 | ||
Ref., referent values.
All adjusted models controlled for baseline total energy intake (continuous), diet pattern at year 3 of follow-up, age (continuous), baseline total mild, moderate and hard physical activity as metabolic equivalents of task-h/week, race/ethnicity, annual family income and baseline smoking status. All categorical variables (race/ethnicity, annual family income and baseline smoking status) were modeled using disjoint indicator variables.
Ptrend corresponds to a Wald test statistic when a linear term for quintile of diet pattern was substituted in the model.
Stratified models
Baseline weight status was found to be a significant (P < 0·10) modifier of the relationship between diet pattern and weight gain. Pooled models therefore included an interaction term for baseline weight with diet pattern to obtain a unified estimate of the odds ratios across categories of baseline weight status. The results of these models are shown in Table 4. High adherence to the low-fat diet was associated with increased risk of weight gain among women who were normal weight (OR 1·28; 95 % CI 1·13, 1·46), overweight (OR 1·60; 95 % CI 1·40, 1·83), obese class I (OR 1·73; 95 % CI 1·43, 2·09) or obese class II (OR 1·44; 95 % CI 1·08, 1·92) at baseline.
Table 4.
Relative odds of weight gain (≥10 % from baseline weight vs. <10 %) by baseline weight status and quintile (Q) of adherence to a low-fat, reduced-carbohydrate, Mediterranean-style or Dietary Guidelines for Americans (DGA) diet pattern in postmenopausal women who participated in the Women’s Health Initiative Observational Study* (Odds ratios and 95 % confidence intervals)
| Normal weight | Overweight | Obese class I | Obese class II | Obese class III | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | |
| Low-fat diet | ||||||||||
| Q1 (high adherence) | 1·28 | 1·13, 1·46 | 1·60 | 1·40, 1·83 | 1·73 | 1·43, 2·09 | 1·44 | 1·08, 1·92 | 1·09 | 0·72, 1·65 |
| Q2 | 1·02 | 0·90, 1·15 | 1·22 | 1·07, 1·39 | 1·34 | 1·12, 1·59 | 1·35 | 1·04, 1·75 | 0·93 | 0·64, 1·35 |
| Q3 | 0·94 | 0·82, 1·07 | 1·19 | 1·04, 1·36 | 1·23 | 1·02, 1·48 | 1·07 | 0·81, 1·40 | 0·81 | 0·56, 1·17 |
| Q5 | 0·89 | 0·78, 1·02 | 1·09 | 0·95, 1·25 | 1·10 | 0·92, 1·31 | 1·06 | 0·82, 1·39 | 0·76 | 0·53, 1·09 |
| Q5 (low adherence) | 1·00 | Ref. | 1·00 | Ref. | 1·00 | Ref. | 1·00 | Ref. | 1·00 | Ref. |
| Ptrend† | <0·001 | <0·001 | <0·001 | 0·005 | 0·727 | |||||
| Reduced-carbohydrate diet | ||||||||||
| Q1 (high adherence) | 0·72 | 0·63, 0·81 | 0·67 | 0·59, 0·76 | 0·63 | 0·53, 0·76 | 0·79 | 0·60, 1·05 | 0·97 | 0·65, 1·46 |
| Q2 | 0·72 | 0·65, 0·81 | 0·73 | 0·65, 0·82 | 0·59 | 0·49, 0·70 | 0·83 | 0·63, 1·11 | 0·65 | 0·43, 0·99 |
