Abstract
Importance
Health outcomes from the Women's Health Initiative Estrogen Plus Progestin and Estrogen-Alone Trials have been reported, but previous publications have generally not focused on all-cause and cause-specific mortality.
Objective
To examine total and cause-specific cumulative mortality, including during the intervention and extended postintervention follow-up, of the 2 Women's Health Initiative hormone therapy trials.
Design, Setting, and Participants
Observational follow-up of US multiethnic postmenopausal women aged 50 to 79 years enrolled in 2 randomized clinical trials between 1993 and 1998 and followed up through December 31,2014.
Interventions
Conjugated equine estrogens (CEE, 0.625 mg/d) plus medroxy progesterone acetate (MPA, 2.5 mg/d) (n = 8506) vs placebo (n = 8102) for 5.6 years (median) or CEE alone (n = 5310) vs placebo (n = 5429) for 7.2 years (median).
Main Outcomes and Measures
All-cause mortality (primary outcome) and cause-specific mortality (cardiovascular disease mortality, cancer mortality, and other major causes of mortality) in the 2 trials pooled and in each trial individually, with prespecified analyses by 10-year age group based on age at time of randomization.
Results
Among 27 347 women who were randomized (baseline mean [SD]age, 63.4 [7.2] years; 80.6% white), mortality follow-up was available for more than 98%. During the cumulative 18-year follow-up, 7489 deaths occurred (1088 deaths during the intervention phase and 6401 deaths during postintervention follow-up). All-cause mortality was 27.1% in the hormone therapy group vs 27.6% in the placebo group (hazard ratio [HR], 0.99 [95% CI, 0.94-1.03]) inthe overall pooled cohort; with CEE plus MPA, the HR was 1.02 (95% CI, 0.96-1.08); and with CEE alone, the HR was 0.94 (95% CI, 0.88-1.01). In the pooled cohort for cardiovascular mortality, the HR was 1.00 (95% CI, 0.92-1.08 [8.9 % with hormone therapy vs 9.0% with placebo]); for total cancer mortality, the HR was 1.03 (95% CI, 0.95-1.12 [8.2% with hormone therapy vs 8.0% with placebo]); and for other causes, the HR was 0.95 (95% CI, 0.88-1.02 [10.0% with hormone therapy vs 10.7% with placebo]), and results did not differ significantly between trials. When examined by 10-year age groups comparing younger women (aged 50-59 years) to older women (aged 70-79 years) in the pooled cohort, the ratio of nominal HRs for all-cause mortality was 0.61 (95% CI, 0.43-0.87) during the intervention phase and the ratio was 0.87 (95% CI, 0.76-1.00) during cumulative 18-year follow-up, without significant heterogeneity between trials.
Conclusions and Relevance
Among postmenopausal women, hormone therapy with CEE plus MPA for a median of 5.6 years or with CEE alone for a median of 7.2 years was not associated with risk of all-cause, cardiovascular, or cancer mortality during a cumulative follow-up of 18 years.
The Women's Health Initiative (WHI) hormone therapy trials were designed to assess the benefits and risks of menopausal hormone therapy taken for chronic disease prevention by predominantly healthy postmenopausal women.1-3 The double-blinded, placebo-controlled, randomized clinical trials, conducted among US postmenopausal women aged 50 to79 years at enrollment, tested the most common formulations of hormone therapy prescribed at the time of study initiation: conjugated equine estrogens (CEE) plus medroxy progesterone acetate (MPA) for women with an intact uterus and CEE alone for women with hysterectomy. The CEE plus MPA trial was stopped early (after 5.6years) due to an increased risk of breast cancer and overall risks exceeding benefits2; the CEE-alone trial was stopped after7.2 years due to an increased risk of stroke.3Postintervention follow up has been ongoing.
Health outcomes, including all-cause mortality from the 2 hormone therapy trials, have been reported,2-7 but previous publications have not focused on all-cause and cause-specific mortality. Moreover, the current report includes extended follow-up and mortality surveillance of the entire randomized cohort using the National Death Index (NDI; 7489 deaths through December 31,2014). Hormone therapy has been shown to have a complex balance of benefits and risks with important effects on numerous outcomes—some of which differ between the 2 hormone therapy formulations.2,3,6 All-cause mortality is a critically important summary measure representing the net effect of hormone therapy on serious and life-threatening health conditions. Thus, we examined total and cause-specific mortality during cumulative 18-year follow-up (intervention plus extended postintervention phases) of the 2 WHI hormone therapy trials, individually and pooled, with attention to potential differences by age.
Methods
Study Design
Details of the 2 WHI hormone therapy trial designs, adherence, and outcome adjudication procedures (including for mortality) have been previously published (trial protocol in Supplement 1).1-3,6 Briefly, 27 347 postmenopausal women ages 50 to 79 years were recruited from 1993 to 1998 at 40 US clinical centers; 16608 women with a uterus were randomized to receive daily oral CEE (0.625 mg) plus MPA (2.5 mg, Prempro) or placebo and 10 739 women with hysterectomy were randomized to receive daily oral CEE (0.625 mg, Premarin) alone or placebo. The primary outcomes of both trials were incident coronary heart disease and invasive breast cancer.1 Institutional review board approval was obtained at each center and all participants provided written informed consent. Race and ethnicity were self-reported. Post intervention follow-up included deaths through December 31, 2014 (median, 18 years cumulatively), with mortality ascertained by regular surveillance of the cohort through the NDI and by reports of next of kin or the postal service. NDI searches were conducted at 7 time points before 2015 for all participants who had unknown vital status. Some hazard ratios (HRs) may differ slightly from those previously reported due to more complete mortality ascertainment in the present report. After the trials were stopped, participants were unmasked to randomization assignment and fewer than 4% of women reported personal posttrial hormone therapy use.
Statistical Analysis
For each trial, cumulative analyses included all randomized participants according to their randomization assignment from time of randomization until death or December 31, 2014, the last date covered by the NDI linkage, based on the intention-to-treat principle. Mortality end points included all-cause mortality (primary analysis); cardiovascular disease (CVD) mortality (further subdivided into deaths from coronary heart disease, stroke, and other known CVD); cancer mortality (subdivided into deaths from breast, colorectal, and other known cancers); and other mortality (based on the other leading causes of death in women including Alzheimer disease or other dementia, chronic obstructive pulmonary disease [COPD], injuries and accidents, and other known causes). HRs were estimated using Cox proportional hazards models stratified by age and randomization status in the WHI Dietary Modification Trial. Preplanned analyses included presentation of cumulative and intervention-phase HRs and forest plots for each trial separately, as well as for the pooled trials unless the P value for heterogeneity between trials was less than .05 (eTables 1-3 in Supplement 2). HRs may exhibit time dependencies within or between phases as previously reported.4,5
Age subgroup analyses were preplanned for the major mortality end points. Interactions between randomization group and age stratum were based on a 1 degree-of-freedom test for linear trend; 10-year age groups (based on age at time of randomization) were assigned a continuous value (0,1, 2). HRs for younger vs older age groups were also directly compared as ratios.
Statistical tests were based on a 2-sided log-rank (score) test. Nominal (unadjusted) P values are provided and those of less than .05 were considered statistically significant. However, P values should be interpreted cautiously due to multiple comparisons. Sensitivity analyses were restricted to participants who took more than 80% of study pills (active or placebo) for at least 2 years or until death. All statistical analyses were conducted using SAS software version 9.3 (SAS Institute Inc) and R software version 2.15(R Foundation for Statistical Computing, http://www.r-project.org/).
Results
Baseline Characteristics and Study Follow-up Periods
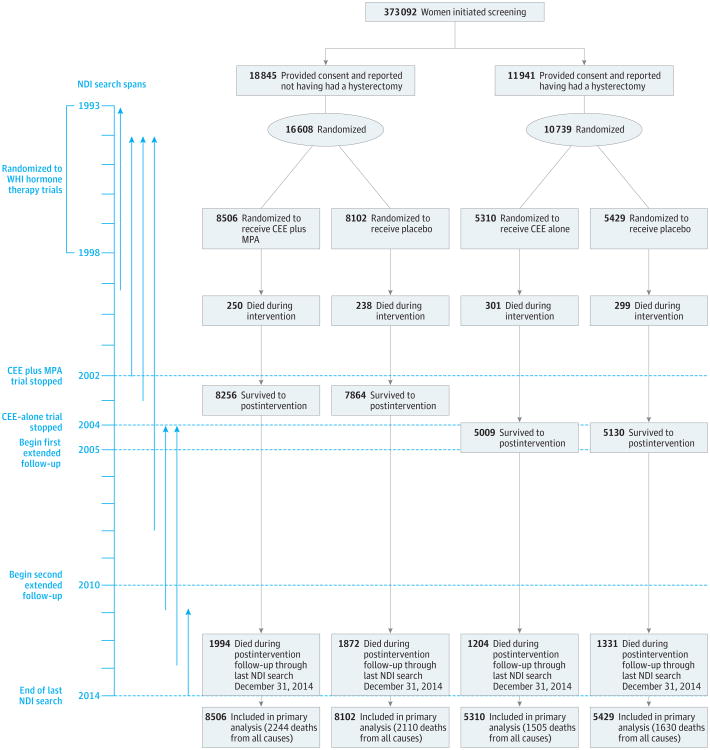
Baseline characteristics for the 2 randomization groups in each trial were well balanced on demographic and clinical risk factors (Table). The CEE plus MPA trial ended in July 2002 (after a median of 5.6 years),2,6 and the CEE-alone trial ended in Feb-ruary2004 (afteramedianof7.2years).3,6 The present report is based on mortality follow-up through December 31, 2014 (end of the last NDI search), and includes 7489 deaths (1088 occurred during the intervention phase and 6401 occurred postintervention; 4083 additional deaths since the last report). In addition to the intervention phases, the results reported in this study include median postintervention follow-up of 12.5 years and cumulative follow-up of 18 years for the CEE plus MPA trial and a median post intervention follow-up of 10.8 years and cumulative follow-up of 18 years for the CEE-alone trial (Figure 1). Mortality is more than 98% complete, based on NDI evaluation.8
Table. Baseline Characteristics of Participants in the Women's Health Initiative Trials of Postmenopausal Hormone Therapy.
| Characteristic | No. (%) of Participantsa | |||
|---|---|---|---|---|
| CEE Plus MPA Trial | CEE-Alone Trial | |||
| Active (n = 8506) | Placebo (n = 8102) | Active (n = 5310) | Placebo (n = 5429) | |
| Age at screening, mean (SD), y | 63.2 (7.1) | 63.3 (7.1) | 63.6 (7.3) | 63.6 (7.3) |
| Age group at screening, y | ||||
| 50-59 | 2837 (33.4) | 2683 (33.1) | 1639 (30.9) | 1674 (30.8) |
| 60-69 | 3854 (45.3) | 3655 (45.1) | 2386 (44.9) | 2465 (45.4) |
| 70-79 | 1815 (21.3) | 1764 (21.8) | 1285 (24.2) | 1290 (23.8) |
| Race/ethnicity | ||||
| White | 7141 (84.0) | 6805 (84.0) | 4009 (75.5) | 4075 (75.1) |
| Black | 548 (6.4) | 574 (7.1) | 781 (14.7) | 835 (15.4) |
| Hispanic | 471 (5.5) | 415 (5.1) | 319 (6.0) | 332 (6.1) |
| American Indian | 25 (0.3) | 30 (0.4) | 41 (0.8) | 34 (0.6) |
| Asian/Pacific Islander | 194 (2.3) | 169 (2.1) | 86 (1.6) | 78 (1.4) |
| Unknown | 127 (1.5) | 109 (1.3) | 74 (1.4) | 75 (1.4) |
| >High school diploma or GED | 6272 (74.1) | 5899 (73.3) | 3488 (66.3) | 3678 (68.3) |
| Family income ≥$50 000 | 2447 (30.4) | 2401 (31.4) | 1148 (22.9) | 1167 (22.9) |