| Q3 | 0·75 | 0·68, 0·83 | 0·74 | 0·66, 0·82 | 0·78 | 0·67, 0·91 | 1·05 | 0·81, 1·37 | 0·99 | 0·66, 1·49 |
| Q5 | 0·82 | 0·76, 0·89 | 0·81 | 0·74, 0·89 | 0·86 | 0·75, 0·99 | 1·05 | 0·83, 1·33 | 0·98 | 0·67, 1·43 |
| Q5 (low adherence) | 1·00 | Ref. | 1·00 | Ref. | 1·00 | Ref. | 1·00 | Ref. | 1·00 | Ref. |
| Ptrend† | <0·001 | <0·001 | <0·001 | 0·040 | 0·416 | |||||
| Mediterranean-style diet | ||||||||||
| Q1 (low adherence) | 1·00 | Ref. | 1·00 | Ref. | 1·00 | Ref. | 1·00 | Ref. | 1·00 | Ref. |
| Q2 | 0·91 | 0·83, 0·99 | 1·02 | 0·93, 1·12 | 1·06 | 0·93, 1·22 | 1·16 | 0·94, 1·43 | 0·79 | 0·57, 1·10 |
| Q3 | 0·99 | 0·90, 1·08 | 0·98 | 0·89, 1·08 | 1·05 | 0·90, 1·21 | 1·11 | 0·88, 1·41 | 1·19 | 0·85, 1·67 |
| Q4 | 0·93 | 0·84, 1·03 | 1·05 | 0·94, 1·18 | 1·09 | 0·92, 1·29 | 0·92 | 0·69, 1·25 | 1·12 | 0·73, 1·72 |
| Q5 (high adherence) | 0·90 | 0·80, 1·01 | 0·95 | 0·83, 1·09 | 1·15 | 0·93, 1·42 | 0·86 | 0·60, 1·23 | 1·08 | 0·61, 1·90 |
| Ptrend† | 0·159 | 0·831 | 0·206 | 0·531 | 0·403 | |||||
| DGA diet | ||||||||||
| Q1 (low adherence) | 1·00 | Ref. | 1·00 | Ref. | 1·00 | Ref. | 1·00 | Ref. | 1·00 | Ref. |
| Q2 | 0·99 | 0·88, 1·12 | 1·06 | 0·94, 1·20 | 1·01 | 0·86, 1·19 | 1·09 | 0·86, 1·38 | 1·04 | 0·75, 1·46 |
| Q3 | 1·00 | 0·89, 1·13 | 1·13 | 1·00, 1·28 | 1·04 | 0·88, 1·23 | 1·09 | 0·84, 1·41 | 1·65 | 1·15, 2·36 |
| Q4 | 1·10 | 0·98, 1·24 | 1·15 | 1·02, 1·30 | 1·28 | 1·08, 1·52 | 1·12 | 0·84, 1·49 | 1·09 | 0·73, 1·65 |
| Q5 (high adherence) | 1·13 | 1·00, 1·28 | 1·30 | 1·15, 1·48 | 1·41 | 1·17, 1·70 | 1·33 | 0·99, 1·80 | 1·86 | 1·18, 2·95 |
| Ptrend† | 0·005 | <0·001 | <0·001 | 0·096 | 0·019 | |||||
Ref., referent values.
Adjusted models included age (continuous) at baseline, alcohol intake at baseline, race/ethnicity, annual family income, physical activity at baseline (continuous), smoking status at baseline and energy intake (continuous) at baseline. All non-continuous variables were modeled using disjoint indicator variables.
Ptrend corresponds to a Wald test statistic when a linear term for quintile of diet pattern was substituted in the model.
High adherence to the reduced-carbohydrate diet was associated with decreased risk of postmenopausal weight gain among women who were normal weight (OR 0·72; 95 % CI 0·63, 0·81), overweight (OR 0·67; 95 % CI 0·59, 0·76) or obese class I (OR 0·63; 95 % CI 0·53, 0·76) at baseline.
Across all categories of baseline weight status, high adherence to the Mediterranean-style diet was not significantly related to risk of weight gain, although the relationship approached significance among women who were normal weight at baseline (OR 0·90, 95 % CI 0·90, 1·01; P = 0·083).