| Hormone use | ||||
| Never | 6277 (73.8) | 6022 (74.4) | 2769 (52.2) | 2769 (51.0) |
| Past | 1671 (19.7) | 1587 (19.6) | 1871 (35.2) | 1947 (35.9) |
| Currentb | 554 (6.5) | 490 (6.1) | 669 (12.6) | 709 (13.1) |
| Vasomotor symptoms | ||||
| None | 5162 (61.3) | 4928 (61.5) | 2962 (56.4) | 3004 (56.0) |
| Mild | 2190 (26.0) | 2115 (26.4) | 1377 (26.2) | 1441 (26.9) |
| Moderate or severe | 1072 (12.7) | 974 (12.1) | 913 (17.4) | 917 (17.1) |
| Body mass index, median (IQR)c | 27.5 (24.2-31.7) | 27.5 (24.3-31.7) | 29.2 (25.7-33.7) | 29.2 (25.7-33. |
| Systolic BP, mm Hg, mean (SD) | 127.6 (17.6) | 127.8 (17.5) | 130.4 (17.5) | 130.2 (17.6) |
| Diastolic BP, mm Hg, mean (SD) | 75.6 (9.1) | 75.8 (9.1) | 76.5 (9.2) | 76.5 (9.4) |
| Smoking | ||||
| Never | 4178 (49.6) | 3999 (50.0) | 2723 (51.9) | 2705 (50.4) |
| Past | 3362 (39.9) | 3157 (39.5) | 1986 (37.8) | 2090 (38.9) |
| Current | 880 (10.5) | 838 (10.5) | 542 (10.3) | 571 (10.6) |
| Bilateral oophorectomy | 29 (0.3) | 24 (0.3) | 1938 (39.5) | 2111 (42.0) |
| Medical treatment received | ||||
| Diabetes | 374 (4.4) | 360 (4.4) | 410 (7.7) | 412 (7.6) |
| Hypertension or BP ≥140/90 | 3377 (43.2) | 3283 (42.7) | 2651 (53.3) | 2647 (52.6) |
| High cholesterol requiring medication | 1018 (12.0) | 1027 (12.7) | 763 (14.4) | 829 (15.3) |
| Statin use at baseline | 580 (6.8) | 535 (6.6) | 397 (7.5) | 430 (7.9) |
| Aspirin use (≥80 mg/d) | 1652 (19.4) | 1654 (20.4) | 1050 (19.8) | 1081 (19.9) |
| Medical history | ||||
| Myocardial infarction | 139 (1.6) | 157 (1.9) | 165 (3.1) | 173 (3.2) |
| Angina | 318 (3.8) | 331 (4.1) | 402 (7.6) | 388 (7.2) |
| CABG or PCI | 95 (1.1) | 120 (1.5) | 120 (2.3) | 114 (2.1) |
| Stroke | 61 (0.7) | 77 (1.0) | 76 (1.4) | 92 (1.7) |
| DVT or pulmonary embolism | 79 (0.9) | 62 (0.8) | 87 (1.6) | 84 (1.5) |
| Family history of breast cancerd | 1286 (16.0) | 1175 (15.3) | 892 (17.9) | 870 (17.1) |
Abbreviations: BP, blood pressure; CABG, coronary artery bypass graft; CEE, conjugated equine estrogens; DVT, deep vein thrombosis; GED, general equivalency diploma; IQR, interquartile range; MPA, medroxy progesterone acetate; PCI, percutaneous coronary intervention.
Values are reported as No. (%) unless otherwise indicated.
Required a 3-month washout period prior to randomization.
Calculated as weight in kilograms divided by height in meters squared.
Indicates occurrence in paticipant's mother, sister, daughter, or grandmother.
Figure 1. Flow of Participants in the Women's Health Initiative Trials of Postmenopausal Hormone Therapy vs Placebo Through Extended Follow-up.
During the postintervention and extension phases, fewer than2%of women in the conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) trial and fewer than 4% of women in the CEE-alone trial reported use of hormone therapy. NDI indicates National Death Index.
All-Cause Mortality
Cumulative Follow-up Phase
During cumulative 18-year follow-up, all-cause mortality in the overall pooled cohort was 27.1% with hormone therapy vs 27.6% with placebo (HR, 0.99 [95% CI, 0.94-1.03]; P = .60). For the individual trials, all-cause mortality was 26.4% for CEE plus MPA vs 26.0% for placebo (HR, 1.02 [95% CI, 0.96-1.08]; P = .51), and for CEE alone it was 28.3% vs 30.0% for placebo (HR, 0.94 [95% CI, 0.88-1.01]; P = .11) (Figure 2; eTable 1 in Supplement 2).
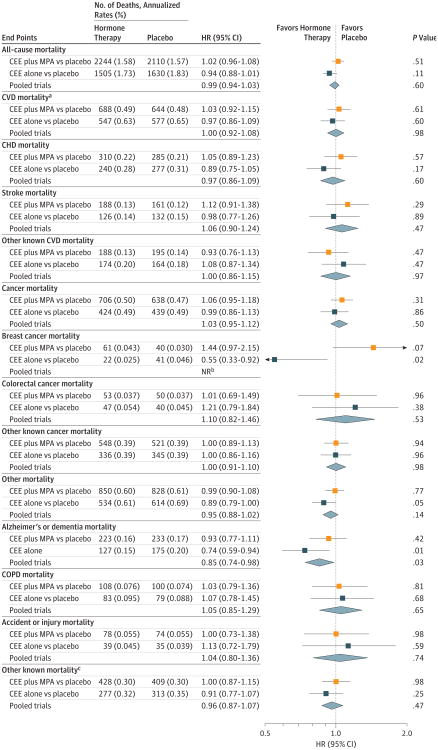
Figure 2. Mortality Outcomes in the Women's Health Initiative Hormone Therapy Trials During the 18-Year Cumulative Follow-up.
The 18-year follow-up is cumulative, indicating the intervention plus extended postintervention phases of the 2 trials (median, 17.7 [interquartile range {IQR}, 16.6-18.6] years in the conjugated equine estrogens [CEE] plus medroxy progesterone acetate [MPA] trial; median, 17.7 [IQR, 16.5-18.7] years in the CEE-alone trial; and median,17.7 [IQR,16.6-18.6] years in the pooled analysis).
aCardiovascular disease (CVD) mortality includes deaths due to myocardial infarction, coronary heart disease, stroke, heart failure, peripheral vascular disease, venous thromboembolism, and other major causes of CVD death.
bThe P value corresponding with a test of heterogeneity between trial-specific hazard ratios (HRs) was .05 or less; therefore, the pooled estimate and HR (95% CI) are not reported.
cIndicates other mortality outcomes that were known but were not due to Alzheimer disease or other dementia, chronic obstructive pulmonary disease (COPD), or accident or injury.
Intervention and Postintervention Phases
During the intervention phase, all-cause mortality in the pooled cohort was 4.0% with hormone therapy vs 4.0% with placebo (HR, 1.01 [95% CI, 0.90-1.14]; P = .86; eFigure and eTable 2 in Supplement2). Compared with placebo, women randomized to receive to CEE plus MPA had an HR of 0.97 (95% CI, 0.82-1.16; P = .77) and women randomized to receive CEE alone had an HR of 1.04 (95% CI, 0.89-1.22; P = .62) (eTable 2 in Supplement 2). During the postintervention period, the HR for all-cause mortality was 1.04 (95% CI, 0.97-1.10; P = .28) for CEE plus MPA and 0.92 (95% CI, 0.85-0.99; P = .03) for CEE alone (eTable 3 in Supplement 2).
Age-Stratified Analyses
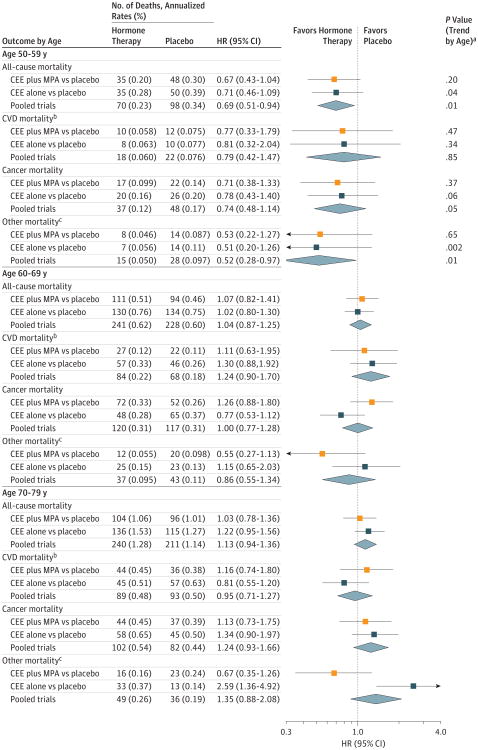
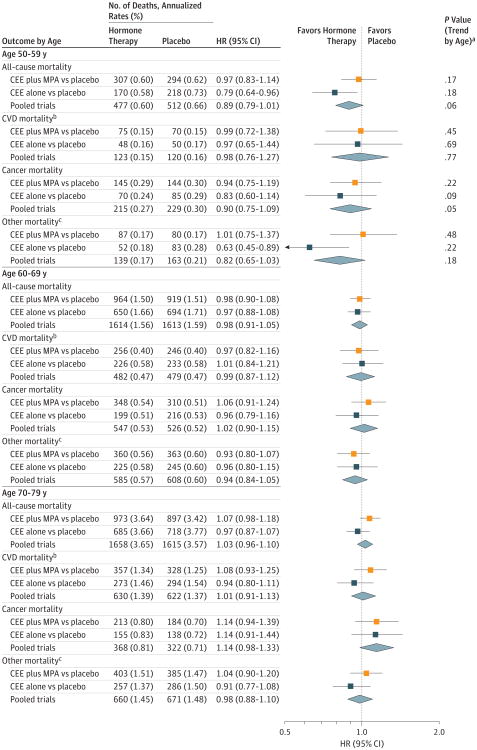
The HRs for all-cause mortality tended to differ by age during the intervention and cumulative follow-up phases. Comparing younger women (aged 50-59 years) with older women (aged 70-79 years), the ratios of nominal HRs for all-cause mortality in the pooled cohort were 0.61 (95% CI, 0.43-0.87) during the intervention phase and 0.87 (95% CI, 0.76-1.00) during the cumulative 18-year follow-up, without significant heterogeneity between trials (eTable 4 in Supplement 2). Comparing hormone therapy with placebo, the HRs in the pooled cohort during the intervention phase were 0.69 (95% CI, 0.51-0.94) for women aged 50 to 59 years, 1.04 (95% CI, 0.87-1.25) for women aged 60 to 69 years, and 1.13 (95% CI, 0.94-1.36) for women aged 70 to 79 years (P value for trend by age = 0.01) (Figure 3; eTable 2 in Supplement 2). The trends by age were not statistically significant during cumulative follow-up (HR, 0.89 [95% CI, 0.79-1.01] for women aged 50 to 59 years; HR, 0.98 [95% CI, 0.91-1.05] for women aged 60 to 69 years; and HR, 1.03 [95% CI, 0.96-1.10] for women aged 70 to 79 years; P value for trend = 0.06) (Figure 4; eTable 1 in Supplement 2).
Figure 3. Mortality Outcomes During the Intervention Phase According to 10-Year Age Groups at Randomization.
Reported values indicate the duration of follow-up for the intervention phase (median, 5.6 [interquartile range {IQR}, 4.9-6.5] years in the conjugated equine estrogens [CEE] plus medroxyprogesterone acetate [MPA] trial; median, 7.2 [IQR, 6.5-8.2] years in the CEE-alone trial; and median, 6.3 [IQR, 5.3-7.3] years in the pooled analysis). Age groups indicate participant ages at randomization. HR indicates hazard ratio.
aP values based on a test for trend of interaction between the randomization group and the age group.
bCardiovascular disease (CVD) mortality includes deaths due to myocardial infarction, coronary heart disease, stroke, heart failure, peripheral vascular disease, venous thromboembolism, and other major causes of CVD death.
c Indicates mortality outcomes not due to CVD or cancer.
Figure 4. Mortality Outcomes During the 18-Year Cumulative Follow-Up According to 10-Year Age Groups at Randomization.
The 18-year follow-up is cumulative, indicating the intervention plus extended postintervention phases of the 2 trials (median, 17.7 [interquartile range {IQR}, 16.6-18.6] years in the conjugated equine estrogens [CEE] plus medroxyprogesterone acetate [MPA] trial; median, 17.7 [IQR, 16.5-18.7] years in the CEE-alone trial; and median,17.7 [IQR,16.6-18.6] years in the pooled analysis). HR indicates hazard ratio.
aP values based on a test for trend of interaction between the randomization group and the age group.
bCardiovascular disease (CVD) mortality includes deaths due to myocardial infarction, coronary heart disease (CHD), stroke, heart failure, peripheral vascular disease, venous thromboembolism, and other major causes of CVD death.
c Indicates mortality outcomes not due to CVD or cancer.