Conversely, high adherence to the DGA diet was associated with increased risk of weight gain in women who were normal weight (OR 1·13; 95 % CI 1·00, 1·28; P = 0·049), overweight (OR·089; 95 % CI 1·15, 1·48), obese class I (OR 1·41; 95 % CI 1·17, 1·70) and obese class III (OR 1·86; 95 % CI 1·18, 2·95). The relationship approached significance among women who were obese class II at baseline (OR 1·33; 95 % CI 0·99, 1·80; P = 0·059).
In sensitivity analyses, a ≥5 % weight gain (as opposed to ≥10 % weight gain) was used as the primary outcome. The pattern and directionality of the findings were similar to those of the primary analyses with only one exception. In our adjusted model, the relationship between the low-fat diet pattern and weight gain was in the opposite direction of our primary analysis (OR 0·85; 95 % CI 0·80, 0·90).
Discussion
Overall, we found that postmenopausal women with high adherence to a reduced-carbohydrate diet, with moderate fat and high protein intake, were at decreased risk for postmenopausal weight gain. This finding is consistent with prior related works. Gardner et al.(27) found that free-living overweight/obese women who consumed reduced-carbohydrate (34·5 % of total energy intake at 12 months) had significantly greater weight loss than those who with higher intake of carbohydrates (range: 45·4–52·4 % of total energy at 12 months). Moreover, those consuming a low-fat diet (29·8 % of total energy intake at 12 months) lost significantly less weight than those consuming diets with higher intakes of fat(27). Similarly, Shai et al.(5) found that, with unrestricted energy intake, respondents aged 40–65 years with obesity who followed a low-carbohydrate diet exhibited greater weight loss than those who followed a low-fat or Mediterranean diet. In each of these studies, respondents consuming the reduced-carbohydrate and low-fat diet patterns had similar macronutrient intake profiles to the respondents in our study with high adherence to the reduced-carbohydrate and low-fat diets, respectively.
Whereas the reduced-carbohydrate diet was protective against weight gain overall, greater adherence to a low-fat diet was associated with markedly increase of postmenopausal weight gain. This relationship persisted in stratified models (by weight status), wherein high adherence to the low-fat diet pattern was associated with greater risk of weight gain in women who were normal weight to obese class II at baseline. The relationship between the low-fat diet and weight gain was also positive among those with class III obesity at baseline, but did not reach statistical significance. This result stands in contrast to findings from long-term (≥2 years) weight loss trials, in which a low-fat diet has been reported to facilitate weight loss(5,28,29). Nonetheless, weight loss trials differ from our study in two important ways that may invalidate comparisons between the two. Foremost, our sample was heterogeneous with the majority of individuals classified as normal weight or overweight by BMI, whereas weight loss trials typically comprise predominantly individuals with obesity(5,28,29). Moreover, achieving an energetic deficit is commonly the goal of weight loss trials, whereas the aim of the current study was to examine the relationship between diet and incident weight gain independent of energetic intake.
Despite these differences, we observed a hierarchical relationship with weight gain among the low-fat, Mediterranean-style and reduced-carbohydrate diets that is consistent with findings from the weight loss trial literature. Shai et al.(5), who compared 2-year weight loss among adults with moderate obesity randomised to a Mediterranean, low-fat, or low-carbohydrate diet, reported that the low-carbohydrate diet was associated with the greatest weight loss, followed by the Mediterranean diet and the low-fat diet (low carbohydrate > Mediterranean > low fat). Similarly, we observed OR of 0·62, 1·24 and 2·05 for the reduced-carbohydrate, Mediterranean-style and low-fat diets, respectively, thereby indicating a hierarchical structure consistent with that reported by Shai et al.(5).
We also found that regardless of diet pattern they followed, postmenopausal women with a BMI ≥35·0 kg/m2 gained ≥10 % of their baseline weight. Although prior studies in adults have found those who were overweight or obese at baseline were more likely to gain weight than those who were normal weight at baseline(30–32), we are unaware of any prior study of weight change over time among adult women in which researchers further stratified their analyses to sub-classify individuals with obesity into class I, II or III. Moreover, our observation that no diet was protective against weight gain among those with a baseline BMI ≥35·0 kg/m2 would suggest the need for intervention in these individuals before their progression from class I to class II obesity. Future studies are needed to identify the point at which this transition occurs in order to inform such intervention efforts.