Cardiovascular Mortality
Cumulative Follow-up Phase
During cumulative follow-up, 2456 deaths from CVD occurred in the pooled cohort. CVD mortality was 8.9% in the hormone therapy group vs 9.0% in the placebo group (HR, 1.00 [95% CI, 0.92-1.08]; P = .98), without differences between trials. The HRs in the pooled cohort for coronary heart disease mortality (0.97 [95% CI, 0.86-1.09]; P = .60) and stroke mortality (1.06 [95% CI, 0.90-1.24]; P = .47) did not differ significantly between hormone therapy and placebo groups or between trials (Figure 2; eTable 1 in Supplement 2).
Intervention and Postintervention Phases
Neither treatment was significantly associated with CVD mortality during the intervention phase (eFigure and eTable 2 in Supplement2). Compared with placebo, women in the CEE plus MPA group had an HR of 1.08 (95% CI, 0.78-1.48; P = .65) and women in the CEE-alone group had an HR of 1.01(95% CI, 0.78-1.31; P = .95). Similarly, the HRs for deaths due to coronary heart disease, stroke, and other known CVD causes were not significantly increased or decreased in either trial during the intervention (eFigure and eTable 2 in Supplement 2) or postintervention phases (eTable 3 in Supplement 2).
Age-Stratified Analyses
No statistically significant trends for CVD with age were observed in either trial during any study phase (Figure 3 and Figure 4; eTables 1-3 in Supplement 2).
Cancer Mortality
Cumulative Follow-up Phase
During cumulative follow-up, 2207 deaths from cancer occurred in the pooled cohort. Cancer mortality rates and HRs in the pooled cohort were similar between the intervention group (8.2%) and the placebo group (8.0%) (HR, 1.03 [95% CI, 0.95-1.12]; P = .50) (Figure 2; eTable 1 in Supplement 2). Mortality was8.3% for CEE plus MPA vs 7.9%for placebo (HR, 1.06 [95% CI, 0.95-1.18]; P = .31) and it was 8.0% for CEE vs 8.1% for placebo (HR, 0.99 [95% CI, 0.86-1.13]; P = .86). For breast cancer mortality, the HRs for cumulative follow-up were 1.44 (95% CI, 0.97-2.15; P = .07) for CEE plus MPA and 0.55 (95% CI, 0.33-0.92; P = .02) for CEE alone; due to heterogeneity between risk estimates (P value for heterogeneity = 0.003), we did not pool these analyses. HRs for deaths from colorectal cancer or other cancers were not statistically significant in either trial (Figure 2).
Intervention and Postintervention Phases
Neither intervention was significantly associated with total cancer mortality (eFigure, eTable 2, and eTable 3 in Supplement 2). During the intervention phase, women receiving CEE plus MPA had an HR of 1.10 (95% CI, 0.86-1.42; P = .44) and those receiving CEE alone had an HR of 0.96 (95% CI, 0.75-1.22; P = .72), compared with placebo. For breast cancer mortality, the HR was 1.08 (95% CI,0.29-4.03;P = .91) for CEE plus MPA and the HR was 0.45 (95% CI, 0.14-1.46; P = .17) for CEE alone, but number of deaths was small; differences became more pronounced during the postintervention phase (eTable 3 in Supplement 2). The interventions were not significantly associated with colorectal cancer or other cancer mortality (eFigure, eTable 2, and eTable 3 in Supplement 2).
Age-Stratified Analyses
In the pooled cohort, the HR for total cancer mortality during the intervention phase was 0.74 (95% CI, 0.48-1.14) for women aged 50 to 59 years and ranged from 1.00 (95% CI, 0.77-1.28) for women aged 60 to 69 years to 1.24 (95% CI, 0.93-1.66) for women aged 70 to 79 years (P value for trend by age = 0.05; Figure 3). These age trends persisted over cumulative follow-up (P value for trend by age = 0.05 for the pooled cohort Figure 4; eTable 1 in Supplement 2). Over cumulative follow-up, HRs for colorectal cancer were elevated for the women aged 70 to 79 years in the CEE-alone trial (HR = 2.13 [95% CI, 1.10-4.12]; P value for trend by age = 0.03; eTable 1 in Supplement 2).
Other Mortality
Cumulative Follow-Up Phases
The HR in the pooled cohort for other (non-CVD, noncancer) mortality (n = 2826 deaths) did not differ between the hormone therapy group (10%) and the placebo group (10.7%) (HR, 0.95 [95% CI, 0.88-1.02]; P = .14). When examined by specific major causes, deaths from Alzheimer disease and other dementia during cumulative follow-up were 2.5% for hormone therapy vs 3.0% for placebo in the pooled cohort (HR, 0.85 [95% CI, 0.74-0.98]; P = .03), 2.6% vs 2.9% for CEE plus MPA vs placebo (HR, 0.93 [95% CI, 0.77-1.11]; P = .42), and 2.4% vs 3.2% for CEE vs placebo (HR, 0.74 [95% CI, 0.59-0.94]; P = .01) (Figure 2; eTable 1 in Supplement 2). HRs for deaths from COPD, accidents or injuries, and other causes were not significantly increased or decreased during cumulative follow-up in either trial (Figure 2).
Intervention and Postintervention Phases
During the intervention phase, the HR for other (non-CVD, noncancer) causes of mortality was 0.59 (95% CI, 0.39-0.90; P = .01) for CEE plus MPA vs placebo but did not differ by treatment group in the CEE-alone trial. Regarding specific causes of death, there were fewer deaths from COPD during the CEE plus MPA intervention (eFigure in Supplement 2), but the number of events was small. During the postintervention phase, a lower risk of mortality from dementia emerged in the pooled cohort (HR, 0.85 [95% CI, 0.74-0.99]; P = .03) and in the CEE-alone trial (HR, 0.73 [95% CI, 0.58-0.92]; P = .008) (eTable 3 in Supplement 2).
Age-Stratified Analyses
HRs for other (non-CVD, noncancer) mortality during the intervention phase were similar across age groups for CEE plus MPA but lower for younger women than for older women in the CEE trial (P value for trend by age = 0.002) (Figure 3; eTable 2 in Supplement 2). However, numbers of events of individual outcomes (COPD, dementia, injuries or accidents, other) were too small to assess cause-specific differences by age during the intervention. During cumulative follow-up, lower risks of non-CVD, noncancer mortality persisted among women aged 50 to 59 years in the CEE-alone trial (HR = 0.63; 95% CI, 0.45-0.89), although the trend for age was no longer significant (P value for trend = 0.22) (Figure 4).
There were fewer deaths from COPD among women aged 50 to 59 years in the CEE trial (eTable 1 in Supplement 2), but the number of events was small.
Additional Analyses
In additional sensitivity analyses censoring participants who took less than 80% of study pills (active or placebo) during the first 2 years, results were generally similar to intention-to-treat results, but statistical power was reduced. For cumulative follow-up, the HR for all-cause mortality was 1.03 (95% CI, 0.95-1.11) for the CEE plus MPA trial and 0.94 (95% CI, 0.86-1.03) for CEE alone. The pattern suggestive of lower mortality risks for younger women when compared with older women during the intervention phase persisted in these analyses (P value for trend by age = 0.05 in pooled analyses), but age trends were not statistically significant during cumulative follow up. In analyses of all-cause and cause-specific mortality with stratification by time since menopause, results were generally similar to the age-stratified analyses, but statistical power was lower due to missing age at menopause for many participants. In addition, sensitivity analyses adjusted for time-varying differences in initiation of statins between treatment group,9 had no appreciable effect on the results.
Discussion
During cumulative 18-year follow-upamong27 347 postmenopausal women in the WHI hormone therapy trials, CEE plus MPA and CEE alone were not associated with increased or decreased risk of all-cause, cardiovascular, or total cancer mortality. Based on 7489 cumulative deaths over 18 years (1088 deaths during the intervention phase and 6401 deaths during postintervention follow-up [Figure 1]; >98% ascertainment of mortality), HRs for all-cause mortality in the hormone therapy group vs the placebo group were 0.99 (95% CI, 0.94-1.03; P = .60) in the overall pooled cohort, 1.02 (95% CI, 0.96-1.08; P = .51) with CEE plus MPA, and 0.94 (95% CI, 0.88-1.01; P = .11) with CEE alone (Table). HRs for all-cause mortality also did not differ between the hormone therapy and placebo groups during the intervention phases of the trials. When examined by 10-year age group (based on age at randomization) comparing women aged 50 to 59 years to those aged 70 to 79 years, the ratios of nominal HRs for all-cause mortality in the pooled cohort were 0.61 (95% CI, 0.43-0.87) during the intervention phase and 0.87 (95% CI, 0.76-1.00) during cumulative 18-year follow-up (eTable 4 in Supplement 2), without significant heterogeneity between trials. The trend by age group was statistically significant during the intervention phase but not during the cumulative follow-up period.
Results for cause-specific mortality should be interpreted cautiously due to multiple comparisons. Although younger women (aged 50-59 years) tended to have lower HRs than older women for mortality due to CVD, cancer, and other (non-CVD, noncancer) causes during the intervention phases of the 2 trials, only the latter outcome in the CEE-alone trial showed a statistically significant trend with age (P value for trend by age = .002), partially influenced by adverse effects of CEE in women aged70to79years. During cumulative follow-up, trends in cause-specific mortality across age groups were not statistically significantly different. For non-CVD, noncancer mortality, the significantly reduced risk among younger women in the pooled trials during the intervention phase (HR, 0.52 [95% CI, 0.28-0.97]; P value for trend by age = .01; eTable 2 in Supplement 2) appeared attributable to small reductions in several outcomes rather than a marked reduction in a single outcome; a risk reduction persisted only for CEE during cumulative follow-up. The observed reduction in deaths from Alzheimer disease or other dementia in the CEE trial and pooled cohort requires particular caution in view of findings of adverse effects of these interventions on cognitive function and incident dementia in the WHI Memory Study.10,11 Although rates of dementia mortality in WHI are well aligned with age-specific national statistics,12 deaths from dementia can be underreported, and competing risks from other causes of death or confounding cannot be excluded. It is unknown whether favorable effects of hormone therapy on insulin resistance and diabetes13,14—major determinants of cognitive decline—15,16 contributed to these findings.
In view of the complex balance of benefits and risks of hormone therapy, the all-cause mortality outcome provides an important summary measure, representing the net effect of hormone therapy use for 5 to 7 years on life-threatening outcomes. Previous WHI reports have focused on incident diagnoses such as coronary heart disease, stroke, breast cancer, hip fracture, and other major outcomes—all of which are serious but predominantly nonfatal and led to fewer than half of the deaths in the cohort. The current analyses include a large number of deaths during 18 years of follow-up (4354 deaths in the CEE plus MPA trial and 3135 in the CEE trial). Given the hormone therapy-related health risks identified in the CEE plus MPA trial2,6,10 and the CEE-only trial3,6, it is noteworthy that no elevations in all-cause mortality were found during either the intervention or cumulative follow-up phases of these trials. Although these findings lend support to practice guidelines endorsing use of hormone therapy for recently menopausal women with moderate-to-severe symptoms, in the absence of contraindications,17-19 the attenuation of age differences with longer follow-up and potential health risksoftreatment6,20,21 would not support use of hormone therapy for reducing chronic disease or mortality. Moreover, it is unclear whether benefits would outweigh risks with longer duration of treatment. In clinical decision making, these considerations must be weighed against the evidence linking untreated vasomotor symptoms in midlife women to impaired health and quality of life, disrupted sleep, reduced work productivity, and increased health care expenditures.17,22,23
No other randomized clinical trial of hormone therapy, to our knowledge, has been large enough to assess a potential modifying effect of age on all-cause mortality, and most previous trials of chronic disease outcomes have focused on older women.17,24 Observational studies, which include primarily women who initiate hormone therapy in early menopause, have generally demonstrated lower mortality among women using hormone therapy compared with nonusers,25-28 although few studies have distinguished between estrogen alone and combination estrogen plus progestin. HRs in most large cohort studies have ranged from 0.40 to 0.80,25-28 but such studies may be susceptible to several potential sources of confounding.17,29 Regarding cause-specific mortality, the most marked risk reductions reported in observational studies have been for coronary or CVD deaths. The potential influence of age on the relation between hormone therapy and vascular disease has received considerable attention, including divergent effects of hormone therapy on atherosclerotic lesions in early vs late menopause.30-33
Total cancer mortality did not differ significantly between intervention and placebo groups in either trial despite the increased incidence of breast cancer with CEE plusMPA34 and concerns about an increased risk of hormone-sensitive cancers with both regimens.29 Hormone therapy has a complex relationship with cancer. Although a significant reduction in breast cancer was seen with CEE,35,36 a significant increase in breast cancer incidence with CEE plus MPA has been documented.6,37 Divergent findings for CEE alone and CEE plus MPA for breast cancer point to an adverse effect of progestin on the breast epithelium,38 but progestins have been linked to favorable effects on the endometrium and a decreased risk of endometrial cancer became apparent with long-term follow-up of the CEE plus MPA trial.6,39 Moreover, these regimens did not appear to alter mortality outcomes for other cancer sites, including lung cancer, and had no significant effect on total cancer incidence.6
Several limitations of this study warrant consideration. Only1 dose, formulation, and route of administration in each trial was assessed; thus, results are not necessarily generalizable to other hormone preparations. The greater than 98% follow-up through the NDI obviates many of the concerns of previous WHI reports covering postintervention follow-up; virtually all cohort deaths are captured in these analyses due to the NDI searches. Nonetheless, specificity of cause of death may vary across outcomes. Finally, the nominal P values presented here should be interpreted cautiously, as multiple outcomes and subgroups were examined. Thus, cause-specific mortality analyses should be considered exploratory.