There are several limitations to our approach that warrant mention. Foremost, it should be noted that our sample comprised women who were predominantly non-Hispanic White (85·1 %), and thus findings may not be generalisable to minority populations. Second, although we found measured weight at baseline to be highly correlated with highest reported weight since last follow-up at year 1, it has been previously shown that self-reported weight is prone to reporting error, and the magnitude and direction with which individuals misreport may vary by sex, age and weight status(33,34). In addition, an epidemiological approach may have missed important confounding variables between dietary intake and weight gain. Additional limitations include the use of FFQ data to characterise diet and self-reported body weight, as measured weight was only available at two time points. Measurement error in diet assessment may have attenuated the relationship between diet and weight gain in our sample(35,36). However, FFQ are better at capturing ‘usual’ diet than other transient methods (e.g. 24-h recall, food record, etc.)(37), and intake from FFQ tend to be stable over time(37). Thus, FFQ are well-suited for our study, in which greater within-person diet class stability over time would enhance our ability to examine the relationship between diet and weight gain. Moreover, it has been previously shown that dietary intake from the WHI FFQ had acceptable correlations with dietary intake from food records(38). The inclusion of covariates related to misreporting of intake via FFQ(39), as well as total energy intake, may have minimised the influence of FFQ-related measurement error on our findings. Fourth, although each of the four diet patterns was characterised using distinct criteria, it was possible for individuals to fall into more than one diet pattern. Nonetheless, diet patterns were modeled separately, thereby eliminating the possibility for an individual to represent more than one diet pattern within a given model. Finally, we chose a weight gain threshold of ≥10 % to characterise weight gain, as the majority of women in our study gained weight during the course of follow-up. In sensitivity analyses, in which we explored the use of ≥5 % weight gain as the outcome, we observed a similar pattern of findings for all but the low-fat diet pattern, thereby suggesting a degree of robustness to our principal findings. Nonetheless, in adjusted models using the lower threshold for weight gain, the relationship between the low-fat diet and risk of weight gain was in the opposite direction of that which we observed in our primary analyses. Notably, the significance of this finding is not clear. A possible explanation is that, because most women in our sample gained weight over time, the lower threshold of ≥5 % weight gain resulted in little heterogeneity in the risk of weight gain between high and low adherers of each diet pattern. If true, then cautious interpretation of these findings would be warranted.
Despite these limitations, this study addresses a gap in research regarding the relationship between diet and long-term weight change among free-living individuals. Unlike weight loss trials, wherein the goal is for subjects to consume fewer energy content than expended, this study provides an examination of the relationship between diet and long-term weight change when subjects were not asked to change their diets. Moreover, whereas most prior studies have focused on weight loss, our focus on prevention of weight gain provides a unique contribution to the literature. Our results address the question ‘which diet is optimal for weight maintenance among free-living postmenopausal women who follow a diet of their own choosing?’ We found that a reduced-carbohydrate diet, high in fat and protein intake, was associated with reduced risk of weight gain in postmenopausal women overall, whereas a low-fat a low-fat diet was associated with increased risk of postmenopausal weight gain.
Conclusion
Consuming a reduced-carbohydrate diet, with moderate fat and high protein intake, may decrease the risk of weight gain in post-menopausal women. However, prevailing dietary recommendations call for limiting fat intake in order to promote optimal health and prevent chronic disease. Our findings therefore challenge prevailing dietary recommendations, suggesting instead that a low-fat may promote rather than prevent weight gain after menopause.
Acknowledgments
Funding for C. F. and S. C. comes from the National Institutes of Health, National Cancer Institute (5 R25 CA057730-24). The Women’s Health Initiative programme is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts HHSN268201100046C, HHSN26801100001C, HHSN 268201100002C, HHSN268201100003C, HHSN268201100004C and HHSN271201100004C.
C. F. designed the study, completed the analyses and drafted and revised the paper. He is the guarantor. S. C. and A. C. F.-W. provided oversight and guidance during the planning phase and were instrumental in writing the manuscript proposal. In addition, S. C. and A. C. F.-W. helped to interpret analytic results, and provided extensive review, edits and feedback on the manuscript. M. Z. V. and J. I. F. also contributed extensively to the manuscript proposal and manuscript by providing critical review, commentary and edits. B. V. H., J. J. R., M. S., B. C., L. S. and R. U. provided additional feedback on the manuscript proposal, as well as extensive feedback, edits and commentary on the manuscript through several rounds of internal revision before the manuscript’s submission to the journal. B. V. H., L. S. and M. S. also had instrumental roles in developing, planning and implementing one or more components of the Women’s Health Initiative Study.
The authors declare that there are no conflicts of interest.
Abbreviations
- 2010-HEI
2010 Healthy Eating Index
- aMed
Alternate Mediterranean Diet
- DGA
Dietary Guidelines for Americans
References
- 1.Wing RR, Matthews KA, Kuller LH, et al. Weight gain at the time of menopause. Arch Intern Med. 1991;151:97–102. [PubMed] [Google Scholar]
- 2.Polotsky HN, Polotsky AJ. Metabolic implications of menopause. Semin Reprod Med. 2010;28:426–434. doi: 10.1055/s-0030-1262902. [DOI] [PubMed] [Google Scholar]
- 3.Masters RK, Reither EN, Powers DA, et al. The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103:1895–1901. doi: 10.2105/AJPH.2013.301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrell LN, Samuel L. Body mass index categories and mortality risk in US adults: the effect of overweight and obesity on advancing death. Am J Public Health. 2014;104:512–519. doi: 10.2105/AJPH.2013.301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 6.Esposito K, Kastorini C-M, Panagiotakos DB, et al. Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Metab Syndr Relat Disord. 2011;9:1–12. doi: 10.1089/met.2010.0031. [DOI] [PubMed] [Google Scholar]
- 7.Pelkman CL, Fishell VK, Maddox DH, et al. Effects of moderate-fat (from monounsaturated fat) and low-fat weight-loss diets on the serum lipid profile in overweight and obese men and women. Am J Clin Nutr. 2004;79:204–212. doi: 10.1093/ajcn/79.2.204. [DOI] [PubMed] [Google Scholar]
- 8.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: the Women’s Health Initiative Dietary Modification Trial. JAMA. 2006;295:39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 9.Avenell A, Brown T, McGee M, et al. What are the long-term benefits of weight reducing diets in adults? A systematic review of randomized controlled trials. J Hum Nutr Diet. 2004;17:317–335. doi: 10.1111/j.1365-277X.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 10.Study TWsHI. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 11.Schakel S, Sievert Y, Buzzard I. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–1271. [PubMed] [Google Scholar]
- 12.Kristal AR, Shattuck AL, Williams AE. Food frequency questionnaires for diet intervention research. Proceedings of the 17th National Nutrient Databank Conference; June 1992; Washington, DC. 1992. pp. 110–125. [Google Scholar]
- 13.Guenther PM, Casavale KO, Reedy J, et al. Update of the healthy eating index: HEI-2010. J Acad Nutr Diet. 2013;113:569–580. doi: 10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowman SA, Friday JE, Moshfegh AJ. MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004: Documentation and User Guide. Beltsville, MD: Food Surveys Research Group, US Department of Agriculture, Beltsville Human Nutrition Research Center, Agricultural Research Service; 2008. [Google Scholar]