Conclusions
Among postmenopausal women in WHI, hormone therapy with CEE plus MPA for a median of 5.6 years or CEE for a median of 7.2 years was not associated with risk of all-cause, cardiovascular, or total cancer mortality during a cumulative follow-up of 18 years.
Supplementary Material
Key Points.
Question
What is the relationship between use of menopausal hormone therapy vs placebo for 5 to 7 years and mortality over 18 years of follow-up?
Findings
Among postmenopausal women who participated in 2 parallel randomized trials of estrogen plus progestin and estrogen alone, all-cause mortality rates for the overall cohort in the pooled trials were not significantly different for the hormone therapy groups vs the placebo groups (27.1% vs 27.6%; hazard ratio, 0.99 [95% CI, 0.94-1.03]).
Meaning
Menopausal hormone therapy for 5 to 7 years was not associated with risk of long-term all-cause mortality.
Acknowledgments
Funding/Support: The Women's Health Initiative is funded by the NHLBI, NIH, and the US Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. Wyeth Ayerst donated the study drugs.
Role of the Sponsor: The Women's Health Initiative investigators and NHLBI representatives had a role in the design and conduct of the study; interpretation of the data and data collection; management, analysis, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Drs Anderson and Prentice report receipt of a grant from the National Heart, Lung, and Blood Institute (NHLBI) during the conduct of the study. Dr LaCroix reports receipt of grants from NHLBI and the National Institutes of Health (NIH) during the conduct of the study; receipt of personal fees from Sermonix for serving on a scientific advisory board and from Pfizer for a consultancy. Dr Chlebowski reports receipt of consulting fees or honoraria from Novartis, Genentech, Amgen, and Astra-Zeneca, Novo Nordisk, and Genomic Health; fees for participation in review activities from Pfizer; payment for lectures from Novartis; and payment for educational activities from Educational Concepts Group.Dr Howard reports receipt of other fees from NIH for a contract for field work. Dr Margolis reports receipt of grants from NHLBI and NIH. Dr Lewis reports receipt of grants to her institution from NIH during the conduct of the study. Dr Espeland reports receipt of grants from NHLBI during the conduct of the study. Dr Wactawski-Wende reports receipt of grants from NHLBI and NIH.No other disclosures were reported.
Additional Contribution: We thank the Women's Health Initiative investigators, staff, and trial participants for their outstanding dedication and commitment.
Glossary
- CEE
conjugated equine estrogens
- COPD
chronic obstructive pulmonary disease
- CVD
cardiovascular disease
- MPA
medroxy progesterone acetate
Footnotes
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT00000611
Author Contributions: Dr Manson and Mr Aragaki had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Manson, Aragaki, Rossouw, Prentice, Chlebowski, Howard, Lewis, Wactawski-Wende.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Manson, Aragaki, Chlebowski.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Manson, Aragaki, Prentice, Chlebowski.
Obtained funding: Manson, Rossouw, Anderson, Prentice, LaCroix, Chlebowski, Howard, Lewis, Stefanick, Johnson, Wactawski-Wende.
Administrative, technical, or material support: Manson, Rossouw, Prentice, Chlebowski, Margolis, Lewis, Jackson, Johnson, Wactawski-Wende.
Supervision: Manson, Chlebowski, Howard, Johnson, Wactawski-Wende.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Disclaimer: The opinions expressed in the manuscript are those of the study authors and do not necessarily represent the views of the Department of Health and Human Services/ National Institutes of Health.
Short List of Women's Health Initiative Investigators: Program Office: Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and NancyGeller (National Heart, Lung, and Blood Institute, Bethesda, Maryland).
Clinical Coordinating Center: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, Washington).
Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts); Barbara V. Howard (MedStar Health Research Institute/ Howard University, Washington, DC); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, California); Rebecca Jackson (The Ohio State University, Columbus); Cynthia A. Thomson (University of Arizona, Tucson/Phoenix); Jean Wactawski-Wende (University at Buffalo, Buffalo, New York); Marian Limacher (University of Florida, Gainesville/Jacksonville); Robert Wallace (University of Iowa, Iowa City/Davenport); Lewis Kuller (University of Pittsburgh, Pittsburgh, Pennsylvania); Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, North Carolina).
Women's Health Initiative Memory Study: Sally Shumaker (Wake Forest University School of Medicine, Winston- Salem, NC).
Additional Information: A full list of all the investigators who have contributed to Women's Health Initiative science appears at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
Contributor Information
JoAnn E. Manson, Division of Preventive Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts.
Aaron K. Aragaki, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington.
Jacques E. Rossouw, National Heart, Lung, and Blood Institute, Bethesda, Maryland.
Garnet L. Anderson, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington.
Ross L. Prentice, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington.
Andrea Z. LaCroix, Department of Family Medicine and Public Health, University of California, San Diego, School of Medicine, San Diego.
Rowan T. Chlebowski, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance.
Barbara V. Howard, MedStar Health Research Institute, Washington DC; Georgetown/Howard Universities Center for Clinical and Translational Sciences, Washington DC.
Cynthia A. Thomson, Department of Health Promotion Sciences, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson.
Karen L. Margolis, HealthPartners Institute for Education and Research, Minneapolis, Minnesota.
Cora E. Lewis, Division of Preventive Medicine, University of Alabama at Birmingham, Birmingham.
Marcia L. Stefanick, Stanford Prevention Research Center, Stanford University School of Medicine, Stanford, California.
Rebecca D. Jackson, Department of Medicine, The Ohio State University, Columbus.
Karen C. Johnson, Department of Preventive Medicine, University of Tennessee Health Science Center, Memphis.
Lisa W. Martin, Cardiology Division, George Washington University School of Medicine and Health Sciences, Washington DC.
Sally A. Shumaker, Department of Social Sciences and Health Policy, Wake Forest School of Medicine, Winston-Salem, North Carolina.
Mark A. Espeland, Department of Biostatistical Sciences, Wake Forest School of Medicine, Winston-Salem, North Carolina.
Jean Wactawski-Wende, Department of Epidemiology and Environmental Health, University at Buffalo, Buffalo, New York.
References
- 1.The Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 2.Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin inhealthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Anderson GL, Limacher M, Assaf AR, et al. Women's Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 4.Heiss G, Wallace R, Anderson GL, et al. WHI Investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 5.LaCroix AZ, Chlebowski RT, Manson JE, et al. WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 8.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am JEpidemiol. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 9.Manson JE, Shufelt CL, Robins JM. The potential for postrandomization confounding in randomized cinical trials. JAMA. 2016;315(21):2273–2274. doi: 10.1001/jama.2016.3676. [DOI] [PubMed] [Google Scholar]
- 10.Shumaker SA, Legault C, Rapp SR, et al. WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: theWomen's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 11.Shumaker SA, Legault C, Kuller L, et al. Women's Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services; Centers for Disease Control and Prevention. National Center for Health Statistics. Deaths: Final Data for 2013. [Accessed July 22, 2016];National Vital Statistics Report. 64(2) http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. [PubMed] [Google Scholar]
- 13.Margolis KL, Bonds DE, Rodabough RJ, et al. Women's Health Initiative Investigators. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women's Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 14.Bonds DE, Lasser N, Qi L, et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women's Health Initiative randomised trial. Diabetologia. 2006;49(3):459–468. doi: 10.1007/s00125-005-0096-0. [DOI] [PubMed] [Google Scholar]
- 15.Reijmer YD, van den Berg E, de Bresser J, et al. Utrecht Diabetic Encephalopathy Study Group. Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes Metab Res Rev. 2011;27(2):195–202. doi: 10.1002/dmrr.1163. [DOI] [PubMed] [Google Scholar]
- 16.Gorelick PB. Risk factors for vascular dementia and Alzheimer disease. Stroke. 2004;35(11) 1:2620–2622. doi: 10.1161/01.STR.0000143318.70292.47. [DOI] [PubMed] [Google Scholar]
- 17.North American Menopause Society. The 2012 hormone therapy position statement of: the North American Menopause Society. Menopause. 2012;19(3):257–271. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975–4011. doi: 10.1210/jc.2015-2236. [DOI] [PubMed] [Google Scholar]
- 19.Goodman NFCR, Cobin RH, Ginzburg SB, Katz IA, Woode DE American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of menopause. Endocr Pract. 2011;17(suppl 6):1–25. doi: 10.4158/ep.17.s6.1. [DOI] [PubMed] [Google Scholar]
- 20.Cirillo DJ, Wallace RB, Rodabough RJ, et al. Effect of estrogen therapy on gallbladder disease. JAMA. 2005;293(3):330–339. doi: 10.1001/jama.293.3.330. [DOI] [PubMed] [Google Scholar]
- 21.Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA. 2005;293(8):935–948. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 22.Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause. 2015;22(3):260–266. doi: 10.1097/GME.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 23.Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806. doi: 10.1056/NEJMp1514242. [DOI] [PubMed] [Google Scholar]
- 24.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women: Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 25.Grodstein F, Stampfer MJ, Colditz GA, et al. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336(25):1769–1775. doi: 10.1056/NEJM199706193362501. [DOI] [PubMed] [Google Scholar]
- 26.Bush TL, Cowan LD, Barrett-Connor E, et al. Estrogen use and all-cause mortality: preliminary results from the Lipid Research Clinics Program Follow-Up Study. JAMA. 1983;249(7):903–906. doi: 10.1001/jama.249.7.903. [DOI] [PubMed] [Google Scholar]
- 27.Henderson BE, Paganini-Hill A, Ross RK. Decreased mortality in users of estrogen replacement therapy. Arch Intern Med. 1991;151(1):75–78. [PubMed] [Google Scholar]
- 28.Folsom AR, Mink PJ, Sellers TA, Hong CP, Zhe W, Potter JD. Hormonal replacement therapy and morbidity and mortality in a prospective study of postmenopausal women. Am J Public Health. 1995;85(8 pt 1):1128–1132. doi: 10.2105/ajph.85.8_pt_1.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santen RJ, Allred DC, Ardoin SP, et al. Endocrine Society. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95(7) 1:s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 31.Writing Group on behalf of Workshop Consensus Group. Aging, menopause, cardiovascular disease and HRT: International Menopause Society Consensus Statement. Climacteric. 2009;12(5):368–377. doi: 10.1080/13697130903195606. [DOI] [PubMed] [Google Scholar]
- 32.Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53(3):605–619. doi: 10.1016/s0008-6363(01)00466-7. [DOI] [PubMed] [Google Scholar]
- 33.Hodis HN, Mack WJ, Henderson VW, et al. ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with Estradiol. N Engl J Med. 2016;374(13):1221–1231. doi: 10.1056/NEJMoa1505241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chlebowski RT, Kuller LH, Prentice RL, et al. WHI Investigators. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefanick ML, Anderson GL, Margolis KL, et al. WHI Investigators. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295(14):1647–1657. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 36.Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women's Health Initiative randomized clinical trials. JAMA Oncol. 2015;1(3):296–305. doi: 10.1001/jamaoncol.2015.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chlebowski RT, Anderson GL, Sarto GE, et al. Continuous Combined Estrogen Plus Progestin and Endometrial Cancer: The Women's Health Initiative Randomized Trial. J Natl Cancer Inst. 2015;108(3) doi: 10.1093/jnci/djv350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.