- 15.Fung TT, McCullough ML, Newby P, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82:163–173. doi: 10.1093/ajcn.82.1.163. [DOI] [PubMed] [Google Scholar]
- 16.Meyer AM, Evenson KR, Morimoto L, et al. Test-retest reliability of the Women’s Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41:530–538. doi: 10.1249/MSS.0b013e31818ace55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuhouser ML, Di C, Tinker LF, et al. Physical activity assessment: biomarkers and self-report of activity-related energy expenditure in the WHI. Am J Epidemiol. 2013;177:576–585. doi: 10.1093/aje/kws269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettee Gabriel K, McClain JJ, Lee CD, et al. Evaluation of physical activity measures used in middle-aged women. Med Sci Sports Exerc. 2009;41:1403–1412. doi: 10.1249/MSS.0b013e31819b2482. [DOI] [PubMed] [Google Scholar]
- 19.Allison PD. Discrete-time methods for the analysis of event histories. Sociol Methodol. 1982;13:61–98. [Google Scholar]
- 20.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belin RJ, Greenland P, Allison M, et al. Diet quality and the risk of cardiovascular disease: the Women’s Health Initiative (WHI) Am J Clin Nutr. 2011;94:49–57. doi: 10.3945/ajcn.110.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beresford SA, Johnson KC, Ritenbaugh C, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:643–654. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- 24.Mendez MA, Popkin BM, Jakszyn P, et al. Adherence to a Mediterranean diet is associated with reduced 3-year incidence of obesity. J Nutr. 2006;136:2934–2938. doi: 10.1093/jn/136.11.2934. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Villegas A, Bes-Rastrollo M, Martinez-Gonzalez M, et al. Adherence to a Mediterranean dietary pattern and weight gain in a follow-up study: the SUN cohort. Int J Obes (Lond) 2006;30:350–358. doi: 10.1038/sj.ijo.0803118. [DOI] [PubMed] [Google Scholar]
- 26.Frazier-Wood AC, Kim J, Davis JS, et al. In cross-sectional observations, dietary quality is not associated with CVD risk in women; in men the positive association is accounted for by BMI. Br J Nutr. 2015;113:1244–1253. doi: 10.1017/S0007114515000185. [DOI] [PubMed] [Google Scholar]
- 27.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and Learn diets for change in weight and related risk factors among overweight premenopausal women: the A to Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 28.Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Inter Med. 2010;153:147–157. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He K, Hu FB, Colditz GA, et al. Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. Int J Obes Relat Metab Disord. 2004;28:1569–1574. doi: 10.1038/sj.ijo.0802795. [DOI] [PubMed] [Google Scholar]
- 31.Field AE, Willett WC, Lissner L, et al. Dietary fat and weight gain among Women in the Nurses’ Health Study. Obesity (Silver Spring) 2007;15:967–976. doi: 10.1038/oby.2007.616. [DOI] [PubMed] [Google Scholar]
- 32.Williamson DF, Kahn HS, Remington PL, et al. The 10-year incidence of overweight and major weight gain in us adults. Arch Intern Med. 1990;150:665–672. [PubMed] [Google Scholar]
- 33.Villanueva EV. The validity of self-reported weight in US adults: a population based cross-sectional study. BMC Public Health. 2001;1:11. doi: 10.1186/1471-2458-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorber SC, Tremblay M, Moher D, et al. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 35.Willett W, Lenart E. Reproducibility and validity of food-frequency questionnaires. In: Willett W, editor. Nutritional Epidemiology. New York: Oxford University Press; 2013. pp. 96–141. [Google Scholar]
- 36.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 37.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 38.Patterson RE, Kristal AR, Tinker LF, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 39.Horner NK, Patterson RE, Neuhouser ML, et al. Participant characteristics associated with errors in self-reported energy intake from the Women’s Health Initiative food-frequency questionnaire. Am J Clin Nutr. 2002;76:766–773. doi: 10.1093/ajcn/76.4.766. [DOI] [PubMed] [Google Scholar]